Abstract
Background
Classical morphological taxonomy places the approximately 1400 recognized species of Scleractinia (hard corals) into 27 families, but many aspects of coral evolution remain unclear despite the application of molecular phylogenetic methods. In part, this may be a consequence of such studies focusing on the reef-building (shallow water and zooxanthellate) Scleractinia, and largely ignoring the large number of deep-sea species. To better understand broad patterns of coral evolution, we generated molecular data for a broad and representative range of deep sea scleractinians collected off New Caledonia and Australia during the last decade, and conducted the most comprehensive molecular phylogenetic analysis to date of the order Scleractinia.
Methodology
Partial (595 bp) sequences of the mitochondrial cytochrome oxidase subunit 1 (CO1) gene were determined for 65 deep-sea (azooxanthellate) scleractinians and 11 shallow-water species. These new data were aligned with 158 published sequences, generating a 234 taxon dataset representing 25 of the 27 currently recognized scleractinian families.
Principal Findings/Conclusions
There was a striking discrepancy between the taxonomic validity of coral families consisting predominantly of deep-sea or shallow-water species. Most families composed predominantly of deep-sea azooxanthellate species were monophyletic in both maximum likelihood and Bayesian analyses but, by contrast (and consistent with previous studies), most families composed predominantly of shallow-water zooxanthellate taxa were polyphyletic, although Acroporidae, Poritidae, Pocilloporidae, and Fungiidae were exceptions to this general pattern. One factor contributing to this inconsistency may be the greater environmental stability of deep-sea environments, effectively removing taxonomic “noise” contributed by phenotypic plasticity. Our phylogenetic analyses imply that the most basal extant scleractinians are azooxanthellate solitary corals from deep-water, their divergence predating that of the robust and complex corals. Deep-sea corals are likely to be critical to understanding anthozoan evolution and the origins of the Scleractinia.
Introduction
Although principally known as the architects of coral reefs, the order Scleractinia, or stony corals, comprises two distinct ecological groups: the zooxanthellate species that live in symbiosis with a photosynthetic dinoflagellate occur in shallow tropical waters; and the azooxanthellate species, which are primarily associated with deeper and colder waters. Of the approximately 1490 valid extant scleractinian species [1], more than 47% are azooxanthellate [1], [2] and occur from polar [3], [4] to equatorial regions, and from shallow to bathyal depths [5].
Scleractinians are first known in the fossil record as shallow-water forms from the Middle Triassic (ca. 245 Ma), but by this time were already highly diverged at the subordinal level [6]. However, the small number of reliable skeletal characteristics and the uncertain impact of environmental variables on these morphological characters [7] have severely hampered attempts to infer relationships among families and suborders [8], [9], [10], [11].
Traditionally the inference of evolutionary relationships among corals has relied heavily on comparing extant and fossil material in terms of micro- and macromorphological skeletal characteristics, but this has resulted in several very different schemes [6], [12], [13], [14]. Attempts to establish phylogenetic relationships within coral families based on skeletal characteristics have proved to be challenging, and as a consequence have been applied to date to only six of the 27 extant families – Fungiidae [15], [16], Mussidae [17], [18], Siderastreidae [17], Turbinoliidae [19], Acroporidae [20] and Dendrophylliidae [21].
During the last two decades, there have been various attempts to infer coral phylogeny based on molecular sequence data independent of skeletal morphology. To date, a wide range of markers have been used, both mitochondrial [8], [10], [11], [22], [23], [24], [25], [26], [27], [28] and nuclear [8], [11], [24], [25], [26], [28], [29], [30], [31], [32]. However, these studies imply quite different evolutionary scenarios for scleractinians, particularly in terms of relationships between suborders and families [8], [22]. Furthermore, solitary azooxanthellate species have rarely been included in these analyses, despite accounting for approximately a third of the extant scleractinian species [1], [33].
In an attempt to address these sampling biases and resolve some of the taxonomic uncertainties, we have undertaken the most comprehensive molecular phylogenetic study of the Scleractinia to date. Molecular sequence data were obtained for a ∼590 bp fragment of the mitochondrial cytochrome oxidase subunit 1 gene for 65 deep-sea azooxanthellate scleractinian species collected off New Caledonia and Australia, representing 25 genera and 9 families. With the inclusion of 11 novel sequences from shallow-water corals kindly provided by Dr. Hironobu Fukami (Kyoto University) and 156 additional sequences from GenBank, the dataset covered all of the scleractinian suborders, comprising a total of 234 species from 104 genera representing 25 of the 27 extant families. Unfortunately, we were unable to include representatives of the families Guyniidae and Schizocyathidae in our analyses; these are small (comprising a total of only four monotypic genera) families of deep-sea corals for which material appropriate for molecular analyses rarely becomes available due to their minute size (sometimes less than 2 mm in calicular diameter). Database sequences for corallimorpharians (11 species), actiniarians (2 species), zoanthids (3 species), an antipatharian, and octocorals (4 species) were also included in the analyses as outgroups. The results imply that most families composed predominantly of deep-sea azooxanthellate taxa (Gardineriidae, Micrabaciidae, Flabellidae, Dendrophylliidae, Fungiacyathidae, and Turbinoliidae) are monophyletic, but the caryophylliids and anthemiphylliids, as well as most of the shallow-water zooxanthellate families, require revision.
Results
The advantage of using CO1 sequence data for coral phylogeny is that, unlike the 16S rDNA, 12S rDNA, and 28S rDNA genes, the sequences are unambiguously alignable because they contain no indels. In addition to 234 scleractinian species, our analysis also included representatives of each of the anthozoan subclasses with the exception of Ceriantharia. The list of all sequences used in the present study is available as File S1. The saturation test showed that there was no significant saturation (P<0.0001; Iss<Iss.c) in the CO1 alignment. Sh-like returned likelihood value of −12912.35, and the Bayesian convergence diagnostic returned a potential scale reduction factor between 1.000 and 1.005, and −13457.44 as the arithmetic mean of the likelihood values between the four runs. Bayesian analyses were also conducted based on the same alignment, but excluding either the third codon position or excluding all transversions, and after translation. All of these kinds of analyses resulted in phylogenies with lower resolution than those based on the full nucleotide sequences, in each case generating large polytomies for the robust shallow water corals. The Bayesian bipartitions of taxon were analyzed for the original run, but none of the generations retrieved a monophyletic Faviidae, Merulinidae, Pectiniidae, or Mussidae family.
Forcing monophyly upon the robust shallow-water coral families resulted in significantly worse likelihood scores than in the absence of constraint (data not shown), implying that, large taxonomical revisions should be carried out.
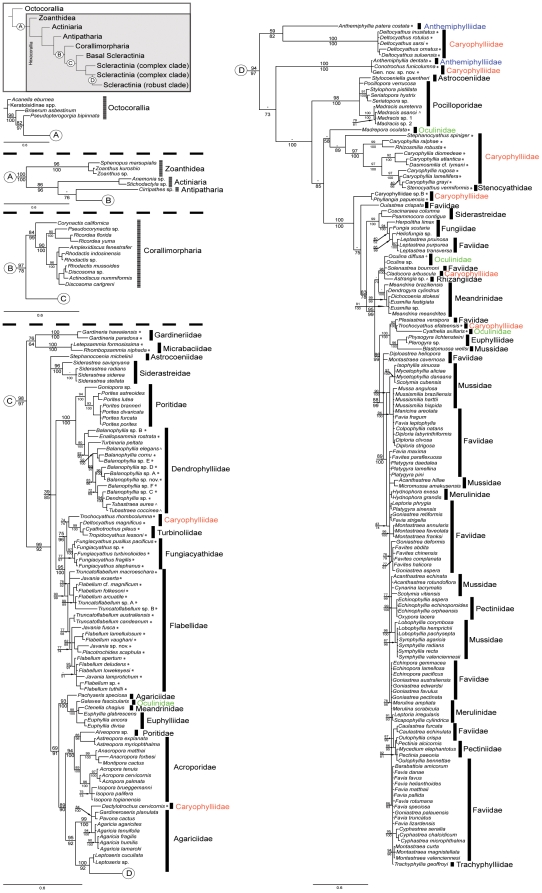
The results of phylogenetic analyses are summarized in figure 1; the (four) octocoral sequences were used to root the phylogenetic tree because of the sister group relationship between hexacorallians and octocorallians [29], [31], [34], [35], [36]. Maximum likelihood and Bayesian analyses strongly supported monophyly of both Scleractinia and Corallimorpharia (Fig. 1–B) [11], [37], and therefore contradict the “naked corals” hypothesis [27], which suggested that corallimorphs are descended from scleractinians via skeleton loss. In contrast to previous studies [24], Antipatharia were not basal within the Hexacorallia in our analysis. Note, however, the relatively weak support for the position of Antipatharia in our tree.
Figure 1. Phylogenetic analyses based on Bayesian inference and Maximum likelihood of the partial mitochondrial CO1 gene from 234 scleractinian species, 11 corallimorpharians, 2 actiniarians, 3 zoanthids, 1 antipatharian, and 4 octocorallians.
Topology was reconstructed under the GTR+I+G model of nucleotide evolution in MrBayes. Numbers on branches show Sh-like support (top) calculated using PhyML, and posterior probability (bottom) calculated using MrBayes. Hyphen (−) indicates no support from the respective method. (A) Zoanthids, actiniarians, and antipatharian clade. (B) Corallimorpharian clade. (C) “Basal” and “complex” scleractinian clades. (D) “Robust” scleractinian clade. Colored names indicate families with azooxanthellate representatives that morphological revisions need to be carried out. Asterisks indicate azooxanthellate deep-water scleractinians, carets indicate azooxanthellate shallow-water scleractinians, and plus signs indicate facultative scleractinians.
Within the Scleractinia, the most deeply diverging clade was composed of members of Gardineriidae and Micrabaciidae, two exclusively solitary and azooxanthellate coral families. The overall shape of the remainder of the scleractinian tree is that the “robust” coral clade branches from within the “complex” corals. However, some morphologically defined families are split between these two major groups as documented in several previous papers [8], [10], [11], [32] - the families Astrocoeniidae, Siderastreidae, Oculinidae, Meandrinidae, Euphylliidae, and Caryophylliidae have representatives within both the “complex” and “robust” corals. In addition to members of these families, the “robust” clade comprises Anthemiphyllidae*, Pocilloporidae, Stenocyathidae, Faviidae*, Fungiidae, Mussidae*, Trachyphylliidae, Merulinidae*, Rhizangiidae, and Pectiniidae*. The “complex” coral clade consists of representatives of families Agariciidae*, Acroporidae, Poritidae*, Dendrophylliidae, Flabellidae, Turbinoliidae and Fungiacyathidae in addition to the six families that are split across the “robust/complex” divide. Some families and suborders appear to urgently require revision; those indicated above by asterisks are paraphyletic within the complex or robust clades, whereas oculinids and caryophylliids are paraphyletic within the robust corals as well as in the complex clade.
Nucleotide composition did not differ significantly between sequences in the “complex” and “robust” clades, with %(A+T) mean composition of 61.7% and 67.9%, respectively, and the basal scleractinian clade likewise did not differ significantly from the “complex” or “robust” clade (Table 1). The average difference between sequences within each scleractinian clade was no more than 8%, and within the corallimorpharian clade was 4%, but between “robust” and “complex”, “robust” and “basal”, and “robust” and corallimorpharian clades the corresponding values were 19.1%, 20.1%, and 19.6%, respectively. Members of the “complex” clade displayed an average of 12.3% differences with those of the “basal” clade, and 13.2% differences with corallimorpharian sequences. In total, 27.4% of bases were invariant across the Scleractinia, the transition: transversion ratio was 2.21, and the average difference compared to corallimorpharian sequences was 17.4% (Table 1).
Table 1. Nucleotide composition, proportion of invariant sites (Pinv), transition vs transversion rate (Ts/Tv), average distance between sequences (DS), and average distance between clades calculated based on GTR+I+G evolution model.
| Clades | Nucleotide composition (%) | Pinv (%) | Ts/Tv | DS (%) | Average distance between clades (%) | ||||||
| A | T | C | G | R | C | B | S | ||||
| R | 22.8 | 39.1 | 15.0 | 22.9 | 32.5 | 2.084 | 8 | - | - | - | - |
| C | 22.7 | 39.0 | 16.8 | 21.3 | 33.6 | 2.565 | 8 | 19.1 | - | - | - |
| B | 22.0 | 35.9 | 18.0 | 23.9 | 69.8 | 2.954 | 8 | 20.1 | 12.3 | - | - |
| S | 22.7 | 38.7 | 15.7 | 22.7 | 27.4 | 2.210 | 13 | - | - | - | - |
| Co | 23.4 | 35.7 | 17.5 | 23.3 | 35.1 | 2.666 | 4 | 19.6 | 13.2 | 13.2 | 17.4 |
| A | 24.1 | 37.6 | 16.7 | 21.5 | 28.8 | 2.354 | 14 | - | - | - | - |
R = “Robust” scleractinian clade.
C = “Complex” scleractinian clade.
B = “Basal” scleractinian clade.
S = Scleractinia clade (robust + complex + basal).
Co = Corallimorpharia clade.
A = All alignment (including Octocorallia, Antipatharia, Zoanthidea, Actiniaria, Corallimorpharia, Scleractinia).
For some genera, the molecular phylogeny is inconsistent with family placements based on classical taxonomy, implying that the positions of these should be re-evaluated. This category includes the azooxanthellate genera Conotrochus, Madrepora, Stenocyathus, Phyllangia, Cladocora, Trochocyathus, and Dactylotrochus, as well as the zooxanthellate genera Pachyseris, Galaxea, Ctenella, Alveopora, and most of the “robust” coral representatives.
Discussion
Both maximum likelihood and Bayesian analyses support the distinction of two major clades (“complex” and “robust” corals) within the Scleractinia (Fig. 1–C and D), as was previously implied by molecular analyses based on mitochondrial 16S rDNA [8], [10], [22], [23], 12S rDNA [25], and CO1 + Cyt B data [11], and on (nuclear) 28S rDNA sequences [11], [29], [30], [32]. However, rather than the deep split between these two groups implied by analyses of ribosomal sequences, our phylogeny places the “robust” coral clade within the “complex” radiation, following the precedent of Fukami et al. [11]. This topology has high Sh-like (ML) and posterior probability (BI) support, and was not significantly affected by weighting the analyses for codon position.
Our analyses imply that the families Gardineriidae and Micrabaciidae, which are exclusively azooxanthellate and contain only solitary species, represent the most basal lineage of modern scleractinians, supporting the concept that deep-sea corals hold important clues regarding the evolutionary history of the order. The evolutionary implications of the basal position of gardineriids and micrabaciids are more fully explored elsewhere (Stolarski et al., in preparation), however, the basal position of these families suggests that the ancestral scleractinian may also have been solitary and azooxanthellate. According to Owens [38] and Squires [39], ancestral micrabaciids probably inhabited shallow-water environments but may have been essentially preadapted for deep-sea life by having auto-mobile coralla [38], and thus been able to gradually invade deeper waters, resulting in an increase of skeleton porosity [39]. Similarly, fossils thought to represent the oldest known gardineriid (Rodinosmilia elegantula) were described from Morocco [40], suggesting that this family may also have first appeared in shallow-water environments. Under this scenario, the early Mesozoic appearance of diverse, highly integrated colonial forms may reflect the advent of symbioses with the dinoflagellate Symbiodinium, as has been suggested based on stable isotope data [41]. Since all early Mesozoic records of Scleractinia represent rather shallow-water ecological settings, it is not yet possible to infer whether the Scleractinia were initially abyssal and then colonized shallow waters (as hypothesized by Lindner et al. [42] for the stylasterid corals), or vice-versa.
Recent molecular analyses are inconsistent with widely used sub-ordinal classification schemes of Vaughan and Wells [43] and Wells [6], which were based on morphology. Although morphological support for the “robust” and “complex” dichotomy is still lacking, it is consistently supported by molecular analyses and the three clades recovered here (“basal”, “complex”, and “robust”) could represent a new sub-ordinal scheme for the classification and evolutionary history of the order.
One general implication of the phylogenetic analyses reported here is that the majority of the azooxanthellate coral families (six of the eight) are monophyletic, whereas only a minority of families (four of seventeen) that are predominantly or exclusively zooxanthellate are supported strongly by the molecular data. Thus many of the morphologically defined families of shallow-water corals do not represent “natural” families. This conclusion is broadly consistent with Fukami et al. [11], although this work was based on more limited sampling of azooxanthellate corals. Below we discuss the status of some individual families based on the overall CO1 phylogeny.
Flabellidae
The flabellids are a large family of exclusively azooxanthellate corals that formed a single well-supported clade in our analyses, which were based on 27 species representing the full morphological spectrum of the family (only missing genera with root-like structures e.g. Rhizotrochus). Interestingly, the CO1 analyses suggest that there may be a major dichotomy within the family, with representatives of many genera examined occurring in both of the resulting clades. The general pattern is that Truncatoflabellum species occupy basal positions in both clades, with different Javania species branching next. These results suggest that the relationship of flabellids with substrata and their mode of reproduction diversified during their evolution. To date we can infer that the most basal form of substrata relationship and reproduction within the extant flabellid genera is fixed (fragile pedicel), with transverse division as the main reproduction mode respectively (as observed in Truncatoflabellum). Subsequently, multiple, concentric layers of sclerenchyme reinforcing the pedicel and the attachment of the corallum to substrate (as observed in Javania and being a trait also shared with the anthocaulus of Placotrochides) appears in our analysis. Later, the substrate relationship became less evident, or present only in very early developmental stages, with adult specimens becoming free-living forms, such as observed within Flabellum. The evolutionary position of different root-like attachment structures present in other flabellid genera (e.g. Monomyces, Polymyces, Rhizotrochus) needs to be further investigated.
The analyses imply a close relationship between the Flabellidae and two other exclusively azooxanthellate coral families – Turbinoliidae and Fungiacyathidae. Both monophyly of Flabellidae and the relationship between this family and Turbinoliidae and Fungiacyathidae are consistent with previous work of Le Goff-Vitry et al. [10].
Fungiacyathidae and Turbinoliidae
The five fungiacyathid representatives sequenced formed a well-supported group notwithstanding the method used, corroborating their family status [13]. In addition to the link with Flabellidae and Fungiacyathidae outlined above, our analyses imply a close relationship of Turbinoliidae with two caryophylliids – Trochocyathus rhombcolumna and Deltocyathus magnificus. Additional material is necessary to better understand the relationships within the turbinoliids, as only two species representing two genera are present in our phylogeny. To collect fresh turbinoliids is particularly challenging because they are among the smallest known scleractinians. The turbinoliids Cyathotrochus pileus and Tropidocyathus lessoni grouped with D. magnificus sharing a common ancestor with T. rhombcolumna. Morphological support for this grouping is, however, lacking. Similarity of the CO1 sequences between Deltocyathus magnificus (four specimens from different collecting stations sequenced) and turbinoliids is difficult to explain although they do share some morphological characters (e.g. lamellar paliform lobes before all but last septal cycle forming a chevron arrangement - not fusing in Tropidocyathus but fusing in Deltocyathus and Cyathotrochus, corallum invested with soft tissue, well developed costae, and a papillose columella). All other Deltocyathus representatives sequenced in the present study grouped in a basal position in the “robust” clade, and could represent a distinctive family once the other caryophylliid species have been separated into five distinct clades.
Dendrophylliidae
With nearly 170 species [21], Dendrophylliidae is the third most speciose family of extant scleractinians and in our analyses was the only well-supported family with substantial representation of both shallow and deep-water species. Within the family, a clade comprising the deep-sea colonial species Enallopsammia rostrata and a solitary deep-sea Balanophyllia sp. diverged most deeply, followed by the shallow-water zooxanthellate colonial genus Turbinaria. Representatives of the azooxanthellate genera Dendrophyllia (identification needs to be re-evaluated), Tubastraea, and Balanophyllia, the first two of which are colonial and the last solitary, appear as most recently diverged. The topology is consistent with an azooxanthellate dendrophylliid ancestor, and the possibility of multiple gains or losses of the colonial state within the family. Dendrophylliids are a particularly interesting group and could be highly informative with respect to the evolution of coloniality and the symbiotic state.
Poritidae and Acroporidae
The families Poritidae and Acroporidae are the most speciose and diverse of shallow-water scleractinians, and are exclusively colonial and zooxanthellate. Our analyses support that the poritid genus Alveopora (the only poritid genus with septa not formed by 3 to 8 nearly vertical trabeculae) should be transferred to the Acroporidae (Fig. 1), as the single Alveopora sequence grouped with those from Astreopora explanata and Astreopora myriophthalma within the well-supported acroporid clade [10], [11]. If Alveopora is transferred to acroporids, Poritidae becomes monophyletic, as the remaining poritid genera (Goniopora and Porites) form a well-supported clade.
The molecular phylogeny (Fig. 1) implies a sister group relationship between dendrophylliids and poritids, the latter of which is one of the few families of zooxanthellate corals to have strong support in our analyses. The common ancestry of Poritidae and Dendrophylliidae implied by our analyses is consistent with previous molecular analyses based on 28S rDNA [30], 16S rDNA [8], [23], and the nuclear rDNA, CO1 and Cyt B [11]. The earliest record of poritids is from the Mid-Cretaceous [6], and for dendrophylliids the Early Cretaceous [44]. Veron [14] suggested that the (Late Cretaceous) Actinacididae might be ancestral to the poritids. However, based on macro and microstructures of the skeleton, Cairns [21] advocated that the actinacidids were probably not the dendrophylliid ancestor.
In common with previous molecular analyses [8], [10], [11], [25], the family Acroporidae was monophyletic in our CO1 analyses. Within the Acroporidae, Anacropora appears to be more related to Montipora, and Acropora to Isopora, which was recently elevated to genus level [45].
Agariciidae
The family Agariciidae occupies a special position in our analyses, as the entire “robust” coral clade branches from within a clade that captures the agariciids (excluding Pachyseris speciosa) together with the caryophylliid genus Dactylotrochus. The Caryophylliidae is not a valid family, its members are scattered throughout the phylogenetic tree (see below). There is morphological support for transferring Dactylotrochus to the Agariciidae–for example, the shared presence of highly developed septal menianae (Kitahara et al., in preparation; also see [46]). Agariciids are shallow water, colonial corals. Whilst this transfer would make Dactylotrochus the only exclusively solitary (and azooxanthellate) extant member of the family, there are precedents from the Cretaceous; the fossil agariciids Vaughanoseris and Trochoseris were solitary. The latter is recorded from the Late Cretaceous and Paleocene of Saudi Arabia and Pakistan respectively [47], and could represent a genus related to Dactylotrochus.
In our analyses, the agariciid clade formed by representatives of Gardineroseris, Pavona, and Agaricia was strongly supported whereas, pending morphological confirmation, Pachyseris speciosa may be transferred to euphyllids. A number of other recent analyses [8], [10], [11], [32], [48] also implied monophyly of the Agariciidae.
Meandrinidae, Astrocoeniidae and Anthemiphylliidae
Unlike the situation with the “complex” corals, where morphology and molecular data are broadly consistent in support of many families, in the case of “robust” corals, the opposite is true. With the sole exception of the Pocilloporidae, every robust family was para- or polyphyletic in our analyses.
In the case of meandrinids, the Atlantic genera (Meandrina, Dichocoenia, Dendrogyra, and Eusmilia) formed a strongly supported clade, but the only non-Atlantic meandrinid that we were able to include in the present analysis (Ctenella chagius) grouped with the euphylliids (see below), challenging the monophyly of this small family. Clarifying the status of Meandrinidae will require data for additional Indo-Pacific genera; Gyrosmilia and Montigyra, both transferred to the family [49] are of particular interest.
Only two members of the family Astrocoeniidae were included in our analyses, the Atlantic species Stephanocoenia michelinii and the Indo-Pacific species Stylocoeniella guentheri; the former fell into the “complex” clade and the latter in the “robust” clade. The fossil record implies an early origin for the family; astrocoeniid-like corals with styliform and vertically continuous columella from the Middle Triassic [6] are amongst the oldest scleractinian fossils yet found. Our analysis supports the idea that Stylocoeniella is related to pocilloporids [11]: S. guentheri forms a strongly supported group with Pocillopora, Stylophora, Seriatopora, and Madracis, and this clade diverges near the base of the radiation of “robust” corals (Fig. 1). To date, no sequence data are available for Palauastrea, which was suggested to belong to astrocoeniids [49], and to pocilloporids by Yabe & Sugiyama [50].
Although Anthemiphylliidae affinities are as yet unclear, our analyses support an early divergence of Anthemiphyllia patera costata in the “robust” coral clade. Described to accommodate the genus Anthemiphyllia, which according to Vaughan [51] “had puzzled every student since its description”, this family is composed of seven species and two subspecies, all with free and solitary growth form, and lobate to laciniate septal edges. Of the eight Anthemiphyllia morphs, only A. patera patera is exclusively Atlantic, the seven other morphs occurring mainly in Pacific waters (with exception of A. dentata, which is recorded also in Indian Ocean waters [52]). If the basal position of A. patera costata (and presumably A. patera patera) holds with other genetic markers, it may represent that the common anthemiphylliid ancestor was morphologically very close to the extant A. patera morphs, and probably inhabited the Tethys Sea 65 Mya. However, it is difficult to understand why Anthemiphyllia dentata did not group with A. p. costata, considering that all anthemiphylliids share skeletal micro-structural characters (Stolarski, unpublished data).
Caryophylliidae
The family Caryophylliidae is the least cohesive of extant coral families, as it is represented in distinct clades in both the complex and robust parts of the tree. The affinity of Dactylotrochus cervicornis with agariciids, and that of Deltocyathus magnificus and Trochocyathus rhombcolumna with turbinoliids and other complex corals have been discussed above. In addition, most members of the genus Deltocyathus form a distinct clade of uncertain affinity.
One substantial grouping within Caryophylliidae comprises all of the Caryophyllia species, Stenocyathus vermiformis, Dasmosmilia cf. lymani, and Rhizosmilia robusta; support for association of Stephanocyathus spiniger with this clade is weak. Interestingly, the genus Stenocyathus, which is one of the two genera assigned to the recently proposed family Stenocyathidae, groups with strong statistical support with Caryophyllia grayi, C. lamellifera, and C. rugosa (also see [53]). This result corroborates the hypothesis that thecal pores originated independently in different scleractinian lineages [54], once S. vermiformis is grouping within the “robust” corals, and Guynia annulata, another species that has pores groups within the “complex” corals in the 16S rDNA phylogeny (Kitahara et al., unpublished data and [22]). As advocated by Stolarski [54], this hypothesis suggests stability of the basic microstructural architecture of the skeleton, and places the family Stenocyathidae in the superfamily Caryophyllioidea rather than Guynioidea.
The clade formed by Caryophyllia diomedeae, Caryophyllia atlantica, and Dasmosmilia cf. lymani also received strong support regardless the method used, and is consistent with the hypothesis that Dasmosmilia is a sister genus of Caryophyllia [53]. The last representative of the genus Caryophyllia, C. ralphae, groups with Rhizosmilia robusta. C. ralphae is one of the most distinctive of Caryophyllia species [53], and resembles three other species (C. capensis, C. paucipalata and C. eltaninae) in terms of the placement of paliform lobes [55]. Morphologically, C. ralphae is distinguished by its highly exsert septa and very deep fossa, but can be confused with R. robusta, both having about the same adult corallum size, septal symmetry and exsertness, colour, and fossa depth. The presence of concentric rings of partitioned chambers in the base cross section of R. robusta is one of the few morphological characters that distinguish it from C. ralphae. However, the CO1 data demonstrate that the morphological similarity of these two species reflects a close evolutionary relationship. According to Zibrowius & Gili [56], C. capensis is not a true Caryophyllia, and Cairns [55] suggested that if this species belongs to a different genus, C. ralphae should be placed with it. If the genetic relationship between C. ralphae and R. robusta stands, the presence of the concentric rings of partitioned chamber in the base cross section in the genus Rhizosmilia was acquired only recently from a solid-based ancestor.
The placement of Stephanocyathus spiniger in the Stenocyathus/Caryophyllia/Dasmosmilia/Rhizosmilia clade is unexpected, since the other two representatives of this genus, S. weberianus and S. coronatus group have quite different affinities on the basis of 16S rDNA sequence analysis (Kitahara et al., unpublished data).
Based on the presence of 12–18 short basal tubercles in Stephanocyathus (Odontocyathus), 6 long costal spines corresponding to each first costae cycle in S. (Acinocyathus), and no tubercles or spines in S. (Stephanocyathus), the genus Stephanocyathus is divided into the above three subgenera. S. weberianus and S. coronatus belonging to S. (Odontocyathus) and S. spiniger to S. (Acinocyathus). If the segregation of Stephanocyathus subgenus is detected with one molecular marker, not the case presented above, which is the comparison between 16S rDNA phylogeny for Odontocyathus (Kitahara et al., unpublished data), and CO1 phylogeny for the Acinocyathus, it may indicate that the subgenus should be elevated to genus status (belonging to different families). Their macro-morphological similarity (if the genetically distance between them is confirmed) could be an evolutionary throwback, such as phenotypic characters preserved in DNA reappearing through different lineages from the same ancestor.
Another caryophylliid genus that needs re-evaluation regarding hierarchical status is the exclusively azooxanthellate Phyllangia. The basal position of this genus regarding almost all “robust” shallow water corals can represent an azooxanthellate shallow-water ancestor for them (the genus Phyllangia is reported exclusively from waters shallower than 100 m [57]).
Siderastreidae
Another family that has representatives within both major clades is the exclusively shallow water Siderastreidae. The genus Siderastrea (represented in our analysis by three Atlantic species: S. radians; S. siderea; and S. stellata, and by the Indo-Pacific S. savignyana) forms a well-supported clade within the “complex” corals. Nonetheless, representatives of Coscinaraea and Psammocora form a clade within the “robust” corals sharing the same common ancestor with the massive faviid genera Leptastrea and large solitary fungiids Heliofungia, Fungia and Herpolitha. Combined CO1 and Cyt-B analysis [11] also recovered this clade, but nuclear phylogenies from the same study did not. The position of Oulastrea crispata within the “robust” corals and its relationship to the “robust” siderastreids and fungiids did not receive good statistical support from ML and BI.
Using 16S rDNA sequences, Romano & Palumbi [23] and Romano & Cairns [8] also found that Coscinaraea, Psammocora, Fungia, and Leptastrea are closely related. Partial 5.8S and ITS2 sequences, and skeletal microstructure analysis clearly suggest that Psammocora and Coscinaraea are closer to fungiids than to siderastreids, however, both genera are not monophyletic [58]. According to the same study, the genus Pseudosiderastrea grouped with the “Siderastrea” clade.
The “siderastreids” that clustered in the “robust” coral clade are distinct from Siderastrea on the basis of both morphology [17] and molecular data, and probably do not belong to this family, once the type genus of this family was established as Siderastrea [43]. Forsman et al. [59] also concluded that the Atlantic species of Siderastrea form a monophyletic group, and S. glynni (the only Eastern Pacific representative of this genus) also appears to be closed related to the Atlantic species.
Oculinidae
As in previous studies [8], [10], the oculinids were polyphyletic in our analyses, with Galaxea falling into the “complex” clade, and Madrepora, Oculina, and Cyathelia occupying distinct positions within the “robust” clade. The strongly-supported grouping of Galaxea with the meandrinid Ctenella chagius and the euphyllids Euphyllia glabrescens, E. ancora, and E. divisa seen in our analyses (Fig. 1) support Fukami's [11] suggestion that Galaxea and Ctenella should be transferred to the Euphylliidae. Le Goff-Vitry et al. [10] suggested that the genus Madrepora should be elevated to family status; the poorly resolved position of M. oculata in our analysis is consistent with this, although the remaining four congeners (M. arbuscula, M. carolina, M. minutiseptum, and M. porcellana) need to be examined. In our analysis, strong support was obtained for a clade containing Oculina and members of three other families-Cladocora, Solenastrea, and Astrangia–but it is unclear whether this clade has morphological support. The significance of the grouping of Cyathelia axillaris with a shallow-water massive faviid and a solitary azooxanthellate caryophylliid is also unclear. Representatives of Bathelia, Petrophyllia, Shizoculina, Sclerhelia, and Simplastrea have not been sequenced to date, and their position within the oculinids needs to be re-evaluated.
Other families
One of the most heterogeneous groups formed in our analysis is composed by five different families: Mussidae (Blastomussa wellsi); Euphylliidae (Physogyra lichtensteini and Plerogyra); Caryophylliidae (in part: Trochocyathus efateensis); Oculinidae (in part: C. axillaris); and Faviidae (Plesiastrea versipora). This clade is strongly supported by all phylogenetic methods and agrees with Fukami et al. [11] who, excluding T. efateensis, recovered the same clade. In fact, the presence of the solitary deep-water azooxanthellate Indo-Pacific species T. efateensis within this clade (otherwise all zooxanthellate and colonial) is difficult to explain and requires further investigation. On the basis of 16S rDNA analyses (Kitahara et al., unpublished data) T. efateensis groups with two other deep-water caryophylliids (Trochocyathus cepula and Tethocyathus virgatus). Kitahara et al. [53] briefly discussed the relationship between the latter two genera.
Most of the remaining species included in our phylogenetic tree are from exclusively zooxanthellate coral families, and the CO1 data imply that these species diverged relatively recently. Our results are consistent with previously analyses [11], [18], [26], [32], which found that most of these coral families are polyphyletic–most strikingly, phylogenetics often splits Pacific and Atlantic representatives of the same genus or family (see [26]). One of the most highly fragmented families in our analyses is Faviidae, which is split into ten different groups (Fig. 1–D). As reported by Fukami et al. [11], the Indo-Pacific faviids appear to be clearly distinct from their Atlantic counterparts, and the latter should probably be transferred to an Atlantic mussid clan/clade, with the following composition: Isophyllia spp. Mycetophyllia spp., Mussismilia spp., Diploria spp., Manicina spp., Colpophyllia spp., Scolymia cubensis, Favia fragum and F. leptophyllia (see also [60]).
According to our results and following Fukami et al. [11], the Trachyphylliidae does not merit recognition at the family level and should be incorporated into the Indo-Pacific “faviid-pectinid-merulinid” clan/clade. Montastraea cavernosa did not group with its congeners, but rather diverged near the base of the “robust” clade, and few conclusions can be drawn concerning the remaining faviids in our phylogeny.
Conclusions
Maximum Likelihood and Bayesian analyses of the CO1 data set indicate that most of the exclusively zooxanthellate coral families are not monophyletic, and require morphological revision. By contrast, the majority of families consisting exclusively or predominantly of azooxanthellate corals appears to be monophyletic. An important exception is the azooxanthellate family Caryophylliidae; here, special attention should be given to the genera Deltocyathus, Trochocyathus, and the heterogeneous group formed by Stephanocyathus, Vaughanella, Conotrochus, Paraconotrochus, Gen. nov. A sensu Stolarski (1996), and Ceratotrochus.
Whereas the deepest dichotomy identified in previous studies was the complex/robust split, our analyses (the present study and Stolarski et al., in preparation) also identified a deeply-diverging clade consisting of members of the exclusively azooxanthellate families Gardineriidae and Micrabaciidae. On the basis of our analyses, these may be the oldest scleractinian families with extant representatives. Although estimates of divergence times among gardineriids/micrabaciids and the complex and robust lineages must be further investigated, the placement of these families as basal to the complex/robust coral lineages implies that scleractinians may have co-existed with rugose corals but, unlike the latter, survived the Permian/Triassic mass-extinction event.
The deep-sea holds important clues to anthozoan evolution, and overall, our phylogenetic reconstruction shows that the most basal extant scleractinians are azooxanthellate corals from deep-water (probably with azooxanthellate shallow-water ancestors), not only in the case of gardineriids/micrabaciids, but also in relation to the “robust” coral clade, and possibly within extant agariciids. Another conclusion can be drawn within the acquisition or loss of solitary/colonial state. Even though most of the groups apparently arouse from solitary life forms, the opposite was also detected (e.g. pocilloporids and M. oculata in relation to Caryophyllia).
Finally, our data supports that the order Corallimorpharia is the sister group of scleractinians [11], [37] and are therefore inconsistent with the “naked coral” hypothesis, which implies that corallimorphs are corals that have undergone skeleton loss.
Materials and Methods
Between 1993 and 2007, French and Australian expeditions collected and preserved in ethanol hundreds of specimens of deep-water scleractinians (ranging in depth from 170 to 1434 m) from off New Caledonia and Australian waters (including Pacific and Indian Ocean). Based on morphological characters all these specimens were identified to the lower taxonomic level possible, and genomic DNA was extracted from most of them. The definition used here to delimit the upper depth boundary of deep-water corals is 50 m [1], since very few zooxanthellate corals occur below this depth.
Tissue was collected from a whole mesentery using a forceps when the species was large, or an entire sector (including the skeleton) was taken when the species was small. However, intending to preserve museum vouchers, if just one specimen of a small solitary species was available, the specimen was completely submerged in the lysis buffer to have its genomic DNA extracted. Genomic DNA was extracted using DNeasy Tissue and Blood Kit (QIAGEN) following the manufacturer's instructions. For each species the concentration of genomic DNA extracted was measured using a Nanodrop 1000 (Thermo Scientific), and when necessary, an aliquot of the genomic DNA was diluted or concentrated to achieve the final concentration of 25 ng/ul.
Using the primers developed by Folmer et al. [61] (LCO1 490-GGTCAACAAATCATAAAGATATTGG and HCO2 198-TAAACTTCAGGGTGACCAAAAAATCA) a fragment of the mitochondrial cytochrome oxidase subunit 1, ranging between 700 and 710 bp according to the species, was amplified. Reactions were carried out in 50 µl, with 5 µl of 10× PCR Buffer, 5 µl of 2 mM dNTPs, 5 µl of 25 mM MgCl2, 2.5 µl of each primer (10 mM each), 0.4 µl of Taq polymerase, and 2 µl of template. PCR conditions used were: a denaturation first step of 95°C for 1 min, followed by 35 cycles of 30 s at 95°C, 30 s at 40°C, and 90 s at 72°C, followed by 10 min at 72°C. If the amplification using this protocol failed, a new reaction using the Advantage-2 kit (Clontech) with the same template, primers and PCR conditions were performed following the manufacturer's instructions. All cycles were performed using Bio-Rad DNA engine (Peltier Thermal Cycler). The PCR products were then purified using Ultra Clean PCR clean up (Mo-Bio) spin columns, and then submitted to Macrogen (Korea) sequencing facility to be sequenced using ABI3730XL (Applied Byosystems). Sequences were verified and manipulated with Sequencher ver. 4.8 (Gene Codes Corporation). A Blast search was performed on GenBank for each sequence and the matching homologous Scleractinian sequences were retained for subsequent alignment. Using this protocol, 158 previously published sequences were added to the alignment (File S1).
All sequences were aligned in ClustalW (EBI) using default settings. The resultant alignment was then checked using JalView ver. 8.0 [62], totaling 595 bp in the final alignment (File S2). The alignment was then submitted to the test of substitution saturation [63] available in DAMBE [64].
Using the final alignment, GTR + Gamma + Proportion Invariant (GTR+G+I) model of DNA evolution was determined by the hierarchical likelihood ratio test implemented in MrModeltest [65] as the best model for the data. The phylogenetic analysis was performed using PhyML for maximum likelihood [66] and MrBayes for Bayesian inference [67], [68].
The most likely topology was calculated based on Shimodaira and Hasegawa (Sh-like) branch support implemented in PhyML. For the Bayesian inference, four runs with 10 million generations each were calculated with topologies saved at each 1000 generations. One fourth of the 10000 topologies were discarded as burnin, and the remaining used to calculate the posterior probability. Additional Bayesian analyses were conducted using BEAST [69] specifically to test the hypothesis that the “robust” shallow water scleractinian families are monophyletic. The BEAST analyses were based on the same alignment as the PhyML and MrBayes phylogenetic analysis, but with and without the constraint of monophyly of the “robust” shallow water scleractinian families.
Supporting Information
Species of Scleractinia sequenced for CO1, including station, location of skeletal voucher, and accession number.
(0.36 MB DOC)
Partial CO1 gene alignment from 255 anthozoans, including 234 scleractinians from 104 genera representing 25 of the 27 extant families.
(9.97 MB TIF)
Acknowledgments
We thank all the Smithsonian Institution Invertebrate Collection Staff (NMNH) for sending the New Caledonian material to Australia, especially Paul Greenhall. Special thanks are due to all Museum of Tropical Queensland staff (MTQ–Queensland), especially Dr. Carden Wallace, Barbara Done, and Dr. Paul Muir for use of facilities at the Museum of Tropical Queensland. We also thank Dr. Philippe Bouchet (MNHN) who generously loaned the New Caledonian material from the Paris Museum to the Smithsonian Institution, and Dr. Bertrand Richer de Forges and IRD-Noumea staff and collaborators for their great effort in collecting and preserving the specimens examined in the present study. We extend our gratitude to Prof. Dr. Karen Miller (UTAS) and Felicity McEnnulty (CSIRO) for the loan of material collected off Australia, which was conducted by the Australian CSIRO Marine Research. We would also like to acknowledge Dr. Hironobu Fukami for providing unpublished sequences used in the present study. The first author is very thankful to Dr. Sylvain Fôret for the technical assistance in the bioinformatics, and to Mr. James Robinson for the laboratorial technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported through funding from the Australian Research Council (through the Centre of Excellence for Coral Reef Studies) grants to DJM. MVK is a recipient of a PhD scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES). The Australian specimens examined in the present study were collected with funding from the Fisheries Research and Development Corporation (FRDC) and Environment Australia (now the Australian Government Department of Environment, Water, Heritage and the Arts); the 2005/2007 cruises (SS102005 and SS022007) were funded by the Australian CSIRO (Commonwealth Scientific and Industrial Research Organization) Wealth from Oceans Flagship with the assistance of the Australian Government Department of the Environment, Water, Heritage and the Arts; and the NORFANZ cruise was a collaboration between Australia's National Oceans Office (now the Australian Government Department of Environment, Water, Heritage and the Arts), Australia's CSIRO Marine and Atmospheric Research, New Zealand's Ministry of Fisheries and New Zealand's National Institute of Water and Atmospheric Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cairns SD. Deep-sea coral ecosystems: Biology and Geology. Bull Mar Sci. 2007;81(3):309–310. [Google Scholar]
- 2.Cairns SD, Hoeksema BW, van der Land J. Appendix: List of Extant Stony Corals. Atoll Res Bull. 1999;459:13–46. [Google Scholar]
- 3.Cairns SD. Antarctic and Subantarctic Scleractinia. 74Antarctic Res Ser. 1982;34(1) [Google Scholar]
- 4.Kerby T, Hall-Spencer JM. Arctic coral reefs, an undiscovered environment. Catalyst, Secondary Science Review. 2007;18:14–16. [Google Scholar]
- 5.Keller NB. The deep-sea madreporarian corals of the genus Fungiacyathus from the Kuril-Kamchatka, Aleutian trenches and other regions of world ocean. Tr Inst Okeanol. 1976;99:31–44. (in Russian) [Google Scholar]
- 6.Wells JW. Scleractinia. In: Moore RC, editor. Treatise on Invertebrate Paleontology. Part F. Lawrence, Geological Society of America; 1956. [Google Scholar]
- 7.Boschma H. The species problem in corals. 15th International Congress of Zoology Section. 1959;3(41):1–2. [Google Scholar]
- 8.Romano SL, Cairns SD. Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bull Mar Sci. 2000;67(3):1043–1068. [Google Scholar]
- 9.Stolarski J, Roniewicz E. Towards a new synthesis of evolutionary relationships and classification of Scleractinia. J Paleontol. 2001;75:1090–1108. [Google Scholar]
- 10.Le Goff-Vitry MC, Rogers AD, Baglow D. A deep-sea slant on the molecular phylogeny of the Scleractinia. Mol Phylogenet Evol. 2004;30:167–177. doi: 10.1016/s1055-7903(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 11.Fukami H, Chen CA, Budd AF, Collins A, Wallace C, et al. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS One. 2008;3:e3222. doi: 10.1371/journal.pone.0003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alloiteau J. Contribution a la Systématiqye des Madréporaires Fossiles. 1957. Tome I. Centre National de la Recherche Scientifique, Paris.
- 13.Chevalier JP, Beauvais Grassé PP, editor. Order des Scléractiniaires. 1987. Traité de Zoologie tome III, fasc. 3. Masson, Paris.
- 14.Veron JEN. Corals in Space and Time: the Biogeography and Evolution of the Scleractinia. Ithaca: Comstock/Cornell paperbacks. 1995 Cornell University Press. [Google Scholar]
- 15.Cairns SD. New records of ahermatypic corals (Scleractinia) from the Hawaiian and Line Islands. Occas pap Bernice P Bishop Mus. 1984;25(10):1–30. [Google Scholar]
- 16.Hoeksema BW. Taxonomy, phylogeny and biogeography of mushroom corals (Scleractinia: Fungiidae). Zool Verh. 1989;254:1–295. [Google Scholar]
- 17.Pandolfi J. Successive isolation rather than evolutionary centres for the origination of Indo-Pacific reef corals. J Biogeogr. 1992;92:593–609. [Google Scholar]
- 18.Budd AF, Stolarski J. Searching for new morphological characters in the systematics of scleractinian reef corals: comparison of septal teeth and granules between Atlantic and Pacific Mussidae. Acta Zool (Stockholm) 2009;90:142–165. [Google Scholar]
- 19.Cairns SD. A generic revision and phylogenetic analysis of the Turbinoliidae (Cnidaria: Scleractinia). Smithson Contrib Zool. 1997;591:1–55. [Google Scholar]
- 20.Wallace CC. Melbourne, Australia: CSIRO Publishing; 1999. Staghorn Corals of the World: A Revision of the Genus Acropora. [Google Scholar]
- 21.Cairns SD. A generic revision and phylogenetic analysis of the Dendrophylliidae (Cnidaria: Scleractinia). Smithson Contrib Zool. 2001;615:75. [Google Scholar]
- 22.Romano SL, Palumbi SR. Evolution of scleractinian corals inferred from molecular systematics. Science. 1996;271:640–642. [Google Scholar]
- 23.Romano SL, Palumbi SR. Molecular evolution of a portion of the mitochondrial 16S ribosomal gene region in scleractinian corals. J Mol Evol. 1997;45:397–411. doi: 10.1007/pl00006245. [DOI] [PubMed] [Google Scholar]
- 24.Daly M, Fautin DG, Cappola VA. Systematics of the Hexacorallia (Cnidaria: Anthozoa). Zool J Linn Soc. 2003;139:419–437. [Google Scholar]
- 25.Chen CA, Wallace CC, Wolstenholme J. Analysis of the mitochondrial 12S rDNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol Phylogenet Evol. 2002;23:137–149. doi: 10.1016/S1055-7903(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Fukami H, Budd AF, Paulay G, Sole-Cava A, Chen CA, et al. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature. 2004;427:832–835. doi: 10.1038/nature02339. [DOI] [PubMed] [Google Scholar]
- 27.Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL. Naked corals: skeleton loss in Scleractinia. PNAS. 2006;103(24):96–100. doi: 10.1073/pnas.0602444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ. Shape-shifting corals: Molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol Biol. 2009;9:45. doi: 10.1186/1471-2148-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CA, Odorico DM, Ten Louis M, Veron JEN, Miller DJ. Systematic relationships within the Anthozoa (Cnidaria Anthozoa) using the 5′-end of the 28S rDNA. Mol Phylogenet Evol. 1995;4(2):175–183. doi: 10.1006/mpev.1995.1017. [DOI] [PubMed] [Google Scholar]
- 30.Veron JEN, Odorico DM, Chen CA, Miller DJ. Reassessing evolutionary relationships of scleractinian corals. Coral Reefs. 1996;15:1–9. [Google Scholar]
- 31.Berntson EA, France SC, Mullineaux LS. Phylogenetic relationships within the class Anthozoa (Phylum Cnidaria) based on nuclear 18S rDNA sequences. Mol Phylogen Evol. 1999;13:417–433. doi: 10.1006/mpev.1999.0649. [DOI] [PubMed] [Google Scholar]
- 32.Cuif JP, Lecointre G, Perrin C, Tillier A, Tillier S. Patterns of septal biomineralization in Scleractinia compared with their 28S rDNA phylogeny: a dual approach for a new taxonomic framework. Zool Scripta. 2003;32:459–473. [Google Scholar]
- 33.Roberts JM, Wheeler AJ, Freiwald A, Cairns SD. Cold water corals: the Biology and Geology of Deep-sea Coral Habitats. 2009. 334p. Cambridge University Press. [DOI] [PubMed]
- 34.Bridge D, Cunningham CW, Schierwater B, DeSalle R, Buss LW. Class-level relationships in the phylum Cnidaria: evidence from mitochondrial genome structure. Evol. 1992;89:8750–8753. doi: 10.1073/pnas.89.18.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.France SC, Rosel PE, Agenbroad JE, Mullineaux LS, Kocher TD. DNA sequence variation of mitochondrial large-subunit rDNA provides support for a two-subclass organization of the Anthozoa (Cnidaria). Mol Mar Biol Biotechnol. 1996;5:15–28. [PubMed] [Google Scholar]
- 36.Song JL, Won JH. Systematic relationship of the anthozoan orders based on the partial nuclear 18S rDNA sequences. Korean J Biol Sci. 1997;1:43–52. [Google Scholar]
- 37.Brugler MR, France SC. The complete mitochondrial genome of the black coral Chrysopathes formosa (Cnidaria:Anthozoa:Antipatharia) supports classification of antipatharians within the subclass Hexacorallia. Mol Phylogen Evol. 2007;42:776–788. doi: 10.1016/j.ympev.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Owens JM. Evolutionary trends in the Micrabaciidae: an argument in favor of preadaptation. Geologos. 1984;11:87–93. [Google Scholar]
- 39.Squires DF. The evolution of the deep-sea coral family Micrabaciidae. Stud Trop Oceanog. 1967;5:502–510. [Google Scholar]
- 40.Beauvais L. Monographie des madréporaires du Jurassique inférieur du Maroc. Palaeontographica Abhandlungen A. 1986;194:1–68. [Google Scholar]
- 41.Stanley GD, Jr, Swart PK. Evolution of the coral-zooxanthellae symbiosis during the Triassic: A geochemical approach. Paleobiol. 1995;21:179–199. [Google Scholar]
- 42.Lindner A, Cairns SD, Cunningham CW. From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE. 2008;3(6):e2429. doi: 10.1371/journal.pone.0002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaughan TW, Wells JW. Revision of the suborders, families and genera of the Scleractinia. GSA Special Papers. 1943;44:1–363. [Google Scholar]
- 44.Filkorn HF, Allor JP. A new early cretaceous coral (Anthozoa; Scleractinia; Dendrophylliina) and its evolutionary significance. J Paleont. 2004;78(3):501–512. [Google Scholar]
- 45.Wallace CC, Chen CAC, Fukami H, Muir PR. Recognition of separate genera within Acropora based on new morphological, reproductive, genetic evidence from A. togianensis, elevation of the subgenus Isopora Studer, 1878 to genus (Scleractinia: Astrocoeniidae; Acroporidae). Coral Reefs. 2007;26:231–239. [Google Scholar]
- 46.Cairns SD. Cnidaria Anthozoa: deep-water azooxanthellate Scleractinia from Vanuatu, and Wallis and Futuna islands. Mem Mus Nat Hist Nat. 1999;180:31–167. [Google Scholar]
- 47.Baron-Szabo RC, Schafhauser A, Götz S, Stinnesbeck W. Scleractinian corals from the Cardenas Formation (Maastrichtian), San Luis Potosí, Mexico. J Paleontol. 2006;80(6):1033–1046. [Google Scholar]
- 48.Kerr AM. Molecular and morphological supertree of stony corals (Anthozoa: Scleractinia) using matrix representation parsimony. Biol Rev. 2005;80:1–16. doi: 10.1017/S1464793105006780. [DOI] [PubMed] [Google Scholar]
- 49.Veron JEN. Corals of the World, vol 3. 2000. Australian Institute of Marine Science, Townsville.
- 50.Yabe H, Sugiyama T. Recent reef building corals from Japan and the South Sea Islands under the Japanese Mandate II, Science Reports Tohoku Imp. 1941;Special vol. II:p. 67–91. University, Sendal, Japan, 3rd ser. (Geology) [Google Scholar]
- 51.Vaughan TW. Recent Madreporaria of the Hawaiian Islands and Laysan. 427Bull US Nat Mus. 1907;59 [Google Scholar]
- 52.Alcock AW. Diagnoses and descriptions of new species of corals. Tijdschrift der Nederlandsche Dierkundige Vereeniging. 1902;7:89–115. [Google Scholar]
- 53.Kitahara MV, Cairns SD, Miller DJ. Monophyletic origin of Caryophyllia (Scleractinia, Caryophylliidae), with description of six new species. Syst Biodivers. 2010;8(1):1–28. [Google Scholar]
- 54.Stolarski J. Origin and phylogeny of Guyniidae (Scleractinia) in the light of microstructural data. Lethaia. 2000;33:13–38. [Google Scholar]
- 55.Cairns SD. The marine fauna of New Zealand: Scleractinia (Cnidaria Anthozoa). 210NZOI Mem. 1995;103 [Google Scholar]
- 56.Zibrowius H, Gili JM. Deep-water Scleractinia (Cnidaria: Anthozoa) from Namibia, South Africa, and Walvts Ridge, southeastern Atlantic. Sci Mar. 1990;54:19–46. [Google Scholar]
- 57.Cairns SD. Roberts JM, Wheeler A, Freiwald A, Cairns SD, editors. Online appendix: Phylogenetic list of 711 valid Recent azooxanthellate scleractinian species, with theirjunior synonyms and depth ranges. Cold water corals: The Biology and Geology of Deep-sea Corals Habitats. 2009. Cambridge University Press.
- 58.Benzoni F, Stefani F, Stolarski J, Pichon M, Mitta G, et al. Debating phylogenetic relationships of the scleractinian Psammocora: molecular and morphological evidences. Contrib Zool. 2007;76:35–54. [Google Scholar]
- 59.Forsman ZH, Guzman HM, Chen AC, Fox GE, Wellington GM. An ITS region phylogeny of Siderastrea (Cnidaria: ANthozoa): is S. glynni endangered or introduced? Coral Reefs. 2005;24:343–347. [Google Scholar]
- 60.Nunes F, Fukami H, Vollmer SV, Norris RD, Knowlton N. Re-evaluation of the systematics of the endemic corals of Brazil by molecular data. Coral Reefs. 2008;27:423–432. [Google Scholar]
- 61.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–297. [PubMed] [Google Scholar]
- 62.Clamp M, Cuff J, Searle SM, Barton GJ. The Jalview Java Alignment Editor. Bioinfo. 2004;20:426–427. doi: 10.1093/bioinformatics/btg430. [DOI] [PubMed] [Google Scholar]
- 63.Xia XH, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylog Evol. 2003;26:1–7. doi: 10.1016/s1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 64.Xia XH, Xie Z. DAMBE: Data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 65.Nylander JAA. MrModeltest 2.0. Program distributed by the author. 2004. Evolutionary Biology Centre, Uppsala University.
- 66.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. System Biol. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 67.Huelsenbeck JP, Ronquist F. MrBAYES: Bayesian inference of phylogenetic trees. Bioinf. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 68.Ronquist F, Huelsenbeck JP. MrBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinf. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 69.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species of Scleractinia sequenced for CO1, including station, location of skeletal voucher, and accession number.
(0.36 MB DOC)
Partial CO1 gene alignment from 255 anthozoans, including 234 scleractinians from 104 genera representing 25 of the 27 extant families.
(9.97 MB TIF)