Abstract
Background:
Recent investigations have shown that leukocyte activation is involved in the pathogenesis of ventilator-associated lung injury. This study was designed to investigate whether the inflammatory responses and deterioration of oxygenation in ventilator-associated lung injury are attenuated by high-frequency oscillatory ventilation (HFO). We analyzed the effects of HFO compared with conventional mechanical ventilation (CMV) on the activation of pulmonary macrophages and neutrophils in 10 female rabbits.
Results:
After surfactant depletion, the rabbits were ventilated by CMV or HFO at the same mean airway pressure. Surfactant-depletion followed by 4 h mechanical ventilation hindered pulmonary oxygenation in both groups. Impairment of oxygenation was less severe in the HFO group than in the CMV group. In the HFO group the infiltration of granulocytes into alveolar spaces occurred more readily than in the CMV group. Compared with CMV, HFO resulted in greater attenuation of β2-integrin expression, not only on granulocytes, but also on macrophages.
Conclusions:
In the surfactant-depleted lung, the activation of leukocytes was attenuated by HFO. Reduced inflammatory response correlated with decreased impairment of oxygenation. HFO may reduce lung injury via the attenuation of pulmonary inflammation.
Keywords: adhesion molecule, high frequency oscillatory ventilation, leukocyte, lung injury, mechanical ventilation
Introduction
Adult respiratory distress syndrome (ARDS), which is characterized by impaired pulmonary gas exchange, large alveolar protein leakage and hyaline membrane formation, is one of the major causes of death in intensive care units (ICU) [1]. Recent investigations have demonstrated that overactivation or improper activation of the immune system plays a central role in the pathogenesis of ARDS [1,2]. A variety of mediators, including active oxygen species [3], arachidonic acid metabolites [4] and proinflammatory cytokines [5] released from granulocytes and macrophages, are responsible for the tissue injury.
Over the past few decades, progress in mechanical ventilation has made a great contribution to the treatment of respiratory failure. Soon after the introduction of mechanical ventilation, however, positive pressure ventilation itself was reported to induce or worsen lung injury [6,7]. Recently, several studies have reported that different ventilatory modes influence the severity and progression of ARDS [8,9,10,11]. High frequency oscillatory ventilation (HFO), a special mode of mechanical ventilation applied only to specific types of diseases in neonates [12], generally produces less lung injury and granulocyte infiltration into alveolar spaces in vivo, compared with conventional mechanical ventilation (CMV) [8,9,13]. These results [8,9,13] suggest that HFO reduces the inflammatory reaction involved in the progression of ventilator-associated lung injury.
Inflammatory conditions may be associated with an increased influx of inflammatory cells into the alveolar spaces, along with marked shifts in the composition of the cell population in bronchoalveolar lavage (BAL) fluid. Analysis of BAL fluid has been found useful in studying the pathophysiology of ARDS [2,14] and other pulmonary diseases [15,16]. In this study, we analyzed the effects of two ventilatory modes, HFO and CMV, on the activation of pulmonary macrophages and neutrophils in rabbits. We demonstrated that pulmonary macrophages and neutrophils were less activated in HFO rather than CMV-treated lungs.
Materials and methods
Animal preparation
The experimental protocol was reviewed and approved by the Animal Research Committee of Osaka University Medical School. Ten adult female New Zealand white rabbits (2.5-3.0 kg) were used in this study. The rabbits were maintained under anesthesia by the iv infusion of 5 ml/kg/h of 5% dextrose in Ringer lactate solution with pancuronium bromide (0.04 mg/ml) and sodium pentobarbital (1 mg/ml). Tracheostomy was performed with a midline incision of the neck. The trachea was intubated with a 3.5 mm inner diameter (ID) endotracheal tube and tied to prevent air leakage. Arterial cannulation was performed via the internal carotid artery. Rectal temperature was monitored and maintained at 38°C using an electric heating blanket.
Removal of lung surfactant
After initial stabilization, the rabbits' lungs were lavaged four times to remove lung surfactant, using a previously described method [34] with minor modifications (first lavage). A dose of 30 ml/kg of warmed normal saline was instilled through the tracheostomy tube with a pressure < 25 cmH2O and drained after 10 s of retention. This procedure provides a surfactant depletion model which is widely recognized as a suitable model for experimental ARDS [34]. At 4 h of mechanical ventilation, soon after the animals had received a lethal infusion of saturated potassium chloride, the final BAL was performed with 20 ml/kg of cold phosphate-buffered saline. The lavage fluid was collected for further analysis.
Mechanical ventilation
The animals were divided into two groups according to ventilation mode (CMV, n = 5; HFO, n = 5). In the CMV group a ventilator for neonates (VIP BIRDTM, Bird Products Corp, Palm Spring, CA, USA) was used [peak inspiratory pressure 20 cmH2O, inspiratory time 0.7 s, respiratory rate 30-40 min, inspiratory flow 20 l/min, positive end expiratory pressure (PEEP) 0 cmH2O, FiO2 0.5]. In the HFO group a piston-pump oscillatory ventilator (Humming VTM, Senko Medical Instrument Manufacturers, Tokyo, Japan) was introduced [oscillatory frequency 15 Hz, stroke volume 5-6 ml/kg, mean airway pressure 7 cmH2O (equivalent to that used in CMV), FiO2 0.5]. The respiratory rate of CMV and stroke volume of HFO were adjusted to maintain PaCO2 between 30 and 50 mmHg.
During BAL, all the animals were ventilated by HFO (oscillatory frequency 15 Hz, stroke volume 5-6 ml/kg, mean airway pressure 7 cmH2O, FiO2 1.0). The animals were then divided into CMV and HFO groups and ventilated for 4 h. Heparinized arterial blood samples were collected just before the first BAL, and 10 min and 4 h afterwards for the analysis of arterial blood gases by ABL 505 (Radiometer Co, Copenhagen, Denmark).
Analysis of total cell counts and cell population of BAL fluid
After 4 h of ventilation the rabbits were killed and a final complete BAL was performed in order to obtain cells that had infiltrated into alveolar spaces. Nucleated cell counts of lavage fluid were done using a hemocytometer (Reichert Co, Buffalo, NY, USA). Cytocentrifuge preparations of lavage fluid were stained with Wright's stain and differential cell counts were conducted.
Flow cytometry
The following monoclonal antibodies (mAbs) (Spring Vally Laboratories Inc, Woodbine, MD, USA) were used for immunofluorescence staining: fluorescein isothio-cyanate(FITC)-conjugated antirabbit CD11a [the α chain of leukocyte function associated antigen-1 (LFA-1)]; CD11b (α chain of Mac-) and CD18 (the β chain of LFA-1 and Mac-1).
For immunofluorescence staining, 106 BAL cells were washed once with phosphate-buffered saline (PBS), and resuspended in 50 μl of PBS containing 2% fetal bovine serum (FBS), 0.05% sodium azide and 5 μg/ml mAbs. After incubation for 30 min at 4 VC in the dark, the cells were washed once with PBS containing 2% FBS and 0.05% sodium azide. The cells were then resuspended in 1 ml of PBS containing 2% FBS and 1% paraformaldehyde, and stored at 4 VC in the dark prior to flow cytometrical analysis.
Flow-cytometric measurements were made with an FACScan (Becton Dickinson, San Jose, CA, USA). A minimum of 20,000 events were analyzed for each sample. Analysis was performed using the software application CELLQuestTM (Becton Dickinson). Neutrophils and alveolar macrophages were separately gated according to their forward and sideward scatter. The expression of cell-surface molecules identified by respective mAbs was assessed in terms of the mean fluorescence intensity (MFI) in arbitrary units. For proper comparison of the fluorescence intensity values, a standard set of FITC-calibrated microbeads (Becton Dickinson), as described previously [15], was included in each experiment.
Statistical analysis
Results were expressed as mean ± standard error. The Mann-Whitney test was used to compare the results between groups. P <0.05 was considered significant.
Results
Gas exchange impairment was greater in CMV
As shown in Table 1, the surfactant depletion procedure performed in this study, consisting of repetitive lung lavage, significantly impaired oxygenation as assessed by respiratory index (the value of PaO2 divided by FiO2). There were no significant differences between the values of the CMV group and those of the HFO group at 15 min after the first BAL. During 4 h of mechanical ventilation, oxygenation was significantly impaired in both groups. The respiratory index of the HFO group was significantly higher, however, than that of the CMV group (P < 0.05; Table 1).
Table 1.
Changes of respiratory index in surfactant-depleted lung injury rabbits ventilated with CMV or HFO
| Mode of | Before first LL | 15 min after LL | 4 h after LL |
| ventilation | |||
| CMV | 450.5 ± 25.2 | 91.8 ± 21.1* | 32.3 ± 6.7†‡ |
| HFO | 430.3 ± 15.2 | 104.0 ± 13.1* | 55.8 ± 4.3† |
After the completion of surfactant depletion, the rabbits were ventilated by conventional mechanical ventilation (CMV) or by high-frequency oscillatory ventilation (HFO) for 4 h at the same mean airway pressure. LL = lung lavage. *P < 0.05 vs the value before the first LL; †P < 0.05 vs the value before and 15 min after the first LL; ‡P < 0.05 vs the values of HFO at 4 h after the first LL.
Granulocyte infiltration was greater in CMV than in HFO
The number of neutrophils, macrophages and lymphocytes in the BAL fluid before and after ventilation is shown in Table 2. There was no significant difference between the groups in the number of cells in the first BAL fluid. The cell composition in the first BAL fluids (before the completion of surfactant-depleted lung injury) for both groups consisted mainly of macrophages. The proportion of neutrophils in the first BAL fluid was <4%. After 4 h of ventilation, the total number of cells in the BAL fluid was significantly greater in the CMV group than in the HFO group.
Table 2.
The number of cells in the BAL fluid before and after ventilation with CMV or HFO
| CMV | HFO | |
| First BAL* | ||
| Total cell count | (7.8 ± 1.2) × 106 | (8.1 ± 1.3) × 106 |
| Last BAL† | ||
| Total cell count | (47.0 ± 8.8) × 105 | (13.6 ± 3.5) × 105 ‡ |
| Neutrophils | (19.7 ± 5.5) × 105 | (1.6 ± 1.0) × 105 ‡ |
| Macrophages | (22.6 ± 3.5) × 105 | (10.9 ± 2.3) × 105 ‡ |
| Lymphocytes | (4.7 ± 0.5) × 105 | (1.1 ± 0.3) × 105 ‡ |
BAL = bronchoalveolar lavage; CMV = conventional mechanical ventilation; HFO = high-frequency oscillatory ventilation. *Before ventilation; †after 4 h ventilation; ‡P <0.05.
After 4 h of ventilation, the number of granulocytes in the BAL fluid was also significantly greater in the CMV group than in HFO group. The CMV group also had more macrophages and lymphocytes in the BAL fluid after 4 h of ventilation.
Pulmonary macrophages and neutrophils were more activated in CMV than in HFO
As shown in Fig 1, the levels of the expression of CD11a, CD11b and CD18 on macrophages were significantly upregulated in both groups after 4 h ventilation. The levels of up-regulation for the expression of CD11a, CD11b and CD18 were significantly higher, however, in the CMV rather than the HFO group (P <0.05; Fig 1b). The expression levels of CD11b on granulocytes in BAL fluids after 4 h of ventilation were higher in CMV than in HFO as measured by MFI (148 ± 12 in the CMV group and 72 ± 10 in the HFO group, P < 0.05). The MFI of CD11b on granulocytes in the first BAL fluid was not analyzed because so few neutrophils were recovered, as mentioned above.
Figure 1.
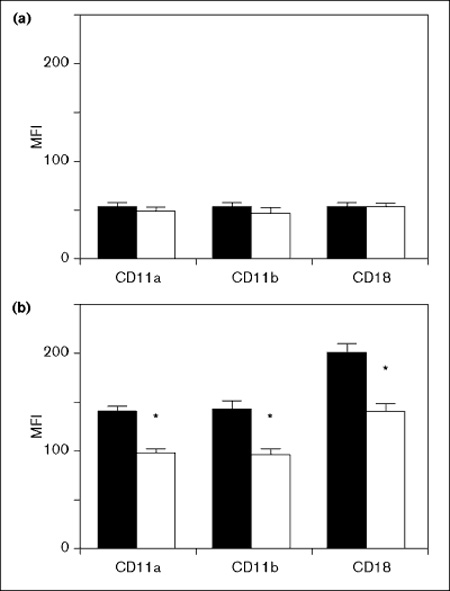
β2-integrin expression on macrophages in the first (a) and final (b) BAL fluid. In the final lavage, the expression of CD11a, CD11b and CD18 was significantly up-regulated (b) in comparison with the values from the first lavage (a), in both groups. However, in the final lavage (b), the levels of up-regulation of CD11a, CD11b and CD18 in the high frequency oscillatory ventilation (HFO) group were significantly lower than those of the conventional mechanical ventilation (CMV) group. The solid column indicates CMV procedure and open column HFO procedure. *P < 0.05 vs CMV-treated. MFI = mean fluorescence intensity (arbitrary units).
Discussion
In the present study, we have shown, using a repeatedly lavaged surfactant-depleted rabbit lung model, that, in comparison with HFO, CMV resulted in greater impairment of oxygenation and increased infiltration of neutrophils, as indicated by their presence in BAL fluids. These findings are consistent with the results described in previous reports [8,9,13]. Sugiura et al reported that the oxidative burst of neutrophils in the lung was attenuated by HFO in a surfactant-depleted rabbit model [8]. Imai et al demonstrated that HFO could reduce the release of inflammatory chemical mediators, platelet-activating factor and thromboxane B2 in the surfactant-depleted rabbit lung [13]. These investigations suggest that HFO may prevent the progression of inflammation in an experimental ARDS model. Consequently, we analyzed the severity of inflammation in injured lungs ventilated with two different modes, CMV and HFO, and measured the expression of adhesion molecules on inflammatory cells through the use of flow cytometry. In comparison with previous studies [8,9,13], the application of flow-cytometric analysis in this study enabled us to examine separately the extent of the activation of neutrophils and macrophages. In this regard, our findings directly demonstrate that the HFO procedure is less harmful than CMV because the expression of adhesion molecules was lower on lung inflammatory cells isolated from the HFO rather than the CMV group.
CD11a, CD11b and CD18 are well known adhesion molecules belonging to the β2-integrin superfamily [17]. Integrins are heterodimers and consist of α and β subunits (CD11a/CD18 = LFA; CD11b/CD18 = Mac-1). β2-integrins have been widely recognized to be important during the recruitment of leukocytes from the peripheral blood to inflamed tissue [17,18]. Inhibition of β2-integrin has been shown to lead to the reduction of neutrophil recruitment in several pulmonary inflammatory states in vivo [19,20]. Clinical investigations have indicated that the expression of β 2-integrin on macrophages and granulocytes is upregulated in pulmonary inflammation [15,21]. Our present study demonstrates that HFO can reduce the activation not only of granulocytes, which has been reported by several investigators [8,9,13], but also of alveolar macrophages, assessed by the expression of β2-integrins. This study provides a first demonstration that the activation of pulmonary macrophages is influenced by the mode of ventilation. However, factors other than ventilation affect the expression of adhesion molecules. There was no significant difference in change in blood pressure between the groups (data not shown). Hypoxia itself may affect the expression of adhesion molecules. Granulocytes and macrophages are both key players in the pathogenesis of ARDS [1,2,3,14]. Proinflammatory cytokines and oxygen radicals released from neutrophils and macrophages seem to be responsible for damaging lung tissue [3,14]. In ARDS patients, high percentages of neutrophils and low percentages of alveolar macrophages in BAL, suggesting sustained alveolar inflammation, have been associated with high mortality [22]. Thus, lower activation of alveolar macrophages and neutrophils in HFO may play a part in the mechanisms by which HFO is responsible for less pulmonary injury, although detailed histological analysis was not performed in this study.
Pulmonary microvascular and parenchymal injury is produced by mechanical ventilation not only in the pathological lung [8,23,24], but also in the normal lung [10,11]. Recent investigations have suggested that high inflation pressure itself is not harmful when overdistension is prevented [10]. High peak-inspiratory pressures (PIP) and volumes cause severe injury when coupled with low end-expiratory lung volume [10,25]. Ventilator-associated lung injury, induced with high PIP combined with high lung volume, is markedly attenuated when alveolar collapse is prevented by the application of PEEP [10,26]. In addition, high tidal volume ventilation activates proinflammatory mediators into circulation, which may contribute to the progression of multiple organ failure [27]. HFO can also reduce ventilator-associated lung injury by preventing cyclic pulmonary distension or avoiding collapse of alveoli, as with PEEP [8,9,13]. Although the precise cellular response of lung parenchymal cells during distension or shear stress remains unknown, several in vitro studies have described single specific aspects of the response. One method of mechanical stimulation (stretching) initiated intracellular signaling via Ca2+ [28] and inositol triphosphate [29], and altered the surfactant production in lung epithelial cells [28]. Another mechanical stimulation (shear stress) induced nitric oxide and arachidonic acid production in endothelial cells [30]. Epithelial and endothelial cells can produce proinflammatory cytokines [31,32] and modulate the process of inflammation. Therefore, altered function of epithelial and endothelial cells induced by mechanical stimulation may lead to the modulation of the activation of neutrophils and macrophages in the lung via proinflammatory cytokines [14] and arachidonic acid metabolites [33]. Thus, the less harmful effects of HFO may be mediated by attenuating the activation of epithelial and endothelial cells. Further investigation will be required to confirm this speculation.
In summary, the activation of macrophages and neutrophils in the lung of a surfactant-depleted rabbit ARDS model was attenuated by ventilating with HFO. Reduced inflammatory response correlated with reduced impairment of oxygenation. The less harmful effects of HFO in minimizing lung injury in ARDS may result from the attenuation of pulmonary inflammation.
References
- Rinaldo JE, Rogers RM. Adult respiratory distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982;306:900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986;133:218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Burger R, Fung D, Bryan AC. Lung injury in a surfactant-deficient lung is modified by indomethacin. J Appl Physiol. 1990;69:2067–2071. doi: 10.1152/jappl.1990.69.6.2067. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- Greenfield LJ, Ebert PA, Benson DW. Effect of positive pressure ventilation on surface tension properties of lung extracts. Anesthesiology. 1964;25:312–316. doi: 10.1097/00000542-196405000-00009. [DOI] [PubMed] [Google Scholar]
- Caldwell EJ, Powell RD, Mullooly JP. Interstitial emphysema: a study of physiologic factors involved in experimental induction of the lesion. Am Rev Respir Dis. 1970;102:516–525. doi: 10.1164/arrd.1970.102.4.516. [DOI] [PubMed] [Google Scholar]
- Sugiura M, McCulloch PR, Wren S, Dawson RH, Froese AB. Ventilator pattern influences neutrophil influx and activation in atelectasis-prone rabbit lung. J Appl Physiol. 1994;77:1355–1365. doi: 10.1152/jappl.1994.77.3.1355. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Kawano T, Miyasaka K. Role of high-frequency ventilation in sufactant-depleted lung injury as measured by granulocytes. J Appl Physiol. 1994;76:539–544. doi: 10.1152/jappl.1994.76.2.539. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- Egan EA. Lung inflation, lung solute permeability, and alveolar edema. J Appl Physiol. 1982;53:121–125. doi: 10.1152/jappl.1982.53.1.121. [DOI] [PubMed] [Google Scholar]
- Martin LD. New approach to ventilation in infants and children. Curr Opin Pediatr. 1995;7:250–261. doi: 10.1097/00008480-199506000-00003. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kawano T, Miyasaka K, Takata M, Imai T. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am J Respir Crit Care Med. 1994;150:1550–1554. doi: 10.1164/ajrccm.150.6.7952613. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu J, Misset B, Lebargy F, Carlet J, Bernaudin JF. Expression of tumor necrosis factor α gene in alveolar macrophages from patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147:1585–1589. doi: 10.1164/ajrccm/147.6_Pt_1.1585. [DOI] [PubMed] [Google Scholar]
- Oosterhoff Y, Hoogsteden HC, Rutgers B, Kauffman HF, Postma DS. Lymphocyte and macrophage activation in bronchoalveolar lavage fluid in nocturnal asthma. Am J Respir Crit Care Med. 1995;15:75–81. doi: 10.1164/ajrccm.151.1.7812576. [DOI] [PubMed] [Google Scholar]
- Mukae H, Kadota J, Kohno S, Matsukura S, Hara K. Increase of activated T-cells in BAL fluid of Japanese patients with bronchiolitis obliterans organizing pneumonia and chronic eosinophilic pneumonia. Chest. 1995;108:123–128. doi: 10.1378/chest.108.1.123. [DOI] [PubMed] [Google Scholar]
- Hamacher J, Schaberg T. Adhesion molecules in lung diseases. Lung. 1994;172:189–213. doi: 10.1007/BF00164437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Doerschuk CM, Winn RK, Coxson HO, Harlan JM. CD18-dependent and -independent mechanism of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- Mulligan MS, Varani J, Warren JS, et al. Roles of β2-integrins of rat neutrophils in complement- and oxygen radical-mediated acute inflammatory injury. J Immunol. 1992;148:1847–1857. [PubMed] [Google Scholar]
- Chanez P, Vignola AM, Lacoste P, Michel FB, Godard P, Bousquet J. Increased expression of adhesion molecules (ICAM-1 and LFA-1) on alveolar macrophages from asthmatic patients. Allergy. 1993;48:576–580. doi: 10.1111/j.1398-9995.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Steinberg KP, Milberg JA, Martin TR, et al. Evolution of bronchoalveolar cell population in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- Cioffi WG, deLemos RA, Coaison JJ, Gerstmann DA, Pruitt BA. Decreased pulmonary damage in primates with inhalation injury treated with high-frequency ventilation. Ann Surg. 1993;3:328–337. doi: 10.1097/00000658-199309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss D, Soler P, Saumon G. Mechanical ventilation-induced pulmonary edema: interaction with previous lung alterations. Am J Respir Crit Care Med. 1995;151:1568–1575. doi: 10.1164/ajrccm.151.5.7735616. [DOI] [PubMed] [Google Scholar]
- Tsuno K, Prato P, Kolobow T. Acute lung injury from mechanical ventilation at moderately high airway pressures. J Appl Physiol. 1990;69:956–961. doi: 10.1152/jappl.1990.69.3.956. [DOI] [PubMed] [Google Scholar]
- Sandhar BK, Niblett DJ, Argiras EP, Dunnill MS, Sykes MK. Effects of end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med. 1988;14:538–546. doi: 10.1007/BF00263527. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and cfos mRNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz HRW, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- Felix JA, Woodruff ML, Dirksen ER. Stretch increases inositol 1,4,5-trisphosphate concentration in airway epithelial cells. Am J Respir Cell Mol Biol. 1996;14:296–301. doi: 10.1165/ajrcmb.14.3.8845181. [DOI] [PubMed] [Google Scholar]
- Tsao PS, Lewis NP, Alpert S, Cooke JP. Exposure to shear stress alters endothelial adhesiveness: role of nitric oxide. Circulation. 1995;92:3513–3519. doi: 10.1161/01.cir.92.12.3513. [DOI] [PubMed] [Google Scholar]
- Adler KB, Fischer BM, Wright DT, Cohn LA, Becker S. Interactions between respiratory epithelial cells and cytokines: relationships to lung inflammation. Ann NY Acad Sci. 1994;725:128–145. doi: 10.1111/j.1749-6632.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Yan SF, Tritto I, Pinsky D, et al. Induction of interleukin 6 by hypoxia in vascular cells: central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- Matuschak GM, Pinsky MR, Klein EC, van Thiel DH, Rinaldo JE. Effect of D-galactosamine-induced acute liver injury on mortality and pulmonary response to Escherichia coli lipopolysaccharide. Modulation by arachidonic acid metabolites. Am Rev Respir Dis. 1990;141:1296–1306. doi: 10.1164/ajrccm/141.5_Pt_1.1296. [DOI] [PubMed] [Google Scholar]
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol. 1983;55:131–138. doi: 10.1152/jappl.1983.55.1.131. [DOI] [PubMed] [Google Scholar]


