Abstract
Background
Cobalamin (vitamin-B12) and cobalamin analogues are present in human feces, but a complete identification has not been established, and the amounts present have not been determined.
Objective
We aimed to develop a liquid chromatography-mass spectrometry method for cobalamin and cobalamin analogues and to identify and quantitiate the amounts present in human feces.
Design
Fecal samples were obtained from 20 human subjects in good general health. The samples were analyzed for the presence and amounts of cobalamin and 12 cobalamin analogues that were synthesized with and without the incorporation of stable isotopes.
Results
Cobalamin and 7 cobalamin analogues were identified and quantitated in human feces. The mean for the total amount present in 18 subjects whose daily intake was ≤ 25 ug cobalamin from vitamin supplements was 1309 ng cobalamin equivalents/g wet wt of feces. Cobalamin (1.4%) and cobinamide (1.8%) (an incomplete corrinoid) represented a small portion of the total amount. Six cobalamin analogues that contain a base other than the 5,6-dimethylbenzimidizidole in cobalamin were present. The bases and their mean amounts (in %) are 2-methyladenine (60.6%), p-cresol (16.3%), adenine (12.5%), 2-(methylthio)adenine (15.5%), 5-hydroxybenzimidazole (1.8%), and phenol (0.1%). One subject ingested 1 mg cobalamin/d and another ingested 2 mg cobalamin/d, and they appeared to convert most of the cobalamin to cobinamide and the 4 analogues that contain the bases – 2-methyladenine, p-cresol, adenine and 2-(methylthio)adenine.
Conclusion
Cobalamin analogues are present in human feces and account for > 98% of the total of cobalamin plus cobalamin analogues. A major portion of large amounts of ingested cobalamin appears to be converted to cobalamin analogues.
Keywords: Cobalamin, vitamin B12, cobalamin analogues, feces, liquid chromatography, mass spectrometry
INTRODUCTION
Cobalamin [vitamin B-12 (Cbl)] is synthesized only by microorganisms, which also synthesize a large number of Cbl analogues that contain a base other than dimethylbenzimidazole (5,6-diMeBZA), which is the base present in Cbl (Figure 1). They consist of benzimidazoles other than 5,6-diMeBZA (items 4–7, 9 and 11 in Figure 1); purines (items 2, 3, and 8), phenols; (items 12 and 13), cobinamide (Cbi) (item 1), which lacks the base, ribose and phosphate moieties present in Cbl. A comprehensive review of Cbl and Cbl analogues was provided by Renz (1).
Figure 1.
Structure of cyanocobalamin (CN-Cbl) (upper section) and the structures (lower section) of the base portion of CN-Cbl [10 (numbers in circles)] and 11 CN-Cbl analogues (2–9) and (11–13) that differ from CN-Cbl only in the base portion. * CN-Cbi (cyanocobalamin) (1) lacks the base, ribose and phosphate moieties of CN-Cbl.
Gastric intrinsic factor, transcobalamin and haptocorrin facilitate the cellular uptake of Cbl by ileal mucosal cells, by all cells in the body, and by hepatocytes, respectively (2–4). These transport systems also appear to protect humans and other animals from the potential deleterious effects of the naturally occurring Cbl analogues. Intrinsic factor binds Cbi and the purine and phenol Cbl analogues with very low affinity, and transcobalamin binds cobinamide and the phenol containing Cbl analogues with very low affinity (3, 5, 6). Haptocorrin binds all of the Cbl analogues with high affinity (3, 5) but delivers them exclusively to hepatocytes (3, 7) where the haptocorrin-Cbl or haptocorrin Cbl analogue complexes are internalized by the asialoglycoprotein receptor-mediated mechanism (7, 8). Within 30 minutes of uptake, haptocorrin is degraded and Cbl is released back into plasma where it is bound to transcobalamin and taken up by all cells in the body (2, 3, 7). Hepatocytes retain Cbl analogues and facilitate their excretion in feces and urine over many days (3).
Feces contain a large number of microorganisms (9, 10) and previous studies have indicated that Cbl and a number of Cbl analogues are present in significant amounts (11–13). Early studies of Cbl analogues used paper chromatography and electrophoresis and the fact that Cbl and a number of naturally occurring Cbl analogues differ in their ability to stimulate the growth of certain microorganisms (11, 12). These studies showed that Cbl analogues were major components of feces from numerous animals. A later study used differences in radiodilution assay results obtained with intrinsic factor and haptocorrin as the Cbl binding proteins and suggested that Cbl accounted for only about 5% of the total of Cbl and Cbl analogues in human feces (13). None of these techniques made it possible to identify with certainty or quantitiate accurately the Cbl and individual Cbl analogues in feces and other biologic materials. We have now modified the liquid chromatography-mass spectrometry (LC-MS) techniques of Alsberg et al (14) in ways that make it possible to identify and quantitiate the amounts of Cbl and individual Cbl analogues in human feces.
SUBJECTS AND METHODS
Control Subjects
The control subject group consisted of 11 men and 9 women who were in good general health and whose mean age was 47 y (range: 19–80 y). They were instructed to place into a 50-mL polypropylene screw-top test tube a stool sample in an amount that approximated a volume of 10–15 mL according to the markings on the tube. The sample was to be stored in a biohazard bag in the home freezer until it was brought to the laboratory, where it was stored at −20° C until the contents were assayed.
Written informed consent was obtained from all subjects. The study was approved by the Institutional Review Board at the University of Colorado Health Sciences Center.
Materials
Cyanocobalamin (CN-Cbl), hydroxycobalamin (OH-Cbl), adenine, benzimidazole, yeast extract, dextrose 1-methylpiperidine, liquefied phenol (~ 90% phenol, 10% H20), and pyridine were purchased from Sigma Chemical Co (St. Louis, MO). 5-Methoxybenzimidazole, 5-methylbenzimidazole, 5,6-dimethylbenzimidazole, 2,3-diaminonapthalene, phenol-D6, and p-cresol-D8 were purchased from Aldrich (St. Louis, MO). 2-Methyladenine-D7 hemisulfate, benzimidazole-4,5,6,7-D4, 3,4-diaminotoluene-2,5,6-D3 and 5,6-dimethylbenzimidazole-4,7-D2 were purchased after custom synthesis from MSD Isotopes [(now C/D/N Isotopes) Montreal, Canada]. 2-methyladenine hemisulfate and 5-methoxybenzimidazole-4,6,7- D3 were purchased after custom synthesis from C/D/N Isotopes. Adenine-1,3-15N2, 2-(methylthio)adenine, 2-(methyl-13C1,D3-thio)adenine, and 2,3-diaminonaphthalene-15N2, were purchased after custom synthesis from Isotech (St. Louis, MO), which was also the source of K13C115N1. C18 (YMC Gel, # AA12S5) was obtained from Seika Corporation of America (Allentown, PA).
Hog R-protein-Sepharose was prepared as described previously (15) except that hog intrinsic factor was not removed. Two final preparations contained CN-Cbl binding capacities of 87 and 394 ug of Cbl/mL Sepharose and contained about 85% R-protein and 15% intrinsic factor. Hydrocyanic acid (HCN) was prepared as described previously (3). CN-Cbi [b,d,e-15N3] was synthesized from CN-Cbi [b,d,e-OH] (3) with the use of 15N1H4Cl (Cambridge Isotope Laboratories, Andover, MA) and N-(3-dimethylaminopropyl)-N1-ethyl carbodiimide (Sigma) as described previously compounds (16). 13C115N1-Cbl was prepared as described previously (17) for 14C1N-Cbl except that C18 was used in place of SM2 B10 Beads (Bio-Rad Laboratories, Hercules, CA).
5-methoxybenzimidazole and 5-methoxybenzimidazole-4,6,7- D3 were converted to 5-hydroxybenzimidazole and 5-hydroxybenzimidiazole-4,6,7- D3, respectively, by a modification of the method of Renz et al (18) that involves refluxing each base with 47% hydrobromic acid for 1–4 h. The hydrobromic acid was then removed by overnight vacuum centrifugation in a vacuum centrifuge (Savant Speedvac Concentrator; Thermo Fisher Scientific, Pittsburgh, PA).
Methods
Concentration of cobalamin and cobalamin analogues
The concentrations of Cbl and Cbl analogues were measured spectrophotometrically at 367.5 nm in 0.1 mol KCN/L. A molar extinction coefficient of 30 800/mol × cm was used in each case (3). All data are presented as CN-Cbl equivalents.
Bacterial synthesis of cobalamin and cobalamin analogues
Cbl and most of the Cbl analogues with alterations in the base portion were prepared by a modification of the biosynthetic technique of Perlman and Barrett (19) with the use of Propionibacterium arabinosum [American Type Culture Collection (ATCC), Manassas, VA 4965]. The culture medium contained the following amounts/L H2O: yeast extract, 20 g; dextrose, 30 g; CoSO4-7H2O, 25 mg; base, 75 mg; and sufficient 10 N NaOH to raise the pH to 7.6–7.8. The following bases were added to cultures to obtain the following respective Cbl analogues: 2-methyladenine hemisulfate to obtain [2-MeAde]CN-Cba; (CBb = cobamide); 2-methyladenine-D7 hemisulfate for [2-(Me-D3]Ade-8-D1]CN-Cba; adenine for [Ade]CN-Cba; adenine-1,3-15N2 for [Ade-1,3-15N2]CN-Cba; benzimidazole for [BZA]CN-Cba; benzimdazole-4,5,6,7- D4 for [BZA-4,5,6,7- D4]CN-Cba; 5-methoxybenzimidazole for [5-MeOBZA]CN-Cba; 5-methoxybenzimidazole-4,6,7- D3 for [5-MeOHBZA-4,6,7- D3]CN-Cba; 5-methylbenzimidazole for [5-MeBZA]CN-Cba; 3,4-diaminotoluene-2,5,6- D3 for [5-MeBZA-4,6,7- D3]CN-Cba; , 2-(methylthio)adenine for [2-MeSAde]CN-Cba; 2-(methyl-13C1,D3-thio)adenine for [2-Me(13C1,D3)SAde]CN-Cba; 5,6-dimethylbenzimidazole-4,7- D2 for [5,6-diMeBZA-4,7- D2]CN-Cbl; 2,3-diaminonapthalene for [NZA]CN-Cba; 2,3-diaminonaphthalene-15N2 for [NZA-15N2]CN-Cba. Cultures were grown at 30° C in Erlenmeyer flasks with gauze and cotton plugs for about 7 d.
With the use of Propionibacterium freudenreichii (ATCC 9614) and the culture media and incubation conditions described above, the following bases were used to obtain their respective Cbl analogues: 5-hydroxybenzimidazole to obtain [5-OHBZA]CN-Cba and 5-hydroxybenzimidazole-4,6,7- D3 to obtain [5-OHBZA-4,6,7- D3]CN-Cba.
Eubacterium limosum (ATCC 10825) was grown anaerobically at 37° C in actinomyces broth (ATCC medium 7) and used to obtain Cbl analogues by using a modification of the method of Renz et al (18). Initially, 500 mL media in a sterile air-tight bottle was inoculated, degassed with N2, stoppered tightly, grown for 7 d at 37° C, and used to inoculate 2 Erlenmeyer flasks, each of which contained 6 L of actinomyces broth that contained 25 mg of CoSo4–7H2O and 75 mg base/L. Before inoculation, the 2 flasks with media were autoclaved and allowed to cool overnight. The next day, the media was boiled for 5 min, placed in an ice bath, aerated with N2 for 20 min, inoculated, and aerated with N2 for an additional 10 min. The flasks were stoppered tightly and incubated at 37° C for 5–7 d. The following bases were added to cultures to obtain their respective Cbl analogues: 5-hydroxybenzimidazole to obtain [5-MeO,6-MeBZA]CN-Cba; and 5-hydroxybenzimidiazole-4,6,7- D3 to obtain [ 5-MeO,6-MeBZA-4,7- D2]CN-Cba.
Sporomusa ovata (ATCC 35899) was grown anaerobically at 30° C in Sporomusa medium (ATCC medium 1425) using a modification of the method of Stupperich et al (20) as described on the ATCC website (21). The modification was that Cbl was not added to the vitamin solution, but the 8% NaHCO3 and 10% glycine betaine solutions and 75 mg/L of the base of interest were added to the initial stock solution. Initial small-scale cultures were obtained in Hungate tubes (Bellco Biotechnology, Vineland, NJ) containing 12 mL liquid media without cobalt or base; 4–5 such tubes were used to inoculate each of two 6-L Erlenmeyer flasks with tight-fitting stoppers containing 5.5 L of media, which were then incubated at 30° C in 80% N2 and 20% CO2 for 12–14 d. The following bases were added to cultures to obtain their respective Cbl analogues: phenol to obtain [Phe]CN-Cba; phenol-D6 for [Phe-D5]CN-Cba; p-cresol for [p-Cre]CN-Cba; and p-cresol-D8 for [p-Cre-D7]CN-Cba.
Purification of cobalamin and cobalamin analogues from bacterial cultures
The total volume of all of the large-scale cultures was 11–12 L. At the end of the incubation period, 5.5–6 L was divided among six 1-L centrifuge tubes and centrifuged at 15 900 × g for 30 min at 20° C (Avanti J-20XP, Beckman Coulter, Fullerton, CA). The supernatants were discarded, the remaining 5.5–6 L was added to the centrifuge tubes that still contained the first pellets, and then centrifugation was repeated under the same conditions. We added 120 mL of 0.1 mol KPO4/L (pH 7.5) containing 0.1 mg freshly prepared KCN/mL to each of the 6 bacterial pellets. The tubes were then frozen in a dry ice-acetone bath for 30 min, thawed in a 20° C water bath, heated in a boiling water bath for 45 min with mixing every 15 min, cooled in an ice water bath, and stored at 4° C overnight. The preparations were combined into 2 of the 1-L centrifuge tubes and centrifuged as described above. The supernatants were then applied to a 2.5 × 20-cm glass column, with organic compatible fittings (Spectrum Chromatography, Houston, TX) and Teflon tubing, that contained 50 mL C18, that had a flow rate of about 600 mL/h, and that had been washed with 100 mL MeOH and then with 200 mL H2O. The column was then washed with 250 mL H2O and eluted with 100 mL MeOH into polypropylene centrifuge tubes. The eluate was dried overnight without heating in a vacuum centrifuge, dissolved in 60 mL H2O in a 50° C water bath, and centrifuged in the Avanti J-20XP centrifuge for 30 min at 75 000 × g at 20° C.
The supernatant from the previous step was applied to a 2.5 × 20 cm column that was identical to one used for the C18 step (see above), except that it contained 40 mL R-protein Sepharose with a total CN-Cbl-binding capacity of 15.8 mg. The column had a flow rate of 250 mL/h and was washed at room temperature with 250 mL H2O just before the supernatant was applied. The column was then washed sequentially with 30 mL H2O, 200 mL of 0.05 mol glycine-NaOH (pH 10.0) containing 1 mol NaCl/L, and 500 mL H2O. The column was eluted in a fume hood with freshly prepared 60% pyridine in H2O at a flow rate of 100 mL/h. The pink color first appeared in the outflow tubing after about 35 mL of 60% pyridine had been applied; at this point the column was turned off for 30 min. The initial 35 mL eluate was discarded. An additional 70 mL eluate was collected in a 200-mL glass Erlenmeyer flask with a 30-min pause at the midway point. One mL of 0.1% HCN was added to the eluate, which was then incubated in the fume hood for 1 h with the light on. The eluate was divided among 4 polypropylene centrifuge tubes and dried overnight in a vacuum centrifuge as described above. The dried material was dissolved in a total volume of 3 mL H2O and stored at −20° C until the final purification by HPLC as described below. The R-protein Sepharose was washed with 40 mL H2O liquefied phenol in an amount such that all of the R-protein Sepharose became translucent (about 100 mL), and 200 mL H20. It was then stored at 4° C until it was used again.
Purification of cobalamin and cobalamin analogues from feces
The scheme for purification of Cbl and Cbl analogues from feces was the same as that used for the large bacterial cultures except the scale was much smaller. Fecal samples were stored at −20° C until they were thawed overnight at 4° C, and 1–10-g amounts were placed in 50-mL Falcon tubes. We added to each tube 30 mL of 0.1 mol KPO4/L (pH 7.5) containing 0.1 mg/mL freshly prepared KCN/mL and 500 uL of the solution containing the labeled CN-Cbl and 12 labeled CN-Cbl analogues and then shook the tubes and placed them in a boiling water bath . The tubes were shaken vigorously every 15 min, and, after 45 min, they were cooled in an ice water bath and stored at 4° C overnight. The tubes were then centrifuged (T J-6 centrifuge; Beckman Coulter) at 1000 × g at 20° C for 30 min. The supernatants were poured into 50-mL polypropylene centrifuge tubes that were centrifuged for 1 h at 20° C and 75 000 × g in the Avanti J-20 XP centrifuge. The supernatants were then poured directly onto 1 × 10-cm polypropylene disposable columns (Econo-Pac columns; Bio-Rad Laboratories) containing 3 mL C18 that had been washed 3 times with 3 mL MeOH and then 3 times with 3 mL H2O. The columns were then washed 3 times with 2.5 mL H2O and eluted 3 times with 2.5 mL MeOH that was collected in 15-mL polypropylene centrifuge tubes. The eluates were dried overnight in the vacuum centrifuge, dissolved in 5 mL H2O, and centrifuged at 20° C and 50 000 × g for 30 min.
The supernatants were applied to 0.7 × 10 cm columns (Econo-Columns, Bio-Rad Laboratories) that had an 11-cm long piece of Teflon tubing (#8050-0187, Thermo Fisher Scientific) attached to chrome-plated stopcocks (#6028 POPPER & SONS, Lincoln, RI), and that contained 1.15 mL R-protein Sepharose with a total Cbl-binding capacity of 100 ug. The columns had been washed 3 times with 2.5 mL H2O, and the sample was applied at a flow rate of about 0.5 mL/min. The columns were then washed sequentially twice with 2.5 mL H2O, 2.5 mL 0.05 mol glycine-NaOH/L (pH 10.0) containing 1 mol NaCl/L, and 6 times with 2.5 mL H2O. The columns were eluted in a fume hood with 5.5 mL of freshly prepared 60% pyridine in H2O with 30 min delays after the first 1.5-mL application and each of 3 subsequent 0.5-mL applications. Fifty uL of 0.1% HCN was added to each eluate, which was then incubated in the fume hood for 1 h with the light on. The eluates were dried overnight by vacuum centrifugation, dissolved in 600 uL H20, and stored at −20° C until they were analyzed by LC-MS. The R-protein Sepharose columns were washed with 2.5 mL H2O, liquefied phenol in an amount such that all of the R-protein Sepharose became translucent (about 3 mL), and 5 times with 2.5 mL H20. They were then stored at 4° C until they were used again.
Final purification of bacterially synthesized cyanocobalamin and cyanocobalamin analogues using liquid chromatography
LC was performed by modification of the methods of Alsberg et al (14) with an Agilent Series 1100 system (Agilent Technologies, Santa Clara, CA) that included a ChemStation, a binary pump, a temperature-controlled autosampler and a fraction collector both set at 6° C, a column thermostat set at 25° C, and a diode array detector set at 361 nm. The final purification of CN-Cbl and the CN-Cbl analogues from the R-protein Sepharose eluates from the large-scale bacterial cultures was performed by using either a Keystone Prism RPN C18 column (Thermo Fisher Scientific) that was 250 × 10 mm and that contained 5-micron particles or an Agilent Extend C18 column (Agilent Technologies) that was 250 × 9.4 mm and that contained 5-micron particles.
The Prism RPN column was used for the large-scale final purification of CN-Cbl and all of the CN-Cbl analogues except CN-Cbi, [Phe]CN-Cba, and [p-Cre]CN-Cba, which eluted as 2 peaks at the acidic pH used in the Prism RPN system described above (Table 1) and [5-MeO,6-MeBZA]CN-Cba. The solvents for LC were solvent A (99% H2O, 1 % acetic acid) and solvent B, (99% acetonitrile, 1 % acetic acid). The flow rate was 3 mL/min, 5-mL fractions were collected, and the injection volume was 0.9 mL. Chromatography was isocratic and the solvent composition was chosen such that the compound of interest eluted 28–32 min after injection. Solvent compositions ranged from a low of 8% B and 92% A for [2-MeAde]-CN-Cba to a high of 13 % B and 87% A for [NZA]CN-Cba. Forty min after injection, the solvent composition was changed to 100% B for 10 min, and this step was followed by a 20-min equilibration at the initial condition before the next injection. A total of 3 injections were made for each compound. Major fractions in the 28–32-min postinjection elution window were analyzed by using the prism RPN analytic LC-MS system, and those that were free of contaminating compounds were pooled, dried overnight in the vacuum centrifuge, dissolved in 1–3 mL H20 to give a concentration of about 1 mg/mL and stored at −20° C.
Table 1.
Ions used for the identification and quantitation of cobalamin (Cbl) and Cbl analogues in the Prism RPN Analytic LC-MS system 1
| Number and item | Base Plus H+ |
Molecular Ion Plus H+ |
Retention Time 2 |
Group | Group Time |
Fragmentor | |
|---|---|---|---|---|---|---|---|
| min | # | min | volts | ||||
| 13 | CN-Cbi | - | 1015.6 | 14.2; 15.5 4 | 1 | 10–17 | 325 |
| CN-Cbi[ b,d,e-15N3 ] | - | 1018.6 | |||||
| 2 | [ 2-MeAde ]CN-Cba | 150.1 | 1358.6 | 18.6 | 2 | 17–20 | 250 |
| [ 2-(Me-D3)Ade-8-D ]CN-Cba | 154.15 | 1362.6 | |||||
| 3 | [ Ade ]CN-Cba | 136.1 | 1344.6 | 21.2 | 3 | 20–31 | 250 |
| [ Ade-1,3-15N2 ]CN-Cba | 138.1 | 1346.1 | |||||
| 4 | [ 5-OHBZA ]CN-Cba | 135.1 | 1343.6 | 22.6 | 3 | 20–31 | 250 |
| [ 5-OHBZA-4,6,7-D3 ]CN-Cba | 138.1 | 1347.6 | |||||
| 5 | [ BZA ]CN-Cba | 119.1 | 1327.6 | 23.8 | 3 | 20–31 | 250 |
| [ BZA-4,5,6,7-D4 ]CN-Cba | 123.1 | 1331.6 | |||||
| 6 | [ 5-MeOBZA ]CN-Cba | 149.1 | 1357.6 | 24.3 | 3 | 20–31 | 250 |
| [ 5-MeOBZA-4,6,7-D3 ]CN-Cba | 152.1 | 1360.6 | |||||
| 7 | [ 5-MeBZA ]CN-Cba | 133.1 | 1341.6 | 25.1 | 3 | 20–31 | 250 |
| [ 5-MeBZA-4,6,7-D3 ]CN-Cba | 136.1 | 1344.6 | |||||
| 8 | [ 2-MeSAde ]CN-Cba | 182.1 | 1390.6 | 26.9 | 3 | 20–31 | 250 |
| [ 2-Me(13C1,D3)SAde ]CN-Cba | 186.1 | 1394.6 | |||||
| 9 | [ 5-MeO,6-MeBZA ]CN-Cba | 163.1 | 1371.6 | 27.3 | 3 | 20–31 | 250 |
| [ 5-MeO,6-MeBZA-4,7-D2 ]CN-Cba | 165.1 | 1373.6 | |||||
| 10 | CN-Cbl | 147.1 | 1355.6 | 27.3 | 3 | 20–31 | 250 |
| [5-Me,6-MeBZA-4,7-D2 ]CN-Cbl | 149.1 | 1357.6 | |||||
| 11 | [ NZA ]CN-Cba | 169.1 | 1377.6 | 30.7 | 3 | 20–31 | 250 |
| [ NZA-15N2 ]CN-Cba | 171.6 | 1379.6 | |||||
| 12 | [ Phe ]CN-Cba | 95.1 | 1303.6 | 32.2; 34.5 4 | 4 | 31–40 | 325 |
| [ Phe-D5 ]CN-Cba | 100.1 | 1308.6 | |||||
| 13 | [ p-Cre ]CN-Cba | 109.1 | 1317.6 | 33.9; 36.8 4 | 4 | 31–40 | 325 |
| [ p-Cre-D7 ]CN-Cba | 116.1 | 1324.6 | |||||
CN-, cyano-; Cbi, cobinamide; Cba, cobamide. Prism RPN Analytic LC-MS (liquid chromatography-mass spectrometry) System; Thermo Fisher Scientific, Pittsburgh, PA. The base plus H+ was used for quantitation of Items 2–11 and the molecular ion plus H+ was used for Items 1,12 and 13.
Retention times for unlabeled and labeled items are virtually identical - see Figure 2 for examples.
Two peaks are seen with these CN-Cbl analogues in which the base is absent (Item 1), or cannot coordinate as the lower axial ligand (Items 12 and 13); thus, the CN can be present as either the upper or the lower axial ligand. The second peak was used for quanitation for each of the 3 CN-Cbl analogues because it was much larger than the first peak in biologic samples.
The sum of 153.1 and 154.1 was used since about 30% of the labeled molecules contained only 3 deuteriums.
The Extend column was used for the large-scale final purification of CN-Cbi, [Phe]CN-Cba and [p-Cre]CN-Cba. The solvents for LC were solvent A (100% H20) and solvent B (100% acetonitrile), both of which contained 10 mmol 1-methylpiperdine/L. Under these conditions of basic pH, all 3 Cbl analogues were in their di-CN forms, and they eluted as single peaks. The flow rate was 2.6 mL/min, 5-mL fractions were collected, and the injection volume was 0.9 mL. Chromatography was isocratic at 12% B and 88% A for CN-Cbi, which had a 26-min retention time. [Phe]CN-Cba and [p-Cre]CN-Cba were always present together in some form in the processed eluates from R-protein Sepharose, and they had retentions times of 14 and 30 min, respectively, with a solvent composition of 14% B and 86% A. Forty min after injection, the solvent composition was changed to 100% B for 10 min, and this step was followed by a 20-min equilibration at the initial condition before the next injection. One injection was made for CN-Cbi, and 3 injections were given for mixtures of [Phe]CN-Cba and [p-Cre]CN-Cba. Major fractions were analyzed with the use of the Prism RPN analytic LC-MS system as described below, and those that were free of contaminating compounds were pooled, dried overnight in the vacuum centrifuge, dissolved in 1–3 mL H20 to give a concentration of about 1 mg/mL and stored at −20° C.
The Extend column was also used for the large-scale final purification of [5-MeO,6-Me]CN-Cba because large amounts of [5-OHBZA]CN-Cba and CN-Cbl were also present (18). The solvents for LC were solvent A, (97% H2O, 3% acetic acid) and solvent B, (97% acetonitrile, 3% acetic acid). The flow rate was 2.05 mL/min, 5-mL fractions were collected, and the injection volume was 0.9 mL. Chromatography was isocratic at 21.5% B, 78.5% A; [5-MeO,6-MeBZA]CN-Cba eluted at 44 min and was well separated from major peaks of [5-OHBZA]CN-Cba and CN-Cbl which eluted at 22 min and 33 min, respectively. Major fractions in the 41–52-min postinjection window were analyzed, pooled, dried overnight, dissolved in H2O and stored at −20° C as described above.
A 100-mL solution of labeled CN-Cbl and the 12 CN-Cbl analogues shown in Figure 1 was prepared in H20; it contained 250 ng of each of the 13 labeled components in 500 uL except that 500 ng labeled [2-MeAde]CN-Cba and labeled [p-Cre]CN-Cba and 101 ng of labeled [Ade] CN-Cba were present in 500 uL. An identical 100 mL solution of unlabeled CN-Cbl and CN-Cbl analogues was also prepared.
Analysis of cyanocobalamin and cyanocobalamin analogues by liquid chromatography-mass spectrometry
LC-MS was performed as described above by using the large-scale Prism RPN system except that the diode array detector was bypassed and a mass selective detector (MSD; Agilent Technologies) was used. The column was 250 × 2.1 mm, the flow rate was 0.3 mL/min, and gradient elution was used. Initial solvent conditions of 1% A and 99% B were held for 3 min, increased to 13% B over 2 min, held at 13% B for 15 min, increased to 25% B over 2 min, held at 25% B for 13 min, increased immediately to 100% B, held there for 10 min, and immediately decreased to 1% B, which was held for 20 min before the next injection. The MSD was operated in positive ion atmospheric pressure ionization-electrospray mode with the following settings: capillary voltage, 5000 V; nitrogen-drying temperature, 350° C; nitrogen-drying gas flow, 10 L min; and nebulizer pressure set point, 50 psi. The instrument was set for high-resolution selected-ion monitoring (SIM).
RESULTS
Synthesis of cobalamin and cobalamin analogues
The structure of CN-Cbl and the partial structure of 12 Cbl analogues are shown in Figure 1. All of the analogues were synthesized by standard techniques as described in the Methods section. The structures of the Cbl analogues have been determined previously using a variety of techniques, including, in some cases, x-ray crystallography (1). Except for [Ade]CN-Cba the unlabeled and labeled forms of the Cbl analogues contained < 0.1% of each other. The final preparation of [Ade-1,3-15N2]CN-Cba contained 19.2% of unlabeled [Ade]CN-Cba. This was not unexpected because yeast extract contains, and P. arabinosum (ATCC 4965) synthesizes, significant amounts of unlabeled adenine.
Major ions observed with the liquid chromatography-mass spectrometry prism system
As shown in Table 2, the major ions observed for CN-Cbl are extremely dependent on the fragmentor voltage, which can be set at values from 0 to 400 V by using the Agilent ChemStation. The molecular ion plus 2H+ (678.3) was the predominant ion in the 0–200-V range, and the base plus H+ (147.1) was the predominant ion in the 250–300 volt range. In the 350–400-V range, the predominant ion was 912.6; it appears to be a plus H+ ion, because there was a moderately prominent 456.8 ion in the 200–300-V range that appears to be the plus 2H+ ion that corresponds to the 912.6 ion. The structure of the 912.6 ion is unknown, but, because it is seen with CN-Cbl and all 12 of the CN-Cbl analogues including cobinamide, it appears to lack the base, ribose, and phosphate moieties of CN-Cbl and the CN-Cbl analogues. It also contains the CN group because 13C115N1-Cbl had 914.6 and 457.8 ions in place of the 912.6 and the 456.8 ions of unlabeled CN-Cbl. CN-Cbl also has a molecular ion plus H+ (1355.6) and a molecular ion plus Na+ (1377.6) that are present throughout the 0–400-V range. The data in Table 2 also indicate that all of the major ions of [Ade]CN-Cba correspond with those of CN-Cbl and show the same dependence on fragmentor voltage in the 0–400-V range. This was true for all of the other Cbl analogues except that significant amounts of the base plus H+ ions were not observed for [Phe]CN-Cba and [p-Cre]CN-Cba at any fragmentor voltage. The major ions for CN-Cbi were a molecular ion plus H+ (1015.6) and a molecular ion plus 2H+ (508.3).
Table 2.
Effect of fragmentor voltage on areas obtained with the major ions observed with 10 ng of cyanocobalamin (CN-Cbl) and [Ade]CN-Cba1
| m/z | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Item and fragmentor (volt) |
147.122 | 456.83 | 678.34 | 912.65 | 1355.66 | 1377.67 | 136.12 | 456.83 | 672.84 | 912.65 | 1344.66 | 1366.67 |
| CN-Cbl | ||||||||||||
| 0 | 1400 | 89 | 14 | |||||||||
| 50 | 6700 | 180 | 22 | |||||||||
| 100 | 9400 | 230 | 33 | |||||||||
| 150 | 37 | 16 | 6300 | 20 | 190 | 26 | ||||||
| 200 | 540 | 150 | 4700 | 120 | 250 | 55 | ||||||
| 250 | 1600 | 610 | 150 | 580 | 340 | 100 | ||||||
| 300 | 1400 | 200 | 1200 | 380 | 190 | |||||||
| 350 | 310 | 1200 | 320 | 300 | ||||||||
| 400 | 760 | 290 | 400 | |||||||||
| [ Ade ]CN-Cba | ||||||||||||
| 0 | 1400 | 11 | ||||||||||
| 50 | 27 | 7100 | 25 | |||||||||
| 100 | 48 | 10 000 | 27 | |||||||||
| 150 | 94 | 38 | 5900 | 14 | 34 | 12 | ||||||
| 200 | 800 | 410 | 3300 | 100 | 43 | 10 | ||||||
| 250 | 1700 | 1000 | 52 | 580 | 52 | 21 | ||||||
| 300 | 970 | 180 | 1100 | 47 | 34 | |||||||
| 350 | 120 | 1100 | 46 | 36 | ||||||||
| 400 | 650 | 22 | 46 | |||||||||
Cba, cobamide; m/z, mass-to-charge ratio. Areas in arbitrary units in 1000’s.
Base plus H+
Ion of unknown structure plus 2H+
Molecular ion plus 2H+
Ion of unknown structure plus H+
Molecular ion plus H+
Molecular ion plus Na+
Quantitation of cyanocobalamin and cyanocobalamin analogues
Table 2 contains the values for the base plus H+ ion and the molecular ion plus H+ that were used for identification and quantitation in the LC-MS Prism system. The base plus H+ ions for the labeled and unlabeled pairs were always used when they were present in prominent amounts because natural isotope abundance was not a problem with ions in the 100–200 range of molecular weight. For example, unlabeled CN-Cbl has a base plus H+ ion of 147.1 and the area observed for its 149.1 ion was < 0.1% of that observed for its 147.1 ion. In contrast, CN-Cbl has a molecular ion plus H+ of 1355.6 and the area observed for its 1357.6 ion was 31.5% of that observed for its 1355.6 ion. The labeled form of CN-Cbl – ie, [5-Me,6-MeBZA-4,7-D2]CN-Cba – has values for the base plus H+ and the molecular ion plus H+ of 149.1 and 1357.6, respectively. Thus, the use of the base plus H+ ions for the unlabeled and labeled forms of CN-Cbl required no correction for natural isotope abundance. The molecular ions plus H+ had to be used for CN-Cbi, [Phe]CN-Cba and [p-Cre]CN-Cba, but the molecular ions plus H+ of their labeled forms were 3, 5, and 7 mass units higher, respectively, than their unlabeled forms.
The amounts of CN-Cbl, and CN-Cbl analogues were calculated by dividing the area of the unlabeled item by the area of the labeled item, multiplying that value by the amount (in ng) of the labeled item that was added to the fecal sample to be analyzed, and then dividing that value by the wet weight (in g) of the fecal sample. Values for the recoveries of Cbl and individual Cbl analogues were estimated to be in the 20–35% range but are not used in our quantification calculation because the recoveries of the labeled forms of a particular item in an individual fecal sample will be essentially the same.
No correction for natural isotope abundance was required for Cbl or any of the Cbl analogues, although a correction was required for the 24 ng of unlabeled [Ade]CN-Cba that was present with the 101 ng labeled [Ade]CN-Cba that was added to the fecal samples. The correction consisted of subtracting 24 from the total amount of calculated [Ade]CN-Cba before the division of that value by the wet weight of the fecal sample.
Samples consisting of 500 uL each of the unlabeled and labeled solutions containing CN-Cbl and all 12 CN-Cbl analogues were analyzed with and without being subjected to the complete purification procedure utilized for fecal samples. The same values were obtained for the ratio of the area for each unlabeled item divided by the area of the corresponding labeled compound, regardless of whether the samples were analyzed with or without being subjected to the fecal purification procedure. This indicates that neither the unlabeled nor the labeled items were differentially altered during the fecal purification procedure. The values for these ratios were usually in the range of 1.02–1.10. The values for fecal CN-Cbl and CN-Cbl analogues calculated above were corrected for this variation by dividing each value by the corresponding ratio just described. The lower limit of detection for CN-Cbl and all of the CN-Cbl analogues was about 5 ng, ie about 1 ng/g wet wt for a 5-g fecal sample.
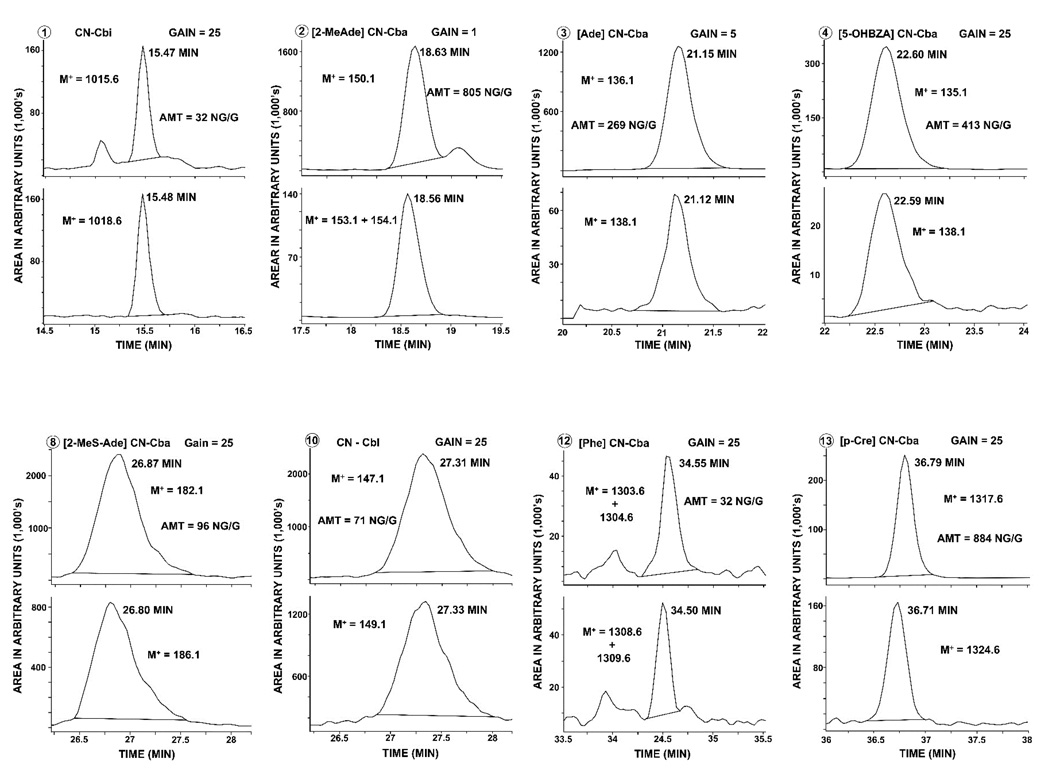
Table 1 also contains the retention times for CN-Cbl and CN-Cbl analogues, which varied from 14.2 to 36.8 min. Peak separations were sufficient to resolve all of the items that had identical values for the ions that were used for quantitation. For example, the labeled forms of [Ade]CN-Cba and [5-OHBZA]CN-Cba both had a base plus H+ ion of 138.1, but there was baseline separation of the 2 peaks (Figure 2), and thus, the use of the 138.1 ion for both compounds was not a problem. Some other peaks, such as those for CN-Cbl and [2-MeSAade]CN-Cba overlapped markedly with each other (Figure 2), but that was not a problem, because the unlabeled and labeled base plus H+ ions for CN-Cbl were 147.1 and 149.1, respectively, whereas those values for [2-MeSAde]CN-Cba were 182.1 and 186.1, respectively.
Figure 2.
Liquid chromatography-mass spectrometry chromatogram of cyanocobalamin (CN-Cbl) and 7 CN-Cbl analogues that were present in a fecal sample from control subject AN01. Cba, cobamide. Each of the 8 panels represents a plot of arbitrary units in 1000’s versus a 2-min time period in which the endogenous unlabeled form was present in the upper half of each panel and the added labeled form was present in the lower half. The number in a circle (top left of each panel) refers to the structure of the base in Figure 1. M+ represents the ion that was monitored. “Gain” refers to the extent to which the electron multiplier voltage was amplified; it was always the same for the upper and lower portions of each panel. The time (min) to the right of each peak is the peak retention time. AMT is the calculated amount (in ng) of endogenous unlabeled CN-Cbl or CN-Cbl analogue, expressed as CN-Cbl equivalents, per g of wet wt of the fecal sample that was analyzed.
Values for the group number in which each item was monitored, the interval during which each group was monitored, and the fragmention voltage for each item are also included in Table 2. The ChemStation can alternate between 4 active signals. Signal 1 was set to monitor ion 912.6 throughout the run to look for new and unexpected CN-Cbl analogues. Signals 2, 3 and 4 monitored all of the group sequences shown in Table 2 at gains of 1, 5 and 25, respectively. The latter 3 signals helped ensure that an abundant CN-Cbl analogue would not be off-scale at gain 1 and that a low-level CN-Cbl analogue could be accurately measured at gain 25. A chromatogram of the CN-Cbl and 7 CN-Cbl analogues that were purified from a sample of human feces from control subject AN01 is presented in Figure 2.
Stability and variability of cobalamin and cobalamin analogues in human feces
The next study consisted of evaluating the stability and variability of Cbl and Cbl analogues in a single human fecal specimen. The data shown in Table 3 indicate that few, if any, changes occurred when fecal samples are incubated at room temperature (22° C) or body temperature (37° C) for periods ranging from 24 to 96 h. Changes related to the position along the length of the fecal specimen also were modest, although they suggested the possibility of a trend toward somewhat higher values at the distal end. Additional experiments showed that Cbl and Cbl analogues in human feces were stable when the samples from the same fecal specimen were assayed again after being stored at −20° C for periods ranging from 1 to 12 mo (data not provided).
Table 3.
Effect of location and incubation at 22° C and 37° C for 24 and 96 h on the content of cobalamin (Cbl) and Cbl analogues in a sample of human feces1
| Incubation | [2-MeAde]- | [Ade}- | [5-OHBZA]- | [2-MeSAde]- | [Phe]- | [pCre] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location2 | Temp | Time | CN-Cbl | CN-Cbi | CN-Cba | CN-Cba | CN-Cba | CN-Cba | CN-Cba | CN-Cba | TOTAL |
| (cm) | (C°) | (Hours) | (ng/g) | (ng/g) | (ng/g) | (ng/g) | (ng/g) | (ng/g) | (ng/g) | (ng/g) | (ng/g) |
| 1 | 17 | 11 | 694 | 150 | 243 | 79 | <1 | 1220 | 2414 | ||
| 3 | 37 | 24 | 24 | 15 | 712 | 143 | 250 | 75 | <1 | 1209 | 2428 |
| 5 | 19 | 12 | 731 | 147 | 242 | 93 | <1 | 1193 | 2437 | ||
| 8 | 22 | 24 | 21 | 20 | 698 | 150 | 243 | 55 | <1 | 1273 | 2460 |
| 10 | 21 | 12 | 749 | 153 | 269 | 94 | <1 | 1364 | 2662 | ||
| 13 | 22 | 96 | 46 | 32 | 916 | 197 | 439 | 143 | <1 | 1402 | 3175 |
| 15 | 37 | 96 | 58 | 54 | 901 | 186 | 437 | 142 | <1 | 1458 | 3236 |
| 17 | 34 | 101 | 907 | 179 | 364 | 125 | <1 | 1313 | 3023 | ||
| Mean | 30 | 32 | 789 | 163 | 311 | 101 | <1 | 1304 | 2729 | ||
CN, cyano; Cbi. Cobinamide; Cba, cobamide. The sample was from subject AN01 and was 17 cm long. Values expressed as ng equivalents of cyanocobalamin (CN-Cbl)/g wet wt of feces.
Location refers to distance from the proximal end of the sample
Fecal content of cyanocobalamin and cyanocobalamin analogues in 20 control subjects
The amount of CN-Cbl and CN-Cbl analogues present in the feces of 18 control subjects whose daily ingestion of CN-Cbl from vitamin supplements was ≤ 25 ug, as shown in Table 4. CN-Cbl was present in all 18 subjects but at very low levels (mean: 19 ng/g wet wt), which represented only 1.4% of the 1309 ng/g wet wt for the mean total of CN-Cbl and CN-Cbl analogues. In contrast, [2-MeAde]CN-Cba was present in all 18 subjects at a mean level of 794 ng/g wet wt. This represented 60.6% of the total of CN-Cbl and CN-Cbl analogues. Also present in all 18 subjects were [p-Cre]CN-Cba, [Ade]CN-Cba, [2-MeSAde]CN-Cba and CN-Cbi at respective levels of 213, 164, 72 and 23 ng/g wet wt and at 16.3%, 12.5%, 15.5%, and 1.8% of the total of CN-Cbl and CN-Cbl analogues. [5-OHBZA]CN-Cba was present in 6 of the 18 subjects, 2 of whom had substantial levels – 308 and 101 ng/g wet wt. [Phe]CN-Cba was present in 3 of the 18 subjects at very low levels of 2–7 ng/g wet wt. These results also confirm the earlier suggestion (13) that Cbl accounts for only a small percentage of the total Cbl and Cbl analogues in human feces.
Table 4.
Cobalamin (Cbl) and Cbl analogues in feces from 20 control subjects1
| Subject | Age | Sex | Daily oral Cbl2 |
CN-Cbl | CN-Cbi | [2-MeAde]- CN-Cba |
[Ade]- CN-Cba |
[5-OHBZA]- CN-Cba |
[2-MeSAde]- CN-Cba |
[Phe]- CN-Cba |
[p-Cre] CN-Cba |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y | ug | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ||
| AN01 | 66 | M | 31 | 8 | 859 | 158 | 308 | 24 | 2 | 1050 | 2442 | |
| AN02 | 51 | F | 5 | 13 | 564 | 96 | 14 | 57 | 162 | 911 | ||
| AN03 | 51 | M | 5 | 4 | 529 | 83 | 4 | 68 | 63 | 756 | ||
| AN05 | 60 | F | 28 | 15 | 887 | 229 | 1 | 81 | 145 | 1386 | ||
| AN06 | 51 | F | 6 | 15 | 3 | 284 | 38 | 75 | 46 | 461 | ||
| AN07 | 56 | M | 6 | 4 | 740 | 206 | 33 | 217 | 1206 | |||
| AN08 | 45 | F | 6 | 22 | 12 | 957 | 156 | 101 | 33 | 132 | 1413 | |
| AN09 | 19 | M | 25 | 66 | 189 | 1800 | 458 | 269 | 6 | 2792 | ||
| AN11 | 52 | F | 12 | 66 | 708 | 102 | 76 | 211 | 1175 | |||
| AN12 | 51 | F | 9 | 4 | 638 | 50 | 59 | 32 | 792 | |||
| AN13 | 53 | M | 10 | 15 | 1020 | 332 | 83 | 2 | 372 | 1833 | ||
| AN14 | 35 | F | 25 | 24 | 5 | 617 | 150 | 41 | 224 | 1061 | ||
| AN15 | 22 | M | 41 | 35 | 587 | 161 | 49 | 356 | 1229 | |||
| AN16 | 57 | M | 25 | 17 | 12 | 742 | 122 | 107 | 283 | 1283 | ||
| AN17 | 80 | F | 2 | 1 | 380 | 34 | 21 | 61 | 499 | |||
| AN18 | 40 | M | 13 | 15 | 1520 | 212 | 1 | 89 | 214 | 2060 | ||
| AN19 | 21 | M | 21 | 4 | 904 | 168 | 49 | 117 | 1263 | |||
| AN20 | 55 | M | 12 | 14 | 12 | 553 | 191 | 83 | 7 | 147 | 1007 | |
| Mean | 19 | 23 | 794 | 164 | 24 | 72 | 1 | 213 | 1309 | |||
| Mean (%)3 | 1.4 | 1.8 | 60.6 | 12.5 | 1.8 | 5.5 | 0.1 | 16.3 | 100.0 | |||
| AN044 | 51 | F | 2000 | 2140 [1]5 | 695 [1] | 1092 [4] | 800 [1] | 13 [5] | 180 [2] | 5 [2] | 492 [2] | 5417 [1] |
| AN104 | 27 | M | 1000 | 1570 [2] | 273 [1] | 2007 [1] | 476 [2] | 130 [2] | 106 [4] | 258 [6] | 4820 [2] | |
| Mean | 1855 | 484 | 1550 | 638 | 72 | 143 | 3 | 375 | 5119 | |||
| Mean (%)3 | 36.2 | 9.4 | 30.3 | 12.5 | 1.4 | 2.8 | 0.1 | 7.3 | 100.0 | |||
CN, cyano; Cbi. Cobinamide; Cba, cobamide. Values are expressed as ng equivalents of cyanocobalamin CN-Cbl/g wet wt of feces.
Amount of CN-Cbl ingested daily from vitamin supplements. This value does not include Cbl intake from foods, some of which eg, some breakfast cereals, are fortified with Cbl.
Percent of the total of CN-Cbl and CN-Cbl analogues.
These two subjects also rank 1 and 2, respectively in the total amount of CN-Cbl analogues present per g wet wt of feces.
The numbers in brackets reflect the ranking from 1 to 20 in the 20 control subjects (all such values). A “1” reflects the highest amount of CN-Cbl or CN-Cbl analogue/g wet wt of feces in the group, and a “20” reflects the lowest amount.
Table 4 also shows the amounts of CN-Cbl and CN-Cbl analogues that were present in the feces of 2 control subjects whose daily ingestion of CN-Cbl from vitamin supplements was 1000 and 2000 ug/d, respectively. The mean values for CN-Cbl and the total of CN-Cbl analogues for the 2 subjects were 1855 and 3264 ng/g wet wt, respectively, and both values were much higher than the mean values of 19 and 1290 ng/g wet wt, respectively, for the other 18 control subjects. Both subjects ranked very high among the 20 control subjects with respect to the amounts of CN-Cbl and each of the 7 CN-Cbl analogues (Table 4, footnote 3), which indicates that these 2 subjects converted significant amounts of the ingested Cbl into a variety of Cbl analogues. The magnitude of the increase in the total of CN-Cbl analogues appears to be caused mainly by increases in the amounts of CN-Cbi and [Ade]CN-Cba together with probable increases in the amounts of [2-MeAde]CN-Cba and [p-Cre]CN-Cba. These observations suggest that microorganisms in the human gastrointestinal tract contain or secrete enzymes or other compounds that can remove the base, ribose and phosphate moieties of Cbl to form Cbi. They also suggest that such Cbi may then be converted to Cbl analogues, such as [Ade]CN-Cba, [2-MeAde]CN-Cba and [p-Cre]CN-Cba, that have bases that differ from the 5,6-diMeBZA base of CN-Cbl. It is also possible that the conversion of Cbl to these Cbl analogues may require only the removal of the base, or removal of the base and the ribose moieties, before these Cbl analogues are formed.
The results of an experiment in which control subject AN01 ingested a single dose of 2 mg of CN-Cbl and fecal CN-Cbl and CN-Cbl analogue analysis was performed on 10 samples collected over a 100-d interval centered around the day of ingestion (designated as day 0) are shown in Table 5. The individual values for CN-Cbl, CN-Cbl analogues, and the total of CN-Cbl and CN-Cbl analogues are very similar from day – 51 to day 3. On day 6, there was an increase in the value of CN-Cbl that is the highest value observed during the 100-d interval. The next highest value occurred on day 7. The values for days 10–49 are in the same lower range observed for days –51 to 3. This pattern of the largest value on day 6 and the next highest value on day 7 was also observed for CN-Cbi, [2-MeAde]CN-Cba, [Ade]CN-Cba, and [p-Cre]CN-Cba but not for [5-OHBZA]CN-Cba, [2-MeSAde]CN-Cba and [Phe]CN-Cba. The 4 CN-Cbl analogues that increased on days 6 and 7 were the same 4 Cbl analogues that appeared to be increased in the 2 control subjects that ingested 1000 and 2000 ug CN-Cbl on a daily basis (Table 4). This observation further supports the concept suggested above: that microorganisms in the gastrointestinal tract can convert CN-Cbl to a number of Cbl analogues including CN-Cbi, [2-MeAde]CN-Cba, [Ade]CN-Cba, and [p-Cre]CN-Cba.
Table 5.
Cobalamin (Cbl) and Cbl analogues in feces from control subject AN01 before and after the oral ingestion of a single 2-mg dose of CN-Cbl in 20 mL of H201
| Time after oral cbl |
CN-Cbl | CN-Cbi | [2-MeAde]- CN-Cba |
[Ade]-CN- Cba |
[5-OHBZA]- CN-Cba |
[2-MeSAde]- CN-Cba |
[Phe]- CN-Cba |
[p-Cre]- CN-Cba |
Total |
|---|---|---|---|---|---|---|---|---|---|
| d | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g | ng/g |
| 51 | 50 | 11 | 859 | 258 | 251 | 44 | 40 | 984 | 2500 |
| − 32 | 71 | 32 | 805 | 269 | 413 | 96 | 32 | 884 | 2600 |
| 0 | 36 | 18 | 801 | 222 | 325 | 40 | 30 | 1070 | 2540 |
| 2 | 48 | 24 | 852 | 180 | 428 | 51 | 25 | 1020 | 2630 |
| 3 | 61 | 49 | 822 | 156 | 467 | 73 | 18 | 1060 | 2710 |
| 6 | 630 | 148 | 1570 | 839 | 362 | 92 | 34 | 1630 | 5310 |
| 7 | 133 | 55 | 1090 | 473 | 533 | 40 | 33 | 1440 | 3800 |
| 10 | 36 | 28 | 853 | 207 | 302 | 35 | 20 | 781 | 2260 |
| 15 | 44 | 16 | 677 | 123 | 271 | 42 | 19 | 632 | 1820 |
| 49 | 52 | 29 | 859 | 196 | 386 | 77 | 17 | 902 | 2520 |
CN-, cyano-; Cbi, cobinamide; Cba, cobamide. Control subject AN01 was taking 25 ug/day of CN-Cbl/d from a vitamin supplement throughout the period of this study, which occurred 6–7 mo after the studies presented in Tables 3 and 4. Values are expressed as ng equivalents of CN-Cbl/g wet wt of feces.
This is the sample illustrated in Figure 2.
The values obtained for Cbl and Cbl analogues for control subject AN01 from day – 51 to day 3 and from day 10 to day 49, shown in Table 5, are very similar to the values obtained for the same control subject 6–7 mo earlier (Tables 3 and 4). The sole exception is [Phe]CN-Cba, which increased from the range of < 1 to 2 ng/g wet wt in the earlier samples to the range of 17 to 40 ng/g wet wt in the later samples when subject AN01 was ingesting a daily vitamin supplement that contained 25 ug CN-Cbl. This pattern of consistency suggests the possibility that a given person maintains a very similar pattern of fecal Cbl and Cbl analogues over a prolonged period. In the 6–7 mo interval between the studies, control subject AN01 took 500 mg ciprofloxacin every 12 h for 1 wk for severe traveler’s diarrhea and, 1 mo later, took 500 mg amoxicillin every 8 h for 1 wk for a sinus infection. These medication regimens are of interest because these 2 antibiotics can have marked effects on microorganisms present in the gastrointestinal tract (22, 23).
DISCUSSION
In the experiments presented in Tables 3–5, we did not detect the presence of the Cbl analogues numbered 5–7, 9 and 11 in Figure 1 and Table 2. Recent experiments, however, indicate that small amounts, 9–56 ng/g wet wt, of item 9-[5-MeO,6-MeBZA]CN-Cba-were present in 3 fecal samples from subject AN01 when a vitamin supplement containing 2 mg CH3-Cbl had been ingested on a daily basis for several months. In addition, small amounts (9–63 ng/g wet wt) of item 6, [5-MeOBZA]CN-Cba, have been observed in 6 samples from a single subject involved in another study.
The review by Renz (1) lists an additional 4 naturally occurring Cbl analogues that we have not synthesized and are not included in Figure 1. These 4 Cbl analogues contain guanine, hypoxanthine, 2-methylsulfinyl-adenine and 2-methylsulfonyl-adenine in place of the 5,6-diMeBZA contained in Cbl. We are in the process of synthesizing labeled and unlabeled forms of these Cbl analogues (24, 25), although we have not encountered peaks with the expected ions for any of them in human feces.
The data obtained with control subject AN01 and presented in Tables 3–5 show that this subject has an unusual CN-Cbl and CN-Cbl analogue distribution, in that it contains a relatively large amount of [5-OHBZA]CN-Cba and often contains detectable amounts of [Phe]CN-Cba. In addition, the CN-Cbl and CN-Cbl analogue distribution persisted over many months. In future studies, it will be interesting to determine whether this observation holds true for longer periods and for other control subjects and, if so, whether individual distribution patterns are due to environmental or genetic differences, or both. It also will be interesting to determine whether patients with various gastrointestinal diseases have distribution patterns of CN-Cbl and CN-Cbl analogue that are specific to a given disease and, if so, whether these patterns differ from those seen in healthy subjects.
It has been estimated that healthy humans secrete 3–9 ug Cbl/d in their bile and consume 5–15 Cbl/d in their diet (5). They reabsorb or absorb 3–9 ug/d to maintain their Cbl homeostasis (5), which leaves 5–15 ug (mean: 10 ug) that could be excreted in the feces each day. On the basis of an average value of 100 g/d for human fecal excretion (26), one would expect a mean value of CN-Cbl in human feces of 100 ng/g wet wt. Our observed value of 19 ng/g wet wt in 18 control human subjects indicates that an average of about 81% of nonabsorbed Cbl is altered or destroyed in the human gastrointestinal tract. We found an average value for Cbl analogues of 1290 mg/g wet wt, which makes it possible that all 81 ng/g wet wt of the missing Cbl is converted to Cbl analogues.
The data in Tables 4 and 5 indicate that the ingestion by healthy subjects of 1–2 mg of CN-Cbl on a daily or one-time basis causes large increases in the fecal content of CN-Cbi, [Ade]CN-Cba, [2-MeAde]CN-Cba and [p-Cre]CN-Cba, as well as an increase in the fecal content of CN-Cbl. This finding suggests that microorganisms in the human gastrointestinal tract can also convert a large amount of ingested CN-Cbl to these 4 CN-Cbl analogues. Preliminary experiments in which CN-Cbl labeled with 15N3–5 in the core, nonbase area was ingested by rats support this possibility, because significant amounts of the 15N3–5 label appeared within 24 h in the feces in CN-Cbl and in each of the 4 CN-Cbl analogues just mentioned (RH Allen, SP Stabler, unpublished observations, 2007). Our results and conclusions are also supported by earlier studies by Brandt et al (27), who described 4 patients with small-bowel overgrowth who had in their intestines bacteria that contained significant amounts of CN-Cbi, [Ade]CN-Cba, [2-MeAde]CN-Cba, and “factor E,” which is a Cbl analogue of unknown structure. More important, they also showed that intestinal bacteria from all 4 patients converted (57Co1)CN-Cbl into the (57Co1)CN-forms of all 4 of these CN-Cbl analogues.
On the basis of our observed means for CN-Cbl and the total of CN-Cbl analogues in 18 control subjects of about 19 and 1290 ng/g wet wt, respectively, and an average value of about 100 g wet wt for daily fecal output (25), we calculate that about 2 ug Cbl is excreted and 130 ug Cbl analogues is formed in and excreted from the human body every 24 h. Thus, about 1 mg Cbl is excreted and 50 mg Cbl analogues is formed and excreted over the course of 1 y. These values are significantly different from estimates for the total human body content of Cbl of only of 2–5 mg (2). It would be interesting to design studies to determine whether significant amounts of these fecal Cbl analogues traverse the gastrointestinal mucosal barrier in healthy human subjects and in patients with various inflammatory bowel diseases, and whether mechanisms exist to prevent their dissemination to other tissues. These studies are particularly intriguing because another study by Brandt et al (28), conducted in dogs with surgically constructed, self-filling high intestinal blind loops, showed that Cbl analogues were present there in much greater amounts than Cbl and that they consisted mainly in the form of CN-Cbi, [Ade]CN-Cba and [2-MeAde]CN-Cba. Furthermore, over the course of 1 y, CN-Cbl was gradually replaced in the liver by the accumulation of mainly CN-Cbi and [2-MeAde]CN-Cba and only relatively small amounts of [Ade]CN-Cbl. It is not known whether [Ade]CN-Cba reached the liver in relatively small amounts or whether it was structurally altered or preferentially excreted once it arrived there.
In summary, we have developed a sensitive and specific assay for Cbl and Cbl analogues and have studied the quantities and patterns of these Cbl analogues in human feces. These assays can be used to study the presence of Cbl and Cbl analogues in food and the environment and to explore the fate of ingested Cbl and Cbl analogues in humans.
ACKNOWLEDGEMENTS
Supported in part by NHS National Institute on Aging AG-09834 (SPS)
We acknowledge the technical assistance of Carla Ray and Bev Raab and the secretarial assistance of Theresa Martinez.
The authors’ responsibilities were as follows – SPS: recruiting the control subjects; RHA: developing the purification procedures used to isolate Cbl and Cbl analogues from bacterial cultures and human feces; and SPS and RHA: the design and performance of the study, the analysis of the data, and the writing and editing of the manuscript. Neither author has any personal or financial conflict of interest.
Footnotes
This is an un-copyedited author manuscript that has been accepted for publication in The American Journal of Clinical Nutrition, copyright American Society of Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://www.ajcn.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or any version derived from it by the National Institutes of Health or other parties.
There are no conflicts of interest.
REFERENCES
- 1.Renz P. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids. In: Banerjee R, editor. Chemistry and Biochemistry of B12. 1st ed. New York: John Wiley & Sons, Inc; 1999. pp. 557–575. [Google Scholar]
- 2.Allen Robert H. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. [PubMed] [Google Scholar]
- 3.Kolhouse JF, Allen RH. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977;60:1381–1392. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seetharam B, Yammani RR. Cobalamin transport proteins and their cell-surface receptors. Expert Rev Mol Med. 2003;5:1–18. doi: 10.1017/S1462399403006422. [DOI] [PubMed] [Google Scholar]
- 5.Stupperich E, Nexo E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur J Biochem. 1991;199:299–303. doi: 10.1111/j.1432-1033.1991.tb16124.x. [DOI] [PubMed] [Google Scholar]
- 6.Fedosov SN, Fedosova NU, Krautler B, Nexo E, Petersen TE. Mechanisms of discrimination between cobalamins and their natural analogues during their binding to the specific B12-transporting proteins. Biochemistry. 2007;46:6446–6458. doi: 10.1021/bi062063l. [DOI] [PubMed] [Google Scholar]
- 7.Burger RL, Schneider RJ, Mehlman CS, Allen RH. Human plasma R-type vitamin B12-binding proteins. II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. J Biol Chem. 1975;250:7707–7713. [PubMed] [Google Scholar]
- 8.Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 9.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 10.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford JE, Holdsworth ES, Kon SK. Biosynthesis of vitamin B12-like compounds. Biochem J. 1954;58:xxiv. [PubMed] [Google Scholar]
- 12.Brown FB, Cain JC, Gant DE, Parker LF, Smith EL. The vitamin B12 group; presence of 2-methyl purines in factors A and H and isolation of new factors. Biochem J. 1955;59:82–86. doi: 10.1042/bj0590082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert V, Drivas G, Manusselis C, Mackler B, Eng J, Schwartz E. Are colon bacteria a major source of cobalamin analogues in human tissues? 24-hr human stool contains only about 5 micrograms of cobalamin but about 100 micrograms of apparent analogue (and 200 micrograms of folate) Trans Assoc Am Physicians. 1984;97:161–171. [PubMed] [Google Scholar]
- 14.Alsberg T, Minten J, Haglund J, Törnqvist M. Determination of hydroxyalkyl derivatives of cobalamin (vitamin B12) using reversed phase high performance liquid chromatography with electrospray tandem mass spectrometry and ultraviolet diode array detection. Rapid Commun Mass Spectrom. 2001;15:2438–2445. doi: 10.1002/rcm.527. [DOI] [PubMed] [Google Scholar]
- 15.Kolhouse JF, Allen RH. Isolation of cobalamin and cobalamin analogs by reverse affinity chromatography. Anal Biochem. 1978;84:486–490. doi: 10.1016/0003-2697(78)90067-2. [DOI] [PubMed] [Google Scholar]
- 16.Stabler SP, Brass EP, Marcell PD, Allen RH. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J Clin Invest. 1991;87:1422–1430. doi: 10.1172/JCI115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolhouse JF, Utley C, Stabler SP, Allen RH. Mechanism of conversion of human apo- to holomethionine synthase by various forms of cobalamin. J Biol Chem. 1991;266:23010–23015. [PubMed] [Google Scholar]
- 18.Renz P, Endres B, Kurz B, Marquart J. Biosynthesis of vitamin B12 in anaerobic bacteria. Transformation of 5-hydroxybenzimidazole and 5-hydroxy-6-methylbenzimidazole into 5,6-dimethylbenzimidazole in Eubacterium limosum. Eur J Biochem. 1993;217:1117–1121. doi: 10.1111/j.1432-1033.1993.tb18344.x. [DOI] [PubMed] [Google Scholar]
- 19.Perlman D, Barrett JB. Biosynthesis of cobalamin analogues by propionibacterium arabinosum. Can J Microbiol. 1958;4:9–15. doi: 10.1139/m58-002. [DOI] [PubMed] [Google Scholar]
- 20.Stupperich E, Eisinger HJ, Kräutler B. Identification of phenolyl cobamide from the homoacetogenic bacterium Sporomusa ovata. Eur J Biochem. 1989;186:657–661. doi: 10.1111/j.1432-1033.1989.tb15256.x. [DOI] [PubMed] [Google Scholar]
- 21.American Type Culture Collection (ATCC) [accessed 6 November 2007]; Internet: http://www.atcc.org/common/documents/mediapdfs/1425.pdf.
- 22.Nord CE. Effect of quinolones on the human intestinal microflora. Drugs. 1995;49 Suppl 2:81–85. doi: 10.2165/00003495-199500492-00013. [DOI] [PubMed] [Google Scholar]
- 23.Brismar B, Edlund C, Nord CE. Impact of cefpodoxime proxetil and amoxicillin on the normal oral and intestinal microflora. Eur J Clin Microbiol Infect Dis. 1993;12:714–719. doi: 10.1007/BF02009388. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes DH, Weber A, Klaiber I, Volger B, Renz P. Guanylcobamide and hypoxanthylcobamide - Corrinoids formed by Desulfovibrio vulgaris. Arch Microbiol. 1994;162:272–276. [Google Scholar]
- 25.Renz P, Blickle S, Friedrich W. Two new vitamin B-12 factors from sewage sludge containing 2-methylsulfinyladenine or 2-methylsulfonyladenine as base component. Eur J Biochem. 1987;163:175–179. doi: 10.1111/j.1432-1033.1987.tb10752.x. [DOI] [PubMed] [Google Scholar]
- 26.Brandt LJ, Bernstein LH, Wagle A. Production of vitamin B 12 analogues in patients with small-bowel bacterial overgrowth. Ann Intern Med. 1977;87:546–551. doi: 10.7326/0003-4819-87-5-546. [DOI] [PubMed] [Google Scholar]
- 27.Hank PB. Practical Physiologic Chemistry. 6th ed. Philadelphia: P. Blankiston’s Son & CO.; 1918. [Google Scholar]
- 28.Brandt LJ, Bernstein LH, Efron G, Wagle A. Vitamin B12 analogue production in the experimental blind loop (abstr) Gastroenterology. 1975;68:863. [Google Scholar]