Abstract
Background:
The present study was undertaken to examine the feasibility of venous oxygen measurements in the inferior vena cava (IVC) via a catheter through the umbilical vein. This may serve as a proxy for mixed venous oxygenation and the complications of right atrial cannulation can be avoided at the same time. It has the added advantage of not being affected by atrial right-left shunting.
Results:
The study included 22 neonates requiring mechanical ventilation for respiratory insufficiency. The success rate of catheterization of the IVC via the umbilical vein was 81% and there was no catheter-related complications. Fifty paired blood samples were obtained and analyzed while the patients were hemodynamically stable. Linear regression analysis showed a poor correlation between arterial oxygen tension (PaO2) and the arterial-venous oxygen content difference [C(a–v)O2], r = -0.005, and between PaO2 and the fractional oxygen extraction (FOE), r = -0.114. There was also a poor correlation between arterial oxygen saturation (SaO2) and C(a–v)O2, r = -0.057, and between SaO2 and FOE, r =-0.139. The correlations between venous oxygen tension (PvO2) and C(a–v)O2 and between PvO2 and FOE were r = -0.528 and r = 0.592, respectively. There were good correlations between various oxygen saturation (SvO2) and C(a–v)O2, r = -0.634, and between SvO2 FOE, r = -0.712.
Conclusion:
Venous oxygen measurement in the IVC via an umbilical vein catheter is a simple and safe procedure and provides information about the tissue oxygenation status of critically ill neonates.
Keywords: venous oxygenation, venous saturation, inferior vena cava, neonates, respiratory failure
Introduction
In neonatal medicine, knowledge about tissue oxygenation is important because hypoxia, as well as hyperoxia, have deleterious effects. For example, unrestricted use of oxygen for low birthweight infants causes retinopathy of prematurity, while extreme lack of oxygen leads to death. Chronic deficiency of oxygen may result in long-term injury to the brain and a subsequent neurodevelopmental handicap. Measurement of oxygenation is, however, often limited to arterial blood, and most clinical decisions regarding oxygen therapy in neonates rely primarily on measurements of arterial oxygen tension (PaO2) and arterial oxygen saturation (SaO2). This approach fails to describe fully the physiological economy of oxygen in terms of supply (systemic oxygen transport), demand (oxygen consumption), or functional reserve (mixed venous oxygen content) [1].
Several authors advocate the use of venous oxygen measurements [1,2,3,4,5]. Monitoring of mixed venous oxygen tension (PvO2) and saturation (SvO2) have been advocated as both an indicator of inadequate tissue perfusion and as means to follow response to therapy. For example, Hirschl et al [5] demonstrated that right atrial SvO2 in an animal model is an excellent way of monitoring the effect of airway pressure or hypovolemia on oxygen delivery, as opposed to using SaO2 alone. The standard reference for a mixed venous oxygen sample is the pulmonary artery; however, because catheterization of the pulmonary artery or right atrium is difficult and hazardous in neonates this procedure is not routinely applied [6].
The present study demonstrates the feasibility of venous oxygen measurements in the inferior vena cava (IVC) via a catheter through the umbilical vein. This may serve as a proxy for mixed venous oxygenation while the complications of right atrial cannulation can be avoided at the same time. This has the added advantage of not being affected by atrial right-left shunting.
Materials and methods
Patient selection
All patients admitted to the neonatal intensive care unit in a 4-week period requiring mechanical ventilation were eligible for the study. Inclusion criteria were:
1. necessity for umbilical arterial and venous catheters;
2. position of the arterial catheter in the aorta with the tip at the level of Th6–Th10 and the venous catheter in the IVC with the tip just above the diaphragm next to the right atruim, confirmed radiographically and by ultrasound [7];
3. no congenital heart disease or shunting of blood on ultrasound evaluation.
Blood gas analysis
Paired blood samples were obtained when the patients were hemodynamically stable (defined as a normal blood pressure, heart rate, and no evidence of peripheral perfusion problems). Blood gas analysis was performed within 5 min of obtaining the samples. Samples were analyzed for hemoglobin, pH, partial pressures of oxygen (PO2) and carbon dioxide (PCO2), and oxygen saturation (ABL 330 Blood gas analyzer and OSM-3 Hemoximeter; Radiometer Co, Copenhagen, Denmark).
Data and statistical analysis
Oxygen content, arterial-venous oxygen content difference [C(a–v)O2], and fractional oxygen extraction (FOE) were calculated using standard formulae [8,9].
oxygen content = (Hb × sat × 1.36) + (PO2 × 0.0031)
FOE = C(a–v)O2/CaO2
where Hb is the measured hemoglobin concentration, sat is the percentage of saturation of hemoglobin, 1.36 represents the oxygen-carrying capacity of normal adult human hemoglobin (1.36 ml O2/g hemoglobin), 0.31 is the solubility coefficient for oxygen in the blood (0.0031 ml O2/100 ml per mmHg) and CaO2 is the arterial oxygen content.
All data are presented as mean ± SEM. Linear regression analysis was used to analyze the blood gas data.
Results
During this period, 45 neonates were admitted to the neonatal intensive care unit. Twenty-seven neonates fulfilled the study criteria; 5 neonates (19%) were excluded because it was impossible to catheterize the IVC via the umbilical vein. There were no catheter-related complications during the time of sampling. Therefore, the study included 22 neonates requiring mechanical ventilation for respiratory insufficient due to respiratory distress syndrome (n = 16), perinatal asphyxia (n = 4), pneumonia (n = 1), and meconoium aspiration (n = 1), Birth weight was 2235 ± 195 g, gestational age 33 ± 2 weeks.
Fifty paired arterial and venous blood samples were analyzed (Table 1). Linear regression analysis showed a poor correlation between PaO2 and C(a–v)O2, r = -0.005, and also between PaO2 and FOE, r = -0.114 (Table 2). There was also a poor correlation between SaO2 and C(a–v)O2, r = -0.057, and between SaO2 and FOE, r = -0.139 (Fig 1a). The correlations between PvO2 and C(a–v)O2 and between PvO2 and FOE were r = -0.528 and r = -0.592, respectively. There were good correlations between SvO2 and C(a–v)O2, r = -0.634, and between SvO2 and FOE, r = -0.712 (Fig 1b).
Table 1.
Arterial and inferior vena cava blood gas values in critically ill neonates
| Arterial | Venous | |
| pH | 7.36 ± 0.01 | 7.36 ± 0.01 |
| PCO2 (kPa) | 5.7 ± 0.2 | 5.6 ± 0.2 |
| PO2 (kPa) | 8.9 ± 0.3 | 5.7 ± 0.2 |
| Saturation (%) | 97.2 ± 0.4 | 88.4 ± 0.8 |
| Oxygen content (ml/dl) | 19.7 ± 0.4 | 17.6 ± 0.4 |
Data are presented as mean ± SEM. PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen.
Table 2.
Correlations by linear regression analysis
| C(a–v)O2 | FOE | |
| PaO2 | -0.005 | -0.114 |
| PvO2 | -0.528 | -0.592 |
| SaO2 | -0.057 | -0.139 |
| SvO2 | -0.634 | -0.712 |
C(a–v) O2, arterial-venous oxygen content difference; FOE, fractional oxygen extraction; PaO2, arterial oxygen tension; PvO2, venous oxygen tension; SaO2, arterial oxygen saturation; SvO2, venous oxygen saturation.
Figure 1.
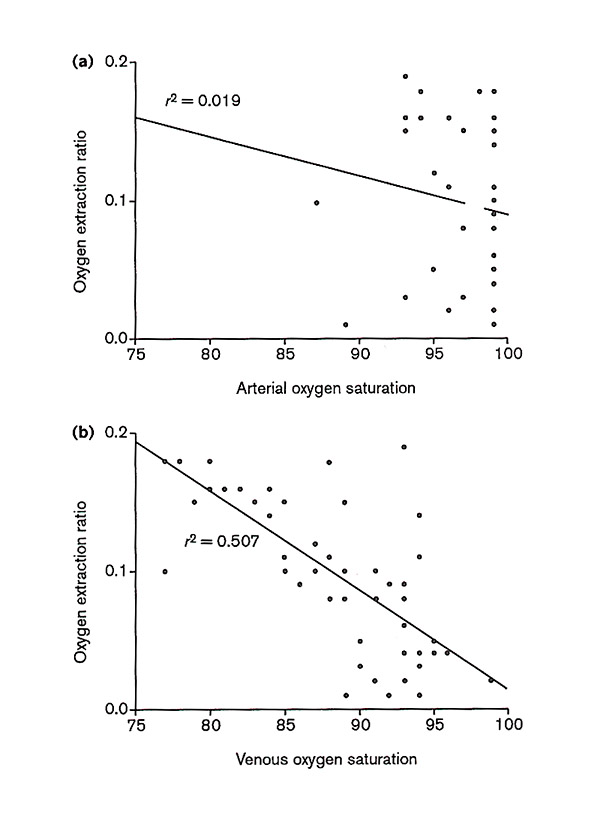
(a) A poor correlation (r = -0.139) was observed between arterial oxygen saturation and the oxygen extraction ratio and (b) a good correlation (r = -0.712) was observed between venous oxygen saturation and the oxygen extraction ratio.
Discussion
We report the feasibility of venous oxygen measurements in the IVC via an umbilical vein catheter. Our success rate of catheterization of the IVC via the umbilical vein was 81% and this was accomplished without catheter-related complications. Therefore, this is a simple and safe procedure when compared with pulmonary or atrial catheterization. In addition, shunting of blood at the atrial level will not affect the venous oxygen content. A left–right atrial shunt may elevate the venous oxygen content in the right atrium and hence may introduce error in the interpretation of the measured values, although usually a right–left shunt is present in newborns with pulmonary hypertension.
We found a higher oxygen content in the IVC (17.6 ± 0.4 ml/dl) compared to the oxygen content in the right atrium in the study of O'Connor and Hall [2] (16.3 ± 22 ml/dl). These observations are in agreement with the normal physiological situation. In normal health, the right atrium receives blood from both the superior and the inferior venae cavae and from the coronary sinus. Blood from the IVC is relatively highly saturated compared to that of the superior vena cava, while blood from the coronary sinus represents the most desaturated blood in the body [10]. This is because organs such as the heart and the brain extract large amounts of oxygen, returning highly desaturated blood compared to that derived from the liver, kidney, and skin [11]. The oxygen content will therefore be lower in the right atrium, as observed by O'connor and Hall [2]. Although the oxygen content in the IVC reflects only a part of the total oxygenation status of a critically ill patient, the importance of complete mixing is lessened if trends in venous oxygenation, rather than absolute values, are used in a given patient.
PvO2 (and, indirectly, SvO2 because of the almost linear relationship between PO2 and saturation) has received much attention recently as the single most reliable indicator currently available in children and infants that is used to detect an imbalance between oxygen supply and demand, and therefore to signal the onset of tissue hypoxie [1,2,3,4,5]. The reason for this assumption is based on the fact that oxygen diffusion from blood to tissue cells is directly proportional to the difference between capillary PO2 and intracellular PO2 [12]. Capillary PO2 is determined by arterial oxygen content, blood flow rate, capillary geometry, and oxygen consumption. The lowest capillary PO2, in other words the PO2 at the end of the capillary, is the critical value for the oxygen diffusion to the cells, and it is this end-capillary value which is reflected by the PvO2. PvO2 can be measured periodically by taking blood samples. Instead of measuring PvO2 periodically, it has become standard practice to measure SvO2 continuously using a fiberoptic catheter [3,4].
The important question remains whether venous oxygen measurements in the IVC provide sufficient information about tissue oxygenation when compared to the right atrium or pulmonary artery? Firstly, we think that although the oxygen content in the IVC reflects only a part of the total oxygenation status of a critically ill patient, the importance of complete mixing is lessened if trends in venous oxygenation, rather than absolute values, are used in a given patient. Secondly, the rate of oxygen consumption is normally drived by the demands of the tissues, which autoregulate the local supply of oxygen [13]. In normal neonates under resting conditions, not only is an adequate amount of oxygen supplied to the tissues but oxygen is provided in great excess of tissue demands. However, in critically ill neonates, not even the resting oxygen demands can be met all the time. In these situations of restricted oxygen supply, a reduction of blood flow through low-extraction tissues, such as the liver and gut, will be rerouted to essential tissues, such as the brain [14]. In this situation, measurements in the IVC will show an early trued towards lower SvO2, thus indicating tissue hypoxia. This study was not designed to describe critical values for PvO2 and SvO2 in the IVC, or its clinical application in neonatal medicine. Before we are able to provide indications as to how the values in the IVC may be interpreted and how therapies may be applied to improve the care of the neonate, it is first necessary to obtain and to compare data in neonates who show respiratory and circulatory instability.
Acknowledgments
Acknowledgements
The authors thank WG Zijlstra for his critical advise in preparing the manuscript.
References
- Whyte RK. Mixed venous oxygen saturation in the newborn. Can we and should we measure it? Scand J Clin Lab Invest. 1990;50:203–211. doi: 10.3109/00365519009087511. [DOI] [PubMed] [Google Scholar]
- O'Connor TA, Hall RT. Mixed venous oxygenatio in critically ill neonates. Crit Care Med. 1994;22:343–346. doi: 10.1097/00003246-199402000-00028. [DOI] [PubMed] [Google Scholar]
- Dudell G, Cornish JD, Barlett RH. What constitutes adequate oxygenation. Pediatrics. 1990;85:39–41. [PubMed] [Google Scholar]
- Van der Hoeven MAHBM, Maertzdorf WJ, Blanco CE. Feasibility and accuracy of a fiberoptic catheter for measurement of venous oxygen saturation in newborn infants. Acta Paediatr. 1995;84:122–127. doi: 10.1111/j.1651-2227.1995.tb13593.x. [DOI] [PubMed] [Google Scholar]
- Hirschl RB, Palmer P, Heiss KF, Hultquist K, Fazzalari F, Bartlett RH. Evaluation of the right atrial venous oxygen saturation as a physiologic monitor in a neonatal model. J Pediatr Surg. 1993;28:901–905. doi: 10.1016/0022-3468(93)90692-e. [DOI] [PubMed] [Google Scholar]
- MacDonald MG, Chou MM. Preventing complications from lines and tubes. Semin Perinatol. 1986;10:224–233. [PubMed] [Google Scholar]
- Madar RJ, Deshpande SA. Reappraisal of ultrasound imaging of neonatal intravascular catheters. Arch Dis Child. 1996;75:F62–F64. doi: 10.1136/fn.75.1.f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeseburg B, Rolfe P, Siggaard Andersen O, Zijlstra WG. Definition and measurement of quantities pertaining to oxygen in blood. In Oxygen Transport to Tissue XV Edited by Vasupel V New York: Plenum Press, 1994. pp. 925–930. [DOI] [PubMed]
- Helfaer MA, Nichols DG, Rogers MC. Developmental physiology of the respiratory system. In Textbook of Pediatric Intensive Care Edited by Rogers MC Baltimore: Williams and Wilkins, 1996. pp. 97–126.
- Scheinman MM, Brown MA, Rapaport E. Critical assessment of use of central venous oxygen saturation as a mirror of mixed venous oxygen in severely ill patients. Circulation. 1989;40:165–172. doi: 10.1161/01.cir.40.2.165. [DOI] [PubMed] [Google Scholar]
- Rudolph AM. Cardiac catheterization and angiography. In Congenital Diseases of the Heart: Clinico-Physiological Considerations in Diagnosis and Management Edited by Gellis SS Chicago: year Book Medical Publishers Inc, 1974. pp. 49–167.
- Fahey JT, Lister G. Oxygen demand, delivery and consumption. In Pediatric Critical Care Edited by Bradley P, Fuhrman BP, Zimmerman JJ St Louis: Mosby Year Book, 1992. pp. 237–248.
- Shepherd AP, Granger HJ, Smith EE, Guyton AC. Local control of tissue oxygen delivery an its contribution to the regulation of cardiac output. Am J Physiol. 1973;225:747–755. doi: 10.1152/ajplegacy.1973.225.3.747. [DOI] [PubMed] [Google Scholar]
- Sidi D, Kuipers JRG, Teitel D, Heymann MA, Rudolph AM. Developmental changes inoxygenation and circulatory responses to hypoxemia in lambs. Am J Physiol. 1983;245:H674–H682. doi: 10.1152/ajpheart.1983.245.4.H674. [DOI] [PubMed] [Google Scholar]


