Abstract
Nanotechnology is finding increasing application in biology and medicine. As with other pharmaceutical formulations and medical devices intended for use in animals and human patients, contamination of nanoparticles with bacterial endotoxins should be thoroughly investigated before preclinical in vitro and in vivo characterization. Traditional methods to study endotoxin contamination include the in vitro quantitative limulus amoebocyte lysate test and the in vivo qualitative rabbit pyrogen test. Both of these tests have long history of use for traditional pharmaceuticals and medical devices and are routinely used in drug development. Here we report that nanoparticles often interfere with these traditional endotoxin detection tests and suggest approaches to detect and overcome such interferences.
Keywords: endotoxin, in vitro assay, interference, limulus amoebocyte lysate, lipopolysaccharide, nanoparticles, rabbit pyrogen test
Lipopolysaccharide (LPS, endotoxin) is the main component of the cell walls of Gram-negative bacteria and is a very potent initiator of inflammatory reactions in mammalians. It is recognized by most cell types in the body, although immune cells (monocytes, macrophages and dendritic cells) are the most sensitive responders. Endotoxin interaction with specialized cellular receptors, CD14 and Toll-like receptor-4/MD2, results in production of a broad panel of inflammatory cytokines [1,2]. The presence of some of these inflammatory cytokines (e.g., TNF-α), may result in tissue damage if produced in large quantities. Others, primarily IL-1β and IL-6, are responsible for febrile reactions and fever [3]. Owing to the potential for severe immune response, endotoxin levels in pharmaceutical products and medical devices are strictly regulated, with allowable maxima of 5 EU/kg/h for most drug products and 0.2 EU/kg/h for intrathecally administered drugs [4,101].
Drug formulations using nanotechnology are finding increasing application in many areas of medicine, especially for the treatment of cancer. Many nanomedicines for targeted drug delivery of chemotherapeutics have matured beyond the discovery phase of research, and a few such products are US FDA approved and on markets. Such targeted nanodrug delivery systems can minimize dosages, reduce systemic toxicity and reduce adverse side effects of chemotherapeutics, increasing the overall therapeutic efficacy. Endotoxin contamination can be a significant hurdle to the preclinical development of nanoparticle formulations. The large surface areas and high reactivity of nanoparticles make them potential targets for contamination with bacterial endotoxins [5]. These factors, along with the fact that nanoparticles are frequently synthesized on equipment that may be novel to the pharmaceutical industry, causes endotoxin contamination to be common among many nanoparticle formulations undergoing preclinical characterization [5]. Such contamination may cause misleading results in toxicity screens (nanoformulations that are not inherently toxic may appear so due to contamination) and in efficacy tests for certain applications (e.g., endotoxin per se has shown anticancer efficacy). Contamination is often difficult to identify due to nanoparticle interference with traditional assays [6]. Many conventional (i.e., not nano) pharmaceutical formulations also interfere with tests for endotoxin detection, but the large variation in nanoparticle physicochemical properties causes the spectrum of nanoparticle interferences to be quite wide, making an individual instance of interference difficult to detect.
In recent studies, endotoxin contamination of gold nanoparticles was shown to be associated with undesired inflammatory reactions, while purified gold nanoparticles did not cause an inflammatory response [5]. Endotoxin itself has been shown to cause tumor regression and was proposed as a drug in clinical oncology trials [7], although later discontinued due to severe immunotoxicity. These data suggest that endotoxin contamination in nanoparticle formulations intended for cancer therapy may exhibit cytotoxic effects on its own and, therefore, confound results of efficacy studies. Since endotoxin may influence the results of toxicity and efficacy studies, it is important to identify endotoxin contamination before such studies, in order to avoid misinterpretation of study results.
Traditionally, medicine and device pyrogenicity (ability to cause fever, which may or may not be linked to endotoxin levels) is assessed with the in vitro limulus amoebocyte lysate (LAL) assay for endotoxin quantification and the qualitative in vivo rabbit pyrogen test (RPT). The LAL test has three formats: chromogenic, turbidity and gel clot. Here we report that certain types of nanoparticles interfere with one or more of the LAL test formats. Even when the US Pharmacopoeia (USP) formal requirements for validity of the LAL test are met, various formats of the LAL test may produce different results for the same nanoformulation. We show that spike recovery controls (i.e., inhibition enhancement controls prepared by spiking known concentration of endotoxin standard into the nanoparticle formulation under investigation) may help identify interference with a particular LAL test format, although such controls cannot conclusively eliminate the possibility of interference. In situations where the disparity between endotoxin levels obtained by the various LAL formats is large, we found the qualitative in vivo RPT to be useful for verification of LAL findings.
Experimental
Reagents
Citrate stabilized gold nanoparticles (average particle diameter: 30 nm) were purchased from TedPella Inc. (Redding, CA, USA); G5 carboxy-terminated polyamidoamine (PAMAM) dendrimers were obtained from Dendritic Nanotechnologies Inc. (Mount Pleasant, MI, USA). Prepharmaceutical and poly(malic acid) (PMLA)–poly(ethylene glycol) (PEG) (PMLA–PEG) nanoparticles were synthesized as previously described [8,9]. Purification of PMLA–PEG nanoparticles from endotoxin was performed according to the procedure described by Aida and Pabst [10] without modifications.
LAL assays
Reagents and kits for LAL tests were from the following sources: chromogenic LAL – end point chromogenic QCL1000 LAL kit and kinetic chromogenic kit, Lonza (Walkersville, MD, USA); turbidity LAL – PyrosKinetix instrument, endotoxin standard, LAL-grade water and lysate were from Associates of Cape Code Inc. (East Falmouth, MA, USA); gel clot LAL – endotoxin standard, LAL water, lysate and PYROTUBE-S gel-clot tubes were from Associates of Cape Code Inc. (East Falmouth, MA, USA). SpectraMax M5 plate reader was used to acquire data from chromogenic LAL. Individual LAL assays were performed according to the manufacturer’s instructions. Assay range for end point chromogenic LAL was from 0.1 to 1.0 EU/ml, for kinetic chromogenic LAL from 0.005 to 5.0 EU/ml, for gel-clot LAL from 0.25 to 2λ (λ is the sensitivity of the lysate provided for each lot of the lysate by the manufacturer), and for kinetic turbidity LAL from 0.001 to 1.0 EU/ml. Standard curves (calibration curves) were prepared by spiking known amounts of USP-certified endotoxin standard into endotoxin-free (LAL-grade) water. In addition to the standard curve, each individual run of each LAL assay included a set of quality controls; positive quality controls (QC) for each assay were prepared by spiking endotoxin standard, at the standard curve midpoint concentration, into LAL-grade water. Inhibition/enhancement controls (IEC) were prepared by spiking the same amount of endotoxin standard used in QC into the nanoparticle formulation. IEC were used within 30 min after preparation, whilst long-term storage was avoided due to unknown stability of endotoxin in the presence of nanoparticles and unknown stability of nanoparticles in the presence of exogenous endotoxin. Results from each individual assay run were not considered valid unless the precision and accuracy of the standard curve and quality control were within 25% and the inhibition/enhancement control exhibited 50–200% spike recovery. These acceptance criteria are in accordance with those mandated by the FDA guideline and USP standard for the LAL test [4,101]. Each nanoparticle sample and IEC was tested in duplicate and repeated three times. For each formulation at least four dilutions of the test sample were tested. If endotoxin results calculated for the sample at different dilutions were more than 25% different, the results did not qualify to be valid and were not reported.
Rabbit pyrogen test
The RPT was conducted according to USP standard, USP 151 [11]. Briefly, New Zealand rabbits were injected via ear vein with 10 ml of nanoparticles per kg of body weight. Animal body temperatures were measured before the injection and every 30 min between 1 and 3 h postinjection. A total of eight animals were tested.
Dynamic light scattering
A Malvern Zetasizer Nano ZS instrument (Southborough, MA, USA) with back scattering detector (173°C, 633 nm laser wavelength) was used for measuring the hydrodynamic size (diameter) in batch mode at 25°C in a low volume quartz cuvette (pathlength: 10 mm). PMLA and PMLA–PEG samples were prepared at a concentration of 2 mg/ml in PBS and filtered through a 0.02 μm filter. Citrate stabilized gold nanoparticles samples were diluted tenfold in 10 mM NaCl and filtered through a 0.45 μm filter. G5 carboxy-terminated PAMAM dendrimer samples were prepared at a concentration of 1 mg/ml in PBS and filtered through a 0.02 μm filter. A minimum of twelve measurements per sample were made. Hydrodynamic size is reported as the intensity-weighted average (Int-Peak).
Zeta potential
Zeta-potential provides a measure of the electrostatic potential at the surface of the electrical double layer and the bulk medium, which is related to the nanoparticle surface charge. A Malvern Zetasizer Nano ZS instrument was used to measure zeta-potential at 25°C. PMLA, PMLA–PEG nanoparticles and G5 carboxy-terminated PAMAM dendrimer samples were prepared at a concentration of 10, 2 and 1 mg/ml, respectively, in 10 mM NaCl. Citrate stabilized gold nanoparticles samples were diluted tenfold in 10 mM NaCl. An applied voltage of 90, 150, 100 and 100 V was used for the PMLA, PMLA–PEG, G5 carboxy-terminated PAMAM dendrimer and citrate stabilized gold nanoparticles samples, respectively. Samples were loaded into prerinsed folded capillary cells and a minimum of three measurements were made per sample.
Results & discussion
Nanoparticle physicochemical properties
Dynamic light scattering (DLS) is a useful way to obtain information about the hydrodynamic size (diameter) of a nanoparticle in solution. Nanoparticle hydrodynamic size is often more relevant to interaction with biological systems than the core size [6]. It has also been observed that hydrodynamic size may change when a nanoparticle is exposed to biological matrix [12,13], as plasma proteins and other molecules may adhere to the particle and increase its effective hydrodynamic diameter. Therefore, the physicochemical characterization of nanoparticles used in this study included measurement of particle size by DLS (hydrodynamic diameter) and measurement of the zeta-potential. The zeta-potential is the electrical potential at the surface separating the molecules that tumble with the nanoparticle in solution and the molecules of the bulk solvent. A nanoparticle’s zeta-potential is related to its surface charge, which has been shown to play a role in nanoparticle uptake and plasma–protein binding [12,13], and may be important for endotoxin adherence. The results of the hydrodynamic size measurement and zeta-potential measurement are summarized in Table 1.
Table 1.
Physicochemical properties of nanoparticles under study.
| Particles | Intensity-weighted hydrodynamic diameter by DLS (nm) | Zeta potential (mV) |
|---|---|---|
| Gold colloids | 34.4 ± 0.3 | −30.2 ± 2.2 |
| PMLA NP | 11.7 ± 0.2 | −22.7 ± 1.1 |
| PMLA–PEG NP | 18.8 ± 6.1 | −6.3 ± 0.6 |
| PAMAM dendrimers | 7.0 ± 0.2 | −36.6 ± 2.2 |
Particle size and zeta-potential were analyzed as described in the experimental section. Shown is mean result plus or minus standard deviation based on 12 data points (n = 12).
DLS: Dynamic light scattering; NP: Nanoparticle; PAMAM: Poly(amidoamine); PEG: Poly(ethylene glycol); PMLA: Prepharmaceutical β-polymalic acid.
Nanoparticles can interfere with one or more LAL assay formats
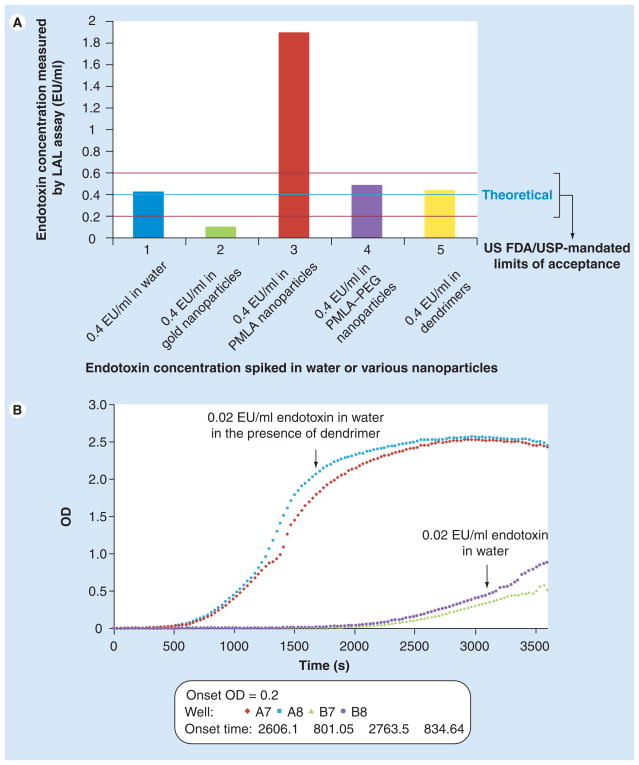
Inhibition/enhancement controls are essential for identifying nanoparticle interference with the LAL test. When the quality control sample (a known amount of endotoxin standard spiked into LAL-grade water) was analyzed, the percent spike recovery was almost 100%, indicating that endotoxin can be reliably quantified in water (Figure 1a, blue bar). However, when the same amount of endotoxin standard was spiked into citrate stabilized colloidal gold nanoparticles, the amount of detected endotoxin was below 50% of the spiked (theoretical) amount (Figure 1a, green bar). Addition of endotoxin into PMLA nanoparticles resulted in a greater than 50% overestimation of the known amount of spiked endotoxin (Figure 1a, red bar). In contrast to PMLA particles, IEC controls for PEG-modified PMLA nanoparticles were within FDA/USP mandated limits of acceptance (Figure 1a, purple bars). No interference was also seen with carboxy-terminated PAMAM dendrimers (Figure 1a, yellow bars). These data show that endotoxin levels obtained from the end point chromogenic LAL assay for PMLA–PEG nanoparticle and PAMAM dendrimers are likely to be accurate, while those for gold colloids and PMLA nanoparticles are not. The endotoxin levels obtained from the end point chromogenic LAL assay for the gold colloids are underestimated due to inhibition and those of the PMLA nanoparticles are overestimated due to enhancement.
Figure 1. Certain nanoparticles interfere with the chromogenic limulus amoebocyte lysate test.
(A) Results of end point chromogenic LAL test. Spike recovery control in water (blue bar), in PMLA–PEG nanoparticles (purple bar) and in dendrimers sample (yellow bar) was within the US FDA/USP mandated criteria for chromogenic LAL assay. PMLA nanoparticles (red bar) enhanced, while gold nanoparticles (green bar) inhibited detection of endotoxin. (B) Results of kinetic chromogenic LAL test. When the same dendrimer formulation tested in end point chromogenic assay was studied in the kinetic chromogenic LAL assay, it resulted in enhancement of endotoxin detection. Endotoxin amount in unspiked nanoparticle formulation could not be accurately evaluated for gold and PMLA nanoparticles in end point assay, and for dendrimers in kinetic assay due to failure of the inhibition/enhancement control. Amounts of endotoxin in PMLA–PEG and dendrimer samples as measured in end point chromogenic assay were 0.53 EU/mg/ml and less than 0.1 EU/mg/ml, respectively. Shown is mean result (n = 6), % coefficient of variation < 25.
LAL: Limulus amoebocyte lysate; OD: Optical density; PEG: Poly(ethylene glycol); PMLA: Prepharmaceutical β-polymalic acid; USP: US Pharmacopoeia.
Exactly what physicochemical property of the nanoparticles causes the inhibition or enhancement is unknown; bacterial LPS is an amphiphilic molecule that hydrophobically interacts with biomembranes, surfaces and proteins [14]. The gold colloids may be binding the endotoxin and, thus, limiting its activity and/or accessibility to proteins in the LAL lysate. Gold has been shown to bind LPS and make it unavailable to LAL [15,16]. Similarly, it is not known why the PMLA nanoparticles cause enhancement of endotoxin detection. This formulation does not contain β-glucans and/or β-glucan-like moieties known to cause false-positive results in LAL test [17,18], and the interference is not removed by incubation in glucashield buffer, a reagent commonly used to reduce β-glucan-mediated interference with LAL assay (data not shown). Perhaps carboxyl groups on the PMLA nanoparticle surface react electrostatically with LAL proteins, as covering these groups on the PMLA nanoparticles with PEG helps to eliminate enhancement. This can be seen from the IEC for the PMLA–PEG particles in the same LAL assay, which yields an accurate spike recovery, indicating no interference with endotoxin detection (Figure 1a).
Inclusion of the IEC in each individual run of the LAL assay is important. It is not safe to assume that a nanoparticle that does not interfere with one format of the LAL test will not necessarily interfere with another. The same formulation of PAMAM dendrimers, which was analyzed in end point chromogenic assay with no perceptible interference (see IEC result for the dendrimer chromogenic LAL in Figure 1a), was then tested in the kinetic chromogenic LAL format. The IEC control for PAMAM dendrimers in the kinetic chromogenic LAL showed enhancement (Figure 1b). Thus, for this particular PAMAM dendrimer formulation, the end point chromogenic, but not kinetic chromogenic LAL assay, can be relied upon for accurate measure of endotoxin levels. The mechanism by which the PAMAM dendrimers enhance the kinetic chromogenic format LAL test is under investigation.
The PAMAM dendrimers (which interfered with the kinetic chromogenic LAL assay) and PMLA nanoparticles (which interfered with the end point chromogenic LAL assay) share a common property (free carboxyl groups on the particle surface). Cationic PAMAM dendrimers have previously been shown to inhibit endotoxin detection due to charge–charge interactions with negatively charged phosphates on the LPS disaccharide backbone [19]. Enhancement of endotoxin detection by the anionic PAMAM dendrimers and PMLA nanoparticles reported in this study could be caused by a similar interaction between the free carboxyl groups and proteins in the LAL lysate. The polymer conformation and density of free carboxyls may differ between the two formulations, causing the interference of the PAMAM dendrimers to be imperceptible in the end point assay, and only manifest in the kinetic assay, which has an increased sensitivity (lower endotoxin working range). It has previously been reported that the higher sensitivity commercial LAL assays are more prone to interference issues [18].
Formal acceptance criteria are met for all tested nanoparticles & kinetic turbidity LAL assay
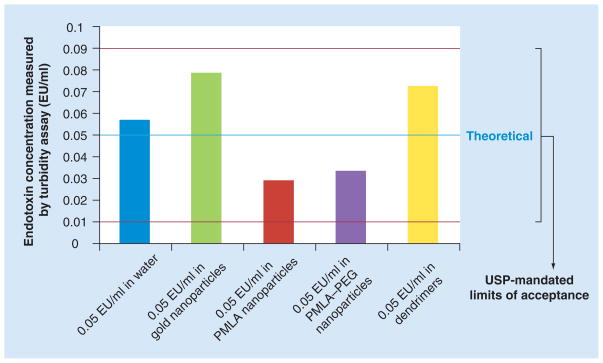
The same nanoparticle formulations at the same concentrations were also evaluated in a kinetic turbidity LAL assay. This format is considered to be the most sensitive commercially available LAL assay, as it has a working detection range from 1 to 0.001 EU/ml [20]. According to the FDA/USP standard for conducting the LAL assay, interference from a product may often be removed by diluting the sample. However, the FDA/USP standard also advises that the dilution should not exceed the maximum valid dilution, which is determined by the assay sensitivity [4,101]. Since the kinetic turbidity assay is more sensitive than the chromogenic LAL assay, its maximum valid dilution is 1:500, while the maximum valid dilution for the chromogenic LAL assay is 1:5. Analysis of the particles in the IECs in the turbidity assay showed no interference for all tested formulations (Figure 2). According to the FDA/USP formal criteria for assay validity, endotoxin levels obtained for the gold colloids, PMLA nanoparticles, PMLA–PEG nanoparticles and PAMAM dendrimers are all valid.
Figure 2. Certain nanoparticles do not interfere with kinetic turbidity limulus amoebocyte lysate test.
Spike recovery controls in water (quality control, blue bar), gold nanoparticles (inhibition enhancement control, green bar), PMLA nanoparticles (red bar), PMLA–PEG nanoparticles (purple bar), and in dendrimers (yellow bar). All tested nanoparticles were within USP mandated acceptance criteria for the turbidity limulus amoebocyte lysate assay (thresholds indicated in red). Shown is mean result (n = 6), % coefficient of variation < 25.
PEG: Poly(ethylene glycol); PMLA: Prepharmaceutical β-polymalic acid; USP: US Pharmacopoeia.
Comparison of endotoxin results from different LAL methods show discrepancy for some nanoparticles
The LAL assay exists in three main formats: chromogenic, turbidity and gel clot. Regardless of the assay format, when LAL methods are used to evaluate endotoxin amounts in traditional pharmaceuticals, they produce consistent results [20]. There are reports in the literature suggesting that the accuracy of the LAL test may be dramatically influenced if a pharmaceutical is encapsulated in a liposome [19–21]. In order to determine whether the same limitations apply to other nanomaterials, we have tested the same formulations we evaluated by chromogenic and turbidity LAL assays in the gel clot assay. Table 2 summarizes the endotoxin levels in various nanoparticle formulations estimated by the three main LAL assay formats. In cases where the IEC did not meet acceptance criteria due to inhibition or enhancement as described in the previous sections, no endotoxin value is reported. The reported values are only for those particles and those LAL assays with acceptable inhibition enhancement controls. Since gold nanoparticles and PMLA nanoparticles interfered with more than one LAL format, there is only one reportable endotoxin value for these particles. The endotoxin values for the PAMAM dendrimers are consistent among the gel clot, end point chromogenic and kinetic turbidity assays. However, the endotoxin results for PMLA–PEG nanoparticles obtained by the chromogenic end point and kinetic turbidity LAL assays are more than 25% different. These data are somewhat consistent with previous observation of liposome-encapsulated hemoglobin product in which turbidity LAL showed higher levels of endotoxin than chromogenic LAL, while IECs in both tests were within FDA/USP acceptable limits [20].
Table 2.
Comparison of endotoxin results from various limulus amoebocyte lysate tests.
| Particle | Gel clot | Chromogenic end point | Turbidity kinetic |
|---|---|---|---|
| Gold NP | IEC not acceptable (inhibition) | IEC not acceptable (inhibition) | 0.799 EU/ml |
| PMLA NP | IEC not acceptable (enhancement) | IEC not acceptable (enhancement) | 16.1 EU/ml |
| PMLA-PEG NP | IEC not acceptable (enhancement) | 0.53 EU/mg | 21.4 EU/mg |
| PMLA-PEG NP after purification | NA | <0.1 EU/mg | 0.0381 EU/mg |
| Dendrimers | 0.03 EU/mg | <0.1 EU/mg | <0.05 EU/mg |
Only endotoxin values from runs in which the IEC spike recovery was within USP/US FDA acceptance criteria are shown. If IEC control for a given nanoparticle formulation did not demonstrate spike recovery within 50–200% limits mandated by the USP standard 85, the results for that formulation are invalid and, therefore, no endotoxin value is possible to calculate. The data are presented as IEC not acceptable with the cause (enhancement or inhibition) shown in parenthesis. While the endotoxin values for dendrimers are consistent among the three different formats of the LAL assay, those for PMLA-PEG are more than 25% different between chromogenic end point and turbidity kinetic LAL assays. The gold and PMLA nanoparticles only had acceptable IECs in the turbidity kinetic LAL format, thus no comparison among the various LAL tests was possible. Each sample was analyzed three times in duplicate. The values shown are the mean of six measurements (% coefficient of variation < 25).
IEC: Inhibition/enhancement controls; NA: Not tested; NP: Nanoparticles; PMLA: Prepharmaceutical β-polymalic acid; USP: US Pharmacopoeia.
RPT confirmed end point chromogenic LAL results for PMLA–PEG nanoparticles
The RPT results for PMLA–PEG formulations showed no major animal distress or death; however, five of eight tested animals showed an increase in body temperature, which qualifies this formulation as pyrogenic under the USP standard (Table 3). Endotoxin level in the PMLA–PEG formulation purified by extraction with Triton X-114 was below the lower limit of quantification of the chromogenic end point LAL and was estimated to be 0.0381 EU/mg by the kinetic turbidity assay (Table 2). The data reported in the current study clearly show that even when formal acceptance requirements for the validity of the LAL test are met, the results for the same nanoparticle formulation from two different LAL tests can differ dramatically. Therefore, in situations where test results from two different LAL assays are more than 25% different, verification by some other method is needed, unless interference with one of the test formats can be conclusively demonstrated. Traditionally, the RPT was used by industry to exclude pyrogenic products from pharmaceutical pipelines. In recent years, in vitro tests have gained greater application, due to their lower costs and to ethical issues associated with animal testing. As such, in the last two decades, LAL testing has almost completely replaced the RPT for drug testing [11]. However, the medical device industry still utilizes both the LAL and RPT tests [22]. We therefore used the RPT to verify the LAL data we obtained for the PMLA–PEG nanoparticles. If the value obtained from the chromogenic assay was correct, we expected this formulation to cause moderate fever responses in the tested animals. However, if the turbidity LAL results were correct, then the formulation would likely cause animal death as the endotoxin dose would exceed the endotoxin LD50 for rabbits. The RPT data in our study are broadly consistent with the findings of the chromogenic end point assay. We also performed the purification of the PMLA–PEG particles by the Triton X-114 extraction method to remove endotoxins and repeated end point chromogenic LAL, kinetic turbidity LAL and RPT of the purified formulation. Endotoxin level in the purified PMLA formulation was below the lower limit of quantification of the chromogenic end point LAL, and RPT of this purified formulation showed no pyrogenic response. Thus, in this case, results of the two different LAL assays were consistent and were confirmed by the RPT.
Table 3.
Results of the rabbit pyrogen test.
| Particle | RPT (rabbits with fever [n]) | RPT conclusion |
|---|---|---|
| PMLA-PEG NP | 5 | Pyrogenic |
| PMLA-PEG NP after purification | 0 | Not pyrogenic |
PMLA–PEG NPs were tested in the RPT. Three animals were injected with 10 ml of NP formulation to achieve the dose of 1.5 mg/kg. Since two of three animals injected with PMLA–PEG NPs showed increase in body temperature, the test was continued with an additional five rabbits (total number of tested animals was eight for PMLA–PEG NPs). When purified PMLA–PEG NP was tested, none of initial three animals showed increase in body temperature; therefore, the test was not continued in additional animals (total number of tested animals was three for purified PMLA–PEG NP). Shown in the middle column is the number of animals whose body temperature increased to 0.5°C and higher above the baseline temperature after administration of the formulation. Conclusion of RPT was based on USP standard 151 (pyrogen test) and is shown in right column.
NP: Nanoparticles; PEG: Poly(ethylene glycol); PMLA: Prepharmaceutical β-polymalic acid; RPT: Rabbit pyrogen test; USP: US Pharmacopoeia.
Conclusion
These data clearly demonstrate the importance of in vitro LAL result verification by in vivo RPT analysis in situations when in vitro results from two different methods disagree, or when only one LAL format is valid (i.e., met USP/FDA acceptance criteria) for a given formulation. This data also suggests the need for an assay for the direct detection of endotoxin in nanoparticle formulations.
Executive summary.
Certain nanoparticles may interfere with one or more formats of the standard limulus amoebocyte lysate (LAL) in vitro test (e.g., gold colloids interfered with gel clot and chromogenic end point LAL).
Inhibition/enhancement controls are critical for detection of nanoparticle interference with the LAL assay, although they may not conclusively eliminate the possibility of interference. Performing more than one LAL assay format on the same formulation may also help to identify the source of interference.
Even when formal requirements for LAL assay validity are met, different LAL protocols may provide contradictory results for the same formulation (e.g., estimated endotoxin concentration in poly(malic acid)–poly(ethylene glycol) nanoparticles by chromogenic end point and kinetic turbidity LAL were more than 25% different).
The qualitative in vivo rabbit pyrogen test may be used to determine the correct result when two formats of the LAL test provide contradictory results.
Acknowledgments
The authors would like to thank Jennifer Hall for help in manuscript preparation.
Footnotes
Financial & competing interests disclosure
The study was supported in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400 and HHSN261200800001E, and by NIH/NCI R01 grant CA123495 (JYL). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Bosshart H, Heinzelmann M. Targeting bacterial endotoxin: two sides of a coin. Ann NY Acad Sci. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- 2.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4(9):903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 3.Brade H, Opal SM, Vogel SN, Morrison DC. Endotoxin in Health and Disease. Marcel Decker Inc; NY, USA: 1999. [Google Scholar]
- 4.USP 30 NF 25. <85> Bacterial endotoxins test. (Ed.^(Eds) (2007)
- 5.Vallhov H, Qin J, Johansson SM, et al. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006;6(8):1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 6.Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Characterization of nanoparticles for therapeutics. Nanomed. 2007;2(6):789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt R, Mackensen A, Galanos C. Phase I trial of intravenously administered endotoxin (Salmonella abortus equi) in cancer patients. Cancer Res. 1991;51(10):2524–2530. [PubMed] [Google Scholar]
- 8.Lee BS, Holler E. Effects of culture conditions on β-poly(L-malate) production by Physarum polycephalum. Appl Microbiol Biotechnol. 1999;51:647–652. [Google Scholar]
- 9.Ljubimova JY, Fujita M, Khazenzon NM, et al. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact. 2008;171:195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132(2):191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 11.Standard procedure <151> Pyrogen test. Vol. 1. The United States Pharmacopeial Convention; Rockville, MD 20852, USA: 2007. United States Pharmacopeia 30th revision and National Formulary 25th edition; pp. 135–136. [Google Scholar]
- 12.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5(2):106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr C, Jr, Morrison DC. Lipopolysaccharide interaction with rabbit erythrocyte membranes. Infect Immun. 1984;43(2):600–606. doi: 10.1128/iai.43.2.600-606.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handley DA, Chien S. Colloidal gold: a pluripotent receptor probe. Proc Soc Exp Biol Med. 1983;174(1):1–11. doi: 10.3181/00379727-174-41697. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Kondo I. Immunoelectron microscopy of Proteus vulgaris by the plasma polymerization metal-extraction replica method: differential staining of flagellar (H) and somatic (O) antigens by colloidal golds. J Electron Microsc (Tokyo) 1989;38(5):382–388. [PubMed] [Google Scholar]
- 17.Roslansky PF, Dawson ME, Novitsky TJ. Plastics, endotoxins, and the Limulus amebocyte lysate test. J Parenter Sci Technol. 1991;45(2):83–87. [PubMed] [Google Scholar]
- 18.Roslansky PF, Novitsky TJ. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J Clin Microbiol. 1991;29(11):2477–2483. doi: 10.1128/jcm.29.11.2477-2483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poxon SW, Hughes JA. Characterization of endotoxin and cationic liposome interaction. Pharm Dev Technol. 1999;4(2):135–143. doi: 10.1081/pdt-100101348. [DOI] [PubMed] [Google Scholar]
- 20.Cliff RO, Kwasiborski V, Rudolph AS. A comparative study of the accurate measurement of endotoxin in liposome-encapsulated hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1995;23(3):331–336. doi: 10.3109/10731199509117949. [DOI] [PubMed] [Google Scholar]
- 21.Harmon P, Cabral-Lilly D, Reed RA, et al. The release and detection of endotoxin from liposomes. Anal Biochem. 1997;250(2):139–146. doi: 10.1006/abio.1997.2216. [DOI] [PubMed] [Google Scholar]
- 22.Ross VC, Twohy CW. Endotoxins and medical devices. Prog Clin Biol Res. 1985;189:267–281. [PubMed] [Google Scholar]
Website
- 101.US FDA CDER, CBER, CDRH, CVM. Rockville, MD, USA 20857: 1987. Guideline on validation of the Limulus Amebocyte Lysate test as an end-product endotoxin test for human and animal parenteral drugs, biological products, and medical devices. CDER, CBER, CDRH, CVM (Eds) http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM080966.pdf. [Google Scholar]