Abstract
Arachidonic acid is metabolized to a number of bioactive eicosanoid molecules by several enzyme, including enzymes of the COX, lipoxygenase and cytochrome P450 (CYP) monooxygenase pathways. Inhibition of the CYP ω-hydroxylase pathway, stimulation of the CYP-epoxygenase pathway and administration of exogenous epoxyeicosatrienoic acids resulted in cardioprotection in animal models of ischemia; contractile function was improved in mouse hearts subjected to global ischemia/reperfusion, and infarct size was reduced in canine and rat hearts. Cardioprotective effects were also achieved when metabolism of the endogenous epoxyeicosatrienoic acids (EETs) by their major enzymatic hydrolysis pathway was blocked in gene knockout mice (EPHX2−/−) or by inhibitors of soluble epoxide hydrolase (sEH), such as 12-(3-odamantan-l-yl-ureido)-dodecanoic acid (AUDA). Pretreatment of canine hearts with AUDA dose-dependently reduced infarct size, and AUDA enhanced the infarct-sparing effect of treatment with exogenous EETs. The preliminary results of studies in rodent hearts have also demonstrated that AUDA and AUDA-butyl ester reduce infarct size. These results and others obtained in models of myocardial stunning and hypertrophy suggest that inhibitors of EPHX2 or sEH have therapeutic potential in a brood range of cardiovascular diseases.
Keywords: Coronary heart disease, cytochrome P450, heart disease, ischemia, pharmacology
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in the developed world. In 2007, the overall cost of CVD in the US alone was approximately $432 billion [1]. One of the most common types of CVD is myocardial infarction (MI), occurring in approximately 800,000 individuals annually [1]. Therefore, it is imperative that new therapies are developed to slow the onset of coronary artery disease, to prevent MI, and to ameliorate the severity of damage to the heart by limiting infarct size after the onset of the attack. In 1986, the discovery of the phenomenon of ischemic preconditioning (IPC) by Murry et al stimulated great hope for the development of novel therapies [2]. It was demonstrated that brief periods of ischemia prior to a more prolonged bout of ischemia could markedly reduce infarct size in dogs and, subsequently, in all animals tested and in humans [2]. Although a number of drugs mimic IPC in animal models, however, no treatment has emerged that is effective in all patients experiencing an acute MI. The primary reason that IPC or pharmacological drugs to mimic IPC are inappropriate as standard treatments for patients suffering MI is that IPC is only effective if administered prior to the ischemic insult, which is almost impossible to predict.
A resurgence of excitement has recently occurred in the field of cardiovascular medicine with of the discovery of the phenomenon of postconditioning, Vinten-Johansen et al demonstrated in dogs that if reperfusion following a prolonged ischemic period is conducted in a 'stuttering' fashion, with alternate cycles of 3- to 30-sec reperfusion and occlusion, infarct size is reduced by a magnitude similar to that observed with IPC [3]. Importantly, the advantage of this technique, or pharmacological means to mimic postconditioning, is that, unlike IPC, the treatment can be administered at the time of reperfusion. These findings caused a paradigm shift in the field of ischemia/reperfusion and fostered efforts to develop a safe drug that can reduce myocardial injury when administered just prior to or at the time of reperfusion. This review discusses the potential use of selective soluble epoxide hydrolase (sEH) inhibitors, such as 12-(3-adamantan-l-yl-ureido) dodecanoic acid (AUDA), as a potential new therapeutic approach in the treatment of reperfusion injury.
Soluble epoxide hydrolase
The cytochrome P-450 (CYP) monooxygenase pathway metabolizes arachidonic acid to produce two types of eicosanoid molecules, hydroxyeicosatetranoic acids (HETEs) resulting from the action of CYP hydroxylases and epoxyeicosatrienoic acids (EETs) resulting from the action of CYP-epoxygenases [4], Four regioisomers of EETs are known - 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET - and these share many biological effects, with the exception of 5,6-EET. EETs and HETEs often exert opposing effects, particularly in the tissues of the heart in which EETs are vasodilators and have several cardioprotective effects [5,6], whereas HETEs (in particularly 20-HETE) produce coronary artery vasoconstriction and increase infarct size in experimental models [7]. An important feature of EETs is that these molecules are metabolized by a specific enzyme, sEH, to the corresponding dihydroxyeicosatrienoic acids (DHETs). DHETs are generally much less efficacious at causing vasodilation than their corresponding precursory EETs in most systems and models studied, although DHETs may exert important effects in some organs [4]. In this regard, Morisseau et al synthesized several urea and carbamate compounds as potent sEH inhibitors, one of which was AUDA [8]. These inhibitors enhanced the cytotoxicity of trans-stilbene oxide and reduced the toxicity of leukotoxin in vitro, reduced the toxicity of leukotoxin in vivo in mice, and prevented the symptoms of acute respiratory distress syndrome. These data suggested that these compounds may have efficacy in treating various inflammatory conditions in which epoxides and diols may be involved. Additional interest in developing selective sEH inhibitors arose as a result of studies in which the genetic knockout of EPHX2 (the gene encoding sEH) in mice caused a decrease in baseline blood pressure compared with corresponding wild-type mice [9]. These findings suggested that selective sEH inhibitors might be useful as treatments for hypertension, and possibly other cardiovascular disorders [9].
New data suggest that selective sEH inhibitors, such as AUDA, are cardioprotective in several models of ischemia/reperfusion injury [10–13]. Compared with wild-type animals, mice in which CYPZJ2 is overexpressed or sEH is inactivated have a superior recovery of contractile function in reversibly injured hearts and in infarct size after ischemia, and a decrease in the incidence of cardiac arrhythmias [10,13]. In dogs, treatment with AUDA reduced infarct size in a dose-dependent manner and enhanced the cardioprotective effects of exogenously administered EETs [11]. Similar results have been observed using the selective sEH inhibitor AUDA-butyl ester (AUDA-BE) in C57BL/6J wild-type mice [12].
The cardioprotective effects of AUDA and sEH expression (sEH knockouts)
Effects of sEH expression on reversible myocardial contractile dysfunction in mice
Seubert et al were the first research group to study the role of sEH on the recovery of contractile function in Langendorff-perfused hearts [10]. In this study, mice with a knockout of the sEH gene had an improved recovery of left ventricular-developed pressure (LVDP) following 20 min of global ischemia and 40 min of reperfusion [10]. In a subset of these knockout mice, a modest but statistically significant reduction in infarct size was demonstrated by tetrazolium staining and by the measurement of lactate dehydrogenase (LDH) release. These beneficial effects were accompanied by an increase in epoxide:diol ratios in the plasma of the knockout mice, which suggested an increase in EETs were present in the sEH knockouts. In further studies, the selective EET antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) blocked the beneficial effects observed in the sEH knockout mice [14]. The perfusion of isolated wild-type murine hearts with physiologically relevant concentrations of 8,9-EET, 11,12-EET and 14,15-EEf resulted in an improvement in the recovery of LVDP that was similar to that observed in the knockout mice [14]. These findings suggest that the beneficial effects observed on contractile function and infarct size were the result of increased EET concentrations in this Langendorff model.
The beneficial effects on contractile function observed in sEH null mice were also blocked by the phosphoinositol-3-kinase (PI3K) inhibitors wortmannin and LY-294002; the KATP channel blockers glibenclamide and 5-hydroxydecanoic acid; and the calcium-activated potassium channel inhibitor paxilline [10]. All of the intracellular events were accompanied by increases in phospho-glycogen synthase kinase-3β (p-GSK3β), which has demonstrated cardioprotective effects in several other studies [15,16]. GSK3β protects the heart from injury by delaying or blocking the opening of the mitochondrial permeability transition pore [16].All of these potential pathways by which the EETs and sEH inhibitors produce cardioprotection are summarized in Figure 1. Taken together the results of these studies suggest that a pharmacological inhibitor of sEH, such as AUDA, should exert cardioprotective activity similar to that observed in sEH null mice.
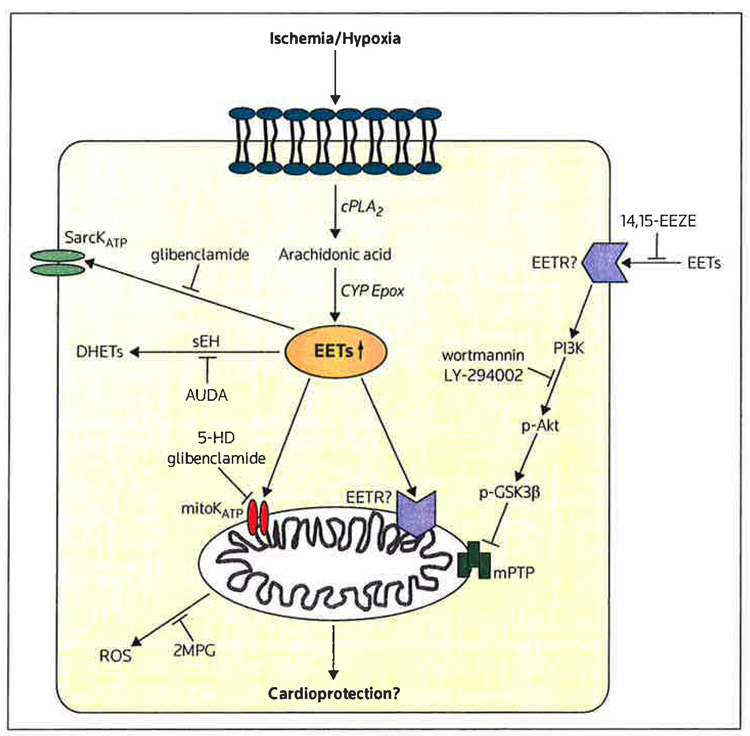
Figure 1. Mechanistic pathways of soluble epoxide hydrolase inhibitors and/or epoxyeicosatrienoic acids in cardioprotection.
Several potential pathways for the activity of soluble epoxide hydrolase (sEH) inhibitors have been identified that may be involved in epoxyeicosatrienoic acid (EET)-induced cardioprotection. Because there is evidence that the EETs may exert both extracellular and intracellular effects, it is postulated that there may be an EET receptor (EETR) at the cell surface or within the mitochondria; however, no EET receptor has been cloned and this hypothesis remains questionable.
2MPG 2-mercaptopropionyl glycine, 5-HD 5-hydroxydecanoic acid, 14,15-EEZE 14,15-epoxyeicosa-5(Z)-enoic acid, AUDA .12-(3-adamantan-1-yt-ureido) dodecanoic acid, cPLA2 cytosolic phosphotipase A2, CYP Epox cytochrome P-450 epoxygenase, DHETs dihydroxyeicosatrienoic acids, mitoKATP mitochondrial ATP-sensitive potassium channel, mPTP mitochondrial permeabitity transitionpore, p-Akt phosphorylated Akt, p-GSK3β phospho-gtycogen synthase kinase-3β, Pl3K phosphoinositol-3-kinase, ROS reactive oxygen species, SarcKATP sarcolemmal ATP-sensitive potassium channel
Effects of AUDA on myocardial infarct size AUDA in dogs
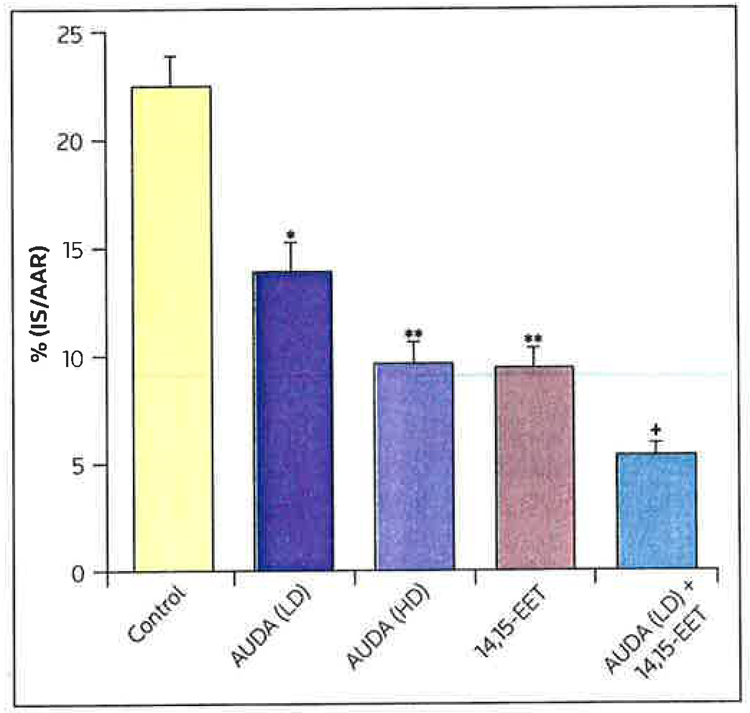
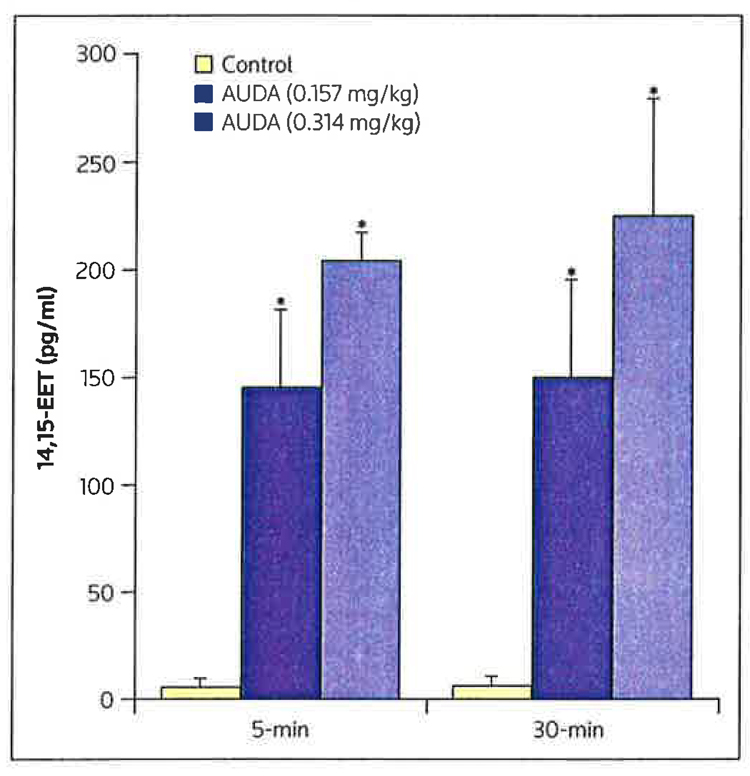
Exogenous EETs produce a marked reduction in infarct size in dogs [17]; however the enhancement of endogenous EET concentration by inhibiting the breakdown of EETs by sEH has not been demonstrated to produce a similar response. Because the cardiovascular effects of EETs on the heart are presumed to be the result of actions on a putative EET receptor on the cell membrane or within the cell, studies were conducted to assess whether selective EET antagonists, such as 14,15-EEZE, could block the effect of several exogenous EETS or the endogenous effect of AUDA [8]. As illustrated in Figure 2, intracoronary administration of AUDA (0.157 or 0.314 mg/kg) produced a dose-related reduction in infarct size (IS), expressed as a percentage of the area at risk (AAR) [11]. The higher dose of AUDA produced a reduction in infarct size that was approximately equivalent to that produced by intracoronary-administered exogenous 14,15-EET [11]. Interestingly, when the lower dose of AUDA was co-administered with 14,15-EET, the reduction in IS/AAR was significantly greater than that observed when the small dose of AUDA was administered alone, and was larger than that observed when 14,15-EET was administered alone (Figure 3) [11]. These data suggest that these effects of AUDA were the result of enhanced EET concentrations in the heart. AUDA caused a dose-related increase in the release of 14,15-EET into the coronary venous blood, draining the previously ischemic area at 5 and 30 min of reperfusion. The selective EET antagonist 14,15-EEZE blocked the effects of both the exogenously administered 14,15-EET and AUDA; the inhibition of AUDA increased endogenous EET concentrations [11]. In contrast, the cardioprotective effects of the mitochondrial KATP opener diazoxide were not inhibited by 14,15-EEZE. These data suggest that 14,15-EEZE is selective in inhibiting the effects of exogenous and endogenous EETs; however, the results obtained thus far do not determine the site of EET action, whether extracellular or intracellular. Perhaps the EETs have several sites of action that result in the overall cardioprotective effect observed. Further experiments are needed to ascertain whether single or multiple receptors are involved in mediating EET-induced cardioprotection.
Figure 2. The effects of AUDA and 14,15-EET on myocardial infarct size.
Effects of Low-dose (LD; 0.157 mg/kg) and high-dose (HD; 0.3,l4 mg/kg) l2-(3-adamantan-l-yl-ureido) dodecanoic acid (AUDA), and 14,15-epoxyeicosatrienoic acid (14,15-EET 0.128 mg/kg) atone or in combination with LD AUDA on myocardial infarct size as a percentage of the area of risk (IS/AAR) in dogs subjected to 60 min of left anterior descending coronary artery occlusion and 3 h of reperfusion.
+p < O.O5 compared with LD AUDA, *p < 0.01 compared with control, **p < 0.00.l compared with control
Figure 3. Changes in 14,15-EET concentrations in the plasma with AUDA treatment.
Concentrations of 14,15-epoxyeicosatrienoic acid (14,15-EET) in the coronary venous plasma at 5 and 30 min of reperfusion following treatment with low or high doses of 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA; 0.157 and 0.314 mg/kg, respectively) compared with control values.
*p < 0.01 compared with controls
AUDA-BE in mice
In studies in male C57BL/6J mice subjected to 40 min of left coronary artery occlusion and 2 h of reperfusion, the IS/RRR was 46 ± 3% [12]. However, in similar wild-type mice pretreated with AUDA-BE (ip) 30 min prior to occlusion, IS/AAR was reduced to 30 ± 5% (p < 0.01) [12]. Although EETs were not measured in these studies, the researchers presume that the cardioprotective effect of AUDA-BE was the result of increases in endogenous EET concentration in the drug-treated mice [12]. This seems likely because the protective effect of AUDA-BE was completely blocked by the EET antagonist 14,15-EEZE, and was mimicked by the intravenous administration of 14,15-EET, a major substrate of sEH [12].
Effect of soluble epoxide hydrolase inhibitors in the prevention and reversal of cardiac hypertrophy: A possible role in heart failure
The most common consequence of an acute MI is the development of ischemic cardiomyopathy and hypertension that results in ventricular remodeling and hypertrophy. Eventually these changes result in severe heart failure and often in sudden death from ventricular fibrillation. To address the role of sEH in the development of left ventricular hypertrophy (LVH), Xu et al studied several groups of C57BL/6J mice with pressure overload-induced LVH [18]. Mice were administered two potent sEH inhibitors, 1-adamantan-1-yl-3-(5-(2-(2-ethoxyethoxy) ethoxy)pentyl)urea (AEPU) and AUDA, either before the onset of LVH or following the establishment of LVH, In both groups, the sEH inhibitors were cardioprotective and prevented the development of LVH or reversed pre-established LVH. Interestingly, both compounds prevented the activation of NFΚB, a mediator of LVH development in cardiac myocytes. Furthermore, these two sEH inhibitors exerted an antiarrhythmic effect, in association with their beneficial effects of preventing LVH [18]. These investigators concluded that the use of sEH inhibitors may increase levels of endogenous lipids, such as the EETs, and may have therapeutic potential in the prevention of LVH and heart failure following activation of NFΚB and its subsequent remodeling pathway.
More recently, Monti et al established that the allelic variation of EPHX2 is associated with heart failure in a rat model of heart failure [19]. Increased expression of EPHX2 transcript and protein, and higher enzyme activity, resulted in the more rapid breakdown of cardioprotective EETs. In EPHX2 gene knockout mice, the mice hearts were protected from developing pressure overload-induced heart failure and cardiac arrhythmias. Taken together, these results suggest that inhibition of sEH may be a new therapeutic approach to add to the increasing pharmacotherapy for heart failure [20]. A more detailed description of the role that sEH inhibitors play in the development of heart failure and the role of NFΚB is included in two reviews by Imig [21] and Harris et al [22].
Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation
Hutchens et al recently tested the hypothesis that sEH knockout mice would be less susceptible to transient whole-body cardiac arrest (10 min) and resuscitation [23]. Surprisingly, survival in the wild-type mice was significantly greater than in the sEH knockout mice. Equivalent studies with selective sEH inhibitors have not been conducted, but might provide a different result because the sEH enzyme is a bifunctional enzyme in which the C-terminal domain contains the hydrolase activity and the N-terminal domain contains a functional phosphatase [24], which is not blocked by the sEH inhibitors. Therefore, it is possible that a sEH inhibitor that only blocks the C-terminal domain might have a beneficial effect in this setting.
Conclusion
Although understanding of the potential of EETs and sEH inhibitors as cardioprotective agents in a number of CVDs is in its infancy, as noted by the modest number of publications at this time, the results that are emerging are encouraging. Findings suggest that methods for increasing levels of endogenous EETs by inhibiting their breakdown through the use of sEH inhibitors produce a number of beneficial effects on the reversibly and/or irreversibly injured heart and in other organs [21,22]. The results obtained thus far suggest that the enhancement of endogenous EET concentrations or inhibition of the breakdown of exogenously administered EETs (by sEH inhibitors or by the administration of novel EET agonists) can have several benefits. These include a reduction in myocardial stunning, myocardial infarct size and inflammatory response, prevention of the onset of LVH and subsequent remodeling, which leads to heart failure, and reductions in the incidence of cardiac arrhythmias associated with heart failure. There are few drugs, such as the PDE5 inhibitors (eg, sildenafil), that seem to possess these wide-ranging beneficial effects on the heart and circulation in the absence of any noticeable side effects. However, this group of compounds should be tested in conscious animal models and models of disease and aging because many compounds that are effective in young healthy animals are less effective in older animals or those with diseases such as diabetes and the metabolic syndrome [25]. Thus far, the beneficial effects of sEH inhibitors occurred in the absence of any significant side effects; however well-controlled clinical trials are needed to further assess the safety of this group of compounds before they can be considered for clinical use. It might be more likely that these compounds are most useful in acute situations, such as coronary angioplasty or bypass surgery, and not in situations in which treatment would be required for a prolonged period of time. The observation that these compounds are effective when administered just prior to reperfusion is a significant advantage because there is the potential for use in patients presenting with an ongoing infarction, where drug treatment can be administered at the time of reperfusion. Further clinical studies are needed to assess the potential use of sEH inhibitors, such as AUDA, in these various cardiovascular disorders.
Acknowledgements
This research was supported by an NIH Grant HL074314 (GJG).
References
•• of outstanding interest
• of special interest
- 1. Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, et al. Heart disease and stroke statistics - 2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. • An excellent summary of the demographics of CVD in the US.
- 2. Murry CE, Jennings RB, Reimer KB. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. • The first paper to describe the phenomenon of IPC and the consequent reduction in infarct size in dogs.
- 3. zhao zQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. • The first paper to describe the phenomenon of ischemic postconditioning during reperfusion and compare its efficacy with that of ischemic IPC.
- 4. Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Ceil Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. • A thorough review of the cellular actions of the EETS in different organs.
- 5.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P45O inhibitors. Proc Natl Acad Sci USA. 2004;101(5):1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42(3):687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nithipatikom K, DiCamelli RF, Kohler S, Gumina RJ, Falck JR, Campbell WB, Gross GJ. Determination of cytochrome P450 metabolites of arachidonic acid in coronary venous plasma during ischemia and reperfusion in dogs. Anal Biochem. 2001;292(1):115–124. doi: 10.1006/abio.2001.5044. • The first evidence that EETs are cardioprotective, and that 20-HETE results in a greater degree of injury in canine heatts.
- 8. Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96(16):8849–8854. doi: 10.1073/pnas.96.16.8849. •• Publication of the first discovery of selective and potent inhibitors of sEHs, including AUDA, and demonstration that these compounds may have anti-inflammatory effects.
- 9.Siinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275(51):40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 10. Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, et al. Role of soluble epoxide hydrolase in Postischemic recovery of heart contractile function. circ Res. 2006;99(4):442–450. doi: 10.1161/01.RES.0000237390.92932.37. •• The first study to demonstrate a cardioprotective effect of knocking-out the sEH gene in mice. sEH null mice demonstrated a greater recovery of contractile function than corresponding wild-type mice, and had a slightly smaller infarct size after 20 min of ischemia, followed by 40 min of reperfusion.
- 11. Gross GJ, Gauthier KM, Moore JM, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETS in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294(6):H2838–H2844. doi: 10.1152/ajpheart.00186.2008. •• The first study to demonstrate that the inhibition of sEH by AUDA produced a marked and dose-related reduction in infarct size in the canine heart, In the presence of a low-dose of AUDA, 14,15-EET produced a greater reduction in infarct size than either AUDA or 14,15-EET provided when administered alone (an additive effect). In addition, the protective effect of AUDA was blocked by the selective EET antagonist 14,15-EEZE, which suggests that AUDA was acting via preservation of endogenous EETs.
- 12.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Phys Heart Circ Physiol. 2008;295(5):H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009;46(1):67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90(9):1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 15.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94(7):960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 16.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3β by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117(21):2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 17. Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: Ischemic versus repefusion injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H537–H542. doi: 10.1152/ajpheart.00071.2006. • The first evidence to suggest that EETS produce benefits on both ischemic and reperfusion injury in a canine model of infarction.
- 18. Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RKP, Singapuri A, Davis BB, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103(49):18733–18738. doi: 10.1073/pnas.0609158103. • The first study to demonstrate that sEH inhibitors can prevent or reverse LVH and abate the arrhythmias that usually accompany cardiac hypertrophy in mice.
- 19.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gösele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, et al. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40(5):529–537. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETS) Prostaglandins Other Lipid Mediat. 2007;82(1–4):42–49. doi: 10.1016/j.prostaglandins.2006.05.004. • A concise review of the results of inhibiting sEH by gene knockout or with selective pharmacological inhibitors in different organ systems, and their potential as therapeutic agents in various diseases.
- 21.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24(2):169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 22.Harris TR, Li N, Chiamvimonvat N, Hammock BD. The potential role of soluble epoxide hydrolase in the treatment of cardiac hypertrophy. Congest Heart Fail. 2008;14(4):219–224. doi: 10.1111/j.1751-7133.2008.08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene depletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2008;76(1):89–94. doi: 10.1016/j.resuscitation.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman JW, Morisseau C, Harris TR, Hammock BD. Thc soluble epoxide hydrolase encoded by EPHX2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100(4):1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: Current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103(6):50l–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]