Abstract
Few studies have specifically examined the outcomes following rhBMP-2 usage in patients 65 years and older. The purpose of this retrospective study is to evaluate the efficacy of rhBMP-2 with allograft versus autograft for posterolateral lumbar fusion in patients 65 years and older. One hundred twenty-seven patients were divided into three groups based on fusion material and age. Subjects in group A (n = 34) consisted of patients 65 years and older who received rhBMP-2 and allograft. Group B (n = 52) was composed of patients under 65 years of age with rhBMP-2 and allograft. Subjects in group C (n = 41) were 65 years and older with autograft use. A comparison was made of fusion rate, fusion time (noticed, solid), clinical outcome, VAS, perioperative complications and revision rate between each group. The fusion rate and fusion time were similar in groups A and C; however, these were lower than that observed in group B. Clinical outcomes were similar amongst the groups. There were no significant differences in VAS and perioperative complication rate between groups A and C. In patients 65 years and older, rhBMP-2 with allograft may lead to acceptable fusion rates and fusion times, good clinical outcomes and reduced perioperative complications. The combination of rhBMP-2 with allograft yields equivalent outcomes as autograft in elderly patients undergoing instrumented posterolateral lumbar fusion. Additionally, when compared to patients under 65 years of age undergoing posterolateral lumbar fusion, the use of rhBMP-2 was not sufficient to overcome all aspects of the age-related weakened osteoinductive capacity encountered in elderly patients.
Keywords: RhBMP-2, Posterior lumbar spine fusion, Elderly patients, Fusion rate
Introduction
Although spinal fusion surgery has become a common treatment for degenerative lumbar spinal disease, many studies have found it difficult to achieve a highly successful fusion rate and good clinical outcomes in the elderly population with autograft, the current gold standard, due to the presence of multiple restricting factors [1–4]. For instance, patients aged 65 years and older require a greater amount of autograft for successful fusion because degenerative lumbar spine diseases usually occur at multiple levels in these patients. However, elderly patients generally have poorer bone quality and potentially less bone available for harvest when compared to their younger counterparts. Elderly patients may also have more overall health issues with multiple medical comorbidities that contribute to the difficulty of achieving a successful fusion with autograft. For these reasons, a number of studies have reported poor surgical outcomes in elderly patients when autograft is used as a bone grafting material [5–7], thereby leading to the exploration of comparable alternatives. One possible alternative is to induce fusion with rhBMP-2.
In July of 2002, the US FDA approved the use of rhBMP-2 for anterior lumbar interbody fusion surgery (ALIF). Since then, many surgeons have used rhBMP-2 in off-label procedures [8–11]. These studies have found that rhBMP-2 in the general patient population has similar or superior fusion outcomes as that of autograft [8–12]. However, few reports have specifically examined the outcomes of rhBMP-2 usage in patients 65 years and older. In this study, we have compared the clinical efficacy of rhBMP-2 with allograft in posterolateral lumbar fusion (PLF) surgery in patients ages 65 years and older to that of patients younger than 65 years undergoing the same procedure. These outcomes were also compared to those of patients in both age groups who underwent the procedure with autologous bone graft.
Materials and methods
After obtaining Institutional Review Board approval we searched our institution’s medical record database for appropriate patients. Patients were included if they underwent instrumented PLF for the treatment of degenerative lumbar spine diseases between 2002 and 2006, if their procedure utilized rhBMP-2 with allograft (rhBMP-2 use group) or autograft only (autograft use group), and if there was at least 2 years of follow-up. Local bone autograft acquired during decompression was added to the fusion mass in all patients. Patients who underwent anterior or posterior lumbar interbody fusion, an uninstrumented PLF, or had less than 2 years of follow-up were excluded. Follow-up was performed postoperatively at 6 weeks, 3, 6, 9 and 12 months and 1 year intervals. Depending on individual patient factors, however, the follow-up period varied by several days.
Based on the above inclusion/exclusion criteria, 127 patients were enrolled in this study and were divided into three groups based on their age at the time of surgery and graft material. Group A (n = 34) was composed of patients 65 years and older who had received rhBMP-2 with allograft [mean age 74.1 ± 5.8 years (min 65, max 91); 18 male and 16 female; 5 smokers; 14 with osteoporosis; 18 with medical comorbidities; 12 revision surgeries; 17 multilevel fusions]. Group B (n = 52) was defined as patients less than 65 years with rhBMP-2 and allograft use [mean age 49.9 ± 11.2 years (min 17, max 64); 20 male and 32 female; 14 smokers; 6 with osteoporosis; 9 with medical comorbidities; 26 revision surgeries; 13 multilevel fusions). Group C (n = 41) was defined as patients 65 years and older with autograft [mean age, 72.4 ± 5.1 years (min 65, max 83); 17 male and 24 female; 7 smokers; 18 with osteoporosis; 24 with medical comorbidities; 8 revision surgeries; 28 multilevel fusions]. The mean follow-up period was 38.3 ± 7.4 months (min 24, max 68) in group A, 39.2 ± 11.7 months (min 24, max 62) in group B and 34.7 ± 8.2 months (min 24, max 58; Table 1) in group C.
Table 1.
Demographic data between groups
| Group A (n = 34) | Group B (n = 52) | Group C (n = 41) | |
|---|---|---|---|
| Sex (M/F) | 18/16 (47.1) | 20/32 (61.5) | 17/24 (58.5) |
| Follow-up (months) | 38.3 ± 7.4 | 39.2 ± 11.7 | 34.7 ± 8.2 |
| Comorbidity (−/+) | 16/18 (52.9) | 43/9 (17.3) | 17/24 (58.5) |
| Osteoporosis (−/+) | 20/14 (41.2) | 46/6 (11.5) | 23/18 (43.9) |
| Smoke (−/+) | 29/5 (14.7) | 38/14 (26.9) | 34/7 (17.1) |
| Fusion level (S/M) | 17/17 (50.0) | 39/13 (25.0) | 13/28 (68.3) |
| Revision (−/+) | 22/12 (35.3) | 26/26 (50.0) | 33/8 (19.5) |
Number in parentheses represents percentage of patients with risk factors for pseudoarthrosis
S single-level fusion, M multilevel fusion
In accordance with WHO guidelines, osteoporosis was defined as having a T score less than −2.5 based on DEXA. All the patients with osteoporosis were taking bisphosphonates during the postoperative follow-up period. Smokers were defined as those who had continuously smoked for at least 1 year prior to surgery, as well as postoperatively. Patients with medical comorbidities were defined as those who were receiving treatment for two or more concurrent medical diseases of Li et al’s [2] comorbidity definition, such as diabetes mellitus, hypertension and thyroid disease, etc. Revision surgeries were restricted to cases in which surgery was performed for pseudoarthrosis; surgeries due to infection and metal removal for irritation pain were excluded. Multilevel fusion was defined as cases in which more than two levels were surgically treated.
Groups A and B received allograft and rhBMP-2 (4.2 mg/2.8 ml for one-level, 8.4 mg/5.6 ml for two level, 12 mg/8.0 ml for three level and over), while groups C received iliac crest bone graft (ICBG). The presence of fusion was determined based on plain radiographs and dynamic lateral radiographs, which were interpreted by a blinded, independent musculoskeletal radiologist. Fusion was defined as the presence of bilateral bridging trabecular bone on plan radiographs with less than 3° of translation and less than 5° of angulation on flexion/extension views. CT scans were used as a secondary measure when bridging trabecular bone was not observed on plain radiographs. Fusion time was divided into two types, noticed fusion time and solid fusion time. Noticed fusion was defined as the first presence of bridging bone between two transverse processes in the fusion segment, while solid fusion was defined as the clear presence of a robust fusion mass with consolidated bridging bone.
In accordance with Kirkaldy-Willis criteria [13], clinical outcomes were assessed based on a 4-grade system: ‘excellent’, ‘good’, ‘fair’ and ‘poor’. ‘Good’ and ‘excellent’ were further classified as good results, and ‘fair’ and ‘poor’ were further classified as poor results. To assess the degree of pain improvement, VAS was measured preoperatively and postoperatively at 6 months, 1 year, and 2 years. The occurrence of perioperative complications was also evaluated.
A comparison was made based on fusion rate, fusion time (noticed, solid), clinical results, VAS, perioperative complication and revision rates. A comparison was also made based on the presence or absence of risk factors for pseudoarthrosis between the rhBMP-2 (group A) and autograft group (group C) in patients 65 years and older.
The Fisher exact test was used to detect significant differences between fusion rates, clinical results and perioperative complication rates. The independent t test detected statistical significance between fusion times (noticed, solid), with a p value of less than 0.05 considered statistically significant. All statistical analyses were performed with SPSS (version 15, SPSS, Chicago, IL).
Results
Comparison of patients 65 years and older and patients under 65 years with rh-BMP2 (groups A and B, respectively)
The fusion rate was 82.4% (28/34) in group A and 94.2% (49/52) in group B (p = 0.146). Noticed fusion time was significantly greater in group A (95.7 ± 24.4 days) than in group B (83.7 ± 32.5 days, p = 0.010). However, there was no significant difference in solid fusion time between group A (259.1 ± 76.9 days) and group B (248.3 ± 77.3 days, p = 0.564; Table 2).
Table 2.
Fusion time and fusion rate in rhBMP-2 groups
| Age | Fusion time | Fusion rate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Noticed fusion [mean ± SD (days)] | Solid fusion [mean ± SD (days)] | Noticed fusion (p value) | Solid fusion (p value) | Fusion (N) | No fusion (N) | % | p value | |
| <65 | 49 | 83.7 ± 32.5 | 248.3 ± 77.3 | 0.010* | 0.564 | 49 | 3 | 94.2 | 0.146 |
| ≥65 | 28 | 95.7 ± 24.4 | 259.1 ± 76.9 | 28 | 6 | 82.4 | |||
* Statistically significant difference
Comparison of rhBMP-2 versus autograft in patients 65 years and older (groups A and C)
There was no significant difference in fusion rate [82.4% (28/34) vs. 78.1% (32/41), p = 0.775], noticed fusion time (95.7 ± 24.4 days vs. 102.5 ± 24.5 days, p = 0.564), or solid fusion time (259.1 ± 76.9 days vs. 291.8 ± 68.8 days, p = 0.296) between groups A and C (Table 3).
Table 3.
Comparison of rhBMP-2 versus autograft in patients over 65 years of age
| Age | Materials | Fusion time | Fusion rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Noticed fusion [mean ± SD (days)] | Solid fusion [mean ± SD (days)] | Noticed fusion (p value) | Solid fusion (p value) | Fusion (N) | No fusion (N) | % | p value | ||
| ≥65 | Autograft | 32 | 102.5 ± 24.5 | 291.8 ± 68.8 | 0.296 | 0.116 | 32 | 9 | 78.1 | 0.775 |
| BMP | 28 | 95.7 ± 24.4 | 259.1 ± 76.9 | 28 | 6 | 82.4 | ||||
* Statistically significant difference
Multivariable analysis of patients 65 years and older with rhBMP-2 versus autograft (groups A and C)
Fusion rates between group A and C were, respectively, 87.5 and 79.2% in females; 82.4 and 75.0% post multilevel spinal fusion; 60.0 and 57.1% in smokers; 85.7 and 77.8% in patients with osteoporosis; 83.4 and 100% post-revision surgery; and 77.8 and 83.4% in patients with multiple comorbidities. There were no statistically significant differences in these fusion rates (p = 0.681, 0.495, 0.719, 1.000, 0.672, 0.706, respectively; Table 4).
Table 4.
Comparison of rhBMP-2 versus autograft in patients over 65 years of age with other risk factors for nonunion
| Factors | Materials | Fusion time | Fusion rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Noticed fusion [mean ± SD (days)] | Solid fusion [mean ± SD (days)] | Noticed fusion (p value) | Solid fusion (p value) | Fusion (N) |
No fusion (N) |
% | p value | ||
| Female | Autograft | 19 | 105.5 ± 26.6 | 285.5 ± 66.7 | 0.393 | 0.247 | 19 | 5 | 79.2 | 0.681 |
| BMP | 14 | 98.1 ± 21.3 | 256.8 ± 71.8 | 14 | 2 | 87.5 | ||||
| Revision | Autograft | 8 | 101.8 ± 24.2 | 301.7 ± 72.9 | 0.588 | 0.411 | 8 | 0 | 100.0 | 0.495 |
| BMP | 10 | 95.1 ± 27.6 | 270.4 ± 82.1 | 10 | 2 | 83.4 | ||||
| Multiple fusion level | Autograft | 21 | 97.5 ± 17.2 | 294.1 ± 62.6 | 0.677 | 0.972 | 21 | 7 | 75.0 | 0.719 |
| BMP | 14 | 100.4 ± 22.9 | 293.2 ± 61.9 | 14 | 3 | 82.4 | ||||
| Smoke | Autograft | 4 | 127.6 ± 33.5 | 319.6 ± 76.9 | 0.801 | 0.745 | 4 | 3 | 57.1 | 1.000 |
| BMP | 3 | 121.1 ± 32.3 | 295.7 ± 99.6 | 3 | 2 | 60.0 | ||||
| Osteoporosis | Autograft | 14 | 103.5 ± 21.1 | 287.4 ± 59.7 | 0.516 | 0.762 | 14 | 4 | 77.8 | 0.672 |
| BMP | 12 | 98.5 ± 17.1 | 279.5 ± 72.2 | 12 | 2 | 85.7 | ||||
| Comorbidity | Autograft | 20 | 103.5 ± 25.1 | 289.8 ± 69.4 | 0.993 | 0.682 | 20 | 4 | 83.4 | 0.706 |
| BMP | 14 | 103.6 ± 19.8 | 299.9 ± 70.6 | 14 | 4 | 77.8 | ||||
Noticed fusion times between group A and C were, respectively, 98.1 ± 21.3 and 105.5 ± 26.6 days in females; 95.1 ± 27.6 and 101.8 ± 24.2 days post-revision surgery; 121.1 ± 32.3 and 127.6 ± 33.5 days in smokers; 98.5 ± 17.1 and 103.5 ± 21.1 days in patients with osteoporosis; 103.6 ± 19.8 and 103.5 ± 25.1 days in patients with multiple comorbidities; and 100.4 ± 22.9 and 97.5 ± 17.2 days post multilevel spinal fusion. These differences were not statistically significant (p = 0.393, 0.588, 0.677, 0.801, 0.516, 0.993, respectively; Table 4).
Solid fusion times between group A and C were, respectively, 256.8 ± 71.8 and 285.5 ± 66.7 days in females; 256.8 ± 71.8 and 256.8 ± 71.8 days post-revision surgery; 293.2 ± 61.9 and 294.1 ± 62.6 days post multilevel spinal fusion; 295.7 ± 99.6 and 319.6 ± 76.9 days in smokers; 279.5 ± 72.2 and 287.4 ± 59.7 days in patients with osteoporosis; and 299.9 ± 70.6 and 289.8 ± 69.4 days in patients with multiple comorbidities. Again, the differences in solid fusion times were not statistically significant (p = 0.247, 0.411, 0.972, 0.745, 0.762, 0.682, respectively; Table 4).
Change in VAS in rhBMP-2 versus autograft groups (groups A, B, C)
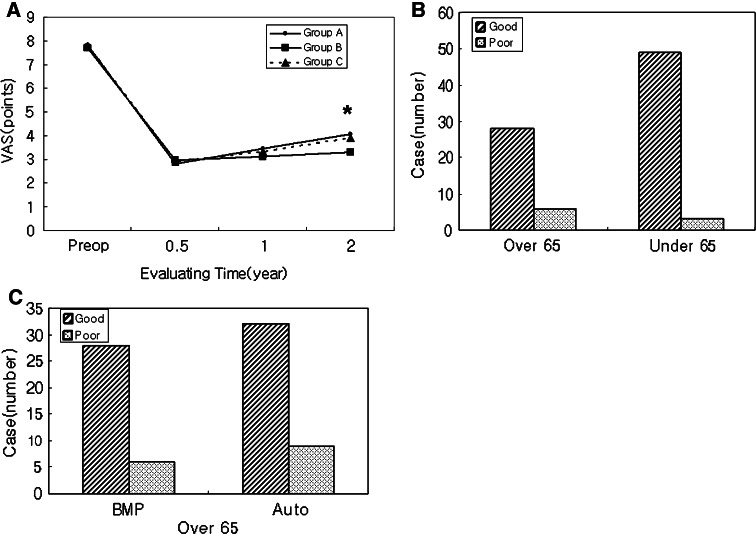
Mean VAS in group A was 7.82 preoperatively, 2.79 at postoperative 6 months, 3.44 at postoperative 1 year and 4.05 at postoperative 2 years. Mean VAS in group B was 7.67, 2.96, 3.11 and 3.28, respectively. Mean VAS in group C was 7.79, 2.91, 3.32 and 3.89, respectively. The only statistically significant difference in mean VAS scores was at the postoperative 2-year mark between groups A and B (p = 0.041, Fig 1a).
Fig. 1.
a Mean VAS scores in each group at the different time intervals. There was a significant difference between subjects over (Group A) and under (Group B) 65 years of age at 2 year follow-up (p = 0.041). Asterisk denotes a statistically significant difference between groups. b Clinical outcomes in the rhBMP-2 groups were better in patients under 65 years of age (92.3% were ‘good’) than in patients greater than 65 years of age (85.3% were ‘good’). This difference was not statistically significant (p = 0.184). c Clinical outcomes in patients over 65 years old were better in the rhBMP-2 group (85.3% were ‘good’) than in the autograft group (73.2% were ‘good’). Again, this difference was not statistically significant (p = 0.414)
Clinical outcomes in rhBMP-2 versus autograft groups (groups A, B, C)
Although groups A and B had good clinical outcomes [85.3% (29/34) and 92.3% (48/52), respectively], they were not significantly different (p = 0.184, Fig 1b). Similarly, no significant difference was seen in the rate of good clinical outcomes for groups A and C [85.3% (29/34) vs. 73.2% (30/41), p = 0.414; Fig 1c].
Perioperative complication rates in patients 65 years and older with rhBMP-2 versus autograft (groups A and C)
Twelve (35.3%) perioperative complications occurred in group A (1 dural tear, 2 cardiac problems, 2 gastrointestinal problems, 1 urinary tract infection, 1 neurological deficit, 3 deep vein thromboses and 1 wound infection). Twenty (48.8%) perioperative complications occurred in group C (3 dural tears, 4 cardiac problems, 4 gastrointestinal problems, 2 urinary tract infections, 1 neurological deficit, 5 deep vein thromboses and 1 wound infection). Although the incidence of perioperative complications was higher in group C, there was no significant difference between groups (p = 0.254, Table 5).
Table 5.
Major complications in patients over 65 years of age
| rhBMP-2 (n = 34) | Autograft (n = 41) | p value | |
|---|---|---|---|
| Dural tear | 1 | 3 | 0.254 |
| Cardiac problem | 2 | 4 | |
| Gastrointestinal problem | 2 | 4 | |
| Urinary tract infection | 1 | 2 | |
| Neurological deficit | 1 | 1 | |
| Deep vein thrombosis | 3 | 5 | |
| Wound infection | 1 | 1 | |
| Total | 12 (35.3%) | 20 (48.8%) |
Revision surgery rates in rhBMP-2 versus autograft groups (groups A, B, C)
The revision surgery rate was 16.7% (1/6) in group A, 0% (0/3) in group B, and 22.2% (2/9) in group C. There was no statistical significant difference between groups A and C (p = 0.799) or groups A and B (p = 0.480).
Discussion
There is still debate in the superiority and efficacy of the use of allograft alone versus autograft for instrumented posterolateral lumbar fusion. Although some surgeons [14, 15] reported that allograft alone may lead to the favorable fusion results in instrumented posterolateral fusion, most surgeons generally accepted [16–18] that allograft was inferior to autograft because allograft has a smaller number of osteocyte, osteoblast, and mesenchymal precursor cells, high possibility of infection and disease transmission. Therefore, there is a consensus that we need to find a material to increase the osteogenic potential as well as to decrease the possibility of infection or disease transmission. One option is to add rhBMPs to allograft.
Although many studies have examined the spinal fusion rate using rhBMP-2 in the general population [8–11], few have specifically evaluated the efficacy in elderly patients. Overall, the use of rhBMP-2 has been found to have similar or slightly better outcomes when compared to the use of autogenous bone graft [8–11, 19, 20]. Multiple studies have additionally found that the success rates of PLF with autograft in patients 65 years and older are lower than those of patients under 65 years [5–7]. However, to our knowledge, only two articles specifically explore the fusion rate of rhBMP-2 in the elderly population [21, 22], with success rates of 83.7 and 86.3%.
There were no statistically significant differences in fusion rates between the groups in our series. The fusion rate was 82.4 in patients 65 years and older with the use of rhBMP-2 with allograft in PLF, which is similar to the rates reported in previous studies [21, 22]. This rate is slightly higher than the 78.1% fusion rate noted in our elderly patients in the autograft group. Nevertheless, it was still less than the 94.2% fusion rate noted in patients under 65 years of age with rhBMP-2 use. Patients 65 years and older with rhBMP-2 exhibited a significantly prolonged noticed fusion time when compared to patients under 65 years of age with rhBMP-2. However, it was shorter than the noticed fusion times of elderly patients with autograft, even though this difference was not statistically significant.
The similarity between our fusion rates and those reported in prior studies suggests that the use of rhBMP-2 with allograft is comparable to autograft, implying that rhBMP-2 with allograft may possibly be used as an effective alternative to autogenous bone graft in elderly patients undergoing instrumented posterolateral lumbar fusions. However, the efficacy of rhBMP-2 in patients 65 years and older is notably reduced when compared to its efficacy in younger patients. One possible explanation for rhBMP-2’s decreased efficacy in older patients is the natural change that occurs with age. rhBMP-2 induces mesenchymal stem cells (MSCs) to differentiate into osteoblasts or osteocytes through a cascade effect. It was thus proposed that rhBMP-2 could lead to bone and fusion formation by inducing the differentiation of osteocytes in elderly patients. However, our results indicate that rhBMP-2, like autograft, has limitations in overcoming the possible physiological changes associated with aging, such as decreased bone metabolism, collagen synthetic activity and alkaline phosphatase synthesis [23], and decreased differentiating capacity of aging osteoprogenitor cells [24, 25]. Adding to the difficulty of inducing bone fusion in elderly patients is the common presence of vascular obstructive disease, secondary to diabetes mellitus and hypertension. Lack of vascular supply likely limits the neogenesis in the fusion bed and subsequently, osteocyte migration to the grafted material. This may be one mechanism whereby rhBMP-2 exhibits lower fusion rates in elderly patients, which cannot be overcome by rhBMP-2 alone. Thus, some authors have suggested the concomitant usage of growth factors to induce angiogenesis in future studies.
Our series also illustrates the limited influence that various factors have on fusion rates when combined with age. When taking into account gender, number of levels being fused, and certain medical and orthopedic comorbidities, similar results were noted in patients without these fusion-related risk factors. The only variable that seemed to greatly influence fusion rates was smoking, which caused a significantly lower fusion rate of 60.0% with rhBMP-2 and 57.1% with autograft. Not only was fusion rate decreased with smoking and advanced age, but time to fusion was also prolonged significantly. This indicates that smoking may lead to poorer outcomes when compared to other risk factors in elderly patients.
Due to the multiple comorbidities commonly found in elderly patients, there have been reportedly mixed clinical outcomes in elderly patients’ post-spinal surgery [1, 26–28]. However, despite a lack of statistical significance in our series, good results were seen in 85.3% of patients 65 years and older who received rhBMP-2. Although this figure was not greater than the 92.3% seen in patients under 65 years, it was greater than the 73.2% seen in patients 65 years and older who received autograft. In contrast to prior studies [26, 27], our study seems to exhibit a correlation between clinical outcomes and fusion rates in elderly patients. Successful fusion may be a positive factor for achieving desirable clinical outcomes in elderly patients. Although not addressed in our current analysis, achieving good clinical outcomes with rhBMP-2 may also be associated with shortened or non-existent bone graft harvesting time, reduced blood loss, and a relatively faster postoperative recovery than are usually associated with autograft usage.
There was no significant difference in VAS scores between the elderly and younger patients at 6 months and 1 year postoperatively. However, at 2 years postoperatively, elderly patients with rhBMP-2 had a significantly greater VAS score than their younger counterparts. This may be due to pain associated with aging or the development or advancement of other concurrent diseases.
One possible limitation of our study involves the controversy over the various methods used to determine the presence of solid fusion [10, 11, 19–21, 29–31], since no definite or reliable methods have yet been established. In recent years, CT has been increasingly utilized in the assessment for fusion because it can clearly depict anatomical structures and connectivity between fusion masses [10, 11, 19–21, 30]. However, some studies have found that CT does not exhibit adequate reliability [29–31], and its regular use in follow-up is further limited by its relatively high radiation exposure. Taking these factors into account, some have argued that surgical exploration is the only accurate modality to determine the presence of spinal fusion [29]. However, this is not a practical recommendation due to the risk of surgical morbidity and cost-effectiveness. We therefore mainly used plain films and dynamic radiographs to determine the presence of successful fusion. This presents another possible limitation of interobserver and intraobserver reliability, which we resolved by utilizing one independent, blind, fellowship-trained musculoskeletal radiologist who was unrelated to the study.
One must also consider the degree as to which the allograft used in the rhBMP-2 group contributed to the successful fusions. Some studies have suggested that allograft alone may lead to favorable fusion results in instrumented posterolateral fusion [14, 15]; however, most surgeons generally find allograft to be inferior to autograft in achieving fusion formation [16–18]. This is likely due to the fact that allograft contains fewer osteocyte, osteoblast, and mesenchymal precursor cells. Thus, the osteoinductive power or rhBMP-2 is evident in the equivalent fusion rates between the rhBMP-2 with allograft and the autograft groups.
Another limitation is the retrospective nature of our study, which may have caused demographic differences between our groups. The variety of diseases in our patients prior to treatment may have therefore limited the reliability of our statistical data. A large, randomized control study may be necessary to overcome these limitations.
Conclusion
In patients 65 years and older, rhBMP-2 may lead to acceptable fusion rates and fusion times, good clinical outcomes and reduced perioperative complications. The combination of rhBMP-2 and allograft yields equivalent outcomes as autograft in elderly patients undergoing instrumented posterolateral lumbar fusion. Additionally, when compared to patients under 65 years of age undergoing posterolateral lumbar fusion, the use of rhBMP-2 was not sufficient to overcome all aspects of the age-related weakened osteoinductive capacity encountered in elderly patients.
References
- 1.Glassman SD, Carreon LY, Dimar JR, et al. Clinical outcomes in older patients after posterolateral lumbar fusion. Spine J. 2007;7:547–551. doi: 10.1016/j.spinee.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Patil CG, Lad SP, et al. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. 2008;33:1250–1255. doi: 10.1097/BRS.0b013e3181714a44. [DOI] [PubMed] [Google Scholar]
- 3.Wang MY, Green BA, Shah S, et al. Complications associated with lumbar stenosis surgery in patients older than 75 years of age. Neurosurg Focus. 2003;14:1–4. doi: 10.3171/foc.2003.14.2.8. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton DK, Jones-Quaidoo SM, Sansur C, et al. Outcomes of bone morphogenetic protein-2 in mature adults: posterolateral non-instrument-assisted lumbar decompression and fusion. Surg Neurol. 2008;69:457–461. doi: 10.1016/j.surneu.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Jt Surg Am. 1991;73:802–808. [PubMed] [Google Scholar]
- 6.Tsutsumimoto T, Shimogata M, Yoshimura Y, et al. Union versus nonunion after posterolateral lumbar fusion: a comparison of long-term surgical outcomes in patients with degenerative lumbar spondylolisthesis. Eur Spine J. 2008;17:1107–1112. doi: 10.1007/s00586-008-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CH, Kao YH, Yang SC, et al. Supplementary pedicle screw fixation in spinal fusion for degenerative spondylolisthesis in patients aged 65 and over. Acta Orthop. 2008;79:67–73. doi: 10.1080/17453670710014789. [DOI] [PubMed] [Google Scholar]
- 8.Bae HW, Stambaugh J, Glassman SD, et al. Level-1 data comparing rhBMP-2/ACS combined with an osteoconductive bulking agent with iliac crest bone graft in posterolateral lumbar fusion. Spine J. 2007;7:9s. doi: 10.1016/j.spinee.2007.07.024. [DOI] [Google Scholar]
- 9.Dimar JR, Glassman SD, Burkus KJ, et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534–2539. doi: 10.1097/01.brs.0000240715.78657.81. [DOI] [PubMed] [Google Scholar]
- 10.Glassman SD, Carreon LY, Djurasovic M, et al. Posterolateral lumbar fusion with INFUSE bone graft. Spine J. 2007;7:44–49. doi: 10.1016/j.spinee.2006.06.381. [DOI] [PubMed] [Google Scholar]
- 11.Singh K, Smucker JD, Boden SD. Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion. A prospective CT-scan analysis at one and 2 years. J Spinal Disord Tech. 2006;19:416–423. doi: 10.1097/00024720-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Carreon LY, Glassman SD, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age. A cost-utility study. Spine. 2009;34:238–243. doi: 10.1097/BRS.0b013e31818ffabe. [DOI] [PubMed] [Google Scholar]
- 13.Kirkaldy-Willis WH, Paine KWE, Cauchoz J. Lumbar spinal stenosis. Clin Orthop Relat Res. 1974;99:30–52. doi: 10.1097/00003086-197403000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gibson S, McLeod I, Wardlaw D, et al. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion. A randomized control trial. Spine. 2002;27:1599–1603. doi: 10.1097/00007632-200208010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nasca RJ, Whelchel ILL. Use of cryopreserved bone in spinal surgery. Spine. 1987;12:222–227. doi: 10.1097/00007632-198704000-00005. [DOI] [PubMed] [Google Scholar]
- 16.An HS, Lynch K, Toth J. Prospective comparison of autograft vs. allograft for posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995;8:131–135. doi: 10.1097/00002517-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Nugent PJ, Dawson EG. Intertransverse process lumbar arthrodesis with allogeneic fresh-frozen bone graft. Clin Orthop Relat Res. 1993;287:107–111. [PubMed] [Google Scholar]
- 18.Weiss LE, Vaccaro AR, Scuderi G, et al. Pseudarthrosis after postoperative wound infection in the lumbar spine. J Spinal Disord. 1997;10:482–487. doi: 10.1097/00002517-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Burkus JK, Sandhu HS, Gornet MF, et al. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Jt Surg Am. 2005;87:1205–1212. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 20.Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301–307. doi: 10.1016/j.spinee.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Glassman SD, Carreon LY, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion. A randomized, controlled trial in patients over sixty years of age. Spine. 2008;33:2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 22.Epstein NE. Fusion rates and SF-36 outcomes after multiple laminectomy and noninstrumented lumbar fusions in a predominantly geriatric population. J Spinal Disord Tech. 2008;21:159–164. doi: 10.1097/BSD.0b013e318074ddaa. [DOI] [PubMed] [Google Scholar]
- 23.Liang CT, Barnes J, Seedor JG, et al. Impaired bone activity in aged rats: alterations at the cellular and molecular levels. Bone. 1992;13:435–441. doi: 10.1016/8756-3282(92)90087-D. [DOI] [PubMed] [Google Scholar]
- 24.Muller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 25.Nyssen-Behets C, Delaere O, Duchesne PY, et al. Aging effect on inductive capacity of human demineralized bone matrix. Arch Orthop Trauma Surg. 1996;115:303–306. doi: 10.1007/BF00420320. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Loeser JD. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis, and procedure. J Bone Jt Surg Am. 1992;74:536–543. [PubMed] [Google Scholar]
- 27.Carreon LY, Puno RM, Dimar JR, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Jt Surg Am. 2003;85:2089–2092. doi: 10.2106/00004623-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Cassinelli EH, Eubanks J, Vogt M, et al. Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine. 2007;32:230–235. doi: 10.1097/01.brs.0000251918.19508.b3. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky ARE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine. 1991;6(Suppl 6):S261–S265. doi: 10.1097/00007632-199106001-00017. [DOI] [PubMed] [Google Scholar]
- 30.Carreon LY, Djurasovic M, Glassman SD, et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32:892–895. doi: 10.1097/01.brs.0000259808.47104.dd. [DOI] [PubMed] [Google Scholar]
- 31.Etminan M, Girardi FP, Khan SN, et al. Revison surgeries for lumbar pseudoarthrosis. Orthop Clin N Am. 2002;33:381–392. doi: 10.1016/S0030-5898(02)00005-6. [DOI] [PubMed] [Google Scholar]