Introduction
Traditional treatment of bacterial infections relies heavily on the use of antibacterial compounds that either kill bacteria (bactericidal) or inhibit their growth (bacteriostatic). Typically, the targets for the main conventional antibiotics are essential cellular processes such as bacterial cell wall biosynthesis, bacterial protein synthesis, and bacterial DNA replication and repair. However, resistance to these drugs arises and spreads very rapidly, even to such an extent that bacteria have been identified that are simultaneously resistant to all available antibiotics [1]. The increasing occurrence of resistant bacteria gradually renders antibiotics ineffective in treating infections and has enormous human and economic consequences worldwide. As a result, the identification of novel drug targets and the development of novel therapeutics constitute an important area of current scientific research. An alternative to killing or inhibiting growth of pathogenic bacteria is the specific attenuation of bacterial virulence, which can be attained by targeting key regulatory systems that mediate the expression of virulence factors. One of the target regulatory systems is quorum sensing (QS), or bacterial cell-to-cell communication. QS is a mechanism of gene regulation in which bacteria coordinate the expression of certain genes in response to the presence or absence of small signal molecules (Figure 1).
Figure 1. General scheme of a quorum sensing system.
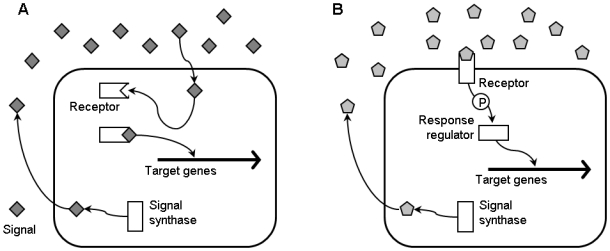
The signal synthase enzyme (a homolog of V. fischeri LuxI in the case of AHL quorum sensing) produces signal molecules, which reach the extracellular environment either via diffusion or transport. At a critical signal molecule concentration, the signal binds to the receptor, which can be located in the cytoplasm (a homolog of V. fischeri LuxR in the case of AHL quorum sensing) (A) or at the cell surface (B). If the receptor is located in the cytoplasm, the signal–receptor complex activates or inactivates transcription of the target genes. If the receptor is located at the cell surface, target gene transcription is modulated through a phosphorylation/dephosphorylation signal transduction cascade with a transcriptional regulator at the end (e.g., a homolog of V. harveyi LuxRVh). P denotes phosphotransfer.
Quorum Sensing: Bacterial Cell-to-Cell Communication
QS was first discovered in the marine bacterium Vibrio fischeri and was thought to be restricted to only a limited series of species. Later on, similar systems were found to be present in many other Gram-negative bacteria. These Gram-negative bacteria use acylated homoserine lactones (AHLs) as signal molecules (for a review see [2]). AHLs are typically produced by a homolog of V. fischeri LuxI and detected by a homolog of V. fischeri LuxR. In addition to the AHL-mediated systems in Gram-negative bacteria, some Gram-positive bacteria also regulate a variety of processes by QS. The QS systems of Streptococcus pneumoniae, Bacillus subtilis, and Staphylococcus aureus, for instance, have been extensively studied (for a review see [3]). A different kind of QS system is found in vibrios. These bacteria use multichannel QS systems in which different types of signal molecules are produced. The signal molecules are detected at the cell surface by membrane-bound, two-component receptor proteins that feed a common phosphorylation/dephosphorylation signal transduction cascade (for a review on QS in vibrios, see [4]). One of the signals produced by vibrios is the so-called autoinducer 2 (AI-2), a furanosyl borate diester [5]. AI-2 activity has been detected in many different species (Gram-negative as well as Gram-positive), although its function as a signal is not generally accepted for all species (for a detailed discussion see [6]). The language of bacteria seems to be even more diversified as new QS systems, using different types of signal molecules, are still being discovered [7].
Disruption of Bacterial Cell-to-Cell Communication
Phenotypes that are controlled by a QS system include bioluminescence, conjugation, nodulation, swarming, sporulation, biocorrosion, antibiotic production, biofilm formation, and the expression of virulence factors such as lytic enzymes, toxins, siderophores, and adhesion molecules [3], [7], [8]. QS systems are found in a still-growing list of bacteria that are pathogenic to plants, animals, and humans [8], [9]. As the importance of QS in virulence development of pathogenic bacteria became clear, about a decade ago, QS disruption was suggested as a new anti-infective strategy [10].
A first major strategy that has been studied is the application of compounds aiming at interfering with signal molecule detection. The red marine alga Delisea pulchra produces halogenated furanones, such as (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. These compounds disrupt QS-regulated gene expression both in AHL QS systems and in multichannel systems of vibrios by interacting with QS transcriptional regulators [11], [12] and the AI-2 biosynthesis enzyme LuxS [13]. The efficacy of halogenated furanones to protect eukaryotic hosts from animal and human pathogenic bacteria such as Pseudomonas aeruginosa, Vibrio anguillarum, Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus has been documented [14]–[16]. Several other macro-algae, micro-algae, and terrestrial plants also produce compounds able to interfere with QS, although in most cases the chemical nature of the signal mimics still has to be elucidated [17], [18]. In addition to these natural compounds, numerous synthetic QS antagonists (mostly AHL and furanone analogs) have been identified and tested (for reviews see [19], [20]).
A second major strategy to disrupt QS is the inactivation of signal molecules. The ability to degrade AHLs seems to be widely distributed in the bacterial kingdom. Enzymes that are able to inactivate AHLs have been discovered in species belonging to the α-proteobacteria, the β-proteobacteria, and the γ-proteobacteria, as well as in some Gram-positive species (for a review see [21]). The actual inactivation of the signal compound can be mediated by two types of enzymes, AHL lactonases and AHL acylases. Lactonases open the lactone ring of AHLs, resulting in the corresponding N-acyl-L-homoserines, whereas acylases cleave the side chain, releasing homoserine lactone and a fatty acid. Signal-degrading bacteria have been effective against plant, animal, and human pathogens such as Erwinia carotovora, V. harveyi, and Vibrio cholerae [22]–[24].
Conventional antibiotics inherently favor the evolution of resistance because they pose a strong selective pressure on bacteria. Indeed, resistant mutants have a large fitness advantage when compared to their susceptible counterparts (which are either killed or inhibited in their growth). In this regard, disruption of QS is generally believed to be unlikely to pose harsh selective pressures, thus minimizing the risk of resistance development [14], [15], [20], [25]–[31]. In the following sections, we argue that this point of view might be too optimistic and discuss the possibility that resistance to QS disruption might occur. Although all mechanisms that lead to resistance to conventional antibiotics also apply for QS disruption, the focus will be on variation in the core genes of QS systems (i.e., the genes involved in signal production, detection, and transduction) since this aspect is specific to the possible development of resistance to QS disruption.
Variability in Quorum Sensing Core Genes
In general, natural selection can only operate on a certain trait if there is (heritable) variation and if this variation is associated with a difference in fitness (i.e., a difference in the amount of offspring that is produced). If these conditions are met, natural selection automatically results. Consequently, there will be a risk for resistance to QS disruption to develop if there is variation in (the expression of) QS genes that can lead to insensitivity towards QS disruption and if this variation results in differences in fitness under QS disrupting conditions. There appears to be variation in the expression of QS core genes among natural bacterial strains. When testing different strains of a certain species for production of QS signal molecules, frequently some of the strains produce signals, whereas others do not. Moreover, variability has been observed in the signal molecule concentration among strains that do produce signals (Table 1). Natural variation in signal molecule levels produced by bacteria might be important when considering QS antagonists that compete with natural signals for receptor binding. Finally, differences among strains of the same species in the specificity of AHL synthases have also been reported. In E. carotovora strain SCC3193, for instance, the main AHL is N-(3-oxooctanoyl)-L-homoserine lactone and only traces of N-(3-oxohexanoyl)-L-homoserine lactone can be detected, whereas in strain SCC1 an inverse profile is observed [32].
Table 1. Examples of inter-strain variability in the production of signal moleculesa in different species.
| Species | Signal Molecule | ΔActivity (Fold)b | nc | References |
| Aeromonas hydrophila | BHL and HHLg | 1.6 | 4 | [33] |
| Aeromonas salmonicida | BHL and HHLg | 1.4 | 7d | [33] |
| Agrobacterium vitis | long-chain AHLs | 15.8 | 12 | [34] |
| Burkholderia vietnamiensis | HHLg | 2.5 | 5 | [35] |
| Erwinia amylovora | AI-2 | 2.5 | 7 | [36] |
| Fusobacterium nucleatum | AI-2 | 9.4 | 4 | [37] |
| Photobacterium phosphoreum | OH-OHLg | 1.8 | 3 | [38] |
| Porphyromonas gingivalis | AI-2 | 2.1 | 6e | [37] |
| Prevotella intermedia | AI-2 | 1.4 | 7d | [37] |
| Pseudomonas aeruginosa | OdDHLg | 65.5 | 28f | [39] |
| Vibrio campbellii | CAI-1 | 2.3 | 7 | [40] |
| AI-2 | 2.3 | 7 | ||
| OH-BHLg | 2.3 | 7 | ||
| Vibrio harveyi | CAI-1 | 2.0 | 5 | [40] |
| AI-2 | 3.1 | 5 | ||
| OH-BHLg | 4.1 | 5 | ||
| Vibrio salmonicida | AHL | 1.7 | 8 | [33] |
| Vibrio vulnificus | AI-2 | 5.5 | 16 | [41] |
Zone of induction on agar plate or TLC, or level of induction in liquid assays of a signal molecule reporter strain.
Ratio between strain-producing maximal and minimal levels, respectively.
Number of signal-producing strains considered in the calculation.
Two additional strains were non-producers.
Three additional strains were non-producers.
Eight additional strains were non-producers.
BHL, N-butanoyl-L-homoserine lactone; HHL, N-hexanoyl-L-homoserine lactone; OH-OHL, N-(3-hydroxyoctanoyl)-L-homoserine lactone; OdDHL, N-(3-oxododecanoyl)-L-homoserine lactone; OH-BHL, N-(3-hydroxybutanoyl)-L-homoserine lactone.
In addition to differences in the presence and activity of signal molecule synthases, there can also be variation in the presence of signal receptors. In a recent survey of the frequency of AHL-driven QS circuits among genome-sequenced bacteria, differences between different strains of the same species in the number of LuxI and LuxR homologs were reported [42]. In Burkholderia mallei, for instance, the number of LuxR homologs varied from two to five. Moreover, a study by Zhu and co-workers indicated that bacteria could simply circumvent the QS blockade by overexpressing signal molecule receptor genes. Indeed, many synthetic AHL analogs were potent QS inhibitors in wild-type Agrobacterium tumefaciens [43], whereas in a transformed strain that overexpressed the luxR homolog traR, inhibition was not detected for any of the analogs [43]. Finally, changes in the specificity of the receptor might also affect the outcome of QS disruption. Indeed, a point mutation of L42→A in the LuxR signal binding site has been shown to render the receptor insensitive to the synthetic antagonist N-(propylsulfanylacetyl)-L-homoserine lactone, which even served as an agonist for this mutant [44]. Importantly, although the mutation rendered the signal receptor insensitive to the inhibitor, it maintained wild type sensitivity to activation by the natural signal.
Variation in QS signal transduction genes has also been documented. Joelsson and co-workers surveyed the QS systems of different V. cholerae strains and observed an unexpectedly high rate of dysfunctional components [45]. Some of the strains showed constitutive expression of QS-regulated genes, and others had frame shift mutations in hapR, a partial deletion in hapR, or even no hapR, resulting in non-functional QS regulation. Interestingly, Defoirdt and co-workers observed differences between closely related vibrios with respect to halogenated furanone-mediated protection of infected brine shrimp larvae [16]. This might reflect differences between the strains in production levels, sequence, or structure of the master regulator LuxRVh, the target of the furanone [12].
Differences between strains in the presence and activity of QS core genes can be caused by horizontal gene transfer. Indeed, the traRI operon (encoding the LuxR and LuxI homologs TraR and TraI) of the plant pathogen Agrobacterium tumefaciens is located on the Ti plasmid [46]. In an exciting report, Wei and co-workers identified a functional QS system in Serratia marcescens that is carried on a transposon [47]. The acquisition of such a mobile QS system might enable bacteria to circumvent specific disruption of their native QS system, provided that the new signal–receptor complex is able to activate target gene expression. Interestingly, Coulthurst and co-workers reported that transfer of the Serratia marcescens smaIR operon (encoding homologs of V. fischeri LuxI and LuxR) into the QS-deficient strain Sma 274 caused a variety of native traits, including pigment production, to become QS regulated [48]. These results suggest that QS core genes can indeed be “plugged into” a strain's existing regulatory systems.
Effect of Quorum Sensing Disruption on Fitness
In the previous section, we provided an overview of data indicating that variation in QS core genes that could result in insensitivity to QS disruption exists or can originate easily (by point mutation). A second important question to answer is whether this insensitivity could lead to increased fitness under QS-disrupting conditions. It is thus critical to correctly evaluate the effect of QS disruption on the fitness of bacteria in order to accurately predict the risk of resistance development. Many reports have shown that QS does not affect bacterial growth [14], [15], [25], [28], [29], and therefore it is generally believed that QS disruption only has a small or even no effect on fitness. However, all these observations were made under conditions where bacteria were growing in nutrient-rich synthetic growth media (where QS-regulated genes are not essential for growth). Importantly, as pointed out by Martinez and colleagues, a crucial and underappreciated aspect of fitness measurements is that they must be performed under conditions that are as similar as possible to the clinical situation [49]. Hence, the question that arises is whether QS disruption poses selective pressure on the bacteria where it really matters—in vivo during infection. If it does, then a mutant that is insensitive to QS disruption will have a selective advantage over the (sensitive) wild type and resistance will develop.
Imamura and co-workers reported that the numbers of viable P. aeruginosa PAO1 in the lungs of infected mice in a respiratory infection model were 3.3-fold lower for the rhlI mutant than for the wild type 2 weeks after infection [50]. Similarly, the levels of P. aeruginosa PAO1 were 3 log units lower in mouse lungs treated with a QS-disrupting furanone when compared to untreated mice in an injection model of infection [14]. According to the authors, this decrease was due to increased clearance of the bacteria by the mouse immune system. However, it might as well reflect a decreased ability of the pathogen to colonize the host. Indeed, Lesic and coworkers found that P. aeruginosa cell counts at the site of infection were not affected by QS inhibitors in a mouse burn wound infection model, whereas cell counts in adjacent muscle and blood were 2–3 log units lower [51]. This indicated that the QS inhibitors blocked systemic dissemination of the pathogen. From the perspective of the bacteria, both increased clearance or decreased colonizing ability would lead to a decrease in fitness, and consequently, a mutant that is insensitive to QS disruption would have a selective advantage over the susceptible wild type (because the capability to colonize the host is undisturbed in the mutant and/or because the host immune system is unable to eliminate the mutant).
It appears that under certain conditions, QS disruption can indeed affect bacterial growth. In a highly interesting report, Diggle and co-workers studied the growth of P. aeruginosa wild type and lasI and lasR mutants in different environmental conditions. In nutrient-rich medium, QS-deficient mutants reached a 1.5-fold higher cell density than the wild type, indicating that under these conditions the costs of signalling were higher than the benefits [52]. In contrast, in a medium in which QS-regulated protease expression is needed for growth, the growth of the mutants was 3- to 4-fold lower than that of the wild type. Hence, under these conditions, QS disruption appeared to strongly decrease the fitness of the pathogen [52]. Under in vivo conditions (i.e., during infection of a host), the fitness advantage of QS might be less pronounced than in the growth medium where QS was essential for growth. In vivo, there will be different nutrient sources present (protein, lipids, phospholipids) and the utilization of some of these nutrients will probably not be controlled by QS. Moreover, the selective pressure for resistance development to QS disruption will be limited to those environmental conditions in which the QS-regulated genes affect bacterial fitness. This is in contrast to conventional (bactericidal and bacteriostatic) antibiotics, which pose strong selective pressure in any environment.
The above mentioned results are in accordance with the work of Sandoz and colleagues, who studied social cheating in P. aeruginosa and found that a lasR mutant was unable to grow in medium containing caseinate as the sole carbon source [53]. Although this cheater mutant showed higher fitness than the wild type when co-cultured in this medium (the mutant could benefit from the nutrients generated by the proteolytic activity of the wild type), total culture density decreased with increasing amounts of mutant cells. This indicated that the presence of the mutants did incur a significant cost to the population as a whole. The authors also reported that during an in vitro evolution experiment in which wild type and lasR mutant were co-cultured for ≈100 generations under conditions that require QS for growth, compensatory mutations emerged that converted cheaters into cooperators. In these novel cooperators, the compensatory mutation resulted in the expression of the QS-regulated phenotype in the lasR mutants, thereby bypassing inactivation of the QS system. The evolution of a cheater to a superior cooperator has also been reported in the fruiting body–forming bacterium Myxococcus xanthus [54]. The capacity for compensatory mutation could be a mechanism of bacteria to overcome QS disruption and as such might be important for possible resistance development.
There is some evidence that QS might affect the elimination of bacteria by the host immune system. Joelsson and co-workers reported that QS enhances the viability of V. cholerae under stress conditions in a HapR-dependent manner [55]. Similarly, McDougald and co-workers found that QS induces stress resistance in Vibrio angustum and Vibrio vulnificus [56]. Inactivation of the QS master regulator SmcR in V. vulnificus resulted in a significantly decreased survival after exposure to hydrogen peroxide, which is a part of the defense of eukaryotic hosts against infections [57]. Hence, QS disruption leading to an increased susceptibility to oxidative immune reactions of the host will reduce the fitness of a pathogen under in vivo conditions.
Conclusions and Further Perspectives
QS disruption has been shown to be an effective anti-infective strategy in different host–microbe systems and is therefore considered to be a promising alternative to antibiotics. It is generally believed (although yet not proven) that pathogens are unlikely to develop resistance to this strategy because it poses no or little selective pressure. In this paper, we critically evaluated the information that is available on competition/adaptive evolution of QS mutants in order to obtain a more balanced view. A number of studies in which QS was investigated under conditions that are different from those in standard laboratory cultures using nutrient-rich synthetic growth media and that are more representative of the conditions pathogens experience during infection of a host indicate that—in contrast to the general perception—disruption of QS can pose selective pressure on bacteria. Hence, although at this moment it is difficult to accurately estimate the risk of resistance development, we argue that scientists need to pay attention to the possibility that it will evolve. Further research in different host–microbe systems is urgently needed in order to obtain a more detailed understanding of the fitness cost of QS disruption under in vivo conditions during infection of a host. In this respect, in vivo competition experiments with wild types versus QS mutants of pathogenic bacteria in infection models with a susceptible host would give highly relevant information on selective pressure posed by QS inhibition under in vivo conditions. In addition to this, in vivo evolution experiments in which QS regulation of virulence is studied over many generations during infection of a host under QS disruption conditions would give direct information on the risk of resistance development. To this end, the pathogen could be re-isolated from the infected host after each round of infection to be used as inoculum for the next round.
Once we have better knowledge of the risk of resistance development to QS disruption, it might be possible to direct further research on QS inhibition preferentially towards strategies that include a lower risk of resistance development. A first strategy might consist of using QS disrupting techniques with a relatively broad activity. AHL lactonase, for instance, is active towards a wide range of AHLs. It hydrolises both short- and long-chain AHLs with similar efficiency, but shows no or little residue activity to other chemicals, including non-acyl lactones and aromatic carboxylic acid esters [58]. Hence, alterations of the type of AHL will not affect the efficacy of lactonases. Apart from that, algae and higher plants have been found to produce several different compounds that interfere with QS, and thus the application of plant or algal extracts or exudates might also reduce the risk of resistance development. The red marine alga D. pulchra, for instance, produces several different, but structurally related, brominated furanones [59]. The production of different QS inhibitory compounds might be an evolutionary adaptation that avoids resistance development by fouling bacteria. Indeed, although there are approximately a million different bacterial species in the marine environment [60], none have developed resistance to the collection of furanones produced by D. pulchra since the alga is not colonized by bacteria. Another strategy might be the development of non-competitive or uncompetitive inhibitors rather than competitive inhibitors. Such inhibitors would not suffer from titration effects due to overexpression of QS core genes (e.g., differences between strains in the production of signal molecules). Further, QS disruption could be combined with other treatments to obtain a synergistic effect. QS-disrupting compounds have been shown, for instance, to increase the susceptibility of biofilm bacteria for antibiotic treatments [14]. Finally, the major virulence factors responsible for infection of the host could be targeted directly instead of blocking their expression by QS disruption (for reviews on this strategy, see [61], [62]). However, resistance to this strategy might also evolve if the virulence factor that is inactivated affects pathogen fitness under in vivo conditions.
Acknowledgments
We thank three anonymous reviewers for their valuable suggestions to improve the manuscript.
Footnotes
The authors have declared that no competing interests exist.
The authors thank the “Fonds voor Wetenschappelijk Onderzoek” (FWO-Vlaanderen) for financial support. TD is a postdoctoral fellow of FWO-Vlaanderen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hall BG. Predicting the evolution of antibiotic resistance genes. Nature Rev Microbiol. 2004;2:430–435. doi: 10.1038/nrmicro888. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 3.Dunny GM, Leonard BAB. Cell-cell communication in Gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 4.Milton DL. Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol. 2006;296:61–71. doi: 10.1016/j.ijmm.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 6.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nature Rev Microbiol. 2005;3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraman A, Wood TK. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145–167. doi: 10.1146/annurev.bioeng.10.061807.160536. [DOI] [PubMed] [Google Scholar]
- 8.De Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams P, Camara M, Hardman A, Swift S, Milton D, Hope VJ, Winzer K, Middleton B, Pritchard DI, Bycroft BW. Quorum sensing and the population-dependent control of virulence. Philos Trans R Soc Lond B Biol Sci. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finch RG, Pritchard DI, Bycroft, BW, Williams P, Stewart GSAB. Quorum sensing: a novel target for anti-infective therapy. J Antimicrob Chemother. 1998;42:569–571. doi: 10.1093/jac/42.5.569. [DOI] [PubMed] [Google Scholar]
- 11.Manefield M, Rasmussen TB, Hentzer M, Andersen JB, Steinberg P, Kjelleberg S, Givskov S. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 12.Defoirdt T, Miyamoto CM, Wood TK, Meighen EA, Sorgeloos P, Verstraete W, Bossier P. The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of of the transcriptional regulator protein LuxR. Environ Microbiol. 2007;9:2486–2495. doi: 10.1111/j.1462-2920.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 13.Zang T, Lee BWK, Cannon LM, Ritter KA, Dai S, Ren D, Wood TK, Zhou ZS. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg Med Chem Lett. 2009;19:6200–6204. doi: 10.1016/j.bmcl.2009.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasch M, Buch C, Austin B, Slierendrecht J, Ekmann KS, Larsen JL, Johansen C, Riedel K, Eberl L, Givskov M, Gram L. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). System Appl Microbiol. 2004;27:350–359. doi: 10.1078/0723-2020-00268. [DOI] [PubMed] [Google Scholar]
- 16.Defoirdt T, Crab R, Wood TK, Sorgeloos P, Verstraete W, Bossier P. Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio harveyi, Vibrio campbellii and Vibrio parahaemolyticus isolates. Appl Environ Microbiol. 2006;72:6419–6423. doi: 10.1128/AEM.00753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skindersoe ME, Ettinger-Epstein P, Rasmussen TB, Bjarnsholt T, de Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol. 2008;10:56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- 18.Teplitski M, Chen H, Rajamani S, Gao M, Merighi M, Sayre RT, Robinson JB, Rolfe BR, Bauer WD. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004;134:137–146. doi: 10.1104/pp.103.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 20.Janssens JCA, De Keersmaecker SCJ, De Vos DE, Vanderleyden J. Small molecules for interference with cell-cell-communication systems in Gram-negative bacteria. Curr Med Chem. 2008;15:2144–2156. doi: 10.2174/092986708785747580. [DOI] [PubMed] [Google Scholar]
- 21.Czajkowski R, Jafra S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol. 2009;56:1–16. [PubMed] [Google Scholar]
- 22.Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 23.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-to-cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinh NTN, Gunasekara RAYSA, Boon N, Dierckens K, Sorgeloos P, Bossier P. N-acyl homoserine lactone-degrading microbial enrichment cultures isolated from Penaeus vannamei shrimp gut and their probiotic properties in Brachionus plicatilis cultures. FEMS Microbiol Ecol. 2007;62:45–53. doi: 10.1111/j.1574-6941.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 25.Lönn-Stensrud J, Petersen FC, Benneche T, Aamdal Scheie A. Synthetic brominated furanone inhibits autoinducer-2-mediated communication and biofilm formation in oral streptococci. Oral Microbiol Immunol. 2007;22:340–346. doi: 10.1111/j.1399-302X.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 26.Peters L, König GM, Wright AD, Pukall R, Stackebrandt E, Eberl L, Riedel K. Secondary metabolites of Flustra foliacea and their influence on bacteria. Appl Environ Microbiol. 2003;69:3469–3475. doi: 10.1128/AEM.69.6.3469-3475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, Hughes DT, Huntley JF, Fina MW, Falck JR, Sperandio V. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321:1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Köte M, Nielsen J, Eberl L, Givskov M. Screening for quorum sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol. 2005;187:1799–1814. doi: 10.1128/JB.187.5.1799-1814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swem LR, Swem DL, O'Loughlin CT, Gatmaitan R, Zhao B, Ullrich SM, Bassler BL. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Bodman SB, Willey JM, Diggle SP. Cell-cell communication in bacteria: united we stand. J Bacteriol. 2008;190:4377–4391. doi: 10.1128/JB.00486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters CM, Bassler BL. Quorum sensing: bacterial cell-to-cell communication. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 32.Brader G, Sjöblom S, Hyytiäinen H, Sims-Huopaniemi K, Palva ET. Altering substrate chain length specificity of an acylhomoserine lactone synthase in bacterial communication. J Biol Chem. 2005;280:10403–10409. doi: 10.1074/jbc.M408603200. [DOI] [PubMed] [Google Scholar]
- 33.Bruhn JB, Dalsgaard I, Nielsen KF, Buchholtz C, Larsen JL, Gram L. Quorum sensing signal molecules (acylated homoserine lactones) in Gram-negative fish pathogenic bacteria. Dis Aquat Org. 2005;65:43–52. doi: 10.3354/dao065043. [DOI] [PubMed] [Google Scholar]
- 34.Lowe N, Gan HM, Chakravartty V, Scott R, Szegedi E, Burr TJ, Savka MA. Quorum-sensing signal production by Agrobacterium vitis strains and theri tumor-inducing and tartrate-catabolic plasmids. FEMS Microbiol Lett. 2009;296:102–109. doi: 10.1111/j.1574-6968.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 35.Poonguzhali S, Madhaiyan M, Sa T. Quorum-sensing signals produced by plant-growth promoting Burkholderia strains under in vitro and in planta conditions. Res Microbiol. 2007;158:287–294. doi: 10.1016/j.resmic.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi M, Geider K. Autoinducer-2 of the fire blight pathogen Arwinia amylovora and other plant-associated bacteria. FEMS Microbiol Lett. 2007;266:34–41. doi: 10.1111/j.1574-6968.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 37.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flodgaard LR, Dalgaard P, Andersen JB, Nielsen KF, Givskov M, Gram L. Nonbioluminescent strains of Photobacterium phosphoreum produce the cell-to-cell communication signal N-(3-hydroxyoctanoyl)homoserine lactone. Appl Environ Microbiol. 2005;71:2113–2120. doi: 10.1128/AEM.71.4.2113-2120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilder CN, Allada G, Schuster M. Instantaneous within-patient diversity of Pseudomonas aeruginosa quorum-sensing populations from cystic fibrosis lung infections. Infect Immun. 2009;77:5631–5639. doi: 10.1128/IAI.00755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Defoirdt T, Verstraete W, Bossier P. Luminescence, virulence and quorum sensing signal production by pathogenic Vibrio campbellii and Vibrio harveyi isolates. J Appl Microbiol. 2008;104:1480–1487. doi: 10.1111/j.1365-2672.2007.03672.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, Choy HE, Chung SS, Rhee JH. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol. 2003;48:1647–1664. doi: 10.1046/j.1365-2958.2003.03536.x. [DOI] [PubMed] [Google Scholar]
- 42.Case RJ, Labbate M, Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2008;2:345–349. doi: 10.1038/ismej.2008.13. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Beaber JW, Moré MI, Fuqua C, Eberhard A, Winans S. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch B, Liljefors T, Persson T, Nielsen J, Kjelleberg S, Givskov M. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors. Microbiology. 2005;151:3589–3602. doi: 10.1099/mic.0.27954-0. [DOI] [PubMed] [Google Scholar]
- 45.Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. . Infect Immun. 2006;74:1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuqua WC, Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei JR, Tsai YH, Horng YT, Soo PC, Hsieh SC, Hsueh PR, Horng JT, Williams P, Lai HC. A mobile quorum-sensing system in Serratia marcescens. . J Bacteriol. 2006;188:1518–1525. doi: 10.1128/JB.188.4.1518-1525.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coulthurst SJ, Williamson NR, Harris AKP, Spring DR, Salmond GPC. Metabolic and regulatory engineering of Serratia marcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology. 2006;152:1899–1911. doi: 10.1099/mic.0.28803-0. [DOI] [PubMed] [Google Scholar]
- 49.Martinez JL, Baquero F, Andersson DI. Predicting antibiotic resistance. Nature Rev Microbiol. 2007;5:958–965. doi: 10.1038/nrmicro1796. [DOI] [PubMed] [Google Scholar]
- 50.Imamura Y, Yanagihara K, Tomono K, Ohno H, Higashiyama Y, Miyazaki Y, Hirakata Y, Mizuta Y, Kadota JI, Tsukamoto K, Kohno S. Role of Pseudomonas aeruginosa quorum-sensing systems in a mouse model of chronic respiratory infection. J Med Microbiol. 2005;54:515–518. doi: 10.1099/jmm.0.46004-0. [DOI] [PubMed] [Google Scholar]
- 51.Lesic B, Lépine F, Déziel E, Zhang J, Zhang Q, Padfield K, Castonguay MH, Milot S, Stachel S, Tzika AA, Tompkins RG, Rahme LG. Inhibitors of pathogen intercellular signals as selective anti-infective compounds. PLoS Pathogens. 2007;3:1229–1239. doi: 10.1371/journal.ppat.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 53.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A. 2007;104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiegna F, Yu YTN, Kadam SV, Velicer GJ. Evolution of an obligate social cheater to a superior cooperator. Nature. 2006;441:310–314. doi: 10.1038/nature04677. [DOI] [PubMed] [Google Scholar]
- 55.Joelsson A, Kan B, Zhu J. Quorum sensing enhances the stress response in Vibrio cholerae. . Appl Environ Microbiol. 2007;73:3742–3746. doi: 10.1128/AEM.02804-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDougald D, Srinivasan S, Rice SA, Kjelleberg S. Signal-mediated cross-talk regulates stress adaptation in Vibrio species. Microbiology. 2003;149:1923–1933. doi: 10.1099/mic.0.26321-0. [DOI] [PubMed] [Google Scholar]
- 57.Murray HW, Cohn ZA. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980;152:1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong YH, Wang LH, Zhang LH. Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:1201–1211. doi: 10.1098/rstb.2007.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtis TP, Sloan WT, Scannell JW. Estimating prokaryotic diversity and its limits. Proc Natl Acad Sci U S A. 2002;99:10494–10499. doi: 10.1073/pnas.142680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nature Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 62.Charkowski AO. Decaying signals: will understanding bacterial-plant communications lead to control of soft rot? Curr Opin Biotechnol. 2009;20:178–184. doi: 10.1016/j.copbio.2009.01.005. [DOI] [PubMed] [Google Scholar]


