Abstract
Context
Studies have shown that the APOE ε4 genotype is associated with cognitive decline and increased risk of Alzheimer’s dementia (AD). Other genes are probably associated with both outcomes. Family history of AD (FhxAD) may represent genetic factors or shared environmental factors that increase the risk of cognitive decline.
Objective
To evaluate the influences of FhxAD and APOE ε4 on cognitive decline.
Design, Setting, and Participants
Residents of Cache County, Utah, aged 65+ were invited to participate. At baseline 2,957 participants provided DNA for genotyping at APOE and a detailed family history of AD. They also completed the Modified Mini-Mental State Examination (3MS). Cognitive status was re-examined after three and seven years. We used mixed models to examine the association between FhxAD, APOE ε4, and cognitive trajectories.
Main Outcome Measure
3MS score trajectories over time.
Results
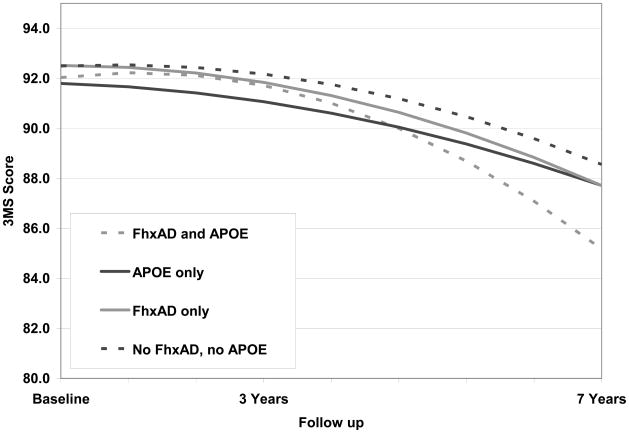
Compared with participants who had neither APOE ε4 nor FhxAD, those with APOE ε4 scored lower at baseline (−0.70 points on 3MS, 95% confidence interval (CI) −1.15 to −0.24). Participants with both FhxAD and APOE ε4 differed less, if at all, in baseline score (−0.46, 95% CI −1.09 to 0.16) but declined faster over 7 years (− 9.75, CI −10.82 -- 8.67) vs.(−2.91, CI −3.37 to −2.44). After exclusion of participants who developed prodromal AD or incident dementia, the group with FhxAD and APOE ε4 declined much less over 7 years (−1.54, CI −2.59 to −0.50).
Conclusions
These results suggest that much of the association between FhxAD, APOE ε4, and cognitive decline is attributed to undetected incipient (latent) disease. Absent this association, the two factors do not appear individually to be associated with cognitive decline although they may be additive.
Keywords: APOE, family history, Alzheimer’s dementia, cognitive decline, cognitive trajectory
Introduction
Alzheimer dementia (AD) is a neurodegenerative condition that begins with mild memory loss and progresses to total memory loss, loss of independence,1 and is a leading cause of death.1, 2 A report from the nationally representative Aging, Demographics, and Memory Study indicated that AD accounts for almost 70% of all dementias in the population.3 Individuals with an expressed family history of AD have a 39% higher lifetime risk of disease by age 96.4 When both parents develop the disease, the risk for offspring is higher still (41.8% by age 70; 54% by age 80).4, 5 Few studies have evaluated the effect of family history of AD (FhxAD) on cognitive trajectories over time. FhxAD may be viewed as an indicator for genetic contributions in the absence of confirmed associations with risk genes, although shared environment may account for a portion of the variance associated with family history.
There are three genes that predispose individuals to an early AD onset, however, these are rare in the general population.6–9 Other, as yet unconfirmed genetic associations will likely account for a much greater proportion of disease.10 The only non-Mendelian risk gene that has been firmly established is the ε4 variant of the apolipoprotein (APOE) gene.11–15 The ε4 allele of this gene is a well known risk factor16 thought to affect the timing of disease.17, 18 Interestingly, the association between APOE ε4 and cognitive decline is somewhat equivocal in the literature. Previous findings have suggested that APOE ε4 is associated with cognitive decline in many19–26 but not all27–30 studies. Some researchers theorize that the difference may be the inclusion of incipient AD cases in the samples.23, 24, 31 One very large study found no differences associated with APOE ε4 on cognitive tests. However, the participants were young, ranging in age from 20 to 64, and the study was cross sectional.32 Few studies have evaluated the influences of both FhxAD and APOE ε4 status on cognitive trajectories in elderly populations. Herein we evaluate the combined effects of the presence or absence FhxAD and the APOE ε4 genetic variant on cognition over time.
Methods
The Cache County Memory Study (CCMS) is an epidemiological investigation of memory in aging in Cache County, Utah.17 Protocols and procedures were approved by the Institutional Review Boards of Utah State University, Duke University, and the Johns Hopkins University. Informed consent was obtained from all study participants at each stage of the study. Spouses or next of kin gave consent when participants were unable to provide it.
Study Sample
Research protocols for the study have been previously described in detail.17, 33 Briefly, at the beginning of the study in early 1995, all residents of the county aged 65+ were invited to participate. A total of 5,092 persons completed baseline screening and a risk factor questionnaire. Surviving members of the cohort who were willing to participate were screened again 3 and 7 years later (1999 and 2003). For the current evaluation, we set aside 357 cases of prevalent all-cause dementia. Another 491 individuals without complete family history reports were excluded as were 95 individuals who did not provide DNA for APOE genotyping. Of 4,149 individuals with the necessary information, a total of 2,957 completed at least one subsequent evaluation and were eligible for inclusion in the analytic sample. Individuals who were excluded (n=2,135) tended to be older but did not differ regarding FhxAD. Of those who provided DNA for APOE genotyping, proportionally more individuals in the excluded group had APOE ε4. This difference was attributed to an over-representation of ε3/4 and ε4/4 genotypes among prevalent dementia cases (χ2 93.70; p-value <.0001).
Cognitive Assessment
At each evaluation point, a cognitive screening test, the Modified Mini-Mental State Examination (3MS)34 adapted for epidemiological studies35 was used to evaluate participants’ cognitive status. The 3MS is frequently used as a screening instrument to detect changes in global cognition. Based on the Mini-Mental State Examination (MMSE), it was adapted for epidemiological studies by changing some questions and adjusting the scoring from a range of 1–30 to 1–100. Demographic questions that are difficult to verify in fieldwork (i.e., date and place of birth) were changed to questions about current and past presidents. With fewer ceiling effects than the MMSE, the 3MS is more sensitive in detection of dementia and milder cognitive syndromes.36 Previously we reported the sensitivity, specificity,37, 38 and normative data on the 3MS in this cohort.39 In the first two evaluations we used the 3MS in combination with the Dementia Questionnaire40 (3MS cutpoint: 86/87); at the second follow-up the 3MS was combined with a brief battery of neuropsychological tests (3MS cutpoint: 90/91). The sensitivities for the detection of dementia at each evaluation were 84.6%, 93.6%, and 96.7% respectively. Individuals who screened positive for cognitive disorder received a full clinical assessment. At the second follow-up evaluation, all individuals aged 85+ were clinically assessed. Dementia cases were diagnosed using standard criteria41–43 as described previously.17, 33 Cases of prodromal AD44 were diagnosed clinically based on a history of mild symptomatic disease (e.g. memory loss or changes in instrumental activities of daily living). Diagnoses were assigned if participants’ history, medical evaluation, and clinical profile indicated subsyndromal AD after excluding competing etiologies.
Assessment of Family History of Disease
FhxAD was determined with a structured questionnaire administered at baseline and the first follow-up evaluation. Participants were asked to list the names of biological parents and siblings and provide information about whether they ever had memory problems. If memory problems were reported, further questions determined the age at which problems began, whether they began suddenly or slowly, progressed over time, if there were limitations with activities of daily living, and whether a doctor’s diagnosis was received. Additionally, a list of problems (AD, Parkinson’s disease, Down’s Syndrome, hardening of the arteries, mini-strokes or TIA’s, arteriosclerosis, and other neurological conditions) was presented as a cue for diagnoses that may have been given. Each relative was classified as having suspected AD if a doctor gave a diagnosis of AD or, lacking a doctor’s diagnosis, the relative’s memory problems worsened over time causing limitations with daily activities. If a first degree relative died prior to age 50 without dementia, their information was coded as missing. Study participants were classified as having a positive FhxAD if at least one first-degree relative was categorized with suspected AD.
Analytic Approach
Initial comparisons were made between individuals who reported a FhxAD to those who reported no FhxAD. Categorical variables were compared with χ2 tests; continuous variables were evaluated with t-tests. To assess the effects of FhxAD and APOE ε4 genotype on cognition over time, we used the 3MS as a measure of global cognitive performance and applied mixed-effects modeling techniques using the SAS PROC MIXED procedure.45 This procedure accommodates both fixed and random effects that account for individual differences in cognitive performance at baseline and at subsequent measurements. All mixed-effects models were adjusted for factors significantly associated with baseline 3MS scores including age, sex, and education. Time was evaluated as a nominal variable (0, 3, and 7 years) corresponding with baseline and average time to each of two follow-up evaluations. A quadratic term for time (time2) was included to allow for nonlinear changes in cognition over time. For the main exposures of interest, we constructed a categorical variable classifying each participant into one of four mutually exclusive groups: those with no FhxAD and no APOE ε4, those with only a FhxAD, those with only APOE ε4, and those with both risk factors. The variable was dummy-coded with the no FhxAD and no APOE ε4 category as the reference group. To assess differences in cognitive trajectories over time, we included interaction terms for linear and quadratic time with each of the FhxAD-by-APOE ε4 groups. Parameterized in this way, main effect terms for each of the three non-reference FhxAD-by-APOE ε4 groups provide estimates of mean differences in 3MS scores compared with the reference group at baseline, while the interaction terms represent differences in the rate of change over time. We evaluated the significance of including the interaction terms with time using an omnibus likelihood ratio test to determine if there were any differences in the rate of change among the FhxAD-by-APOE ε4 groups. Post hoc comparisons of the differences in the rate of change between each individual group and the reference were made separately using multivariate Wald tests. An additional model was constructed with an alternate parameterization including separate dichotomous indicator terms for the presence/absence of FhxAD, of APOE ε4, and another term for the product between the two to test for multiplicative interaction.
Results
Demographic characteristics of the sample are reported in Table 1. From a total sample of 2,957 individuals participating in at least two evaluations, 28% had FhxAD and 30% had APOE ε4; 10% had both. Most participants were female (57.8%), mean age 74 years, and most had a high school education or more. The average baseline 3MS score was 92. Groups with APOE ε4 alone or FhxAD plus APOE ε4 were significantly younger. The average follow-up time was 6 years (standard deviation 2.1, range 2–9 years).
Table 1.
Demographic Characteristics of 2,957 Study Participants
| Characteristic | FhxAD(−) APOE(−) (n=1532) | FhxAD(+) APOE(−) (n=533) | FhxAD(−) APOE(+) (n=585) | FhxAD(+) APOE(+) (n=307) | Total (n=2,957) |
|---|---|---|---|---|---|
| Baseline Age, mean (SD)* | 74.3 (6.6) | 74.6 (6.7) | 73.0 (6.1) | 73.1 (5.5) | 74.0 (6.4) |
| Female Sex, No. (%) | 867 (56.6) | 333 (62.5) | 335 (57.3) | 175 (57.0) | 1,710 (57.8) |
| Education, mean (SD) | 13.4 (2.9) | 13.4 (2.9) | 13.5 (2.8) | 13.6 (2.9) | 13.4 (2.9) |
| Baseline 3MS score, mean (SD) | 92.1 (5.7) | 92.2 (5.7) | 91.9 (5.8) | 92.2 (5.1) | 92.1(5.7) |
p<0.01
Values are presented as means and standard deviations (SD) unless indicated as number and percent (%).
Modeling cognitive trajectories based on 3MS scores showed that the inclusion of interaction terms between time (linear and quadratic components) and each of the FhxAD-by-APOE ε4 groups was significant (Likelihood ratio test χ2=17.8, df=6, p-value=0.007). In the model with these interactions (Table 2, model 1), there was no evidence of differences in the rate of change on the 3MS over time for those with only FhxAD (multivariate Wald p-value = 0.91). APOE ε4 was significantly associated with a lower baseline score (−0.70, 95% confidence interval (CI) −1.15 to −0.24) and a faster decline over time than the reference group (−6.64, 95% CI −7.38 to −5.89; multivariate Wald p-value <0.0001). Participants with both FhxAD and APOE ε4 declined significantly faster than those with neither risk factor (multivariate Wald p-value <0.0001). We estimated that those with both FhxAD and APOE ε4 declined on average 9.75 points on the 3MS over 7 years (95% CI −10.82 to −8.67) compared with 2.91 points (95% CI −3.37 to −2.44) for the reference group. Results presented in Figure 1 represent the average participant in each group after adjustment for baseline age (centered at age 74), sex, and education (centered at 13.4 years). A separate model designed to test multiplicative interaction between FhxAD and APOE ε4 revealed no significant interaction between these two risk factors (p-value =0.59; data not shown).
Table 2.
Means and Cumulative Declines in 3MS scores over 7 years of Observation*
| Model 1 | Baseline (n=2,957) | 3 years (n=2,957) | 7 years (n=1,936) |
|---|---|---|---|
| FhxAD (−), APOE(−) | (reference) | −0.32 (−0.62 to −0.03) | −2.91 (−3.37 to −2.44) |
| FhxAD (+), APOE(−) | 0.02 (−0.49 to 0.53) | −0.25 (−0.81 to 0.31) | −2.69 (−3.55 to −1.83) |
| FhxAD (−), APOE(+) | −0.70 (−1.15 to −0.24)a | −2.21 (−2.69 to −1.72) | −6.64 (−7.38 to −5.90)b |
| FhxAD (+), APOE(+) | −0.46 (−1.09 to 0.16) | −2.37 (−3.09 to −1.66) | −9.75 (−10.82 to −8.67)b |
| Model 2 | Baseline (n=2,145) | 3 years (n=2,145) | 7 years (n=1,369) |
| FhxAD (−), APOE(−) | (reference) | 0.37 (0.09 to 0.66) | −1.03 (−1.43 to −0.63) |
| FhxAD (+), APOE(−) | −0.26 (−0.80 to 0.28) | 0.19 (−0.36 to 0.74) | −1.30 (−2.06 to −0.55) |
| FhxAD (−), APOE(+) | −0.42 (−0.91 to 0.07) | 0.44 (−0.05 to 0.92) | −0.82 (−1.46 to −0.19) |
| FhxAD (+), APOE(+) | −0.58 (−1.30 to 0.13) | 1.37 (0.60 to 2.15) | −1.54 (−2.59 to −0.50)c |
Models are adjusted for baseline age, sex, education, time, time2, and interactions between each group with time and time2.
Model 1 includes all study participants.
Model 2 excludes individuals diagnosed with prodromal AD or dementia.
p-value=0.003 compared with reference group
p-value<0.0001 compared with reference group
p-value=0.007 compared with reference group
Figure 1.
Trajectories of cognitive change by FhxAD and APOE status
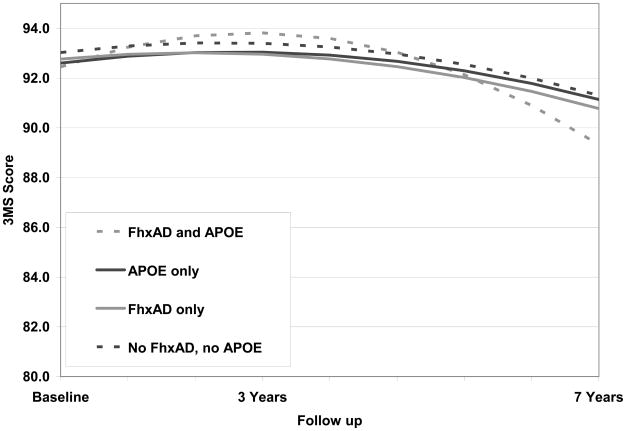
We then undertook a second evaluation of cognitive trajectories after removing from the sample. 348 individuals who eventually received a diagnosis of dementia (72.7% were AD cases) and 464 individuals who at their last observation point were diagnosed with prodromal AD. A total of n=2,145 individuals at the first follow-up and n=1,369 at the second follow-up remained in the sample. These results are presented in Table 2, model 2. As a result of removing both dementia and prodromal AD cases, the slope of the curves decreased (Figure 2). A post hoc multivariate Wald test revealed that a statistically significant difference between the reference group and the group with FxhAD and APOE ε4 remained (p=0.007) although the change over time was not clinically meaningful (1.54 points over 7 years).
Figure 2.
Trajectories of cognitive change by FhxAD and APOE status among cognitively normal individuals
Comment
We evaluated the associations between FhxAD, APOE ε4, and their combination on cognitive change over time as compared with participants with neither risk factor. The cumulative decline over 7 years in the group with both risk factors roughly approximates the sum of the declines for the groups with FhxAD alone and APOE ε4 alone, suggesting that the two factors are additive. A formal test for a multiplicative interaction between FhxAD and APOE ε4 revealed none. After removing incident cases of dementia and prodromal AD, trajectories of the groups became more similar, but a significant effect remained for the FhxAD plus APOE ε4 group. These findings imply that the greater cognitive decline found in those with APOE ε4 reflects the known relation of APOE ε4 to AD, and not to another, independent phenotype of poor cognition.
These analyses may help explain the lack of consensus in the literature on the effects of APOE ε4 on cognitive change over time. Sources of variation in results have been attributed to different study designs, study populations, follow-up periods, and age distributions of the samples under study. In some instances, prior research did not take future dementia diagnoses into account. For example in the MacArthur Study of Successful Aging, a population based multi-cite study of 965 individuals over 7 years, incident dementia cases were not removed from the sample and the authors concluded that ε4 was related to cognitive decline.19 In studies where incident cases are removed from the sample 27, 30 or groups are analyzed separately according to cognitive status, 28, 29 no decline has been found in cognitively normal individuals with APOE ε4. However, a careful study by Christensen et al, 20 found that individuals with APOE ε4 had poorer scores on the MMSE and the Symbol Digit Modalities test after controlling for other risk factors. When mild cases of cognitive disorder were set aside, the results were unchanged. Similarly, Hofer et al found a significant effect of APOE ε4 on cognitive decline over 7 years using latent growth curve modeling.21 In this study, dementia cases were identified only at the first two evaluations and not the third. Cases of mild impairment or family history of AD were not considered. Indeed, most studies of APOE ε4 and cognitive decline do not consider family history in their analyses.
In our study, the difference between groups was attenuated with the removal of prodromal AD and incident dementia cases suggesting that much of the decline was due to incipient disease. Nonetheless, a clinically small but statistically significant difference remained. There are several possible explanations for this phenomenon. It may be that we did not identify all incident cases in the sample and the resulting decline is due to unidentified cases. We doubt this is the case, because the CCMS protocol called for close scrutiny of those with APOEε4. A more likely explanation is that much of the residual decline represents early change that has not yet revealed itself as a recognizable cognitive syndrome. Yet another explanation, suggested by others, is that individuals with APOE ε4 decline at a faster rate in a way that is unrelated to development of symptomatic conditions. In our sample, once incident dementia and prodromal AD cases were removed, the group with APOE ε4 and no family history did not show significantly greater decline over time than the reference group. Finally, we note the effects of the combination of family history and APOE ε4, suggesting that the former may act independently, possibly reflecting other genetic influences or shared environment, and these influences may result in cognitive decline that does not reflect incipient AD.
Very few studies have evaluated the combined effects of APOE ε4 and family history. In a six year follow-up of the Kungsholmen Project cohort, Huang et al,46 concluded that a family history of dementia was only associated with an increased risk of dementia among APOE ε4 carriers. Duara et al,47 evaluated the combined effects of family history and APOE ε4 on time to AD onset in a sample of 197 individuals and concluded that APOE ε4 and family history of disease are independent risk factors that operate in an additive manner. Our results extend these findings and suggest a similar additive effect of APOE ε4 and FhxAD on cognitive decline.
This study has several strengths and limitations. Our determination of FhxAD was based on self-reported data. We acknowledge that with the advent of widespread genetic testing, self-reported family history information may soon become obsolete. However, the method of data collection applied here was a structured detailed interview similar to those commonly used in genetic studies. This method has been successfully used in numerous studies and found to be reasonably reliable40 albeit with some measurement error. In the current study, measurement error may bias our results in favor of those who have APOE ε4 as it is associated with earlier disease onset. Individuals with APOE ε4 and FhxAD were younger than those with FhxAD only (p<0.01). Information about FhxAD the present analysis was updated through the second data point but was not available for the full cohort at the third evaluation. Therefore, it is possible that some individuals with FhxAD were misclassified, however, the effect of such a misclassification would tend to make our results more conservative. Our measure of cognitive performance, the 3MS, mainly reflects global cognition rather than specific cognitive domains. For this reason it is possible that we were unable to detect subtle changes that may be specific to a particular cognitive domains. The strengths of the study include the large sample size and long-term follow-up. We implemented a comprehensive evaluation of cognitive status at all three time points although it is possible that some incident cases of cognitive decline may have been misclassified. This may explain the remaining observed decline among those with FhxAD and APOE ε4 when incident cases were removed. While this finding was statistically significant, the decline itself was not clinically significant.48
There may appear to be some tautology inherent in this study in that the removal of individuals with cognitive impairment from the analysis yields a sample of individuals with little or no cognitive decline. While this is true, the objective of the study was to evaluate the performance of individuals with FhxAD and APOE ε4 compared with individuals with neither risk factor to see if decline could be observed prior to any clinical manifestation of disease. We have shown that both FhxAD and APOE ε4 have deleterious effects on cognition over time. This effect is significant for those who have both risk factors and suggests, as we know, that genes beyond APOE have influences on AD risk and expression. When prodromal AD and incident dementia cases are removed from the sample, the effects of genes on cognitive decline is mitigated implying that much of the decline can be attributed to early expression of disease. Observable decline in cognitive screening scores over time (among individuals classified as normal) may be a useful indicator for subgroups at risk for AD or already expressing mild symptomatic disease. Further work is needed to elucidate more fully the cognitive patterns that precede and predict cognitive decline for each of these groups.
Acknowledgments
Funding/Support: This work was supported by NIH grants R01-AG-11380 and K01- AG-029336 (Dr. Hayden).
We wish to thank the Steering Committee for their thoughtful review of this manuscript. We are grateful to the neurogenetics laboratory of the Bryan Alzheimer’s Disease Research Center at Duke University for the APOE ε4 genotyping, and to Cara Brewer, BA, Tony Calvert, BSC, Michelle McCart, BA, Tiffany Newman, BA, Roxane Pfister, M.S., Nancy Sassano, PhD, Sarah Schwartz, M.S., and Joslin Werstak, BA for expert technical assistance. Other Cache County Study of Memory, Health, and Aging Investigators include: James Anthony, PhD, Erin Bigler, PhD, Ron Brookmeyer, PhD, James Burke, MD, PhD, Eric Christopher, MD, Jane Gagliardi, MD, Robert Green, MD, Michael Helms, Christine Hulette, MD, Ara Khachaturian, PhD, Liz Klein, MPH, Carol Leslie, MS, Constantine Lyketsos, MD, MHS, Lawrence Mayer, MD, John Morris, MD, Ron Munger, PhD, MPH, Chiadi Onyike, MD, MHS, Truls Ostbye, MD, PhD, MPH, Ron Petersen, MD, Kathy Piercy, PhD, Carl Pieper, DrPH, Brenda Plassman, PhD, Peter Rabins, MD, Pritham Raj, MD, Russell Ray, MS, Linda Sanders, MPH, Ingmar Skoog, MD, Ph.D., David Steffens, MD, MHS, Martin Steinberg, MD, Marty Toohill, PhD, Leslie Toone, MS, Jeannette Townsend, MD, Lauren Warren, PhD, Heidi Wengreen, PhD, Michael Williams, MD, and Bonita Wyse, PhD.
Neuropsychological testing and clinical assessment procedures were developed by Dr. Welsh-Bohmer and Dr. Breitner. Dr. Tschanz provided training and oversight of all field staff and reviewed all individual neuropsychological test results to render professional diagnoses. The board-certified or board-eligible geriatric psychiatrists or neurologists who examined the study members included Drs Steinberg, Breitner, Steffens, Lyketsos, Gagliardi, Raj, Christopher, and Green. Dr. Williams also examined several subjects and provided expert neurologic consultation. Autopsy examinations were conducted by Dr. Townsend. Ms. Leslie coordinated the autopsy enrollment program. Diagnosticians at the expert consensus conferences included Drs Breitner, Burke, Lyketsos, Plassman, Steffens, Steinberg, Toohill, Tschanz, and Welsh-Bohmer.
Footnotes
Financial Disclosures: The authors have no disclosures.
Author Contributions: Study concept and design: Hayden, Zandi, West, Welsh-Bohmer. Acquisition of data: Welsh-Bohmer, Breitner, Tschanz, Norton, West. Analysis and interpretation of data: Hayden, Zandi, Corcoran. Drafting of the manuscript: Hayden, Zandi. Critical revision of the manuscript for important intellectual content: Hayden, Zandi, West, Tschanz, Norton, Corcoran, Breitner, Welsh-Bohmer. Statistical analysis: Hayden, Zandi, Corcoran. Obtained funding: Welsh-Bohmer, Breitner. Administrative, technical, or material support: Welsh-Bohmer, Breitner. Study supervision: Welsh-Bohmer. Dr. Hayden had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.2008 Alzheimer’s disease facts and figures. Alzheimers Dement. 2008;4(2):110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.US Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports Deaths: preliminary data for 2005 health E-stats. 2007. [Google Scholar]
- 3.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lautenschlager NT, Cupples LA, Rao VS, et al. Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: What is in store for the oldest old? Neurology. 1996;46(3):641–650. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- 5.Jayadev S, Steinbart EJ, Chi YY, Kukull WA, Schellenberg GD, Bird TD. Conjugal Alzheimer disease: risk in children when both parents have Alzheimer disease. Arch Neurol. 2008;65(3):373–378. doi: 10.1001/archneurol.2007.61. [DOI] [PubMed] [Google Scholar]
- 6.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 7.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 8.Levy-Lahad E, Wijsman EM, Nemens E, et al. A familial Alzheimer’s disease locus on chromosome 1. Science. 1995;269(5226):970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 9.Schellenberg GD, Bird TD, Wijsman EM, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 10.Daw EW, Payami H, Nemens EJ, Nochlin D, Bird TD, Schellenberg GD, Wijsman EM. The number of trait loci in late-onset Alzheimer disease. Am J Hum Genet. 2000;66(1):196–204. doi: 10.1086/302710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 12.Pericak-Vance MA, Bebout JL, Gaskell PC, Jr, et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 14.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 17.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 18.Meyer MR, Tschanz JT, Norton MC, et al. APOE genotype predicts when--not whether--one is predisposed to develop Alzheimer disease. Nat Genet. 1998;19(4):321–322. doi: 10.1038/1206. [DOI] [PubMed] [Google Scholar]
- 19.Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60(7):1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- 20.Christensen H, Batterham PJ, Mackinnon AJ, et al. The association of APOE genotype and cognitive decline in interaction with risk factors in a 65–69 year old community sample. BMC Geriatr. 2008;8:14. doi: 10.1186/1471-2318-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofer SM, Christensen H, Mackinnon AJ, et al. Change in cognitive functioning associated with apoE genotype in a community sample of older adults. Psychol Aging. 2002;17(2):194–208. [PubMed] [Google Scholar]
- 22.Jonker C, Schmand B, Lindeboom J, Havekes LM, Launer LJ. Association between apolipoprotein E epsilon4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Arch Neurol. 1998;55(8):1065–1069. doi: 10.1001/archneur.55.8.1065. [DOI] [PubMed] [Google Scholar]
- 23.Mayeux R, Small SA, Tang M-X, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E*1. Neurobiology of Aging. 2001;22(4):683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 24.Small BJ, Basun H, Backman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: evidence from very old adults without dementia. Psychol Aging. 1998;13(1):80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RS, Schneider JA, Barnes LL, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54(9):1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 27.Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63(5):816–821. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 28.Dik MG, Jonker C, Bouter LM, Geerlings MI, van Kamp GJ, Deeg DJ. APOE-epsilon4 is associated with memory decline in cognitively impaired elderly. Neurology. 2000;54(7):1492–1497. doi: 10.1212/wnl.54.7.1492. [DOI] [PubMed] [Google Scholar]
- 29.Lange KL, Bondi MW, Salmon DP, et al. Decline in verbal memory during preclinical Alzheimer’s disease: examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8(7):943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of apolipoprotein E epsilon4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry. 2002;72(6):794–797. doi: 10.1136/jnnp.72.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol Aging. 1999;14(2):295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- 32.Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21(1):1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58(2):209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 34.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 35.Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: The Cache County Study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(1):28–38. [PubMed] [Google Scholar]
- 36.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46(6):506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 37.Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two-stage dementia screen in a population sample. J Clin Epidemiol. 2000;53(5):531–540. doi: 10.1016/s0895-4356(99)00196-1. [DOI] [PubMed] [Google Scholar]
- 38.Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC. Characteristics of a two-stage screen for incident dementia. J Clin Epidemiol. 2003;56(11):1038–1045. doi: 10.1016/s0895-4356(03)00247-6. [DOI] [PubMed] [Google Scholar]
- 39.Welsh-Bohmer KA, Ostbye T, Sanders L, Pieper CF, Hayden KM, Tschanz JT, Norton MC. Neuropsychological performance in advanced age: Influences of Demographic factors and Apolipoprotein E: Findings from the Cache County Memory Study. Clin Neuropsychol. 2009;23(1):77–99. doi: 10.1080/13854040801894730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am J Psychiatry. 1986;143(10):1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 41.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 42.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. Revised: DSM-III-R. [Google Scholar]
- 44.Breitner JC, Welsh KA, Gau BA, et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch Neurol. 1995;52(8):763–771. doi: 10.1001/archneur.1995.00540320035011. [DOI] [PubMed] [Google Scholar]
- 45.SAS/STAT. [computer program]. Version 9.1. Cary, NC: 2003. [Google Scholar]
- 46.Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: a 6-year follow-up study. Arch Neurol. 2004;61(12):1930–1934. doi: 10.1001/archneur.61.12.1930. [DOI] [PubMed] [Google Scholar]
- 47.Duara R, Barker WW, Lopez-Alberola R, et al. Alzheimer’s disease: interaction of apolipoprotein E genotype, family history of dementia, gender, education, ethnicity, and age of onset. Neurology. 1996;46(6):1575–1579. doi: 10.1212/wnl.46.6.1575. [DOI] [PubMed] [Google Scholar]
- 48.Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61(8):827–831. doi: 10.1016/j.jclinepi.2007.10.022. [DOI] [PubMed] [Google Scholar]