Abstract
Methods
In this study we determined, for the first time, the ability of microorganisms to traverse microneedle-induced holes using two different in vitro models.
Results
When employing Silescol® membranes, the numbers of Candida albicans, Pseudomonas aeruginosa and Staphylococcus epidermidis crossing the membranes were an order of magnitude lower when the membranes were punctured by microneedles rather than a 21G hypodermic needle. Apart from the movement of C. albicans across hypodermic needle-punctured membranes, where 40.2% of the microbial load on control membranes permeated the barrier over 24 h, the numbers of permeating microorganisms was less than 5% of the original microbial load on control membranes. Experiments employing excised porcine skin and radiolabelled microorganisms showed that the numbers of microorganisms penetrating skin beyond the stratum corneum were approximately an order of magnitude greater than the numbers crossing Silescol® membranes in the corresponding experiments. Approximately 103cfu of each microorganism adhered to hypodermic needles during insertion. The numbers of microorganisms adhering to MN arrays were an order of magnitude higher in each case.
Conclusion
We have shown here that microneedle puncture resulted in significantly less microbial penetration than did hypodermic needle puncture and that no microorganisms crossed the viable epidermis in microneedle—punctured skin, in contrast to needle-punctured skin. Given the antimicrobial properties of skin, it is, therefore, likely that application of microneedle arrays to skin in an appropriate manner would not cause either local or systemic infection in normal circumstances in immune-competent patients. In supporting widespread clinical use of microneedle-based delivery systems, appropriate animal studies are now needed to conclusively demonstrate this in vivo. Safety in patients will be enhanced by aseptic or sterile manufacture and by fabricating microneedles from self-disabling materials (e.g. dissolving or biodegradable polymers) to prevent inappropriate or accidental reuse.
Keywords: infection, microneedle arrays, microorganisms, stratum corneum
INTRODUCTION
Microneedle arrays (MN) are minimally-invasive devices that can be used to bypass the stratum corneum barrier of the skin and thus permit enhanced transdermal drug delivery. MN typically consist of a plurality of microprojections, made from silicon, metal or polymeric materials, attached to a support which also serves as a means of preventing the apparatus from penetrating the skin beyond a pre-determined distance. The MN themselves can be in the form of blunt projections, microblades or needle-like structures and can be solid or have a hollow bore through the centre. Importantly, the application of such devices to the skin is painless and causes no bleeding; their short lengths reduce the likelihood of encountering and/or stimulating a dermal nerve and/or reaching a dermal blood vessel (1). Solid MN puncture skin prior to application of a drug-loaded patch or are pre-coated with drug prior to insertion. Hollow bore microneedles allow diffusion or pressure-driven flow of drugs through a central lumen, while polymeric drug-containing microneedles release their payload as they dissolve or biodegrade in the viable skin layers. The Macroflux® system (Zosano Pharma) incorporates a 2.0 cm2 array of titanium MN, which can be coated with drug for bolus administration or used in combination with a drug reservoir. This system does, however, require a specialized device to stretch the skin, allowing drug to diffuse down spaces in the SC created around the MN.
MN technology has been shown to enhance transdermal delivery of a wide range of molecules, including anthrax vaccine (2), β-galactosidase (3), calcein (4), bovine serum albumin (BSA) (5), desmospressin (6), plasmid DNA (3), and, most commonly, insulin (7-9). We have previously used solid silicon microneedle arrays to enhance photosensitiser delivery into the skin for use in photodynamic therapy (10,11). Although MN puncture of the skin breaches the stratum corneum barrier that usually prevents ingress of microorganisms, there have been no reports of this technology causing skin or systemic infection (1). However, to date, this has never been properly investigated, and if MN-based transdermal drug delivery systems are to gain widespread acceptance, their safety, as well as their utility, must initially be demonstrated. Consequently, in this study we determined, for the first time, the ability of microorganisms to traverse microneedle-induced holes using two different in vitro models.
MATERIALS AND METHODS
Microorganisms and Chemicals
Stock cultures of Staphylococcus epidermis (NCTC 11047) were obtained from the National Collection of Type Cultures, London, UK. Stock cultures of Candida albicans (NCYC 1467) were obtained from the National Collection of Yeast Cultures, Norwich, UK. Stock cultures of Pseudomonas aeruginosa, strain PA01 (NCIMB 10548), were obtained from the National Collection of Industrial & Marine Bacteria, Aberdeen, UK. Virkon®, used as a disinfectant, was purchased from Antec International (a DuPont Company), Suffolk, UK. Dimethylsulfoxide was purchased from Honey-well Riedel-de Haën, Seelze, Germany. A 1.0 mCi vial of tritium-labelled [5′-3H] thymidine was purchased form GE Healthcare, Buckinghamshire, UK. All other chemicals used were of analytical reagent grade. MN2-type Mixing Needles (100 mm long) were purchased from Henley Medical Supplies (Hertfordshire, UK). Scotch® Magic™ 25 mm×66 m tape, used to strip the stratum corneum, was purchased from 3M (Bracknell, UK).
Microneedle Arrays
Silicon microneedle (MN) arrays were prepared using a previously reported approach (18). Briefly, a silicon wafer was coated with a nitride and oxide layer using a low-pressure chemical vapour deposition. This layer was then lithographically patterned using plasma etching. The patterned wafer was subsequently etched using 29% w/v potassium hydroxide solution at a temperature of 79°C in a water bath with constant agitation. The aspect ratio of the resulting microneedles was 3:2 (height:base diameter). In a final step, the microneedles were surface-coated with titanium, as an adhesive layer, and then platinum by evaporation.
Clearly, individual MNs could not be seen using the naked eye. SEM imaging (JEOL JSM840 scanning electron microscope, Jeol, Tokyo, Japan) revealed that the MNs were arranged on the silicon chips as 6×5 arrays, meaning there were 30 MNs in each array (Fig. 1 (a) and (c). Each MN was approximately 280μm in height, with a diameter of 250μm at the base and an interspacing of 750μm between rows of MNs (Fig. 1 (b) and (d)). Fig. 1 (e) shows a digital photograph of a typical silicon MN array. Using a digital micrometer, the dimensions of each array were determined to be approximately 5.6 mm in length by 4.8 mm in breadth.
Fig. 1.
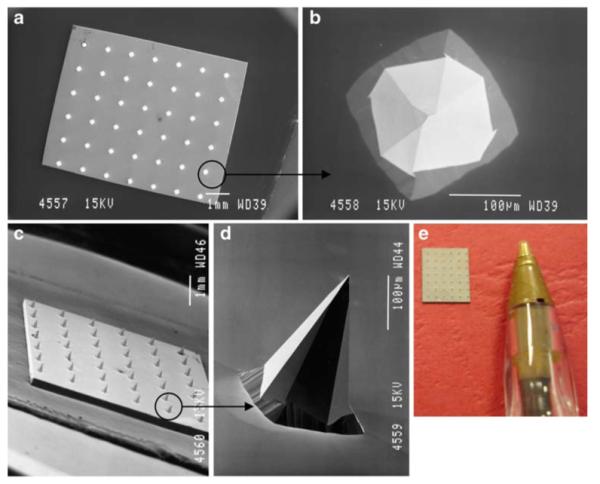
SEM images taken of a typical silicon MN array from directly above (a), of a single MN from above (b), of an array from the side (c) and of an individual MN from the side (d). Digital photograph of a typical silicon MN array (e).
Preparation of Microbial Suspensions
Microbial isolates were cultured in Mueller-Hinton broth (MHB) for 18 h in an incubator at 37°C. The resulting microbial suspensions were then centrifuged at 3000 rpm for 10 min and the pellets reconstituted in phosphate-buffered saline with pH 7.4 (PBS) by vortex mixing. The absorbance of S. epidermis and P. aeruginosa suspensions at 550 nm were adjusted to 0.1, and that of C. albicans suspension was adjusted to 1.0 to give a final inoculum of 1×107cfu ml−1 for each microorganism, which was verified by total viable count.
Microbial Permeation Across Silescol® Membranes
The permeation of microorganisms across Silescol®, a membrane used to mimic the intercellular lipid transport pathway taken by most drug substances crossing the stratum corneum, was investigated using a modified Franz-cell apparatus (PermeGear Inc, Bethlehem, PA, USA), as shown in Fig. 2. The silicone membrane (Silescol®, Barloworld Scientific Ltd, Staffordshire, UK) had a thickness of 50μm. The orifice diameter in both donor and receptor compartments was 15 mm. Receptor compartment volumes, approximately 12 ml, were exactly determined by triplicate measurements of the weights of water they could accommodate. Account was taken for the volumes occupied by Teflon-coated magnetic stirring bars. Compartment temperatures were kept constant at 37°C by re-circulating water from a thermostatically controlled bath. Silescol® membranes were sandwiched between the receptor and donor compartment using sterile forceps. The receptor phase was sterile PBS, stirred synchronously at 600 rpm. The buffer was degassed prior to use by sonication.
Fig. 2.
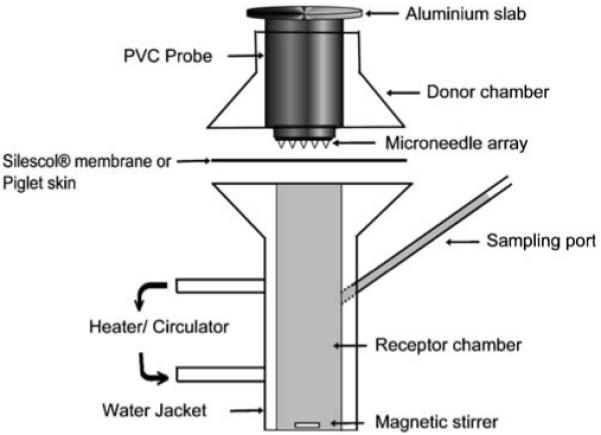
Schematic representation of the Franz-cell apparatus used to study MO penetration across Silescol® or Piglet skin.
An aliquot (1.0 ml) of microbial suspension containing 1×107cfu ml−1 was placed in the donor compartments of the Franz-cells and left in place for 24 h. Donor compartments were covered with sterilised lids to prevent buffer evaporation and avoid contamination. After 24 h, suspensions containing microorganisms not adhered to the membrane were removed from the donor compartments, and the membranes were either not punctured (Control) or punctured (Test) in one of three different ways: punctured with MN which were left in place for 24 h, punctured with MN which were removed, or punctured with a hypodermic needle (21G) which was removed. Control experiments were also run in a similar fashion, but without puncturing the membranes.
A poly (vinyl chloride) (PVC) probe (Fig. 2) with MNs attached to the base and an aluminium slab attached at the top was used for membrane puncture. Prior to use, MNs were sterilised by sonication in 95% v/v ethanol followed by three washings in sterile PBS. Where MN were left in place after puncture, the membrane was punctured with the help of stainless steel clamps that pressed the PVC probe assembly against the membrane, creating holes on the surface. The entire assembly was then left in place for 24 h. When MNs were removed after puncture, the probe assembly was removed within 30 s after application. When a 21G sterile hypodermic needle (21G×1 ½″, Terumo Neolus, Leuven, Belgium) was used, the membrane was punctured slowly by hand, with the full length of the needle inserted and then removed within 30 s. A digital microscope (GXMGE-5 USB Digital Microscope, Laboratory Analysis Ltd, Devon, UK) was used to confirm the presence of holes in the membranes in preliminary studies.
Using a long sterile mixing needle, samples (0.1 ml) were removed from the sampling port of the receptor compartment at defined time intervals (0.25, 1, 2, 3, 4, 5, 6, and 24 h). This volume was immediately replaced using sterile PBS. Each sample was spread plated onto Mueller-Hinton Agar (MHA) which was incubated at 37°C for 24–48 h. Following incubation, the total viable count was determined.
Upon removal, MNs and hypodermic needles were immersed in sterile PBS (5.0 ml) and bacteria attached to the surface dislodged by mild ultrasonication (5 min). Serial ten-fold dilutions were performed, and after plating on MHA plates and incubation for 24–48 h, the total number of colony-forming units on each MN array and hypodermic needle was counted.
As a control, the total number of each microorganism attached to the membrane surface after 24 h contact with the microbial suspensions and prior to membrane puncture was determined. After incubation of the microbial suspensions in the donor compartments of the Franz-cells for 24 h, microbial suspensions and membranes were removed. Each membrane was washed 3 times in PBS and bacteria attached to the surface dislodged by mild ultrasonication (10 min) followed by rapid vortex mixing (2 min) in 10 ml sterile PBS. Serial ten-fold dilutions were performed as described above, and the total number of colony-forming units on each membrane determined as before.
Radiolabelling of Microorganisms
One colony of each isolate was inoculated into 25 ml of MHB-containing 100μl of [3H] thymidine (1.0 mCi ml−1) and incubated for 18 h at 37°C. The suspension was centrifuged (3000 rpm, 10 min), washed twice in cold PBS, and resuspended, as previously described, to an optical density at 550 nm equivalent to 1.0×107cfu ml−1.
To determine the relationship between disintegrations per minute (dpm) and viable count, 1.0 ml aliquots of each labelled suspension were added separately to vials containing 5.0 ml of liquid scintillation cocktail (Ultima gold™, Perkin Elmer, Boston, USA). Vials were stored in darkness for 2.0 h prior to analysis to reduce chemiluminescence to less than 1% in respect of the total count. Samples were then counted in a scintillation counter (Tricarb 1900 TR, LS Analyzer, Packard, USA) for 30 min. Conversion to dpm was achieved against quench correction curves. Mean dpm values from three replicate experiments were then related to the total viable count of microorganisms present.
Permeation of Radiolabelled Microorganisms Across Porcine Skin
Neonate porcine skin, a good model for human skin in terms of hair sparseness and distribution of blood vessels (12,13), was obtained from stillborn piglets and immediately (<24 h after birth) excised, trimmed to a thickness of 400μm using a dermatome (Integra Life Sciences™, Padgett Instruments, NJ, USA) and frozen in liquid nitrogen vapour. Skin was then stored in aluminium foil at −20°C for no more than 7 days prior to use. The porcine skin was then sandwiched between the receptor and donor compartments of the modified Franz cells using sterile forceps. The procedures described in section 2.4 were then repeated, with the radiolabelled microbial suspensions replacing their unlabelled counterparts and the excised porcine skin replacing the Silescol® membranes. Puncturing of the skin was done in exactly the same fashion, with the same variables and controls. However, the sampling protocol was different.
Twenty-four hours after puncture, the Franz cells were dismantled. Skin samples were then placed on an aluminium stub and covered with an aluminium ring, exposing only the diffusional area of 15 mm2. The skin was then subjected to tape-stripping to remove the stratum corneum (14). The skin was dabbed dry with the first tape strip (15×18 mm), and this and the following 12±3 tape strips were placed in a scintillation vial. To ensure intimate contact between the tape and skin, forceps were used to apply gentle pressure for each tape strip. Complete removal of the stratum corneum was indicated by the glistening of the viable epidermal layer after removal of the final tape strip (15). An aliquot (5.0 ml) of dimethyl sulfoxide was then added to the vial to dissolve the tape and stratum corneum. The viable tissue remaining was dissolved in 1.0 ml of tissue solubilizer (NCS® -II, Amersham Biosciences, England, UK) in a scintillation vial. Both sample types were then incubated at 60°C for 12 h.
Liquid samples (1.0 ml) of the tape-stripped stratum corneum, viable tissue and the receptor solution removed at the end of the experiment were then added separately to vials containing 5.0 ml of liquid scintillation cocktail, which were again stored in darkness for 2.0 h prior to analysis. Samples were then counted in the scintillation counter for 30 min. Mean dpm values from three replicate experiments were converted to total viable count of microorganisms present.
Scanning Electron Microscopy Studies
Silescol® membranes, porcine skin and MNs left in place for 24 h after puncture were all examined by scanning electron microscopy (SEM) upon completion of the experiments. Samples were dehydrated in graded alcohol, transferred to a critical-point-drier and fixed on circular aluminium stubs and coated at 2.5 Kv, 18 mA with gold for 45 s (POLARON E5150, Gold Sputter Coater, Quorum Technologies, Ringmer, UK) and examined using a JEOL JSM-840A Scanning Electron Microscope (Jeol, Tokyo, Japan).
Assessment of the Influence of Hypodermic Needle and Microneedle Puncture on Barrier Function of Silescol® and Excised Skin
Transepidermal water loss (TEWL) and transmembrane water loss (TMWL) were measured using a Delfin VapoMeter® (Delfin Technologies Ltd, Kuopio, Finland) by application of the device for 15 s to non-microorganism-loaded porcine skin and Silescol® membrane, respectively, immediately following removal of an inserted hypodermic needle, immediately following removal of a MN array that was left in place for 24 h following puncture, and immediately following removal of a MN array that was removed immediately following puncture. In each case, the membrane or excised skin was mounted on the Franz cell as described in 2.4 and 2.6, respectively.
Statistical Analysis
Where appropriate, data was analysed using the Mann-Whitney U-test. In all cases p<0.05 denoted significance.
RESULTS
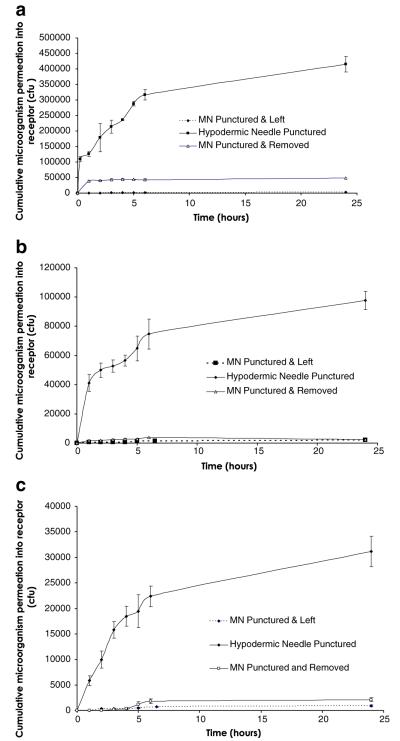
As can be seen from Fig. 3, microorganisms permeated punctured Silescol® membranes in a manner similar to a burst drug release pattern, with most microorganisms having crossed the membranes after 6 h in each case. Table I shows that C. albicans moved across the membrane most effectively following hypodermic needle puncture, with approximately 4.3×105 colony-forming units (cfu) traversing the membrane over 24 h. The total viable count of P. aeruginosa and S. epidermidis crossing the punctured membranes were around an order of magnitude lower. In addition, the total viable count of each microorganism moving across MN-punctured membranes was a further order of magnitude lower, with slightly greater numbers permeating membranes that were punctured with MNs that were immediately removed. The only exception to this was C. albicans, where significantly greater numbers permeated across membranes where MNs were removed immediately rather than remaining in place for 24 h (p=0.0495). Apart from the movement of C. albicans across hypodermic needle-punctured membranes, where 40.2% of the microbial load on control membranes permeated the barrier over 24 h, the numbers of microorganisms permeating the membrane was less than 5% of the original microbial load on control membranes, significantly so in the majority of cases (Table I).
Fig. 3.
Cumulative permeation of microorganisms across Silescol® membranes following puncture with a microneedle (MN) array or a hypodermic needle. The permeating microorganisms were C. albicans (a), P. aeruginosa (b) and S. epidermidis (c). Means ± S.D., n=3.
Table I.
Influence of Puncture Protocol on the Cumulative Number of Microorganisms Crossing Silescol® Membranes after 24 h and the Numbers of Microorganisms Adherent to Hypodermic needles or Microneedle Arrays on Removal. For Both Sets of Data, Numbers are Compared with the Total Microbial Load on Unpunctured Control Membranes
| Cumulative number of microorganisms crossing Silescol® membrane after 24 h (cfu) |
Number of microorganisms adherent to hypodermic needle or MN array on removal (cfu) |
||
|---|---|---|---|
|
|
|||
| (% of original count on control membranes) |
(% of original count on control membranes) |
||
|
|
|||
| Treatment | Microorganism | (means ± S.D., n=3) | (means ± S.D., n=3) |
| Hypodermic Needle | C. albicans | 4.3±0.2×105 (40.2%) |
2.4±0.9×103 (0.23%) |
| P. aeruginosa | 9.8±0.6×104 (4.5%) |
4.7±0.6×103 (0.21%) |
|
| S. epidermidis | 3.1±0.2×104 (0.5%) |
2.4±1.0×103 (0.03%) |
|
| MN Punctured & Left | C. albicans | 2.1±0.4×103 (0.2%) |
5.6±1.1×104 (5.23%) |
| P. aeruginosa | 2.0±0.2×103 (0.1%) |
5.3±1.0×104 (2.38%) |
|
| S. epidermidis | 0.9±0.2×103 (0.01%) |
7.7±1.2×104 (1.04%) |
|
| MN Punctured & Removed | C. albicans | 4.8±0.3×104 (4.5%) |
4.6±0.6×104 (4.30%) |
| P. aeruginosa | 2.5±0.3×103 (0.1%) |
2.9±1.0×104 (1.30%) |
|
| S. epidermidis | 2.1±0.4×103 (0.03%) |
2.7±1.1×104 (0.36%) |
|
As can be seen from Table I, approximately 103cfu of each microorganism adhered to hypodermic needles during insertion. The number of microorganisms adhering to MN arrays was an order of magnitude higher, regardless of puncture protocol. However, only in the case of C. albicans when MNs were inserted into Silescol® membranes and left in place for 24 h, were the number of microorganisms removed on the MN array greater than 5% of the original microbial loading on control membranes.
As can be seen from Table II, experiments employing excised porcine skin and radiolabelled microorganisms, showed that the numbers of microorganisms penetrating skin beyond the stratum corneum were approximately an order of magnitude greater than the numbers crossing Silescol® membranes in the corresponding experiments. While P. aeruginosa did not take up enough radiolabelled thymidine to allow reliable results to be obtained, studies with C. albicans and S. epidermidis showed that the majority of the microbial load which initially attached to the stratum corneum remained attached and did not penetrate the skin, regardless of puncture protocol. Microbial movement into viable tissue was of the order of 104cfu following puncture with hypodermic needles for both C. albicans and S. epidermidis. However, in both cases, the total viable count of microorganisms entering viable epidermal skin was less than 1% of the total number attached to control stratum corneum. Interestingly, approximately 106cfu crossed the epidermal skin completely into the receiver fluid in both cases, amounting to 48% and 3.51% of the original microbial load on control stratum corneums for C. albicans and S. epidermidis, respectively. The permeation of C. albicans and S. epidermidis into viable epidermal skin was of the order of 105, regardless of MN puncture protocol. However, the numbers of microorganisms moving into the viable epidermal skin was less than 5% of the original microbial loading on control stratum corneums in all cases. Significantly, no microorganisms were detectable beyond the epidermal skin in the receptor fluid when MN were used to puncture the skin.
Table II.
Influence of Puncture Protocol on the Number of Microorganisms Found on the Stratum Corneum or in the Viable Tissue of Neonate Porcine Skin on the Number of Microorganisms Completely Traversing the Skin to the Receptor Fluid in the Receiver Compartments of the Franz Cells and the Numbers of Microorganisms Removed on Hypodermic Needles or Microneedle Arrays and Comparison of these Numbers with the Number of Microorganisms Found on Unpunctured Stratum Corneums. Means ± S.D., n=3
| Total viable count of microorganisms (cfu) found in: (% of original count on control stratum corneum) |
Number of microorganisms adherent to hypodermic needle or MN array on removal (cfu) (% of original count on control stratum corneum) |
||||
|---|---|---|---|---|---|
| Treatment | Microorganism | Stratum corneum | Viable tissue | Receptor fluid | |
| Control | C. albicans | 1.5±0.1×107 | ND | ND | NA |
| P. aeruginosa | 7.0±2.8×107 | ND | ND | NA | |
| S. epidermidis | 3.7±0.6×107 | ND | ND | NA | |
| Hypodermic Needle | C. albicans | 5.8±1.4×106 (38.67%) |
9.7±2.2×104 (0.65%) |
7.2±0.6×106 (48.00%) |
1.00±0.03×103 (0.01%) |
| P. aeruginosa | 3.1±2.7×107 (44.29%) |
ND | ND | 4.6±0.9×103 (0.01%) |
|
| S. epidermidis | 2.2±0.5×107 (59.46%) |
3.1±0.9×105 (0.84%) |
1.3±0.6×106 (3.51%) |
4.3±0.7×103 (0.01%) |
|
| MN Punctured & Left |
C. albicans | 4.2±0.9×106 (28.00%) |
5.3±1.1×105 (3.50%) |
ND | 4.1±0.3×104 (0.27%) |
| P. aeruginosa | 4.9±4.8×107 (70.00%) |
ND | ND | 4.7±0.5×104 (0.07%) |
|
| S. epidermidis | 1.2±0.2×107 (32.43%) |
6.9±1.3×105 (1.87%) |
ND | 6.0±0.5×104 (0.16%) |
|
| MN Punctured & Removed |
C. albicans | 4.8±0.1×106 (32.00%) |
3.2±0.9×105 (2.13%) |
ND | 2.9±0.2×104 (0.19%) |
| P. aeruginosa | 5.3±1.6×107 (75.71%) |
ND | ND | 2.8±0.4×104 (0.04%) |
|
| S. epidermidis | 2.2±0.3×107 (59.46%) |
4.1±1.3×105 (1.11%) |
ND | 2.9±0.6×104 (0.08%) |
|
NA=Not applicable.
ND=No microorganisms detectable. P. aeruginosa did not take up the radiolabel to the same extent as the other microorganisms, thus compromising experiments using it.
As with the Silescol® experiments, approximately 103cfu of each microorganism adhered to hypodermic needles during insertion. Similarly, the total viable count of microorganisms adhering to MN arrays was also an order of magnitude higher, regardless of puncture protocol. In all cases, the total viable count of microorganisms adherent to the needle or MN array on removal were less than 0.3% of the original microbial load attached to control stratum corneum, with C. albicans again showing the greatest adherence to MN arrays. Fig. 4 shows adherent C. albicans on the surface of a MN following removal from skin.
Fig. 4.

Adherent C. albicans on the surface of a single microneedle following removal from neonate porcine skin.
DISCUSSION
Since the first study on their use for enhanced transdermal drug delivery in the late 1990s (4), microneedle (MN) arrays have been extensively investigated. Currently, this technology represents one of the most promising strategies for enabling transdermal delivery of the macromolecular drugs produced by the biotechnology boom. Central to their success is their ability to puncture the outermost barrier of the skin, the stratum corneum, creating aqueous pathways for drug diffusion. However, the stratum corneum is a vital barrier, contributing to thermoregulation and homeostasis and preventing entrance of foreign particles and, importantly, microorganisms. Until recently, studies involving MNs were limited to in vitro membrane permeation experiments and animal trials, where the subjects were killed shortly after completion of the investigation (2-11). However, a number of clinical studies involving human participants have now been published (16-18) and MNs have been proposed as a means to prevent transmission of infection during vaccination in the developing world (18,19).
Given that patient safety is equally as important for the pharmaceutical industry as product efficacy, it is suprising that the present study is the first to investigate whether microorganisms can traverse through MN-induced holes. We have clearly shown, using two different models, that representative Gram positive (S. epidermidis), Gram negative (P. aeruginosa) and fungi (C. albicans) can indeed traverse MN-induced holes. Silescol® is a silicone membrane used to mimic the intercellular lipid transport pathways of the stratum corneum in in vitro experiments. Employing this model allowed us to obtain time-resolved permeation profiles for three representative microorganisms. It was obvious that hypodermic needle puncture led to significantly greater microbial penetration across Silescol® than MN puncture in each case. This was despite the cross-sectional area of the needle employed (0.5 mm2) being less than the combined cross-sectional area (at bases) of the 30 MNs of the array (1.5 mm2). It was likely, however, that the inherent elasticity of the membrane allowed the individual MN-induced holes to close over to a greater extent than the much larger single hole created by hypodermic needle puncture. Notably, microbial penetration appeared to continue over at least the first 6 h, as evidenced by the permeation profiles obtained. This suggests that the microorganisms studied were not simply pushed across the membranes during the act of puncture.
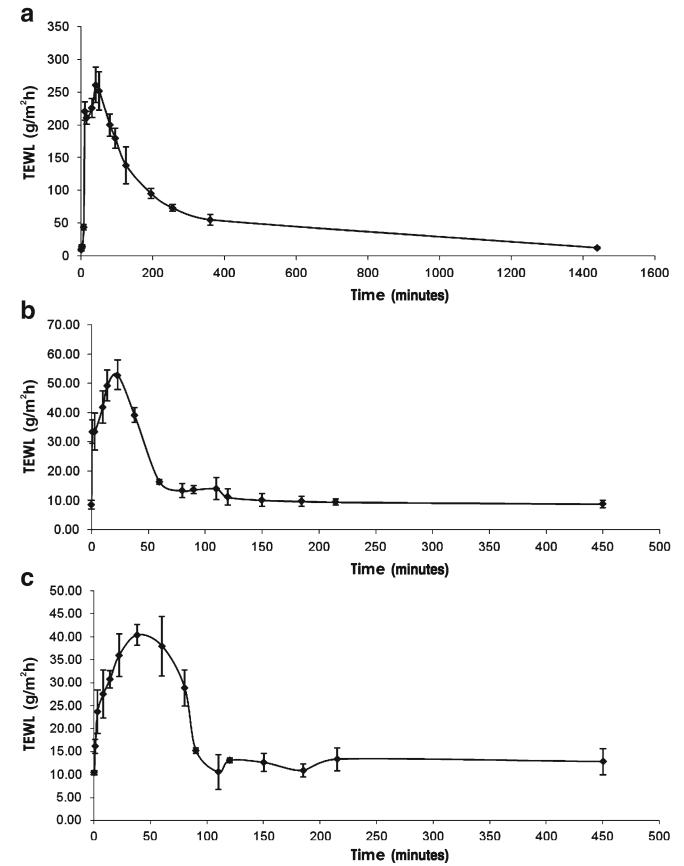
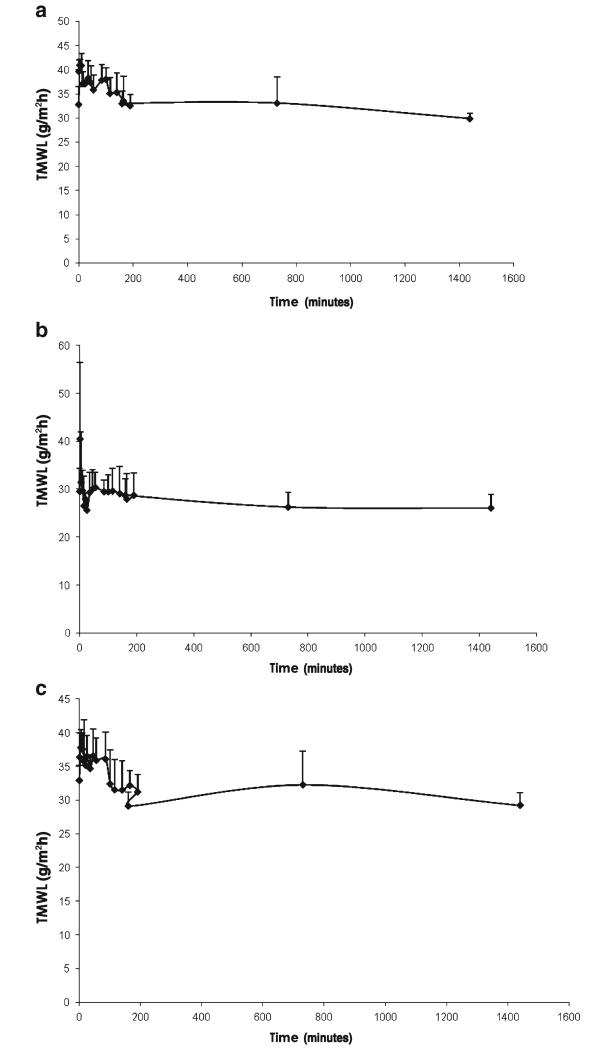
The Silescol® model is limited in that it mimics only the intercellular lipid barrier of the stratum corneum and takes no account of naturally-occurring antimicrobial substances in skin, the immune components of viable skin or, importantly, the obstacle to microbial movement offered by the cellular structures in the underlying viable epidermis. Indeed, our studies using excised porcine skin showed that C. albicans and S. epidermidis did not move beyond the viable epidermis following MN puncture. This model, while incapable of following microbial movement over time, did show again that hypodermic needle puncture led to significantly greater microbial penetration into skin, even allowing microorganisms to penetrate beyond the viable epidermis. This finding was supported by transepidermal water loss studies (Fig. 5), which revealed that, following hypodermic needle puncture, water loss across skin was much greater and remained elevated for longer time, periods than when MN puncture was employed, regardless of protocol. The number of microorganisms penetrating the stratum corneum in the skin model was significantly greater than that penetrating Silescol® membranes in each case. This is likely due to the greater elasticity of the silicone membrane, meaning it tended to close over to a greater extent following puncture (Figs. 5 and 6).
Fig. 5.
Transepidermal water loss (TEWL), as measured using a Delfin VapoMeter® (Delfin Technologies Ltd, Kuopio, Finland) by application of the device for 15 s to nonmicroorganism-loaded porcine skin immediately following removal of an inserted hypodermic needle (a), immediately following removal of a microneedle array that was left in place for 24 h following puncture (b) and immediately following removal of a microneedle array that was removed immediately following puncture (c).
Fig. 6.
Transmembrane water loss (TMWL), as measured using a Delfin VapoMeter® (Delfin Technologies Ltd, Kuopio, Finland) by application of the device for 15 s to nonmicroorganism-loaded Silescol® membrane immediately following removal of an inserted hypodermic needle (a), immediately following removal of a microneedle array that was left in place for 24 h following puncture (b) and immediately following removal of a microneedle array that was removed immediately following puncture (c).
The numbers of microorganisms crossing both Silescol® membranes and stratum corneum following MN puncture were greater than 103cfu in each case. It is likely that this number would increase if the density of MNs on the array was increased and/or if MNs of greater length or diameter at base were employed. The number of invading microorganisms required to cause an infection has been estimated as 104 (20), but ultimately depends on pathogen and host response factors. We have shown here that, with a microbial load of approximately 6.7×106 cfu cm−2 on Silescol® membranes and stratum corneum, hypodermic needle puncture allowed more than 104 microorganisms to penetrate both with 106cfu of C. albicans and S. epidermidis completely crossing the epidermal skin in 24 h following needle puncture. However, in the developed world, appropriate use of hypodermic syringes does not routinely cause infection (21) despite the fact that bacteria are found as part of the normal skin flora in numbers ranging from 102–106 cfu cm−2 depending on location and that alcohol decontamination of skin prior to injection is not completely effective (22, 23). This suggests that bacterial numbers entering skin following hypodermic needle puncture are not sufficient to cause infection in normal circumstances, which may be due to the fact that skin has a number of highly effective innate defence mechanisms. Normal skin has a pH of 5–6, which inhibits the growth of pathogenic organisms with proliferation of pathogens also prevented by secreted lipids with inhibitory properties. Furthermore, antibiotic-style peptides, defensins, play a role in protection, while peptidoglycan recognition proteins 3 and 4 have been shown to impede the growth of transient skin flora (24). Finally, the skin is a major immune-competent organ. Foreign agents and antigens that penetrate the stratum corneum encounter a dense network of potent antigen-presenting cells, the epidermal Langerhans cells and the dermal dendritic cells. These cells readily take up foreign antigens, migrate to the draining lymph node to present antigen fragments to resting T lymphocytes and initiate antigen-specific immune responses (25).
In this study, we have shown in vitro that MN puncture resulted in significantly less microbial penetration than did hypodermic needle puncture and that no microorganisms crossed the viable epidermis in MN-punctured skin, in contrast to hypodermic needle-punctured skin. Given the antimicrobial properties of skin, it is, therefore, likely that application of MN arrays to skin in an appropriate manner would not cause either local or systemic infection in normal circumstances in immune-competent patients. However, we have clearly shown that microorganisms can successfully adhere to MN arrays, with the contact of the large surface-area baseplate with the skin the likely reason. Consequently, MNs should be sterilised during manufacture and should not be re-used after application.
In conclusion, this study has shown, for the first time, that although microorganisms can traverse microneedle-induced holes in the stratum corneum in vitro, the infection risk associated with skin application of microneedles is likely to be less than that associated with hypodermic needles. In supporting widespread clinical use of microneedle-based delivery systems, appropriate animal studies are now needed to conclusively demonstrate this in vivo. Safety in patients will be enhanced by aseptic or sterile manufacture and by fabricating microneedles from self-disabling materials (e.g. dissolving or biodegradable polymers) to prevent inappropriate or accidental reuse.
ACKNOWLEDGEMENTS
This work was supported by BBSRC grant number: BB/E020534/1 and the Science Foundation Ireland Tyndall National Access Programme project number NAP 156.
REFERENCES
- 1.Prausniz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–7. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Mikszta JA, Dekker JP, Harvey NG, Dean CH, Brittingham JM. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74:6806–10. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3:65–75. doi: 10.2174/156720106775197510. [DOI] [PubMed] [Google Scholar]
- 4.Henry S, McAllister DV, Allen MG, Prausnitz MR. Micro-fabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–5. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Cont Rel. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Cont Rel. 2004;97:503–11. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise micro-injection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–7. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 8.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–60. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdev. 2005;7:47–52. doi: 10.1007/s10544-005-6171-y. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: potential for enhanced topical photodynamic therapy. J Cont Rel. 2008;129:154–62. doi: 10.1016/j.jconrel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle arrays permit true intradermal delivery of a preformed photosensitiser. Photochem Photobiol. 2009;85:195–204. doi: 10.1111/j.1751-1097.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 12.Woolfson AD, McCafferty DF, McCallion CR, McAdams ET, Anderson JMcC. Moisture-activated, electrically conducting bio-adhesive hydrogels as interfaces for bioelectrodes: effect of film hydration on cutaneous adherence in wet environments. J Appl Polym Sci. 1995;58:1291–6. [Google Scholar]
- 13.Fourtanier A, Berrebi C. Miniature pig as an animal model to study photoaging. Photochem Photobiol. 1989;50:771–84. doi: 10.1111/j.1751-1097.1989.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 14.Moser K, Kriwet K, Froehlich C, Kalia YN, Guy RH. Supersaturation: enhancement of skin penetration and permeation of a lipophilic drug. Pharm Res. 2001;18:1006–11. doi: 10.1023/a:1010948630296. [DOI] [PubMed] [Google Scholar]
- 15.Pellett MA, Roberts MS, Hadgraft J. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm. 1997;151:91–8. [Google Scholar]
- 16.Wermeling DP, Banks SL, Huclson DA, Prausnitz M. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Nat Acad Sci USA. 2008;105:2058–63. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdev. 2009;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 19.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vacc Immun. 2007;14:375–81. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seal DV, Hay RJ, Middleton KR. Skin and wound infection: investigation and treatment in practice. Informa Healthcare; London: 2000. pp. 5–8. [Google Scholar]
- 21. [Accessed 16th April 2009]. www.who.int/en.
- 22.Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6:641–52. doi: 10.1016/S1473-3099(06)70600-4. [DOI] [PubMed] [Google Scholar]
- 23.Del Mar CB, Glasziou PP, Spinks AB, Sanders SL. Is isopropyl alcohol swabbing before injection really necessary? Med J Aust. 2001;175:341–2. doi: 10.5694/j.1326-5377.2001.tb143609.x. [DOI] [PubMed] [Google Scholar]
- 24.Elsner P. Antimicrobials and the skin physiological and pathological flora. Curr Prob Derm. 2006;33:35–41. doi: 10.1159/000093929. [DOI] [PubMed] [Google Scholar]
- 25.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–64. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]