Abstract
The objectives of the study were to evaluate the association between lumbar paraspinal muscle density, evaluated on computed tomography (CT) and age, sex and BMI; and to evaluate the association of those changes with low back pain (LBP) and spinal degeneration features in a community-based sample. This study was an ancillary project to the Framingham Study. A sample of 3,529 participants aged 40–80 years had a CT scan performed to assess aortic calcification. 187 individuals were randomly enrolled in this study. LBP in the last 12 months was evaluated using self-report questionnaire. Density (in Hounsfield units) of multifidus and erector spinae was evaluated on CT. The prevalence of intervertebral disc narrowing, facet joint osteoarthritis (FJOA), spondylolysis, spondylolisthesis and spinal stenosis were also evaluated. We used linear regression models to examine the association of paraspinal muscles density with age, sex, BMI, LBP, and spinal degeneration features. The results show that in our study, men have higher density of paraspinal muscles than women, younger individuals have higher density than older ones and individuals with lower weight have higher muscle density than overweight. No differences between individuals with and without LBP were found. Significant association was found between L4 multifidus/erector spinae density and FJOA at L4–L5; between multifidus at L4 and spondylolisthesis at L4–5; and between erector spinae at L4 and L5 with disc narrowing at L4–5 and L5–S1, respectively. We conclude that the paraspinal muscle density decreases with age, and increases BMI. It is associated with at some levels FJOA, spondylolisthesis and disc narrowing at the same level, but not associated with occurrence of LBP.
Keywords: Low back pain, Paraspinal muscles, Multifidus, Erector spinae, Computed tomography
Introduction
Low back pain (LBP) is one of the main contributing factors to disability and time lost from work in western countries [57]. Despite the high prevalence of back pain and intensive study in this area, our understanding of the pathogenesis of some types of LBP is still insufficient. There is very modest information about the role of the back muscles in the etiology of LBP.
Medical imaging techniques provide non-invasive and reproducible [6, 29], information on muscle density, cross-sectional surface area (CSA), and other characteristics of muscles such as fatty infiltration. In healthy (non-symptomatic) individuals, computerized tomography (CT) may show disc degeneration or nerve root compression, spondylolysis, spondylolisthesis, and spinal stenosis. However, these findings neither predict the risk of LBP nor correlate with clinical symptoms [49]. Although the lack of correlation between pathology and pain is largely argued to be due to the multifactorial nature of the report of pain (including other biologic, psychologic and social factors); it is also possible that the poor relationship is explained by other factors that have not been evaluated in a routine CT evaluation. Among such factors are degenerative changes in facet joints [25], ligamentous damage [43] and changes (traumatic or degenerative) in paraspinal muscles [7, 37].
The CSA and density of the paraspinal muscles are affected by several variables such as age, physical condition, diet, weight, and back pain. With increasing age, there is a reduction in muscle volume [13, 35]. Current evidence suggests that the paraspinal muscles are smaller in patients with chronic LBP [4, 6, 40, 50] than in healthy individuals of similar age. Reduced size of multifidus has also been reported in acute LBP [16] and recent animal work has shown rapid changes in CSA in the multifidus muscle following discrete injury to an intervertebral disc [17]. In several imaging studies, chronic LBP was found to be associated with CT-evaluated muscle density (measured in Hounsfield units) [14, 19, 28]. Despite the range of studies that have investigated the association between back pain and muscle changes, none has aimed to relate muscle changes to specific radiologically identified pathology of the spine.
The aims of this study were (1) to evaluate the association between lumbar paraspinal muscle density, evaluated on CT and age, sex and BMI; and (2) to evaluate the association of those changes with LBP and spinal degeneration features in a community-based population.
Methods
Study design
Cross-sectional study.
Sample
This project was an ancillary project to the Framingham Study; a study that began in 1948 as a longitudinal population-based cohort study of the causes of heart disease. Initially, 5,209 men and women between the ages of 30 and 60 years living in Framingham, Massachusetts were enrolled. In 1971, 5,124 offspring (and their spouses) of the original cohort were entered into a prospective cohort study. More recently, the third generation (Gen3) of participants was recruited. A sample of 3,529 participants of the Framingham study (from the Offspring and Gen3 cohorts) aged 40–80 years had a CT scan performed to assess aortic calcification. The recruitment and conduct of CT scanning have been previously reported [18, 45]. During the latter part of the CT study, 191 participants were consecutively enrolled in this ancillary study to assess the association between radiographic features of the lumbosacral spine and LBP. Four individuals were not analyzed because of insufficient CT data.
LBP evaluation
All study participants undergoing multi-detector CT scan were asked to complete the modified Nordic Low Back Questionnaire [33]. The first question on this questionnaire was: “Have you had low back pain on most days of at least one month in the last 12 months?” Individuals’ who answered “yes” or “no” on the above question, were categorized in the present study as either having back pain or not (dichotomous index). Similar methods have been widely used in studies of work-related LBP [8, 11].
Imaging parameters
All eligible participants were imaged with an eight-slice multidetector CT (Lightspeed Ultra, GE, Milwaukee, WI, USA). Each subject underwent unenhanced abdominal CT that was performed using a sequential scan protocol with a slice collimation of 8 × 2.5 mm (120 KVp, 320/400 mA for 0.220 lbs body weight, respectively) during a single end inspiratory breath hold (typical duration 18 s). For the abdominal scan, 30 contiguous 5-mm thick slices of the abdomen were acquired covering 150 mm above the level of S1. CT scans were evaluated in blinded fashion with respect to clinical and personal data. To assess the reliability of spinal degeneration features evaluations on CT two readers (LK and AG) read and re-read 20 CTs separately and 2 weeks apart to assess the intra- and inter-rater reliability (kappa statistic). One musculoskeletal radiologist (AG) trained one reader (LK) that read all of the CTs, blinded to patient identifiers.
Paraspinal muscles density evaluation
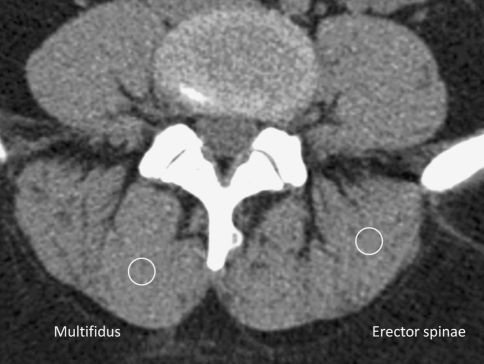
The density of the multifidus and erector spinae muscles were measured in Hounsfield units (HU) on both the left and right side at level of middle part of L3, L4, and L5 vertebrae. It was evaluated by measuring the mean density in the region of interest, using a 6-mm circle in the center of the muscle mass of the studied muscles, without visible fat deposits, if possible (Fig. 1). The measurement was done three times with slightly different location of the circle and the highest density value was used in the further analyses. A similar method was previously used in Keller et al. [29] study of paraspinal muscle density.
Fig. 1.
The CT image of paraspinal muscles. The circles illustrate how the density measurements were performed
Evaluation of spinal degeneration features
All spinal degeneration features were evaluated between L2 and S1 spinal levels.
Intervertebral disc narrowing
Disc narrowing was estimated using sagittal reformations using semiquantitative method proposed by Videman et al. [55, 56]. This method uses the following grades of disc narrowing: 0 = normal, disc higher than the upper disc (except disc L5–S1); 1 = slight, disc as high as the upper disc if it is normal; 2 = moderate, disc narrower than the upper disc if it is normal; 3 = severe, endplates almost in contact. For the present study, we collapsed this 4-grade score to dichotomous index: grades 0 and 1 were considered as no disc space narrowing and grades 2 and 3 as narrowed disc space.
Facet joint osteoarthritis (FJOA)
Four grades of FJOA were defined using criteria described in Kalichman et al. [27] and similar to those published by Weishaupt et al. [58]. In the part of this study we used the dichotomized index that was constructed on the basis the presence or absence of FJOA (≥grade 2) on any side at any level.
Spondylolysis and spondylolisthesis
The entire lumbar spine was reviewed for each case, using bone windows. Spondylolysis and spondylolisthesis were defined as present or absent (dichotomous indices) for each subject.
Spinal stenosis
Lumbar spine was reviewed using bone and soft tissue windows. For measurements of congenital spinal stenosis, the saggital measure of spinal canal was taken at the level of the middle of the vertebra. For measurements of acquired spinal stenosis, the saggital measure of spinal canal (between intervertebral disc, anteriorly and meeting point of two ligamenta flava, posteriorly) was taken at the level of intervertebral disc. We used 10-mm cut-points in the definition of spinal stenosis [2, 51, 54]. For this study, this index was dichotomized on the basis the presence or absence of lumbar spinal stenosis of any type and at any level.
Body mass index (BMI)
BMI was computed as the ratio of weight (in kg) divided by height (in m2).
Statistical analysis
To evaluate the level and side specific association between spinal degeneration features and density of paraspinal muscles we used the original measurements of density. At the first stage, we compared the muscle density between three measured spinal levels and between the right and left sides. To summarize and reduce the original variables into fewer composite variables, without loss of information, we performed a principal component analysis, using the density measurements of three levels, and if both sides, for multifidus and erector spinae separately. The first principal components (DenMult, density of multifidus; and DenES, density of erector spinae) were used as the outcomes in further analyses.
Second, to evaluate the association between paraspinal muscle density and classical epidemiological covariates, we performed linear regression analyses with muscle density indices as dependent variables and age, sex and BMI as independent variables.
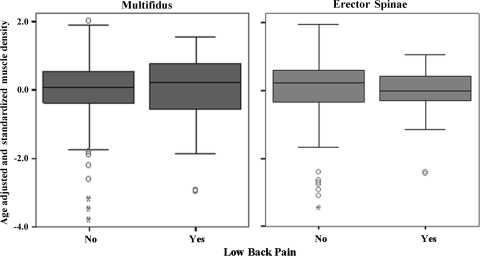
Third, we assessed the difference in muscle density between individuals with and without LBP, after adjusting for age, sex and BMI, and presented it graphically on a box-plot.
Finally, we evaluated if the density of paraspinal muscles was associated with the presence of spinal degeneration features. Using multiple linear regression models, we evaluated side and spinal level specific associations between densities of multifidus and erector spinae and FJOA, adjusting for age, sex and BMI. We also evaluated the spinal level specific associations between paraspinal muscles density and spinal degeneration features such as disc height, spondylolysis, spondylolisthesis and spinal stenosis at the corresponding levels. After that, we performed multiple regression analyses with muscle density indices as outcomes and spinal degeneration features as predictors, after adjusting for age, sex, and BMI. All statistical analyses were performed using SAS software, (SAS Institute Inc, Cary, NC, release 9.1).
Results
Results of reliability tests (kappa statistics) were as follows: the intra-observer reliability for muscle density of multifidus and erector spinae evaluation at different levels varied between 0.94 and 0.99. The inter-observer reliability ranged from 0.70 to 0.97, respectively. The intra-observer reliability for disc narrowing varied at different spinal levels between 0.84 and 0.90. The inter-observer reliability for disc narrowing ranged from 0.78 to 0.88. The intra-observer reliability for grading different FJ OA indices varied between 0.64 and 0.91. The inter-observer reliability ranged from 0.59 to 0.94. The intra-observer reliability for identification of spondylolysis was 1.00. The inter-observer reliability was 0.98. For spondylolisthesis, the intra-observer reliability varied at different levels between 0.95 and 1.00, and the inter-observer reliability ranged from 0.75 to 0.98. The intra-observer reliability varied at different spinal levels between 0.95 and 0.98 for congenital stenosis and between 0.92 and 0.98 for acquired stenosis. The inter-observer reliability ranged from 0.80 to 0.92 and from 0.86 to 0.96, respectively. This range of kappa statistics represents good to excellent reproducibility.
Table 1 lists the demographic characteristics of the 187 study participants. The study sample included 104 males and 83 females. The mean age was 52.6 ± 10.8 (age range 32–79). The mean BMI was 27.8 ± 5.0. Thirty-seven individuals, 18 (17%) men and 19 (23%) women suffered from LBP on most days in at least 1 month of the year that preceded the study.
Table 1.
Descriptive statistics of the studied sample (n = 187)
| Frequencies | Males | Females |
|---|---|---|
| N | 104 | 83 |
| LBP | 18 (17%) | 19 (23%) |
| Mean values | ||
| Age (years) | 51.9 ± 11.3 | 53.5 ± 10.2 |
| BMI (kg/m2) | 28.0 ± 4.2 | 27.69 ± 6.0 |
| Mean density of multifidus (HU) | 64.80 ± 11.05 | 57.12 ± 9.98 |
| Mean density of erector spinae (HU) | 56.84 ± 11.54 | 52.55 ± 7.41 |
The Pearson correlations between paraspinal muscle densities of right and left sides were very high, 0.83–0.90 (p < 0.0001) at different levels. The correlations between paraspinal muscle densities of the same side, but different spinal levels were between moderate to high, 0.65–0.84 (p < 0.0001). To summarize the dozen variables that were highly correlated, we performed principal component analysis and the first principal components that indicate the density of multifidus (DenMult) and density of erector spinae (DenES) were used in subsequent analyses. The corresponding scores were high and only one principal component with an Eigen-value >1.0 was identified. 78.3% of the total variation of density of multifidus muscles and 83.0% of the variation of density of erector spinae were retained in the principal components.
The association between DenMult and DenES and age, sex and BMI are shown in Table 2. There are significant negative associations between paraspinal muscle density and age, sex and BMI. Men have higher density of paraspinal muscles than women, younger individuals higher than older ones and individuals with lower weight have higher density of paraspinal muscles than those who are overweight. The standardized residuals from these regression analyses were used for the comparison between individuals with and without LBP. A substantial proportion of the variability remains unexplained with the R2 for DenMult and DenES models being 0.48 and 0.34, respectively.
Table 2.
Relation of muscle density to sex, age and BMI
| Variables | Parameter estimate | Standard error | t value | p | R2 of the model |
|---|---|---|---|---|---|
| DenMult | |||||
| Sex | −0.6840 | 0.094 | −7.2470 | 0.0000 | 0.480 |
| Age | −0.0410 | 0.004 | −9.2550 | 0.000 | |
| BMI | −0.0250 | 0.009 | −2.6550 | 0.009 | |
| DenES | |||||
| Sex | −0.4420 | 0.109 | −4.0430 | 0.0000 | 0.338 |
| Age | −0.0370 | 0.005 | −7.1860 | 0.000 | |
| BMI | −0.0360 | 0.011 | −3.3210 | 0.001 | |
The box-plots depicted in Fig. 2 show the comparison of density of paraspinal muscles between groups of individuals with and without LBP, after adjusting for age, sex and BMI. No significant differences between groups were found (p = 0.20 for multifidus; p = 0.14 for erector spinae).
Fig. 2.
Box-plot of comparison of age adjusted muscle density of Multifidus (left plot) and Erector Spinae (right plot) between groups of individuals with and without LBP
Analysis of side and spinal level specific association between FJOA and density of paraspinal muscles (Table 3) showed that after adjustment for age, sex and BMI, there are statistically significant associations mainly between DenES and DenMult at L4 level and FJOA at L4–L5 level. Statistically significant association (after adjustment for age, sex and BMI) were found also between DenES at L4 and L5 levels and disc narrowing at L4–L5 and L5–S1 levels, correspondingly; and between DenMult at L4 level and spondylolisthesis at L4–L5 level (Table 4).
Table 3.
Side and spinal level specific associations between paraspinal muscles density and facet joint OA, after adjustment for age, sex and BMI
| Facet joint OA | ||||||
|---|---|---|---|---|---|---|
| Grade 1 versus 0 | Grade 2 versus 0 | Grade 3 versus 0 | ||||
| Estimate | p value | Estimate | p value | Estimate | p value | |
| Density multifidus left L3 | −0.583 | 0.702 | −3.562 | 0.079 | −2.757 | 0.228 |
| Density multifidus left L4 | −1.326 | 0.412 | −2.129 | 0.254 | −7.169 | 0.0002 |
| Density multifidus left L5 | −2.753 | 0.176 | −2.863 | 0.269 | −5.467 | 0.078 |
| Density multifidus right L3 | 2.492 | 0.178 | −0.859 | 0.670 | −2.806 | 0.271 |
| Density multifidus right L4 | −3.420 | 0.049 | −4.430 | 0.026 | −4.334 | 0.029 |
| Density multifidus right L5 | 0.495 | 0.814 | 0.811 | 0.743 | −0.059 | 0.983 |
| Density erector spinae left L3 | 0.074 | 0.947 | −0.157 | 0.916 | −1.128 | 0.500 |
| Density erector spinae left L4 | −0.743 | 0.623 | 0.291 | 0.868 | −4.664 | 0.010 |
| Density erector spinae left L5 | −4.261 | 0.023 | −4.125 | 0.084 | −3.294 | 0.246 |
| Density erector spinae right L3 | 0.869 | 0.456 | −0.873 | 0.491 | −2.048 | 0.203 |
| Density erector spinae right L4 | −1.650 | 0.308 | −1.273 | 0.489 | −3.529 | 0.056 |
| Density erector spinae right L5 | −3.674 | 0.123 | −3.525 | 0.209 | −1.482 | 0.633 |
Statistically significant association at p < 0.05 marked bold; statistically significant association at p < 0.1 marked italic
Table 4.
Spinal level specific associations between paraspinal muscles density and spinal degeneration features, after adjustment for age, sex and BMI
| Disc narrowing | Spondylolysis | Spondylolisthesis | Spinal stenosis | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | |
| Density multifidus L3 | −1.411 | 0.385 | 0.242 | 0.975 | 0.372 | 0.937 | 2.816 | 0.443 |
| Density multifidus L4 | −2.734 | 0.101 | −2.793 | 0.484 | −7.500 | 0.011 | −0.515 | 0.899 |
| Density multifidus L5 | −2.940 | 0.109 | 2.515 | 0.345 | −3.208 | 0.205 | 0.316 | 0.931 |
| Density erector spinae L3 | −1.435 | 0.195 | −5.030 | 0.352 | −2.521 | 0.432 | −0.090 | 0.971 |
| Density erector spinae L4 | −2.809 | 0.045 | −6.261 | 0.099 | −2.310 | 0.354 | −2.721 | 0.424 |
| Density erector spinae L5 | −6.229 | 0.004 | 2.518 | 0.429 | −2.790 | 0.347 | −1.515 | 0.721 |
Statistically significant association at p < 0.05 marked bold; statistically significant association at p < 0.1 marked italic
In a multiple regression analyses where muscle density indices were used as outcomes and spinal degeneration features as predictors, after adjusting for age, sex, and BMI, only spondylolisthesis showed statistically significant negative association with DenMult (p = 0.0225); and disc narrowing (p = 0.0579) and spondylolisthesis (p = 0.0844) showed a trend for negative association with DenES.
Discussion
A variety of muscles, including the long and short paraspinal muscles, psoas, and quadratus lumborum play essential roles in stabilization and movement of the spine [5, 44] and are potentially implicated in the genesis of back pain. There is a growing body of evidence for association between paraspinal muscles degeneration and LBP. In several imaging studies, LBP has been investigated in relation to paraspinal muscle CSA [1, 6, 12, 14, 16, 28, 41, 47, 48, 52], MRI signal intensity [10], and fat infiltration [1, 6, 12, 14, 19, 21, 23, 28, 32, 41, 47, 52]. In patients with chronic LBP, studies consistently show a decrease in CSA [6, 12, 23], which may reach 10% compared to healthy individuals [19]. However, the extent of muscle changes is not necessarily related to symptom duration [39]. Danneels et al. [6] found the atrophy was selective for multifidus, as neither the psoas nor erector spinae muscle masses were significantly smaller compared with matched controls. Hides et al. [16] found marked wasting of multifidus on the symptomatic side, isolated to one vertebral level in patients with acute LBP. They proposed that the wasting was not likely to be due to disuse atrophy because of the rapidity of onset and localized distribution. Animal studies have shown atrophy to develop rapidly after experimental injury to an intervertebral disc [17]. Again these changes were localized to the level of injury and were associated with rapid changes in intra-muscular fat.
Several studies evaluated the association between CT-evaluated paraspinal muscles density and LBP [14, 15, 19, 28, 29, 34, 40]. Muscle density is an expression of degeneration of the muscles and reflects the number of muscle fibers, the area of the individual muscle fiber, and the packing of the contractile material [22], whereas the CSA is mainly determined by the total number of muscle fibers, to a lesser degree, the size of the fibers [35, 36], and the quantity of fat in the muscle [6, 9]. Laasonen [34] more than two decades ago described the decrease in CT-evaluated density of sacrospinal muscles after spinal surgery. In a study of Hultman et al. [19], CT-evaluated density of erector spinae was significantly decreased in males with chronic (more than 3 years) LBP compared to males without chronic LBP. However, there was no association between muscle density and strength and endurance parameters. Hicks et al. [14] in a large population-based sample (n = 1,527) also found that lower paraspinal muscle density was associated with higher LBP severity during the past year. The study demonstrated that paraspinal muscle density was more consistently associated with physical function than CSA.
In the present community-based study, we found no association between paraspinal muscles density and the occurrence of LBP in the last year. Therefore, we cannot support the hypothesis of association between paraspinal muscle density and LBP. However, this could be related to our criteria for back pain, or because our measure combined data across levels, which may have reduced the sensitivity. This is important to consider as several earlier studies have identified changes only at specific levels of the spine [6, 16]. In this study, we could not investigate changes specifically associated with the level of pain as it was not possible to determine which was the symptomatic level(s) in this population. It is also possible that the study sample was not large enough to detect an association between LBP and muscle density as we had only 38 individuals with LBP. Furthermore, the variables used in this study for LBP estimation may not have had sufficient sensitivity; it is possible that changes in muscle may be more prevalent in those with greater intensity or duration of pain.
Our study is the first to show that CT-evaluated paraspinal muscle density is associated with specific features of spinal degeneration such as FJOA, disc narrowing and spondylolisthesis. The data about prevalence of spinal degeneration features in this studied sample and their association with LBP were previously published [24, 26, 27]. Although we were only able to identify a significant relationship between multifidus density and spondylolisthesis when we used the summative measure from the principle component analysis, when data were considered separately for each level we identified an association between muscle density of multifidus and/or erector spinae at a specific lumbar level and FJOA, disc narrowing, and spondylolisthesis and related levels. This is consistent with data of Hides [16], and Hodges [17] following acute pain/injury and Danneels [6] in chronic LBP, L4 multifidus density was reduced in association with FJOA or spondylolisthesis at L4–5. As multifidus fibers arising from L4 arise from the caudal border of the spinous process and adjacent lamina [38], their bulk lies adjacent to L5. Taken together with the location of sampling (in the lateral region of the muscle related to longer more superficial fibers of the muscle), this suggests greatest density change in the long fibers arising from proximal segments. This also applies to the erector spinae data.
A recent MRI study [20] showed asymmetry in MRI-evaluated density of pure muscle CSA in 78.6% patients with unilateral lumbosacral radiculopathy in comparison with 24% in individuals with herniated intervertebral disc or 10% in a healthy control group. The presence of paraspinal muscle changes in people with spondylolisthesis, as it was shown in the present study, provides further understanding of the possible mechanisms for efficacy of exercise in interventions in LBP. For instance, O’Sullivan et al. [42] reported good clinical efficacy (decreased pain intensity and functional disability) of specific training of muscles surrounding the spine in individuals with spondylolysis and spondylolisthesis. Our data that also show a relationship of FJOA (multifidus and erector spinae) and disc space narrowing (erector spinae) with muscle changes provides justification for trials of training in other populations.
Results of our study demonstrated that paraspinal muscle density is higher in men than in women and it decreases with age. Surprisingly, we found only one publication that described the sex differences in CT-evaluated muscle density [3] and one about the change of the density with age [53]. Previous studies of healthy individuals have found smaller CSA of paraspinal muscles in women than men [30] and decreased CSA with advancing age [39, 46]. We believe that additional studies are needed to establish the normal parameters of paraspinal muscle density for males and females and for different age groups. This would enable identification of pathological deviation in parameters of muscle density and development of prevention and treatment strategies for spinal degeneration conditions.
In the present study, we found a statistically significant negative correlation between paraspinal muscle density and BMI. Similar negative association has been found between CT-evaluated muscle density measured in the mid thigh and BMI [31]. On the other hand, no association has been found between fat deposits in the back muscles, evaluated by MRI, and weight [32, 46].
There are some limitations of our study that warrant further discussion and investigation. The relation between muscle density and some of the spinal degeneration features were not found consistently at all levels on both sides could be a consequence of multiple testing. These findings need to be replicated and ideally done so in larger study samples than our own.
Conclusions
Results of our community-based study demonstrated that CT-evaluated paraspinal muscle density decreases with age, and increasing BMI. We found some side and spinal level specific association between FJOA and density of multifidus and erector spinae, and spinal level specific association between density of multifidus and spondylolisthesis and between density of erector spinae and intervertebral disc narrowing. No association was found between paraspinal muscle density and occurrence of LBP. Additional studies, especially longitudinal ones, are needed to establish the association between LBP, spinal degeneration features and change in paraspinal muscle density.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study contract (No. N01-HC-25195) for the recruitment, enrollment, and examination of the Offspring and Third Generation Cohort and the imaging by computed tomography scan.
Conflict of interest statement None of the authors have any conflict of interest regarding the contents of this article. L. K. is supported by an Arthritis Foundation Postdoctoral Grant. P. H. is supported by a Research Fellowship from the National Health and Medical Research Council of Australia.
References
- 1.Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine. 2004;29:E515–E519. doi: 10.1097/01.brs.0000144405.11661.eb. [DOI] [PubMed] [Google Scholar]
- 2.Bolender NF, Schonstrom NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985;67:240–246. [PubMed] [Google Scholar]
- 3.Bulcke JA, Termote JL, Palmers Y, Crolla D. Computed tomography of the human skeletal muscular system. Neuroradiology. 1979;17:127–136. doi: 10.1007/BF00339869. [DOI] [PubMed] [Google Scholar]
- 4.Cooper RG, St Clair Forbes W, Jayson MI. Radiographic demonstration of paraspinal muscle wasting in patients with chronic low back pain. Br J Rheumatol. 1992;31:389–394. doi: 10.1093/rheumatology/31.6.389. [DOI] [PubMed] [Google Scholar]
- 5.Crisco JJ, 3rd, Panjabi MM. The intersegmental and multisegmental muscles of the lumbar spine. A biomechanical model comparing lateral stabilizing potential. Spine. 1991;16:793–799. doi: 10.1097/00007632-199107000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demoulin C, Crielaard JM, Vanderthommen M. Spinal muscle evaluation in healthy individuals and low-back-pain patients: a literature review. Joint Bone Spine. 2007;74:9–13. doi: 10.1016/j.jbspin.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Dovrat E, Katz-Leurer M. Cold exposure and low back pain in store workers in Israel. Am J Ind Med. 2007;50:626–631. doi: 10.1002/ajim.20488. [DOI] [PubMed] [Google Scholar]
- 9.Elliott JM, Jull GA, Noteboom JT, Durbridge GL, Gibbon WW. Magnetic resonance imaging study of cross-sectional area of the cervical extensor musculature in an asymptomatic cohort. Clin Anat. 2007;20:35–40. doi: 10.1002/ca.20252. [DOI] [PubMed] [Google Scholar]
- 10.Flicker PL, Fleckenstein JL, Ferry K, Payne J, Ward C, Mayer T, Parkey RW, Peshock RM. Lumbar muscle usage in chronic low back pain. Magnetic resonance image evaluation. Spine. 1993;18:582–586. doi: 10.1097/00007632-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ghaffari M, Alipour A, Jensen I, Farshad AA, Vingard E. Low back pain among Iranian industrial workers. Occup Med (Lond) 2006;56:455–460. doi: 10.1093/occmed/kql062. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons LE, Latikka P, Videman T, Manninen H, Battie MC. The association of trunk muscle cross-sectional area and magnetic resonance image parameters with isokinetic and psychophysical lifting strength and static back muscle endurance in men. J Spinal Disord. 1997;10:398–403. doi: 10.1097/00002517-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097X.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 14.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 15.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–1424. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 16.Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine. 2006;31:2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Siebert U, Bull-Stewart A, Achenbach S, Ferencik M, Moselewski F, Brady TJ, Massaro JM, O’Donnell CJ. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort-consequences for progression studies. Eur J Radiol. 2006;57:396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Hultman G, Nordin M, Saraste H, Ohlsen H. Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. J Spinal Disord. 1993;6:114–123. doi: 10.1097/00002517-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine. 2007;32:E598–E602. doi: 10.1097/BRS.0b013e318155837b. [DOI] [PubMed] [Google Scholar]
- 21.Jinkins JR. Lumbosacral interspinous ligament rupture associated with acute intrinsic spinal muscle degeneration. JBR-BTR. 2003;86:226–230. [PubMed] [Google Scholar]
- 22.Jones DA, Rutherford OM, Parker DF. Physiological changes in skeletal muscle as a result of strength training. Q J Exp Physiol. 1989;74:233–256. doi: 10.1113/expphysiol.1989.sp003268. [DOI] [PubMed] [Google Scholar]
- 23.Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–149. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 24.Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalichman L, Hunter D. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37:69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34:199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalichman L, Li L, Kim D, Guermazi A, Berkin V, O’Donnell C, Hoffmann U, Cole R, Hunter D. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller A, Brox JI, Gunderson R, Holm I, Friis A, Reikeras O. Trunk muscle strength, cross-sectional area, and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine. 2004;29:3–8. doi: 10.1097/01.BRS.0000103946.26548.EB. [DOI] [PubMed] [Google Scholar]
- 29.Keller A, Gunderson R, Reikeras O, Brox JI. Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine. 2003;28:1455–1460. doi: 10.1097/00007632-200307010-00017. [DOI] [PubMed] [Google Scholar]
- 30.Keller A, Johansen JG, Hellesnes J, Brox JI. Predictors of isokinetic back muscle strength in patients with low back pain. Spine. 1999;24:275–280. doi: 10.1097/00007632-199902010-00016. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 32.Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2. doi: 10.1186/1741-7015-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sorensen F, Andersson G, Jorgensen K. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-X. [DOI] [PubMed] [Google Scholar]
- 34.Laasonen EM. Atrophy of sacrospinal muscle groups in patients with chronic, diffusely radiating lumbar back pain. Neuroradiology. 1984;26:9–13. doi: 10.1007/BF00328195. [DOI] [PubMed] [Google Scholar]
- 35.Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No:11–16 [DOI] [PubMed]
- 36.Lexell J, Downham D. What determines the muscle cross-sectional area? J Neurol Sci. 1992;111:113–114. doi: 10.1016/0022-510X(92)90119-6. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11:254–263. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine. 1987;12:658–668. doi: 10.1097/00007632-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Mannion AF, Kaser L, Weber E, Rhyner A, Dvorak J, Muntener M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9:273–281. doi: 10.1007/s005860000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer TG, Vanharanta H, Gatchel RJ, Mooney V, Barnes D, Judge L, Smith S, Terry A. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine. 1989;14:33–36. doi: 10.1097/00007632-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 41.McLoughlin RF, D’Arcy EM, Brittain MM, Fitzgerald O, Masterson JB. The significance of fat and muscle areas in the lumbar paraspinal space: a CT study. J Comput Assist Tomogr. 1994;18:275–278. doi: 10.1097/00004728-199403000-00021. [DOI] [PubMed] [Google Scholar]
- 42.O’Sullivan PB, Phyty GD, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997;22:2959–2967. doi: 10.1097/00007632-199712150-00020. [DOI] [PubMed] [Google Scholar]
- 43.Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, Murabito JM, Massaro JM, Hoffmann U, O’Donnell CJ. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 46.Parkkola R, Kormano M. Lumbar disc and back muscle degeneration on MRI: correlation to age and body mass. J Spinal Disord. 1992;5:86–92. doi: 10.1097/00002517-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18:830–836. doi: 10.1097/00007632-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Peltonen JE, Taimela S, Erkintalo M, Salminen JJ, Oksanen A, Kujala UM. Back extensor and psoas muscle cross-sectional area, prior physical training, and trunk muscle strength—a longitudinal study in adolescent girls. Eur J Appl Physiol Occup Physiol. 1998;77:66–71. doi: 10.1007/s004210050301. [DOI] [PubMed] [Google Scholar]
- 49.Savage RA, Whitehouse GH, Roberts N. The relationship between the magnetic resonance imaging appearance of the lumbar spine and low back pain, age and occupation in males. Eur Spine J. 1997;6:106–114. doi: 10.1007/BF01358742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Sortland O, Magnaes B, Hauge T. Functional myelography with metrizamide in the diagnosis of lumbar spinal stenosis. Acta Radiol Suppl. 1977;355:42–54. [PubMed] [Google Scholar]
- 52.Storheim K, Holm I, Gunderson R, Brox JI, Bo K. The effect of comprehensive group training on cross-sectional area, density, and strength of paraspinal muscles in patients sick-listed for subacute low back pain. J Spinal Disord Tech. 2003;16:271–279. doi: 10.1097/00024720-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Termote JL, Baert A, Crolla D, Palmers Y, Bulcke JA. Computed tomography of the normal and pathologic muscular system. Radiology. 1980;137:439–444. doi: 10.1148/radiology.137.2.7433677. [DOI] [PubMed] [Google Scholar]
- 54.Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine. 1979;4:369–378. doi: 10.1097/00007632-197907000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Videman T, Battie MC, Gibbons LE, Maravilla K, Manninen H, Kaprio J. Associations between back pain history and lumbar MRI findings. Spine. 2003;28:582–588. doi: 10.1097/00007632-200303150-00013. [DOI] [PubMed] [Google Scholar]
- 56.Videman T, Battie MC, Ripatti S, Gill K, Manninen H, Kaprio J. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine. 2006;31:671–678. doi: 10.1097/01.brs.0000202558.86309.ea. [DOI] [PubMed] [Google Scholar]
- 57.Waddell G. Low back pain: a twentieth century health care enigma. Spine. 1996;21:2820–2825. doi: 10.1097/00007632-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 58.Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28:215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]