Abstract
The race- and sex-specific reference values for vertebral shape are important to determine the prevalence of osteoporotic vertebral fracture. However, these reference values are absent in Chinese women. In the present study, the anterior, middle and posterior heights and the ratios of these heights were measured from 14 vertebral bodies (T4–L5) in 60 premenopausal Chinese women (aged 19–25 years). Cutoff values were set as standard deviations (3 and 3.5 SD) and percentages (15 and 20%) below the means of vertebral height (VH) ratios to define vertebral deformities. The number of subjects with a VH ratio lower than −15% cutoff were significantly more than those with a VH ratio lower than −3 SD cutoff (p < 0.05), but this difference did not occur when a −20% cutoff was selected. A few VH ratios were distributed below −20% and −3 SD cutoffs, and none was below −3.5 SD. The vertebral shape defined by VH ratios was different between Chinese and European women. We conclude that 3.5 SD below the reference mean is an ideal cutoff value for the definition of prevalent vertebral fractures in Chinese women, and reference data should be obtained from young premenopausal women.
Keywords: Vertebral shape, Cutoff values, Premenopausal, Chinese women
Introduction
Osteoporotic vertebral fracture will cause serious health consequence associated with increased mortality and morbidity, particularly in the elderly postmenopausal women [1–5]. The diagnosis of vertebral fracture is based on the degree of vertebral deformity in lateral radiographs of the thoracolumbar spine [6–10]. However, the definition of vertebral deformity is controversial. Non-fracture deformity may cause false-positive identification of osteoporotic vertebral fracture [11–13]. Therefore, a standardized and reproducible approach is required to differentiate true osteoporotic fracture from normal variation or nonfracture deformity [10, 13–16]. Since qualitative assessment of vertebral deformity is limited by poor interobserver agreement and reproducibility, quantitative vertebral morphometry has been introduced into the diagnosis of vertebral fracture [9, 15, 17]. Melton et al. [16, 18] defined vertebral deformities by measuring reduction (percentages or standard deviations) in ratios of anterior, middle and posterior heights of a vertebral body compared with normal values for that particular vertebra. Since then, this method has been widely employed in clinical and epidemiologic studies on vertebral fracture [6, 19, 20].
Comparison to normal or reference values is essential for the quantitative assessment of vertebral fractures. There are several ways to obtain reference values: (1) from radiographs that have been assessed qualitatively as normal by a clinician or radiologist [15]; (2) using radiographs from normal subjects in whom the risk of vertebral fracture is very low [11, 21] and (3) using radiographs from population samples, in which the cases with vertebral deformity need to be excluded by statistical manipulation [6, 16, 22]. The different sources may yield different reference values [13]. Therefore, selection of population is important to obtain optimal reference data for the determination of vertebral deformity [13]. We propose that normal young adults, who are supposed to have low incidence of vertebral deformity and osteoporotic fractures, is an ideal population for obtaining reference values. In addition, the reference values for vertebral dimensions vary among people of different races and sexes [21–24]. Accordingly, a set of specific reference values is required for each race- and sex-specific population. Most documented reference values are derived from American, European and Japanese [18, 22, 25]. To our knowledge, there is lack of vertebral reference data for the Chinese.
In the present study, we measured the vertebral heights of 60 normal Chinese women (aged 19–25 years) to provide reference and cutoff values for the definition of vertebral deformity and osteoporotic vertebral fracture, both of which are prone to occur in postmenopausal women.
Materials and methods
Eighty young premenopausal Chinese women (aged 19–25 years) were recruited in this study. Two women were excluded because their mild scoliosis had been detected by physical examination. The remaining subjects were examined with thoracic and lumbar spine radiography. Lateral thoracic and lumbar spinal radiographs were taken in the lateral decubitus position (X-ray machine: Definium 6000, GE Healthcare). Tube-to-bed distance was 105 cm, with the X-ray beam centered at T8 for thoracic spinal radiograph and at L3 for the lumbar spine radiograph. All radiographs were reviewed by two senior orthopedic surgeons (authors: LG and YC). There were two criteria for subject selection: (1) absence of obvious vertebral deformity and (2) good enough quality of radiograph for quantitative measurement. Finally, 60 young women were included in the study with consensus between two reviewers. Thus, the prevalence of vertebral deformity was presumed to be 0 in this cohort of women.
The digital radiographic images were imported into a personal computer and analyzed using an NIH image program (NIH image J). As reported by Genant et al. [26], six points were marked on each vertebral body from T4 to L5. The six points correspond to the four corners of the vertebral body and the midpoints of the endplates. If the view was not true lateral, the points were chosen that defined the area of the midplane of the vertebral body; the midpoints were chosen so that they fell on a line bisecting the distal and proximal projections of the superior and inferior endplates [26].
A normal vertebral body is approximately rectangular in lateral profile. Three heights, anterior (Ha), middle (Hm) and posterior (Hp), were measured and the ratios of these heights were used to define the normal shape of the vertebral body. Four ratios were calculated for each vertebra: Ha/Hp, Hm/Hp, Hp/Hp+1, Hp/Hp−1; the last two ratios are related to the upper and lower vertebral posterior height. Vertebral deformities were classified into: wedge (reduction in Ha/Hp), concave (reduction in Hm/Hp) and crush (reduction in Hp/Hp+1 or Hp/Hp−1) [16, 18]. Cutoff values were set as standard deviation (3, 3.5 and 4 SD) or percentage (15 and 20%) below the means of vertebral height (VH) ratios. To discriminate racial disparity in vertebral shape, we compared our data with those from normal European women (age <50 years) [11, 21].
The percentage difference in subject numbers below different cutoff values was compared using the z test. In 60 subjects, the difference between cutoff values (percentage vs. SD) for each VH ratio was compared using paired t test. Intraobserver reproducibility was assessed by calculating the coefficient of variance (CV%) of two repeated measurements for 12 radiographs (168 vertebral bodies).
Results
Sixty premenopausal Chinese women, 22 ± 1.5 years old (range 19–25 years), were recruited for this study. The average body height was 162 ± 5.4 cm and body weight was 52 ± 7.2 kg. The age at menarche was 13.6 ± 0.88 years.
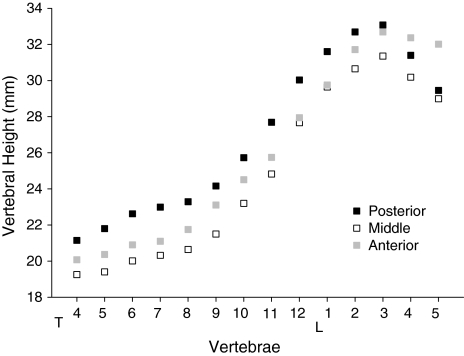
The mean ± SD values of vertebral heights and their ratios for 14 vertebral bodies (T4–L5) in 60 premenopausal Chinese women are shown in Table 1. Three vertebral heights varied with the same trend between different vertebral levels. These heights progressively increased from T4 to L3, in which Hp was the highest and Hm was the lowest. Thereafter, the vertebral heights decreased. In L4 and L5, Hp became shorter than Ha (Fig. 1). The increase in vertebral heights from T4 to T8 was remarkably lesser than that from T8 to L3. For VH ratios, Ha/Hp and Hm/Hp varied with a similar trend between vertebral levels, but Hm/Hp varied with a smaller range. At each vertebral level, Hm/Hp was lower than Ha/Hp (Table 1). The CV% between two repeat measurements of 168 vertebrae was 3.67, 3.94 and 3.91, respectively, for Ha, Hm and Hp.
Table 1.
Values of vertebral heights and height ratios
| Vertebral height (mm) | Vertebral height ratios (%) | ||||||
|---|---|---|---|---|---|---|---|
| Anterior | Middle | Posterior | Ha/Hp | Hm/Hp | Hp/Hp+1 | Hp/Hp−1 | |
| T4 | 20.1 (1.56) | 19.3 (1.54) | 21.1 (1.72) | 95.1 (4.99) | 91.2 (5.08) | 97.1 (6.63) | Null |
| T5 | 20.4 (1.57) | 19.4 (1.47) | 21.8 (1.65) | 93.6 (4.98) | 89.1 (4.35) | 96.4 (4.23) | 103.5 (7.04) |
| T6 | 20.9 (1.46) | 20.0 (1.44) | 22.6 (1.62) | 92.6 (5.09) | 88.6 (5.27) | 98.4 (4.96) | 103.9 (4.66) |
| T7 | 21.1 (1.60) | 20.3 (1.39) | 23.0 (1.80) | 91.9 (5.01) | 88.6 (4.54) | 98.8 (5.01) | 101.7 (5.23) |
| T8 | 21.7 (1.87) | 20.6 (1.49) | 23.3 (1.77) | 93.5 (6.31) | 88.8 (5.03) | 96.6 (4.39) | 101.3 (5.01) |
| T9 | 23.1 (1.75) | 21.5 (1.65) | 24.2 (1.78) | 95.8 (5.62) | 89.1 (5.04) | 93.9 (5.98) | 103.7 (4.83) |
| T10 | 24.5 (1.92) | 23.2 (1.96) | 25.7 (2.50) | 95.6 (5.75) | 90.4 (5.34) | 93.1 (5.41) | 106.9 (6.59) |
| T11 | 25.7 (1.97) | 24.8 (2.32) | 27.7 (2.57) | 93.3 (5.77) | 89.9 (6.04) | 92.7 (6.48) | 107.8 (6.41) |
| T12 | 27.9 (2.26) | 27.7 (2.16) | 30.0 (2.33) | 93.2 (5.09) | 92.2 (4.51) | 95.2 (5.03) | 108.4 (7.54) |
| L1 | 29.8 (2.53) | 29.6 (2.17) | 31.6 (2.32) | 94.2 (5.87) | 93.9 (4.84) | 96.8 (4.91) | 105.3 (5.52) |
| L2 | 31.7 (2.63) | 30.6 (1.99) | 32.7 (2.20) | 97.1 (6.39) | 93.9 (4.7) | 99.0 (5.51) | 103.5 (5.25) |
| L3 | 32.7 (2.37) | 31.4 (2.03) | 33.1 (2.22) | 99.0 (6.15) | 94.9 (4.41) | 105.5 (5.00) | 101.3 (5.74) |
| L4 | 32.4 (2.42) | 30.2 (2.26) | 31.4 (2.17) | 103.2 (6.13) | 96.2 (4.83) | 107.1 (6.50) | 95.0 (4.58) |
| L5 | 32.0 (2.83) | 29.0 (2.48) | 29.5 (2.41) | 109.0 (9.21) | 98.6 (7.21) | Null | 93.7 (5.57) |
The data expressed as mean ± (SD)
Fig. 1.
The anterior, middle and posterior vertebral heights (Ha, Hm and Hp) for 14 vertebral bodies (T4–L5). All the heights progressively increased from T4 to L3, in which posterior height was the highest and middle height was the lowest. Thereafter, the vertebral heights were decreased. In L4 and L5, posterior height became shorter than anterior height
For each VH ratio, we compared four cutoff values, 15%, 20%, 3 SD and 3.5 SD below the mean level (−15%, −20%, −3 SD, and −3.5 SD), in 60 young premenopausal Chinese women. Eleven subjects (18.3%) had >1 VH ratio below −15% cutoff, whereas only 2 (3.3%) and 3 (5.0%) subjects, respectively, had ≥1 VH ratio below −20% and −3 SD cutoffs. No subject had a VH ratio lower than −3.5 SD cutoff. Compared to −3 SD, there were significantly more number of subjects with VH ratios below −15% cutoff (p < 0.05), but this difference did not appear when a −20% cutoff was selected.
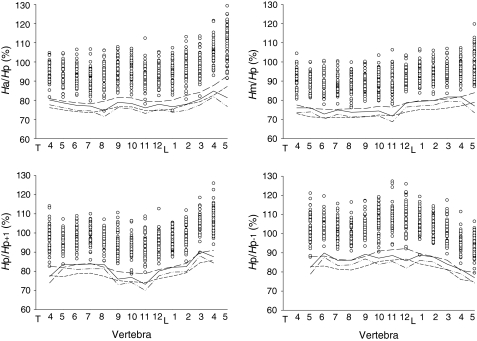
Figure 2 shows the distribution and cutoff levels of four VH ratios from T4 to L5. A few VH ratios were lower than −20% and −3 SD cutoffs (Fig. 2), but none of the VH ratios was below −3.5 SD. Since the −3.5 SD cutoff was already lower than all observed VH ratios, the result of −4 SD was not needed. We compared the cutoff values for each VH ratio. Of the total 54 VH ratios in 14 vertebrae, only 8 VH ratios (~15%) showed their −3 SD cutoff to be lower than −20% cutoff (Table 2). Of these eight ratios, seven had a difference lower than 2%. For the −3.5 SD cutoff, 23 VH ratios (~43%) had lower values than the −20% cutoff (Table 2). Comparing the mean values of each type of VH ratio (Ha/Hp, Hm/Hp, Hp/Hp+1 and Hp/Hp−1 in 14 vertebrae) at −20% and −3 SD cutoff levels, we found that the values defined by −20% were all significantly lower than that defined by −3 SD (p < 0.05 to 0.001) (Table 3). However, there was no significant difference in any type of VH ratio between −3.5 SD and −20% cutoffs (Table 3).
Fig. 2.
The difference between four cutoffs (−15%, −20%, −3 SD and −3.5 SD from mean) for various vertebral height (VH) ratios (Ha/Hp, Hm/Hp, Hp/Hp−1 and Hp/Hp+1). Open circles show the distribution of VH ratios in each vertebra. Long dashed line shows −15% cutoff; short dashed line −20% cutoff; solid line −3 SD cutoff; dashed and dotted line −3.5 SD cutoff
Table 2.
Values of vertebral height ratios defined by various cutoffs
| Ha/Hp (%) | Hm/Hp (%) | Hp/Hp+1 (%) | Hp/Hp−1 (%) | Ha/Hp (%) | Hm/Hp (%) | Hp/Hp+1 (%) | Hp/Hp−1 (%) | |
|---|---|---|---|---|---|---|---|---|
| 3 SD below mean (−3 SD) | 3.5 SD below mean (−3.5 SD) | |||||||
| T4 | 80.1 | 76.0 | 77.2 | Null | 77.6 | 73.4 | 73.9 | Null |
| T5 | 78.6 | 76.0 | 83.7 | 82.4 | 76.1 | 73.9 | 81.6 | 78.9 |
| T6 | 77.3 | 72.8 | 83.5 | 89.9 | 74.7 | 70.2 | 81.0 | 87.6 |
| T7 | 76.9 | 74.9 | 83.8 | 86.0 | 74.4 | 72.7 | 81.3 | 83.4 |
| T8 | 74.6 | 73.7 | 83.5 | 86.2 | 71.4 | 71.2 | 81.3 | 83.7 |
| T9 | 78.9 | 74.0 | 75.9 | 89.2 | 76.1 | 71.5 | 73.0 | 86.8 |
| T10 | 78.3 | 74.4 | 76.8 | 87.2 | 75.4 | 71.7 | 74.1 | 83.9 |
| T11 | 76.0 | 71.7 | 73.2 | 88.6 | 73.1 | 68.7 | 70.0 | 85.4 |
| T12 | 77.9 | 78.7 | 80.1 | 85.8 | 75.4 | 76.4 | 77.6 | 82.0 |
| L1 | 76.6 | 79.4 | 82.1 | 88.8 | 73.7 | 77.0 | 79.6 | 86.0 |
| L2 | 77.9 | 79.8 | 82.4 | 87.8 | 74.7 | 77.4 | 79.7 | 85.2 |
| L3 | 80.5 | 81.7 | 90.5 | 84.1 | 77.5 | 79.5 | 88.0 | 81.2 |
| L4 | 84.8 | 81.7 | 87.6 | 81.3 | 81.8 | 79.3 | 84.3 | 79.0 |
| L5 | 81.3 | 77.0 | Null | 77.0 | 76.7 | 73.4 | Null | 74.2 |
| 15% below mean (−15%) | 20% below mean (20%) | |||||||
| T4 | 80.8 | 77.5 | 82.5 | Null | 76.1 | 73.0 | 77.7 | Null |
| T5 | 79.5 | 75.7 | 82.0 | 88.0 | 74.9 | 71.3 | 77.2 | 82.8 |
| T6 | 78.7 | 75.3 | 83.6 | 88.3 | 74.0 | 70.9 | 78.7 | 83.1 |
| T7 | 78.1 | 75.3 | 84.0 | 86.5 | 73.5 | 70.9 | 79.0 | 81.4 |
| T8 | 79.5 | 75.5 | 82.1 | 86.1 | 74.8 | 71.0 | 77.3 | 81.0 |
| T9 | 81.4 | 75.7 | 79.8 | 88.1 | 76.6 | 71.3 | 75.1 | 83.0 |
| T10 | 81.2 | 76.8 | 79.1 | 90.9 | 76.5 | 72.3 | 74.5 | 85.5 |
| T11 | 79.3 | 76.4 | 78.8 | 91.6 | 74.6 | 71.9 | 74.1 | 86.2 |
| T12 | 79.2 | 78.4 | 80.9 | 92.2 | 74.5 | 73.8 | 76.2 | 86.7 |
| L1 | 80.1 | 79.8 | 82.3 | 89.5 | 75.4 | 75.1 | 77.5 | 84.2 |
| L2 | 82.5 | 79.8 | 84.1 | 88.0 | 77.7 | 75.1 | 79.2 | 82.8 |
| L3 | 84.1 | 80.7 | 89.7 | 86.1 | 79.2 | 75.9 | 84.4 | 81.1 |
| L4 | 87.7 | 81.8 | 91.0 | 80.8 | 82.6 | 77.0 | 85.7 | 76.0 |
| L5 | 92.6 | 83.9 | Null | 79.7 | 87.2 | 78.9 | Null | 75.0 |
Highlighted VH ratios defined by −3 SD and −3.5 SD cutoffs are lower than those defined by −20% cutoff
Table 3.
Comparison of vertebral height ratios at different cutoffs
| Cutoffs | −15% | −20% | −3 SD | −3.5 SD |
|---|---|---|---|---|
| Ha/Hp | 81.8 (4.01)c,f | 77.0 (3.78)a | 78.6 (2.56) | 75.6 (2.45) |
| Hm/Hp | 78.0 (2.47)a,f | 73.5 (2.58)c | 76.6 (3.23) | 74.0 (3.39) |
| Hp/Hp+1 | 83.1 (3.66)a,f | 78.2 (3.45)c | 81.6 (4.81) | 78.9 (5.00) |
| Hp/Hp−1 | 87.4 (3.73)a,f | 82.2 (3.51)c | 85.7 (3.68) | 82.9 (3.76) |
The data expressed as mean ± (SD)
a, b, c: compared to −3 SD; ap < 0.05, bp < 0.01, cp < 0.001
d, e, f: compared to −3.5 SD; dp < 0.05, ep < 0.01, fp < 0.001
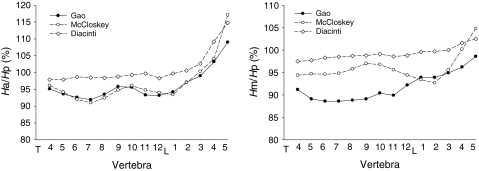
Figure 3 shows the average Ha/Hp and Hm/Hp ratios for each vertebral body in the present study compared with the data obtained from normal European women by McCloskey et al. [11] and Diacinti et al. [21] The Ha/Hp ratio varied with a similar trend between different vertebrae in all three groups. The values were very close between our group and McCloskey’s. However, the values of Ha/Hp ratio of Diacinti et al. were all obviously higher than those in our group (Fig. 3). For Hm/Hp ratio, the values of Diacinti et al. were also significantly higher than ours. The difference in Hm/Hp ratio was relatively smaller between our group and McCloskey et al. (Fig. 3). Both Ha/Hp and Hm/Hp ratios were higher in Diacinti et al. than in McCloskey et al.
Fig. 3.
The racial difference in vertebral height ratios between Chinese, English (McCloskey et al. [11]) and Italian women (Diacinti et al. [21])
Discussion
Lateral radiography is a standard approach for the diagnosis of vertebral fracture, which is based on the assessment of vertebral deformity [27]. However, there is no agreed gold standard for the definition of vertebral fracture through the interpretation of radiographic appearance [17, 27]. Traditionally, clinical diagnosis has been made by radiologists or experienced clinicians through qualitative assessment of conventional radiographs [28]. Because the qualitative approach is an entirely subjective method, its validity is limited by poor inter-observer agreement and reproducibility [27]. Additionally, the conditions in epidemiologic investigation differ considerably than that in clinical diagnosis. In epidemiologic study, the assessment is often performed without specific clinical indications, and a large number of radiographs are viewed by a variety of observers with different levels of experience. Therefore, it is necessary to develop a definable, reproducible and objective method to detect vertebral fracture. Recently, various quantitative approaches have been employed in clinical and epidemiologic studies [17, 27]. A widely accepted quantitative method for identification of vertebral deformity is to compare the VH ratios of the affected vertebra with the reference values of that particular vertebra [11, 15, 16, 18]. Accordingly, determination of reference values of VH ratios is essential for the assessment of vertebral deformity [13].
The reference values of VH ratios for women were derived from either premenopausal population [13, 21] or a trimming procedure for the data from elderly postmenopausal women [6, 16, 22], which iteratively removed the number from the upper and lower tails of a distribution of VH ratio until the remaining data became normally distributed [6, 14, 22]. For both methods, any VH ratio lower than a given percentage or SD (e.g., 15% or 3 SD) below the mean can be regarded as vertebral deformity [6, 11, 13, 15, 16, 29]. However, the prevalence of vertebral deformity is likely to be overestimated by using the trimming method [11, 13], because VH ratios are often not normally distributed, leading to the cutoff values falling within the observed distribution of the data [13]. It suggests that the reference values of VH ratios for women should be collected from the premenopausal population (to avoid trimming) [13].
In the present study, we measured VH ratios in premenopausal women aged 19–25 years. The women within this age range are expected to have peak bone mass and vertebral height [30–33]. Before measurement, radiologists and experienced orthopedic surgeons reviewed all radiographs to exclude spinal deformities and congenital or degenerative abnormalities. For these women, the prevalence of osteoporotic vertebral fracture was presumed to be zero. Therefore, young premenopausal women can provide optimal reference data for the assessment of vertebral deformity, which may effectively reduce the false positives in the epidemiologic studies on vertebral fractures [13].
In premenopausal Chinese women, the vertebral heights increase from T4 to L3, in which the middle height is the lowest and the posterior is the highest; Ha/Hp and Hm/Hp ratios for these vertebrae range from 92 to 99% and 89 to 95%, respectively. These data suggest that vertebral bodies from T4 to L3 are in wedged and biconcave shape. The heights of L4 and L5 are lower than L3 and their shape becomes contra-wedged, in which Ha is greater than Hp. The variation of vertebral shape at different levels forms spinal curvatures, an anatomical feature conferring stability in the upright posture by reducing bending moments [34]. Vertebral deformity would change the smooth curvature of the spine, modifying the distribution of vertebral loading and increasing postural instability [21, 34–37]. Kyphosis, usually occurring in older persons, is likely to affect spinal inclination and postural balance, increasing the likelihood of falls [35, 38]. This can explain why vertebral deformity likely contributes to subsequent fracture [19]. It is well known that previous vertebral fracture is a major risk factor to increase the incidence of new vertebral fracture [36, 39, 40]. A possible mechanism is that osteoporotic vertebral fracture, especially multiple vertebral fractures, may cause severe thoracic and lumbar kyphosis [38]. The abnormal load distribution in vertebrae and posture instability would significantly increase the risk of new vertebral fracture.
A variety of methods have been developed for the assessment of vertebral deformities [11, 15, 16, 18]. Several comprehensive studies have compared different methods in the same population to determine the impact of methodology on the estimates of prevalent vertebral fractures and on the identification of individual patient or individual vertebra as being fractured [13, 15, 37]. Black et al. [15] reported that three commonly used methods developed by Melton et al. [18, 29], McClosky et al. [11] and themselves [15] could provide similar conclusions in the assessment of prevalent vertebral deformities in the same population. These methods are not suitable to define mild vertebral deformities [11, 15] because the mild deformities often confound with normal anatomical variation. It is difficult to find an optimal cutoff value for the definition of vertebral deformity. Any cutoff value is a trade-off between sensitivity and specificity [22]. The prevailing cutoff points are −15%, −20%, −3 SD and −4 SD from the reference mean values [11, 15, 16, 18]. The present study compared different cutoff values to determine their validity on the estimate of prevalent vertebral deformity in premenopausal Chinese women. The prevalence of vertebral deformity in young premenopausal women is presumed to be zero. Therefore, an ideal cutoff value is to allow the prevalence of vertebral deformity closing to zero in this population. The data demonstrated that −15% cutoff resulted in 18.3% of the subjects being associated with deformed vertebrae, which was significantly higher than other cutoffs. Thus, −15% is apparently not an ideal cutoff value for the assessment of vertebral deformity. However, the prevalence of vertebral deformity had no significant difference between cutoffs of −20% (3.3%) and −3 SD (5%), but most of VH ratios at −3 SD had a higher value than that at −20% (Table 2), suggesting that as a cutoff value −3 SD may be more sensitive than −20% in the definition of vertebral deformity. We also found that VH ratios at −3.5 SD cutoff point were not significantly decreased as compared with that at −20% cutoff point. Additionally, none of the subjects had a VH ratio lower than −3.5 SD cutoff point. Taken together, declined SD is probably more sensitive than declined percentage as a cutoff value for determining vertebral deformity. According to our data, −3.5 SD is an appreciable cutoff value for the definition of vertebral fracture in Chinese women. The prevalence of vertebral fracture may be underestimated if −4 SD is used.
Our data showed that the shape of the vertebral body was different between Chinese and European premenopausal women [11, 21]. It is likely that there are significant differences in related values between English and Italian women (Fig. 3) [11, 21]. These shape differences in vertebrae may be attributed to different races and ages of the participants. There are sufficient data to conclude that the shape of the vertebral body is apparently different among people of different ages, genders and races [22, 24, 25]. Interestingly, the values obtained from different communities may be different though the age, gender and race are the same [22]. The application of different inclusion criteria might be a contributor to this phenomenon. Therefore, the use of externally derived reference data may result in an inaccurate estimate of the prevalent vertebral fracture [22]. Based on these data, we propose that the reference values for vertebral shape should be obtained from an individual population, and that values from males and females should be separated [22].
Acknowledgments
This research was supported by the key discipline (Orthopedics and Traumatology) of Traditional Chinese Medicine in Shanghai (05 III 027-005).
Contributor Information
Yongqiang Chen, Email: chenyongqiang@medmail.com.cn.
Shijing Qiu, Email: qiu@bjc.hfh.edu.
References
- 1.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 2.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 3.Jalava T, Sarna S, Pylkkanen L, Mawer B, Kanis JA, Selby P, Davies M, Adams J, Francis RM, Robinson J, McCloskey E. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res. 2003;18:1254–1260. doi: 10.1359/jbmr.2003.18.7.1254. [DOI] [PubMed] [Google Scholar]
- 4.Nevitt MC, Thompson DE, Black DM, Rubin SR, Ensrud K, Yates AJ, Cummings SR. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med. 2000;160:77–85. doi: 10.1001/archinte.160.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SA, Tenenhouse A, Robertson L. Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:680–687. doi: 10.1007/s001980070066. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Palermo L, Nevitt MC, Genant HK, Christensen L, Cummings SR. Defining incident vertebral deformity: a prospective comparison of several approaches. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:90–101. doi: 10.1359/jbmr.1999.14.1.90. [DOI] [PubMed] [Google Scholar]
- 8.Kleerekoper M, Parfitt AM (1984) Measurement of vertebral fracture rates in osteoporosis. In: Christiansen C, Arnaud CD, Nordin BE, Parfitt AM, Peck WA, Riggs BL (eds) Proc Copenhagen International Symposium on Osteoporosis. Aalborg Stiftsbogtrykkeri, pp 103–109
- 9.Wu CY, Li J, Jergas M, Genant HK. Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int. 1995;5:354–370. doi: 10.1007/BF01622258. [DOI] [PubMed] [Google Scholar]
- 10.Rea JA, Chen MB, Li J, Potts E, Fan B, Blake GM, Steiger P, Smith IG, Genant HK, Fogelman I. Morphometric X-ray absorptiometry and morphometric radiography of the spine: a comparison of analysis precision in normal and osteoporotic subjects. Osteoporos Int. 1999;9:536–544. doi: 10.1007/s001980050182. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey EV, Spector TD, Eyres KS, Fern ED, O’Rourke N, Vasikaran S, Kanis JA. The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int. 1993;3:138–147. doi: 10.1007/BF01623275. [DOI] [PubMed] [Google Scholar]
- 12.Wasnich RD. Vertebral fracture epidemiology. Bone. 1996;18:179S–183S. doi: 10.1016/8756-3282(95)00499-8. [DOI] [PubMed] [Google Scholar]
- 13.Zebaze RM, Maalouf G, Wehbe J, Nehme A, Maalouf N, Seeman E. The varying distribution of intra- and inter-vertebral height ratios determines the prevalence of vertebral fractures. Bone. 2004;35:348–356. doi: 10.1016/j.bone.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Black DM, Cummings SR, Stone K, Hudes E, Palermo L, Steiger P. A new approach to defining normal vertebral dimensions. J Bone Miner Res. 1991;6:883–892. doi: 10.1002/jbmr.5650060814. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San Valentin R, Cummings SR. Comparison of methods for defining prevalent vertebral deformities: the Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:890–902. doi: 10.1002/jbmr.5650100610. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ, 3rd, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119. doi: 10.1007/BF01623271. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmi G, Diacinti D, Kuijk C, Aparisi F, Krestan C, Adams JE, Link TM. Vertebral morphometry: current methods and recent advances. Eur Radiol. 2008;18:1484–1496. doi: 10.1007/s00330-008-0899-8. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ, 3rd, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129:1000–1011. doi: 10.1093/oxfordjournals.aje.a115204. [DOI] [PubMed] [Google Scholar]
- 19.Pongchaiyakul C, Nguyen ND, Jones G, Center JR, Eisman JA, Nguyen TV. Asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective study. J Bone Miner Res. 2005;20:1349–1355. doi: 10.1359/JBMR.050317. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, Palermo L, Vogt M, Ensrud KE, Ewing S, Hochberg M, Nevitt MC, Black DM. Prevalent vertebral fractures in black women and white women. J Bone Miner Res. 2008;23:1458–1467. doi: 10.1359/jbmr.080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diacinti D, Acca M, D’Erasmo E, Tomei E, Mazzuoli GF. Aging changes in vertebral morphometry. Calcif Tissue Int. 1995;57:426–429. doi: 10.1007/BF00301945. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill TW, Varlow J, Felsenberg D, Johnell O, Weber K, Marchant F, Delmas PD, Cooper C, Kanis J, Silman AJ. Variation in vertebral height ratios in population studies. European Vertebral Osteoporosis Study Group. J Bone Miner Res. 1994;9:1895–1907. doi: 10.1002/jbmr.5650091209. [DOI] [PubMed] [Google Scholar]
- 23.Grados F, Fardellone P, Benammar M, Muller C, Roux C, Sebert JL. Influence of age and sex on vertebral shape indices assessed by radiographic morphometry. Osteoporos Int. 1999;10:450–455. doi: 10.1007/s001980050253. [DOI] [PubMed] [Google Scholar]
- 24.Salimzadeh A, Moghaddassi M, Alishiri GH, Owlia MB, Kohan L. Vertebral morphometry reference data by X-ray absorptiometry (MXA) in Iranian women. Clin Rheumatol. 2007;26:704–709. doi: 10.1007/s10067-006-0379-y. [DOI] [PubMed] [Google Scholar]
- 25.Ross PD, Wasnich RD, Davis JW, Vogel JM. Vertebral dimension differences between Caucasian populations, and between Caucasians and Japanese. Bone. 1991;12:107–112. doi: 10.1016/8756-3282(91)90008-7. [DOI] [PubMed] [Google Scholar]
- 26.Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 27.Ferrar L, Jiang G, Adams J, Eastell R. Identification of vertebral fractures: an update. Osteoporos Int. 2005;16:717–728. doi: 10.1007/s00198-005-1880-x. [DOI] [PubMed] [Google Scholar]
- 28.Kleerekoper M, Peterson EL, Nelson DA, Phillips E, Schork MA, Tilley BC, Parfitt AM. A randomized trial of sodium fluoride as a treatment for postmenopausal osteoporosis. Osteoporos Int. 1991;1:155–161. doi: 10.1007/BF01625446. [DOI] [PubMed] [Google Scholar]
- 29.Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ., 3rd Classification of vertebral fractures. J Bone Miner Res. 1991;6:207–215. doi: 10.1002/jbmr.5650060302. [DOI] [PubMed] [Google Scholar]
- 30.Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporos Int. 1994;4:7–13. doi: 10.1007/BF01623429. [DOI] [PubMed] [Google Scholar]
- 31.Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93:799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosekilde L. Age-related changes in bone mass, structure, and strength: effects of loading. Z Rheumatol. 2000;59:1–9. doi: 10.1007/s003930070031. [DOI] [PubMed] [Google Scholar]
- 33.Anderson M, Hwang SC, Green WT. Growth of the normal trunk in boys and girls during the second decade of life; related to age, maturity, and ossification of the iliac epiphyses. J Bone Joint Surg Am. 1965;47:1554–1564. [PubMed] [Google Scholar]
- 34.Zebaze RM, Maalouf G, Maalouf N, Seeman E. Loss of regularity in the curvature of the thoracolumbar spine: a measure of structural failure. J Bone Miner Res. 2004;19:1099–1104. doi: 10.1359/JBMR.040320. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa Y, Miyakoshi N, Kasukawa Y, Hongo M, Shimada Y (2009) Spinal curvature and postural balance in patients with osteoporosis. Osteoporos Int [Epub ahead of print] [DOI] [PubMed]
- 36.Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR. Long-term risk of incident vertebral fractures. JAMA. 2007;298:2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 37.Melton LJ, 3rd, Wenger DE, Atkinson EJ, Achenbach SJ, Berquist TH, Riggs BL, Jiang G, Eastell R. Influence of baseline deformity definition on subsequent vertebral fracture risk in postmenopausal women. Osteoporos Int. 2006;17:978–985. doi: 10.1007/s00198-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 38.Kado DM, Prenovost K, Crandall C. Narrative review: hyperkyphosis in older persons. Ann Intern Med. 2007;147:330–338. doi: 10.7326/0003-4819-147-5-200709040-00008. [DOI] [PubMed] [Google Scholar]
- 39.Ross PD, Genant HK, Davis JW, Miller PD, Wasnich RD. Predicting vertebral fracture incidence from prevalent fractures and bone density among non-black, osteoporotic women. Osteoporos Int. 1993;3:120–126. doi: 10.1007/BF01623272. [DOI] [PubMed] [Google Scholar]
- 40.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]