Abstract
Kyphoplasty has become a standard procedure in the treatment of painful osteoporotic compression fractures. According to current guidelines, involvement of the posterior wall of the vertebral body is a relative contraindication. From February 2002 until January 2008, 97 patients with at least one AO classification A 3.1 fracture were treated by kyphoplasty. There was a structured follow-up for the medium-term evaluation of the patients’ outcome. Ninety-seven patients (68 of whom were females and 29 of whom were males) with involvement of the vertebra’s posterior margin averaging 76.1 ± 12.36 (59–98) years were treated by kyphoplasty. The fractures of 75 patients were caused by falls from little height, 5 patients had suffered traffic accidents and in the case of 17 patients, no type of trauma was remembered. According to the AO classification, there were 109 A 3.1.1 and one A3.1.3 injuries. Prior to surgery, all patients were neurologically without pathological findings. Seventy-nine fractures were accompanied by a narrowing of the spinal canal [average of 15% (10–40)]. Overall, 134 vertebras were treated by Balloon kyphoplasty (81 × 1 segment, 22 × 2 segments, 3 × 3 segments). In 47.4% of the patients, cement leakage was observed after surgery. All patients with cement extravasation, however, were clinically unremarkable. Using the visual analog scale, patients stated that prior to surgery their pain averaged 8.1, whereas after surgery it significantly decreased and averaged 1.6 (p < 0.001). In geriatric patients with osteoporotic vertebral fractures with partial inclusion of the posterior wall of the vertebral body, kyphoplasty is an effective procedure with few complications.
Keywords: Kyphoplasty, Burst fracture, Posterior wall, Vertebroplasty, Vertebral compression fractures
Introduction
Vertebroplasty and kyphoplasty are minimally invasive accepted procedures in the therapy of painful osteoporotic compression fractures. According to the guidelines [1], vertebroplasty and kyphoplasty are indicated after inefficient conservative pain therapy in “stable” vertebral compression fractures. Involvement of the vertebra’s posterior wall is supposed to be a relative contraindication. If there is an involvement of the posterior wall, cement might leak into the spinal canal or the balloon might further dislocate bone fragments into the spinal canal. Both incidents could lead to neurological deficits. The relevance of Balloon kyphoplasty for the treatment of incomplete, fresh burst fractures remains unclear.
The purpose of this study is to evaluate the effectiveness of kyphoplasty in the treatment of osteoporotic burst fractures as well as to point out any possible risks.
Patients and methodology
Due to the great effectiveness of the kyphoplasty procedure in cases of osteoporotic stable fractures of the vertebras, our department has been treating patients with incomplete burst fractures and osteoporosis that did not show any neurological deficits using kyphoplasty since 2002. All patients undergoing vertebroplasty or kyphoplasty were prospectively documented in a standardized database.
The data of all 97 patients who suffered at least from one vertebral fracture with involvement of the posterior wall and who were only undergoing kyphoplasty between January 2002 and January 2008 in our department were retrospectively evaluated. The fractures were classified according to the AO classification.
The patients’ perioperative risks were documented and rated according to the ASA score (American Society of Anesthesiologists).
Intraoperative data (duration of surgery, exposure time to radiation, and the amount of cement applied) were also ascertained. Postoperatively and prior to discharge from the hospital, spinal radiographs were taken in two planes.
Particular attention was paid to the ascertainment of cement extravasation and to neurological deficits. In all patients, pain intensity was measured using the visual analog scale (VAS) prior to surgery as well as on the day of discharge. There was a structured follow-up for the medium-term evaluation of the patients’ clinical outcome that took place via telephone 20.2 ± 9.79 (5–48) months after surgery.
The demographic data of the patients with involvement of the posterior wall were compared with the data of the patients that been treated in our department with vertebral compression fractures without involvement of the posterior wall. This demographic data were supposed to serve as a control group.
For the descriptive statistics, means and standard deviations were calculated and minimum and maximum values were noted. Comparison within the groups was carried out with the Mann–Whitney U, exact Wilcoxon and χ2 test using the SPSS software.
Radiological inclusion criteria of the examined patient population
Initially, radiological diagnostics included conventional spinal radiographs in two planes. In cases of suspected spinal canal compromise, a CT was also performed, which, as long as there were no contraindications, was complemented by a magnet resonance imaging (MRI, STIR sequence). MRI was used to differentiate between acute and older fractures. Using the stabilization criteria, we have set the following radiological inclusion criteria for our examination:
- Involvement of the posterior wall of the vertebral body.
- Discontinuation of the cortical substance in the CT.
Clear involvement of the posterior wall of the vertebral body if there was no CT.
No fractures or injuries of the facet joints.
Spinal canal compromise <40%.
Edema of the bone shown in the MRI (STIR sequence).
Spinal radiographs in two planes were taken at baseline and after kyphoplasty. Height restoration of the vertebral bodies was assessed using the Beck index (comparing the height of the anterior face of the fractured vertebral body with that of the posterior face of the fractured vertebral body) and the local kyphotic angle was measured both before and after the surgical procedure.
Surgical technique
Kyphoplasty took place under general anesthesia. The reduction of the fractures was supported by positioning of the patients prone on a special mattress on the operating table to aggravate the lordosis. We used the bipedicular approach under fluoroscopic imaging with one image intensifier that was moved 90°.
Patient positioning during surgery is essential to achieve a partial height restoration and spontaneous reduction of the posterior fragment via ligamentotaxis. In addition, the positioning of the balloon is important to prevent any secondary fragment dislocation.
The objective was to place the balloons close to the injured superior endplate and/or the base plate in the anterior two-thirds of the vertebral body in order to perform a good repositioning of the endplate and to stabilize the anterior part of the vertebra. In contrast to the technique used in painful osteoporotic fractures that only insufficiently respond to conservative therapy and in which the amount of the cement used is not directly crucial for the pain therapy, we have tried to almost completely fill the anterior two-thirds of the vertebra with cement in order to achieve a good mechanical stability of the vertebra, which is in compliance with the three-column theory by Louis.
High balloon volumes were necessary to augment the vertebras. In order to avoid a peripheral cement leakage, it is essential to wait until the cement has become highly viscous.
Follow-up
Pain and neurological status were recorded before and after surgery as well as on the day of discharge from the hospital. Furthermore, there was a standardized telephone interview, which included the questionnaire suggested by Wiggins. The questions were either answered by the patients personally and, when that was impossible, by relatives or other people close to the patient. Apart from the patients’ subjective satisfaction, the questionnaire investigates the activity level and the need for pain medication. There were several possible answers provided in the different subgroups.
Results
Ninety-seven patients (68 females and 29 males) with fractured vertebras with involvement of the posterior wall were treated with kyphoplasty. The average age was 76.1 ± 12.36 (59–98) years (Table 1). There was partially a remarkable amount of comorbidities, which resulted in an average ASA score of 3 (ASA II = 19; ASA III = 60; ASA IV = 18). In 75 patients, the fractures were caused by falls from little height, 5 patients had suffered traffic accidents. Seventeen patients did not remember a trauma. According to the AO classification, there were 109 A 3.1.1 and one A3.1.3 injuries (Figs. 1, 2). In addition, 16 A1.2.1 and 8 A1.1 fractures were treated. Prior to surgery, no patient exhibited neurological deficits. 79 fractures (72.5%) included a constriction of the spinal canal [average of 15% ± 9.21 (10–40%)].
Table 1.
Demographics and statistical comparison between the two groups
| With involvement of the posterior wall | Without involvement of the posterior wall | Mann–Whitney U test (p) | χ² test (p) | |
|---|---|---|---|---|
| Age (years) | 76.1 ± 12.36 (59–98) | 74.5 ± 12.53 (43–93) | 0.419 | |
| Number of patients | 97 | 98 | ||
| Ratio females: males | 68:29 | 77:21 | 0.070 | |
| ASA score | 2.98 | 3.03 | ||
| Inpatient stay (days) | 11.3 ± 5.8 (2–37) | 10.2 ± 5.8 (4–40) | 0.051 | |
| Duration of surgery (min) | 46 ± 18.2 (25–86) | 43 ± 19.2 (21–95) | 0.636 | |
| Fluoroscopy (s) | 178 ± 108.79 (40–300) | 183 ± 123.01 (36–348) | 0.839 | |
| Cement volume (ml) | 8.62 ± 4.48 (3–20) | 8.23 ± 3.51 (2.5–14.5) | 0.807 | |
| Cement extravasation | 47.4% | 42.86% | 0.620 | |
| VAS preoperatively | 8.09 (±0.815) | 8.38 (±0.929) | 0.822 | |
| VAS postoperatively | 1.57 (±1.02) | 1.84 (±0.968) | 0.10 |
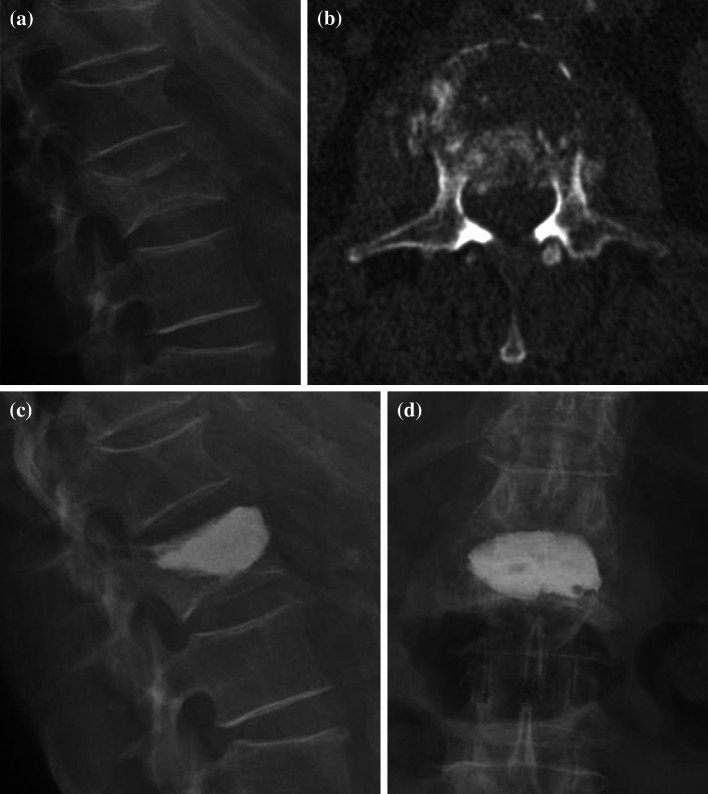
Fig. 1.
Incomplete burst fracture of the second lumbar vertebral body vertebra a initial X-rays lateral, b computed tomography axial plane showing the damage of the posterior wall and narrowing of the spinal canal of approximately 30%, c, d X-rays in two planes after kyphoplasty
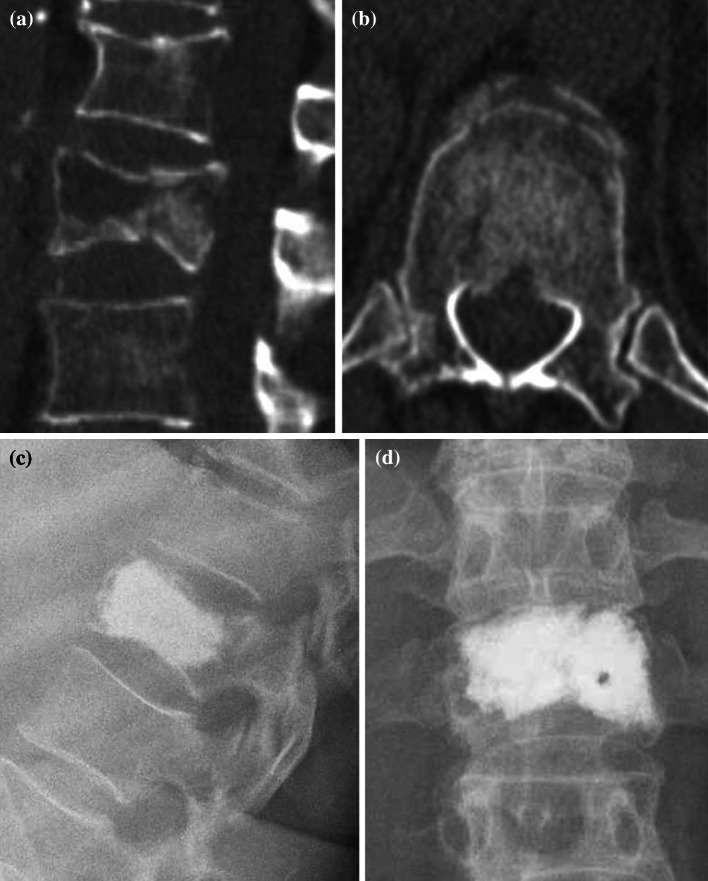
Fig. 2.
Burst fracture of the first vertebra a computed tomography sagittal plane, b computed tomography axial plane showing the damage of the posterior wall and narrowing of the spinal canal of approximately 15%, c, d X-rays in two planes after Kyphoplasty
One hundred thirty-four vertebras (1.38 per patient) were augmented (81 × 1 segment, 22 × 2, 3 × 3 segments). The procedures were performed or assisted by three trained surgeons. 70% of the fractures were located in the area of the thoracic–lumbar junction. The surgical procedure lasted 46 ± 18.22 (25–86) min, the duration of fluoroscopy was 148 ± 108.8 (40–300) s. On average, 8.62 ± 4.5 (3–20) ml of cement (KyphX HV-R©) was injected into the vertebras.
Complications
Postoperatively, it became necessary to release a subcutaneous hematoma in two cases. Both patients were clinical inconspicuous. The ultrasound scan showed subcutaneous hematomas extending 2 × 2 × 4 cm and 3 × 2 × 5 cm. The wound closure was removed early postoperative on days 3 and 5 under sterile conditions. After evacuation of the hematomas and flushing of the cavity, the wound was closed again and healed without complications in both cases. During their hospital stay, six patients were diagnosed with a urinary tract infection. Two patients died during their hospital stay 12 and 14 days after surgery. One patient’s death was caused by a mitral valve endocarditis; another patient’s death was caused by a cardiac insufficiency NYHA 4 and a severe obstructive pulmonary disease. Both deaths were not related to the operative procedure.
The inpatient treatment lasted an average of 11.3 ± 5.84 (2–37) days. Thirty-three patients were transferred for inpatient rehabilitation. On average, the VAS value decreased from 8.1 ± 0.815 prior to surgery to 1.6 ± 1.02 (p < 0.005) on the day of discharge (Nineteen patients were not able to score their pain according to the VAS, e.g. dementia.). These patients were also excluded from the follow-up group.
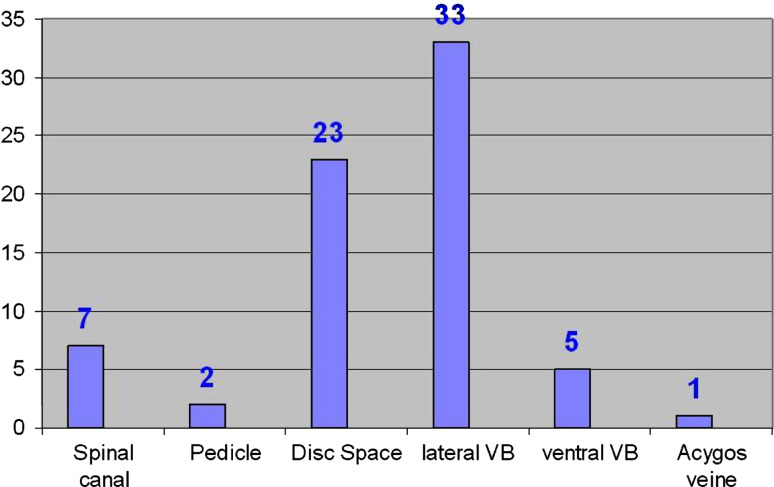
In 47.4% of the patients, cement leakage outside the vertebras [23× intervertebral disk spaces, 7× basivertebral plexus, 38× paravertebral, 2× pedicle, 1× v. renalis (Fig. 3)] was detected on the postoperative radiographs. All cases of extravasation were clinically unremarkable. The Beck index increased from 0.808 ± 0.182 (0.4–1.12) before kyphoplasty to 0.875 ± 0.118 (0.5–1.03) (p < 0.005) after surgery. The local kyphotic angle measured both before and after the surgical procedure decreased from 8.53° ± 6.30 (−5° to −27°) to 4.77° ± 3.97 (−2° to 14°) (p < 005). There was no radiologic follow-up since we decided to collect follow-up data via telephone interview.
Fig. 3.
Localization of the cement extravasations (multiple locations possible)
During the telephone interviews, we were able to collect information from 90 out of 97 patients. At the time of follow-up, 13 patients had died (13.4%). 19 other patients (19.6%) were not able to answer the interview due to demential illnesses. In those cases, we interviewed the patients’ relatives and/or care givers at the respective nursing homes.
In regard to pain reduction, 35% of the patients were free of pain. In 56.5% of the cases, the pain had decreased manifestly. Overall, 91.5% of the patients profited from the kyphoplasty. At the time of the follow-up, however, 8.5% of the patients were still not free of pain. The activity level of 69.8% of the patients was either the same or higher than compared to prior to the fracture. 30.2% of the patients were not able to achieve the same activity level as prior to the fracture.
When asked about their subjective satisfaction with the treatment, 84.9% of the respondents declared that they were very satisfied and 11.6% were moderately satisfied with the treatment. 3.5% of the respondents were dissatisfied with the treatment.
There was no standardized radiological follow-up of the patients. Five patients returned because of new back pain. Five patients (5.1%) started suffering from a new, painful fracture that was successfully treated performing kyphoplasty. There was only one fracture directly beside the vertebra that was initially treated. The other symptomatic fractures were satellite fractures and at least two segments away from the augmented vertebras.
The results of the patients suffering from a fracture with involvement of the posterior wall were compared to the ones with no involvement of the posterior wall. At the same time, between January 2002 and January 2008, 98 patients averaging 74.46 ± 12.53 (43–92) years were treated. According to the AO, there were 37 superior endplate impression fractures and 92 wedge compression fractures A 1.2.1. After conservative therapy, all patients continued suffering from persistently strong pain. The average ASA score was 3.03. In 42.86% of the patients, there were cement extravasations. The difference between the cement extravasation in both groups was insignificant (p = 0.662). After surgery, the pain score on the VAS was reduced to 1.84 (±0.968) compared to 8.38 (±0.929) (p < 0.005) preoperatively.
Discussion
For several years, vertebroplasty and kyphoplasty have been successfully used in the treatment of painful osteoporotic compression fractures of the vertebras. According to the criteria of evidence-based medicine, there is only one level Ib evidence [22] that shows the benefits of kyphoplasty compared to conservative treatment especially in the early course after osteoporotic fractures. In addition, in several systematic reviews, it was possible to observe the efficacy in regard to a significant pain reduction in 87% of the patients undergoing vertebroplasty and in 92% of the patients undergoing kyphoplasty [6]. Overall, the complication rate of both procedures is considered to be low [6, 17, 18]. Main complication is the leakage of cement occurring in 41% of the vertebroplasties and in up to 30% of the kyphoplasties [6].
In cases of fractures involving the posterior wall of the vertebral body, there is a potential risk of cement flowing along the fracture line into the spinal canal and that the inflation of the balloon might dislodge retropulsed bone fragments dorsally into the spinal canal, which could result in neurological deficits.
The fractures were classified according to the AO classification [14]. Table 2 provides an overview over the classification of burst fractures. The AO classification can only be used to a certain extent in the case of acute fractures caused by osteoporosis. The classification does, however, provide a good description of the fractures’ morphology. In patients with osteoporotically changed vertebras, fractures can be caused by considerably lower force than in young patients where more force is needed.
Table 2.
Classification of burst fractures according to the AO
| Burst fracture according to AO | |
|---|---|
| A3 | |
| A 3.1 Incomplete burst fracture | A 3.1.1 cranial incomplete |
| A 3.1.2 lateral incomplete | |
| A 3.1.3 caudal incomplete | |
| A 3.2 Burst-split fracture | A 3.2.1 cranial |
| A 3.2.2 lateral | |
| A 3.2.2 caudal | |
| A 3.3 Complete burst fracture | A 3.3.1 Pincer burst fracture |
| A 3.3.2 Flexion burst fracture | |
| A 3.3.3 Axial burst fracture | |
Osteoporotic (pathological) fractures
Almost all trauma-related fracture classifications, especially the one established by the AO primarily targets the classification of fresh traumatic injuries. Classifying osteoporotic fractures, which generally occur without reason or which cannot be remembered, is more difficult. The involvement of the posterior wall is a hint for instability.
Genant et al. [4] suggest a semi-quantitative, morphological classification of osteoporotic compression fractures. The author differentiates between three main groups (wedge, biconcave und crush deformity), which according to their plastic deformity is grouped into mild (20–25%), moderate (25–40%) and severe (>40%). Fractures of the vertebral body with involvement of the posterior wall cannot be categorized according to Genant.
Faciszewski and McKiernan [3] suggest a classification adapted to vertebroplasty, which apart from the fracture’s morphology also includes the fracture’s age (acute < 3 months, chronic > 3 months), the reparative activity (presence of an edema in the MRI), the dynamic mobility (differences when standing up or lying down), discontinuation of the trabeculae or a vacuum phenomenon in the vertebras as well as the presence of involvement of the posterior wall.
We categorized the osteoporotic fractures according to the AO classification (Table 2) being aware that the classification has shortcomings in osteoporotic compression fractures.
Treatment of thoracic–lumbar fractures remains controversial. Different types of therapies are favored in the treatment of acute traumatic injuries, which in younger patients is usually a sign of crude violence. Current systematic reviews document the low evidence level of the studies that can be used for the determination of treatment strategies [19, 20]. In patients with burst fractures without neurological deficits, long-term results according to the criteria of evidence-based medicine in the group of surgically treated patients are not necessarily superior to the one of patients treated conservatively [16]. Wood et al. [24] were not able to show better long-term results in the surgical group. In a prospective randomized multi-center study, Siebenga et al. [15] were able to point out a better functional outcome in the group of patients treated with dorsal instrumentation.
There is also no agreement on what type of surgical stabilization should follow. In the study performed by the Task Force “Wirbelsäule” (spinal column) of the Deutsche Gesellschaft für Unfallchirurgie (German Society of Trauma Surgery), heterogeneity was shown in the types of approaches as well as in the implants used. The complication rates (recessing and non-recessing complications) of the different procedures varied between 14.1 and 29.7% [14, 15]. The average age of the patients participating in the study of the workshop “Wirbelsäule” of the Deutsche Gesellschaft für Unfallchirurgie was 39.5 years.
Using stand-alone kyphoplasty for the treatment of cranial burst fractures in younger patients is not a standard procedure. Preliminary results from small series such as the ones from Maestretti et al. [13] are very promising. Maestretti also reported about the use of calcium phosphate in addition to ballon kyphoplasty in young patients. Dorsal instrumentation, possibly combined with ventral vertebral fusion, remains the standard treatment in cases of fresh traumatic burst fractures in young patients.
The treatment objective in older patients has to be defined differently than the one in young patients. In cases of limited activity level and limited life expectancy, the physiological axial skeleton is less important than a fast mobilization that is low in risk as well as in pain. Another goal is a prompt reintegration in the outer environment the patient is used to. In old as well as very old patients, conservative treatment is also recommended in cases of strong defective positions in order to avoid the patient being exposed to surgical complications. Furthermore, the surgical treatment possibilities are also limited due to a decrease in the bones’ quality. Dorsal stabilizations partly require extensive exposure of the spine and sometimes an additional cement augmentation of the transpedicular screws. Due to the general risks of patients averaging 76 years and an average ASA score of 3, higher complication rates than the one described by Knop et al. [7] can be anticipated.
Postoperatively, the pain intensity, measured on the VAS, which prior to surgery amounted to an average of 8.1 ± 0.815, decreased significantly immediately to 1.6 ± 1.02 (p < 0.005) after surgery. No sensomotoric deficits or radicular neurological symptoms were observed.
Compared to the complications in dorsal or ventral procedures, the postoperative rate of complications that had to be revised in our collective was quite low. There were two patients that required evacuation of a hematoma.
The main complication in the patients we treated was the high rate of cement extravasation (47.4%). The average rate of cement leakage after kyphoplasty is quoted to be 9% [6], whereas some authors experienced in kyphoplasty state a percentage of up to 33% [2, 8, 21]. The reason for 47.4% leakage could be the cementing technique we used. We believe that besides pain reduction, the objective of a kyphoplasty is a mechanical stability of the anterior two-thirds of the vertebral body. Louis [12] defines an anterior column as corresponding to the vertebral bodies and two posterior columns corresponding to the facet joints, which together carry the load. In contrast to the two-column model of the AO, the posterior column has also a carrying function.
In order to achieve a mechanical stability in the area of the anterior column, it is necessary to almost completely fill the vertebral bodies with cement. The vertebras’ posterior wall has to be looked after exceedingly to avoid dorsal cement extravasation. As long as the cement does not leak dorsally, cement can continue being injected carefully. If lateral cement extravasation or leaking into the intervertebral space has occurred, further filling is stopped. All cement extravasations we observed were clinically irrelevant, which corresponds to the data provided in the literature [9]. We did not use the Eggshell technique described by Greene et al. [5], which includes another balloon placement once the cement has been inserted in order to distribute the cement more viscously. Using this technique, however, might have reduced the amount of extravasations.
There are different types of cements that can be used for kyphoplasty and vertebroplasty [10]. PMMA (polymethylmethacrylate) and calcium phosphate cements are the main groups. Compared to the PMMA, the calcium phosphate cements are not as mechanically firm [23] as PMMA and there is a lack of long-term observations, both of which are considered to be disadvantages. For years, PMMA has been successfully used in the fields of orthopedics and traumatology. In our patients that averaged an age of 76.1 years, we almost exclusively used PMMA. We used calcium phosphate (KyphOS) in three younger patients (<50 years). The idea of an ideal mechanically stable, osteoinductive and non-toxic cement is still intensely researched. Until then, PMMA cements can continue to be used safely in older patients.
There is a discussion about an increased risk of follow-up fractures after a kyphoplasty. In case of our patients, there was no standardized radiological follow-up, since an additional exposure to radiation in cases of symptom-free patients did not seem to be indicated. We had to treat six new painful vertebral fractures. The relative risk of patients suffering another fracture in the first year after the initial vertebra fracture is 20% [11]. A prospective randomized study [1] that studied whether following fractures appear less often, if bordering, not fractured vertebras are also treated with kyphoplasty had neither a positive nor a negative effect in regards to following fractures.
Possible points of critique of this present study are that the evaluation took place retrospectively, by means of a prospective data base and that the follow-up did not take place at the hospital on clearly defined days, but cross-sectionally via telephone. We made the conscious decision of undertaking an interview via telephone since clinical follow-ups are often associated with transporting the patients via ambulance and the additional information that we could have gained through the clinical examination would not have been relevant in this case. As described above, our treatment objectives in old patients were defined as follows:
Fast, low-risk, painless mobilization.
Prompt reintegration into the habitual outer environment.
Avoiding extensive surgeries.
The deciding factors in the follow-up were items that were easy to inquire about by telephone: satisfaction with the treatment, pain reduction, reintegration into the patient’s normal environment and recovery of mobility. Defective positions, loss of reposition and asymptomatic following fractures were not evaluated in this study, since they play a minor role in the elderly patients we treated.
Thirty-five percent of the patients were pain free. 56.5% of the patients reported a significant reduction of pain. Overall, 91.5% of the patients clearly profited from the kyphoplasties performed. Those numbers coincide with the general experience [18] with kyphoplasty and underline the immediate efficacy of the kyphoplasty in regard to a reduction in pain. 8.5% of the patients continued suffering from pain at the time of the follow-up, but it did not cause the patients to return to the clinic.
Conclusion
In cases of limited activity level and limited life expectancy, the restoration of the physiological axial skeleton is less important than a fast mobilization. Another goal is a prompt reintegration into the outer environment the patient is used to.
Kyphoplasty is also an effective, low-risk procedure for geriatric patients with osteoporotic vertebral fractures with partial inclusion of the posterior wall of the vertebral body. Pain reduction is achieved immediately after surgery. Patient satisfaction is very high.
We were not able to observe suspected neurological complications in our patients. Main complications included asymptomatic cement extravasation, which were caused by the cementation technique and not by the procedure itself.
References
- 1.Becker S, Garoscio M, Meissner J, Tuschel A, Ogon M (2007) Is There an Indication for Prophylactic Balloon Kyphoplasty? A Pilot Study. Clin Orthop Relat Res 458:83–89 [DOI] [PubMed]
- 2.Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomized study. Eur Spine J. 2004;13:496–501. doi: 10.1007/s00586-004-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faciszewski T, McKiernan F. Calling all vertebral fractures classification of vertebral compression fractures: a consensus for comparison of treatment and outcome. J Bone Miner Res. 2002;17:185–191. doi: 10.1359/jbmr.2002.17.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 5.Greene DL, Isaac R, Neuwirth M, Bitan FD. The eggshell technique for prevention of cement leakage during kyphoplasty. J Spinal Disord Tech. 2007;20:229–232. doi: 10.1097/01.bsd.0000211276.76024.30. [DOI] [PubMed] [Google Scholar]
- 6.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 7.Knop C, Bastian L, Lange U, Oeser M, Zdichavsky M, Blauth M. Complications in surgical treatment of thoracolumbar injuries. Eur Spine J. 2002;11:214–226. doi: 10.1007/s00586-001-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane JM, Hong R, Koob J et al. (2004) Kyphoplasty enhances function and structural alignment in multiple myeloma. Clin Orthop Relat Res 426:49–53 [DOI] [PubMed]
- 9.Lavelle W, Carl A, Lavelle ED, Khaleel MA. Vertebroplasty and kyphoplasty. Anesthesiol Clin. 2007;25:913–928. doi: 10.1016/j.anclin.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5:305S–316S. doi: 10.1016/j.spinee.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 12.Louis R (1985) Die stabilisierende Funktion der Wirbelsäule. In: Die Chirurgie der Wirbelsäule. Springer, Berlin
- 13.Maestretti G, Cremer C, Otten P, Jakob RP. Prospective study of standalone balloon kyphoplasty with calcium phosphate cement augmentation in traumatic fractures. Eur Spine J. 2007;16:601–610. doi: 10.1007/s00586-006-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 15.Siebenga J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881–2890. doi: 10.1097/01.brs.0000247804.91869.1e. [DOI] [PubMed] [Google Scholar]
- 16.Stadhouder A, Buskens E, de Klerk LW, et al. Traumatic thoracic and lumbar spinal fractures: operative or nonoperative treatment: comparison of two treatment strategies by means of surgeon equipoise. Spine. 2008;33:1006–1017. doi: 10.1097/BRS.0b013e31816c8b32. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. 2006;31:2747–2755. doi: 10.1097/01.brs.0000244639.71656.7d. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4:351–358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 20.van der RN, de Lange ES, Bakker FC, de Vet HC, van Tulder MW. Management of traumatic thoracolumbar fractures: a systematic review of the literature. Eur.Spine J. 2005;14:527–534. doi: 10.1007/s00586-004-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voggenreiter G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine. 2005;30:2806–2812. doi: 10.1097/01.brs.0000190885.85675.a0. [DOI] [PubMed] [Google Scholar]
- 22.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 23.Wilke HJ, Mehnert U, Claes LE, Bierschneider MM, Jaksche H, Boszczyk BM. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine. 2006;31:2934–2941. doi: 10.1097/01.brs.0000248423.28511.44. [DOI] [PubMed] [Google Scholar]
- 24.Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85-A:773–781. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]