Abstract
Multiple sclerosis (MS) is an inflammatory, demyelinating, central nervous system disease mediated by myelin-specific T cells. Environmental triggers that cause a breakdown of myelin-specific T cell tolerance are unknown. We found that CD8+ myelin basic protein (MBP)-specific T cell tolerance can be broken and autoimmunity induced by infection with a virus that does not express MBP cross-reactive epitopes and does not depend on bystander activation. Instead, the virus activated dual T cell receptor (TCR)-expressing T cells capable of recognizing both MBP and viral antigens. These results demonstrate the importance of dual TCR T cells in autoimmunity and suggest a mechanism by which a ubiquitous viral infection could trigger autoimmunity in a subset of infected individuals, as hypothesized in the etiology of MS.
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS) that affects more than one million people worldwide. It is believed to be an autoimmune disease in which exposure of genetically predisposed individuals to environmental factors triggers a breakdown in T cell tolerance to myelin antigens. The specific types of myelin-specific T cells that contribute to the pathogenesis of MS are not known. Most studies have focused on the pathogenic role of myelin-specific CD4+ T cells because of the relatively strong association of MS susceptibility with major histocompatibility complex (MHC) class II alleles. In addition, CD4+ T cells are the primary effector T cells in experimental autoimmune encephalomyelitis (EAE), a widely used animal model of MS. However, there has been increasing recognition of the potential importance of CD8+ T cells in the pathogenesis of MS. CD8+ T cells typically outnumber CD4+ T cells in acute and chronic lesions in MS patients, and the CD8+ T cell subset exhibits more evidence of antigen-driven activation compared to CD4+ T cells in the CNS and blood of MS patients1, 2. The frequency of CNS antigen-specific CD8+, but not CD4+, T cells is also higher in MS patients compared to healthy controls3. Furthermore, depletion of CD4+ T cells was not beneficial in MS patients, while depletion of a broader spectrum of leukocytes, including both CD4+ and CD8+ T cells, reduced lesion formation and relapses4. Together these observations support a role for both CD4+ and CD8+ myelin-specific T cells in the pathogenesis of MS.
Conditions leading to a loss of tolerance in either myelin-specific CD4+ or CD8+ T cells are not known. Genome-wide association studies have identified numerous MS susceptibility alleles, each of which (apart from HLA DR2) appears to contribute only slightly to the risk of developing MS5. This genetic complexity, together with the variability in the pathology, symptoms and clinical course of MS, suggest the possibility of multiple disease-initiating pathways. Disease heterogeneity may account for the difficulty in identifying environmental triggers of MS. While viral infections have long been proposed to initiate the disease process6–9, linking a particular virus to MS pathogenesis has not yet been achieved. Association with a specific infection is particularly difficult for a multifactorial disease like MS because a ubiquitous infection may trigger disease in only a small fraction of infected individuals depending upon the diverse interactions of their particular susceptibility alleles with the environment.
Few animal models exist in which infectious triggers of CNS autoimmunity can be investigated. Theilers murine encephalomyelitis virus (TMEV) infection, another model for MS, has been shown to induce CNS autoimmunity by causing bystander activation of myelin specific CD4+ T cells10. However, no model has been described in which an infectious agent abrogates tolerance in myelin-specific CD8+ T cells. Here we utilized a MHC class I-restricted TCR transgenic model that generates CD8+ T cells specific for myelin basic protein (MBP) to investigate conditions that break CD8+ T cell tolerance and induce CNS autoimmunity. We previously generated two TCR transgenic models expressing distinct TCRs specific for MBP79-87 associated with the H-2Kk MHC molecule11. Mice expressing a transgenic TCR comprised of Vα8 and Vβ6 (referred to as 8.6 mice) exhibit both central and peripheral tolerance, consistent with the constitutive presentation of MBP in lymphoid and other tissues. In contrast, T cells expressing a transgenic TCR comprised of Vα8 and Vβ8 (called 8.8 mice) escape central and peripheral tolerance, although they proliferate vigorously to MBP79-87 peptide in vitro. This split tolerance has also been observed for several CD4+ MBP-specific TCR transgenic models in which the low avidity of the interactions between the MBP-specific T cells and their ligand prevents responses to endogenous MBP in vivo, although the T cells can respond to MBP peptide in vitro. Tolerance in CD4+ TCR transgenic models is broken at some stochastic frequency as spontaneous EAE can occur, especially in the absence of regulatory T cells12, 13. Disease is also easily induced in CD4+ TCR transgenic models using immunization protocols that induce CD4+ T cell-mediated EAE in wild-type mice. The tolerance exhibited by CD8+ T cells in 8.8 mice differs from that of MBP-specific CD4+ T cells in that 8.8 T cells exhibit high avidity for their ligand and appear to remove the ligand from APCs without triggering T cell activation11. This observation suggested that circumstances leading to the loss of tolerance may differ for CD8+ and CD4+ MBP-specific T cells.
Our studies here show that many conditions that induce disease in CD4+ myelin-specific TCR transgenic models failed to break tolerance in CD8+ 8.8 T cells. CNS autoimmunity in 8.8 mice was triggered by infection with a recombinant vaccinia virus encoding MBP, consistent with the ability of viruses to efficiently prime MHC class I-restricted T cells. Surprisingly, infection with wild-type vaccinia virus also triggered CNS autoimmunity as efficiently as recombinant virus expressing MBP despite a lack of cross-reactivity between the 8.8 TCR and viral epitopes. Disease induction by wild-type virus required expression of endogenous TCR chains on the 8.8 T cells. Our results demonstrate a role for dual TCRs in the initiation of CNS autoimmune disease and suggest a novel mechanism by which a ubiquitous viral infection may trigger disease in only a subset of infected individuals.
Results
Tolerance differs in CD8+ and CD4+ MBP-specific T cells
In contrast to MBP CD4+ TCR transgenic models, MBP-specific 8.8 mice did not develop spontaneous EAE, even on the Rag2−/− background (0/198 Rag2+/+ and 0/24 Rag2−/− 8.8 mice observed for more than 12 weeks). This result indicated that regulatory T cells, which are absent in Rag2−/− 8.8 mice (data not shown), are not required to prevent 8.8 T cells from responding to endogenous MBP. We investigated the susceptibility of 8.8 mice to active disease induction using a protocol that efficiently induces EAE in CD4+ MBP-specific TCR transgenic models and also induces autoimmune disease in a previously described CD8+ TCR transgenic humanized mouse model of MS in which the transgenic T cells recognize a MHC class I-restricted epitope of proteolipid protein (PLP)14. No neurological signs were observed in 8.8 mice immunized with MBP79-87 in complete Freunds adjuvant (CFA) with or without pertussis toxin injections (Supplementary Table 1). To assess whether peptide immunization is an efficient protocol for activating CD8+ 8.8 T cells, CFSE-labeled 8.8 and 8.6 T cells were transferred into Mbp−/− mice that had been previously immunized with MBP79-87 in CFA and T cell proliferation was analyzed three days later. Although both 8.8 and 8.6 T cells proliferated equally well upon stimulation with MBP peptide in vitro, 8.8 T cells barely proliferated in vivo in response to adjuvant-activated antigen-presenting cells (APCs) presenting exogenous MBP peptide while 8.6 T cells proliferated strongly (Supplementary Fig. 1). To determine if 8.8 T cell tolerance could be abrogated by strong, widespread activation of the APCs that present endogenous MBP throughout the animal, we administered lipopolysaccharide (LPS) and agonistic CD40 antibody to 8.8 mice. Neither reagent, alone or in combination, induced disease in 8.8 mice. Likewise, no disease was observed in 8.8 mice treated with poly(I:C) (Supplementary Table 1). However, weight loss and mild neurological signs were observed in 8.8 mice when MBP peptide was simultaneously injected with both LPS and the CD40 antibody (Supplementary Fig. 2 and Supplementary Table 1). Injection of MBP peptide alone had no effect. These results suggest that both strongly activating APCs in multiple tissues and increasing the concentration of ligand above the amount generated from endogenous MBP are required to break 8.8 T cell tolerance in vivo.
Viral infection triggers autoimmunity in 8.8 mice
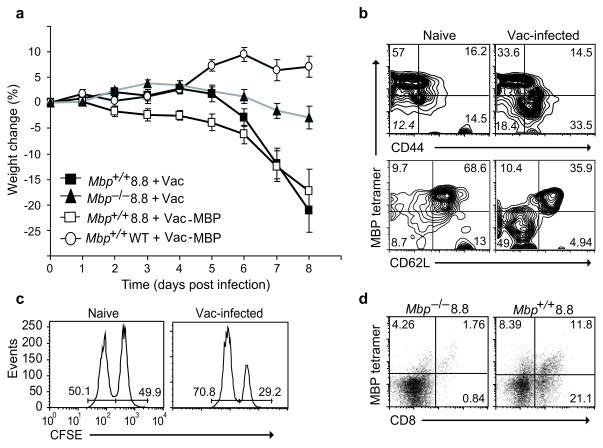
The conditions for breaking 8.8 T cell tolerance described above suggested that CD8+ T cell-mediated autoimmunity might be triggered by a viral infection that causes both widespread APC activation and generates de novo expression of a self-antigen mimic. Consistent with this hypothesis, we found that 8.8 mice exhibited a 100% incidence of autoimmune disease following infection with a recombinant vaccinia virus expressing MBP (Vac-MBP). Unexpectedly, we also found that 8.8 mice infected with wild-type vaccinia virus exhibited the same incidence and severity of disease (Fig. 1a and Supplementary Table 1). In both groups of mice, the disease was characterized by weight loss and clinical signs such as ataxia, knuckling, difficulty walking and tail weakness. The clinical course of disease is depicted as increasing weight loss as this is the most quantitative measure of disease progression; however, immunochemical analyses demonstrated infiltration of CD8+ T cells and F4/80+ macrophage and activated microglia in both the brain and spinal cord of wild-type vaccinia-infected 8.8 mice as expected in autoimmune disease targeting the CNS (Supplementary Fig. 3). The disease progressed rapidly and most mice were sacrificed nine days post infection. In some experiments, mice survived this acute disease and developed chronic neurological symptoms such as walking difficulty and tail weakness (Supplementary Video 1). Analyses of viral titers in lymphoid organs and the CNS following infection showed that 8.8 mice cleared vaccinia virus as efficiently as wild-type mice, indicating that the disease was not due to poor viral clearance in the TCR transgenic mice (data not shown). Effective viral clearance in 8.8 TCR transgenic mice was supported by the absence of clinical signs in Mbp−/− 8.8 mice infected with wild-type virus (Fig. 1a and Supplementary Table 1). Wild-type vaccinia virus infection activated a population of 8.8 T cells in vivo identified by decreased MBP–H-2Kk tetramer and CD62L staining and increased CD44 expression compared to uninfected mice (Fig. 1b). These cells acquired effector function because MBP-pulsed splenocytes were specifically lysed when transferred into vaccinia-infected but not naïve 8.8 mice (Fig. 1c). Accumulation of 8.8 T cells was observed in the CNS of vaccinia-infected Mbp+/+ but not Mbp−/− 8.8 mice and correlated with neurological symptoms (Fig. 1d). These data indicate that wild-type vaccinia virus infection breaks 8.8 T cell tolerance and promotes an autoimmune response directed against endogenous MBP in the CNS.
Figure 1.
Wild-type vaccinia virus infection induces autoimmune disease in 8.8 mice. (a) Mbp+/+ 8.8, Mbp−/− 8.8, and wild-type mice (7–11 mice/group) were infected with Vac-MBP or wild-type vaccinia virus on day 0. Mice were weighed daily and the percent weight loss relative to day 0 is shown. The difference in weight loss is significant between MBP+/+ 8.8 and either Mbp−/− 8.8 (P <0.0001) or wild-type mice (P <0.001) infected with wild-type vaccinia virus. Data are compiled from three independent experiments. (b) Splenocytes from 8.8 naïve or wild-type vaccinia virus-infected mice (seven days post-infection) were stained with MBP79-87–H-2Kk tetramer and antibodies specific for CD8, CD44, and CD62L. Flow cytometry analyses are gated on CD8+ cells. Down-regulation of MBP79-87–H-2Kk tetramer staining routinely occurs on activated 8.8 T cells. (c) 8.8 naïve or wild-type vaccinia virus-infected mice (seven days post-infection) were injected with equal numbers of wild-type splenocytes pulsed with MBP79-87 peptide (CFSE-bright) and non-pulsed (CFSE-dim). Mice were sacrificed 20 h later and CFSE-labeled cells analyzed by flow cytometry. Data are representative of two experiments. (d) Mononuclear CNS cells isolated from Mbp+/+ 8.8 and Mbp−/− 8.8 mice seven days post infection with wild-type vaccinia virus were stained with MBP79-87/H-2Kk tetramer and anti-CD8.
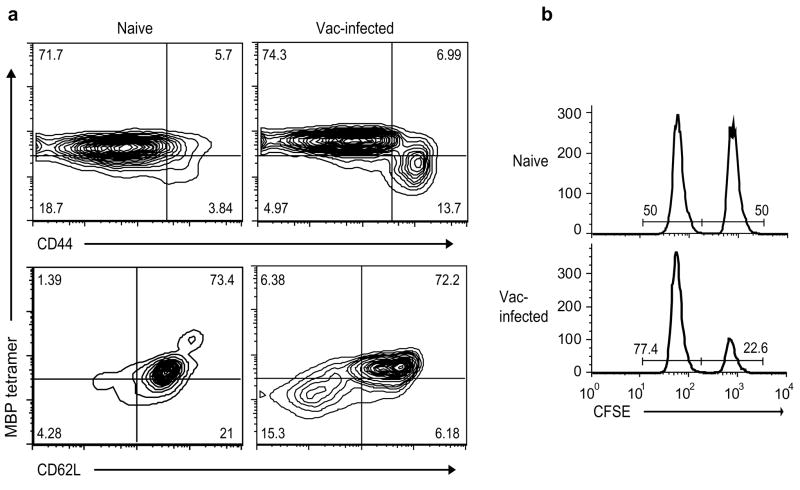
Viral infections have been proposed to trigger autoimmune disease via several mechanisms. Bystander activation of self-reactive T cells might occur if a viral infection causes the release of sequestered autoantigens into an inflammatory milieu. To test this possibility, Mbp−/− 8.8 mice were infected with wild-type vaccinia virus and T cells were analyzed for activation markers and the ability to lyse MBP-pulsed splenocytes in vivo. As observed for Mbp+/+ 8.8 mice, vaccinia infection induced a population of activated 8.8 T cells in Mbp−/− mice that specifically lysed MBP-pulsed target cells in vivo (Fig. 2). These results indicate that vaccinia infection does not activate 8.8 T cells via a bystander mechanism because the 8.8 T cells were activated in the absence of endogenous MBP.
Figure 2.
Wild-type vaccinia virus does not stimulate 8.8 T cells via bystander activation. (a) Splenocytes from Mbp−/− 8.8 mice, either naïve or infected seven days earlier with wild-type vaccinia virus, were stained with MBP79-87–H-2Kk tetramer and antibodies specific for CD8, CD44, and CD62L. Flow cytometry analyses are gated on CD8+ cells. Data are representative of two experiments. (b) Naïve and infected (seven day post infection) Mbp−/− 8.8 mice were injected with equal numbers of wild-type splenocytes pulsed with MBP79-87 (CFSE-bright) and non-pulsed (CFSE-dim). Mice were sacrificed 20 h later and CFSE-labeled cells were analyzed by flow cytometry. Data are representative of three experiments.
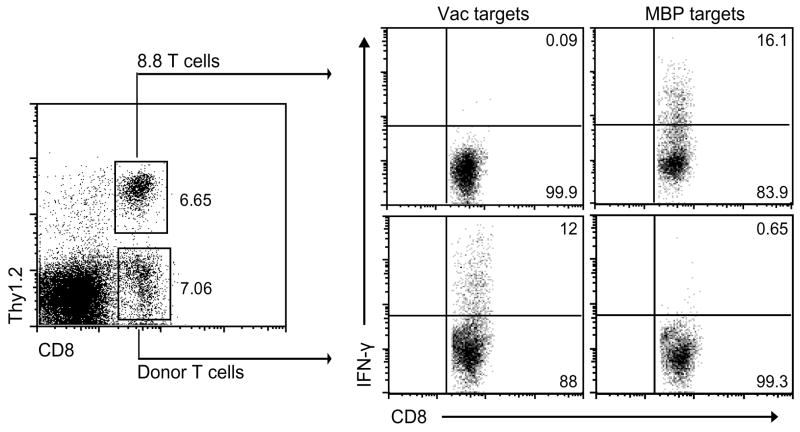
Molecular mimicry could also account for the ability of wild-type vaccinia virus to induce disease in 8.8 mice if the 8.8 TCR exhibits cross-reactivity to a viral antigen. In vitro experiments did not support this possibility as 8.8 T cells proliferated in response to splenocytes infected with Vac-MBP but not wild-type vaccinia virus (data not shown). To explore this possibility in vivo, genetically marked, wild-type splenocytes were transferred into Rag2−/− 8.8 mice prior to infection with wild-type vaccinia virus to provide B cells and non-transgenic T cells needed for viral clearance. Seven days post infection, splenocytes from the infected mice were stimulated in vitro with vaccinia virus-infected target cells and T cell responses were analyzed by intracellular staining for interferon-γ IFN-γ) (Fig. 3). IFN-γ secretion was detected only in the CD8+ T cell population derived from donor non-transgenic splenocytes and not in the host Rag2−/− 8.8 T cells, demonstrating the inability of the 8.8 TCR to recognize viral antigens.
Figure 3.
The 8.8 TCR is not cross-reactive to wild-type vaccinia virus epitopes. Non-transgenic splenocytes (2.5 × 106) from Thy1.1+ C3HeB/Fej mice were transferred into Thy1.2+ Rag2−/− 8.8 mice two weeks prior to infection with wild-type vaccinia virus. Seven days post infection, splenocytes were harvested and stimulated in vitro for 18 h with either vaccinia-infected or MBP79-87-pulsed Thy1.1+ splenocytes. Cells were stained with antibodies specific for CD8 and Thy1.2, then permeabilized and stained with anti-IFN-γ. Data are representative of two experiments.
8.8 T cells are activated via endogenous TCR chains
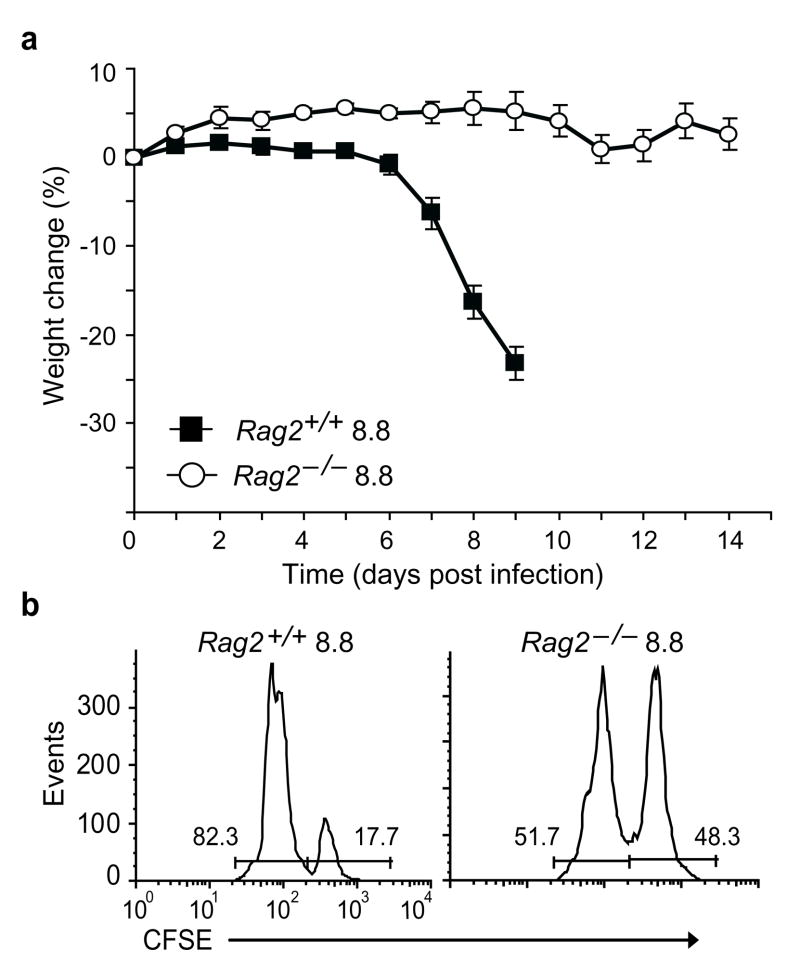
The lack of IFN-γ production by Rag2−/− 8.8 T cells in response to vaccinia-infected target cells suggested that Rag2−/− 8.8 T cells differed from Rag2+/+ 8.8 T cells in that they were not activated during vaccinia infection. Therefore, we asked if Rag2−/− 8.8 mice were susceptible to autoimmune disease induced by wild-type vaccinia infection. Wild-type splenocytes were transferred into Rag2−/− 8.8 mice prior to infection with wild-type vaccinia virus and the recipients were monitored for clinical signs. While all control Rag2+/+ 8.8 mice developed autoimmune disease, none of the Rag2−/− 8.8 recipients developed disease (Fig. 4a and Supplementary Table 1). Consistent with the lack of disease induction, Rag2−/− 8.8 T cells did not acquire effector function as a result of wild-type vaccinia infection as MBP-pulsed splenocytes were lysed only after transfer into Rag2+/+ and not Rag2−/− 8.8 infected recipients (Fig. 4b). This result, together with the fact that the Rag2−/− 8.8 mice contained non-transgenic T and B cells prior to infection, implicates an intrinsic difference in Rag2−/− versus Rag2+/+ 8.8 T cells in conferring susceptibility to virus-induced autoimmunity.
Figure 4.
Activation of Rag2+/+ 8.8 T cells by wild-type vaccinia virus requires expression of endogenous TCR chains. (a) Splenocytes (2.5 × 106) from wild-type mice were transferred into Rag2+/+ and Rag2−/− 8.8 mice (five mice/group) and the mice were infected with wild-type vaccinia virus two weeks later. There is a significant difference in weight loss (P <0.0001) between infected Rag2+/+ compared to Rag−/− 8.8 mice. Error bars represent s.e.m. (b) The ability of infected Rag2+/+ and Rag2−/− 8.8 mice to specifically lyse MBP peptide-pulsed splenocytes in vivo was assessed as described Fig. 1c. Data are representative of four experiments.
Peripheral T cells in Rag2+/+ 8.8 mice are skewed toward the CD8+ subset, however, some CD4+ Vα8+Vβ8+ T cells also develop. In contrast, T cells in Rag2−/− 8.8 mice are all CD4−CD8+. To determine if the inability of wild-type vaccinia virus to induce autoimmunity in Rag2−/− 8.8 mice was due to the loss of CD4+ 8.8 T cells, Rag2+/+ 8.8 mice were treated with anti-CD4 depleting antibody prior to vaccinia virus induction. CD4+ T cell-depleted 8.8 mice exhibited a delayed onset and somewhat milder disease, but the incidence of autoimmunity was not decreased by the lack of CD4+ T cells (Supplementary Fig. 4a and Supplementary Table 1). The decrease in disease severity in CD4+ T cell-depleted vaccinia-infected 8.8 mice could be due either to a loss of pathogenic CD4+ 8.8 T cells or to a loss of help provided by CD4+ T cells to activated CD8+ 8.8 T cells. The later possibility is supported by the fact that adoptive transfer of 8.8 T cells that were stimulated with MBP peptide in vitro induced autoimmunity only when interleukin 2 (IL-2) was administered following T cell transfer (unpublished results). To determine if CD4+ 8.8 T cells were pathogenic, CD4+ and CD8+ T cells were purified from Rag2+/+ 8.8 mice, stimulated in vitro with MBP peptide and adoptively transferred into naïve recipients accompanied by injections of IL-2. While all CD8+ 8.8 T cell recipients succumbed to autoimmunity, none of the CD4+ 8.8 T cell recipients exhibited weight loss or clinical signs (Supplementary Fig. 4b). These results indicate that the ability of vaccinia virus to induce disease in Rag2+/+ 8.8 mice does not depend on the presence of CD4+ 8.8 T cells.
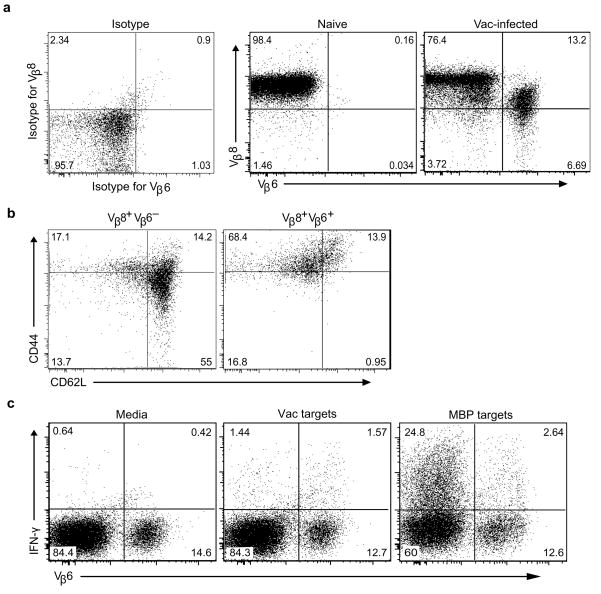
The other major difference between Rag2+/+ and Rag2−/− 8.8 T cells is the potential for Rag2+/+ 8.8 T cells to express endogenous TCR chains because of the incomplete allelic exclusion of Tcra and Tcrb gene rearrangements. Indeed, expression of dual TCRs containing endogenous TCR β-chains paired with transgenic TCR α-chains or endogenous TCR α-chains paired with transgenic TCR β-chains has been observed on peripheral T cells in several TCR transgenic models15–18. The possibility that expression of endogenous TCR chains on 8.8 T cells is required for susceptibility to wild-type virus-induced autoimmune disease suggested the hypothesis that vaccinia virus breaks 8.8 T cell tolerance by triggering T cell activation via a virus-specific TCR co-expressed with the MBP-specific TCR on 8.8 T cells. If this mechanism is correct, CD8+ T cells co-expressing the 8.8 TCR with particular endogenous TCR α- and/or β-chains that confer specificity to viral antigens should be enriched in infected compared to uninfected 8.8 mice. To test this hypothesis, we compared the expression of a panel of TCR Vβ chains (see Methods) on CD8+ T cells isolated from naive versus wild-type vaccinia-infected Rag2+/+ 8.8 mice. While none of the antibodies specific for endogenous Vβ chains detected populations >1% of CD8+ T cells in uninfected 8.8 mice (data not shown), T cells co-expressing Vβ8 and Vβ6 expanded following vaccinia infection (Fig. 5a). The absolute number of Vβ8+Vβ6+ T cells in the spleen increased >40-fold in infected (n = 4) compared to naïve (n = 8) 8.8 mice (P = 0.004). In contrast to Vβ8hiVβ6− T cells, Vβ8+Vβ6+ T cells exhibited an activated phenotype in infected mice (Fig. 5b). To investigate the antigen specificity associated with Vβ6 expression, T cells isolated from vaccinia-infected 8.8 mice were restimulated in vitro with vaccinia-infected splenocytes or MBP-pulsed splenocytes and analyzed for IFN-γ production and Vβ6 expression. More than 50% of the T cells that produced IFN-γ in response to wild-type vaccinia-infected splenocytes expressed Vβ6, while only 9.6% of T cells that produced IFN-γ in response to MBP-pulsed splenocytes were Vβ6+, indicating that co-expression of Vβ6+ preferentially conferred specificity for viral antigens (Fig. 5c). To determine if 8.8 T cells co-expressing particular Vα chains also expanded in response to vaccinia infection, T cells exhibiting either a naïve or activated phenotype were sorted from infected 8.8 mice and expression of different Vα chains was analyzed by real-time PCR. Expression of Vα11, 13 and 14 was enriched in activated compared to naïve T cells isolated from infected mice (Supplementary Fig. 5). Together these data indicate that T cells co-expressing the 8.8 TCR and specific endogenous TCR chains are expanded in response to vaccinia infection and activation of these T cells correlates with loss of 8.8 T cell tolerance and induction of autoimmune disease. This mechanism predicts that other viral infections would trigger autoimmunity in 8.8 mice, which is supported by our finding that adenovirus also induces autoimmunity in Rag2+/+ 8.8 mice (Supplementary Table 1).
Figure 5.
Wild-type vaccinia infection of 8.8 mice preferentially expands CD8+Vβ6+Vβ8 T cells that respond to vaccinia virus epitopes. (a) Splenocytes from Rag2+/+ 8.8 mice were harvested seven days after wild-type vaccinia virus infection and stained with antibodies specific for CD8, Thy1.2, Vβ6, Vβ8, CD44 and CD62L. Splenocytes from naïve 8.8 mice were stained with antibodies specific for CD8, Thy1.2, Vβ8 and Vβ6. Flow cytometry analyses show expression of Vβ6 and Vβ8 on CD8+-gated cells. Data are representative of five experiments with more than 10 mice. (b) CD44 and CD62L expression is shown for the CD8+Vβ6−Vβ8+ and CD8+Vβ6+Vβ8+ cells identified in vaccinia-infected mice depicted in (a). (c) Splenocytes from the infected mice in (a) were cultured in vitro overnight with either unmanipulated, vaccinia-infected or MBP peptide-pulsed splenocytes from Thy1.1 C3HeB/Fej mice. Cells were then analyzed for CD8, Thy1.2, Vβ6, Vβ8 and IFN-γ expression. The data shown is gated on CD8+Thy1.2+ cells and are representative of three experiments.
Vac-MBP activates 8.8 T cells via the MBP-specific TCR
Our experiments administering MBP peptide, LPS and anti-CD40 in vivo demonstrated that the 8.8 T cells can be activated via the MBP-specific TCR if the APCs are strongly activated and the dose of MBP increases above endogenous amounts. Therefore, we asked whether infection with Vac-MBP could induce disease in Rag2−/− 8.8 mice. Rag2−/− 8.8 T cells proliferated in response to Vac-MBP-infected, but not Vac-infected, cells in vivo (Fig. 6b). In contrast to our results with wild-type vaccinia infection, Rag2−/− 8.8 mice that received wild-type splenocytes needed to clear the virus succumbed to autoimmune disease following Vac-MBP infection (Fig. 6b and Supplementary Table 1). This result demonstrates that viral infection can break 8.8 T cell tolerance via signaling through the MBP-specific TCR if the infection causes both increased expression of MBP and widespread APC activation.
Figure 6.
Vac-MBP but not wild-type vaccinia virus activates Rag2−/− 8.8 T cells to induce autoimmunity. (a) CFSE-labeled Rag2−/− 8.8 splenocytes (2 × 106) were transferred into Mbp−/− mice that were either uninfected or infected one day earlier with either wild-type vaccinia or Vac-MBP virus. Splenocytes were harvested three days later and stained with anti-CD8, Thy1.2, Vα8 and Vβ8 antibodies. Dilution of CFSE is shown for CD8+Thy1.2+Vα8+Vβ8+ cells (black line) and overlaid with that from non-infected mice (gray line). (b) Rag2+/+ 8.8 or Rag2−/− 8.8 mice were reconstituted with 2.5 × 106 splenocytes from naïve wild-type mice and infected with Vac-MBP virus two weeks later. Weight loss was monitored as described in Fig. 1a. Numbers in parentheses represent disease incidence of total mice. Data are representative of two experiments. Error bars represent s.e.m.
Discussion
In the current study, we demonstrate that viral infection triggers CD8+ T cell-mediated autoimmune disease in the CNS by two distinct mechanisms. Infection with Vac-MBP induces disease via a “molecular identity” mechanism in which the virus encodes an epitope recognized directly by the MBP-specific TCR. In contrast, infection with wild-type vaccinia virus breaks tolerance in T cells that express dual TCRs due to incomplete allelic exclusion of the Tcra or Tcrb loci. Activation via a co-expressed virus-specific TCR overcomes the lack of response of the MBP-specific 8.8 TCR to endogenous MBP such that the T cell is able to respond to both the viral epitope and endogenous MBP.
Viral infection has long been postulated to be an environmental factor contributing to the etiology of MS. Although several different viruses have been implicated over the years19, 20, no specific virus has been confirmed as a causative agent in the pathogenesis MS. Because MS is a multifactorial disease5, it is possible that multiple viruses influence MS susceptibility, and the ability of any particular virus to contribute to the pathogenesis of MS may be dependent on the repertoire of susceptibility alleles each individual carries and their exposure to other pre-disposing environmental factors. Alternatively, MS may be triggered by a common infection that initiates disease in only a small fraction of infected people, as suggested by the geographical distribution of MS and the change in risk observed in migrants. In particular, a large body of evidence accumulated over the past two decades strongly implicates the human herpes Epstein-Barr virus (EBV) as a risk factor in the development of MS, operating independently of the risk contributed by the MHC DR15 allele20–23. Data from many studies show that the risk of developing MS is 15 times higher in EBV-positive compared to EBV-negative individuals and is two-threefold higher in individuals with a history if infectious mononucleosis compared to individuals that experienced asymptomatic infection24, 25.
Animal models of MS induced by viral infection have identified some mechanisms by which viruses could trigger CNS autoimmunity. Murine hepatitis virus (MHV) infection induces chronic, demyelinating disease that depends only on the activity of virus-specific T cells rather than the emergence of myelin-specific T cells during the course of infection26. In contrast, TMEV induces CNS autoimmune disease in susceptible mouse strains via bystander activation of myelin antigen-specific CD4+ T cells. Bystander activation is facilitated by myelin damage that occurs during the initial clearance of virus by CD8+ T cells, resulting in presentation of myelin epitopes by APCs to CD4+ myelin antigen-specific T cells that were non-specifically recruited to the CNS. This phenomenon of epitope spreading from viral antigen-specific CD8+ T cells to self-reactive, myelin-specific CD4+ T cells results in a chronic disease resembling MS27. A different mechanism has been demonstrated for recombinant TMEVs encoding either a peptide derived from Haemophilus influenzae sharing six of 13 amino acids with a peptide from proteolipoprotein (PLP)139–151, or a peptide derived from MHV sharing only three amino acids with PLP139-151 (refs. 28, 29). In these cases, the molecular mimicry between H. influenzae or MHV peptide and PLP peptide is sufficient to prime CD4+ PLP139-151-specific T cells, which then initiate chronic disease. Autoreactive CD8+ T cell clones have also been isolated from mice infected with the DA strain of TMEV, and these clones induce CNS pathology upon adoptive transfer into uninfected mice30. However, the self-antigen recognized by these CD8+ T cell clones has not been identified. Infection with Semliki Forest virus (SFV) also induces an inflammatory, demyelinating disease in the CNS in which both virus-specific T cells and antibodies are generated that cross-react with myelin epitopes31.
In contrast to these models, our studies revealed mechanisms by which viral infection breaks tolerance directly in CD8+ MBP-specific T cells. Vac-MBP triggers autoimmunity in Rag2−/− 8.8 mice by a mechanism analogous to molecular mimicry in that an epitope encoded by the virus is specifically recognized by the MBP-specific TCR. The context of viral infection was important to break the tolerance that is normally maintained in vivo when the 8.8 TCR engages endogenous MBP ligand as immunization with MBP79-87 in CFA was not sufficient to induce disease in 8.8 mice. This result differs from findings in a CD8+ PLP-specific TCR transgenic mice in which neurological signs could be induced by immunization with PLP peptide14. The clinical signs induced by PLP immunization were very mild and CD4+ T cell activity was required for further disease progression and relapses. The differences in the requirement for CD4+ T cells and the severity of disease seen in this model compared to our model may reflect differences in the tolerance mechanisms that allow the PLP-specific and the MBP-specific 8.8 T cells to circulate in the periphery as naïve T cells. Furthermore, CD4+ T cell help is not required to activate naïve CD8+ T cells during a viral infection. Administration of LPS and agonistic anti-CD40 also did not induce disease in 8.8 mice, even though this method of activating APCs presenting endogenous MBP breaks tolerance in some CD4+ MBP-specific TCR transgenic T cells32. Mild disease was induced by co-administration of MBP peptide with LPS and agonistic anti-CD40, indicating that the epitope stripping that normally maintains 8.8 T cell tolerance can be overcome when there is both wide-spread activation of APCs and an increase in the concentration of the MBP ligand. These conditions are achieved more efficiently with Vac-MBP infection, accounting for the stronger ability of Vac-MBP infection to induce disease in 8.8 mice compared to co-administration of MBP peptide with LPS plus the CD40 antibody.
Unexpectedly, we found that infection of Rag2+/+ 8.8 mice with wild-type vaccinia virus also induced autoimmunity. In contrast to TMEV, this viral infection does not induce CNS autoimmunity by bystander activation or by molecular mimicry. Importantly, wild-type vaccinia-infected Rag2−/− 8.8 mice were not susceptible to autoimmune disease. The lack of disease in Rag2−/− 8.8 mice does not reflect a requirement for CD4+ 8.8 T cells that are eliminated on the Rag2−/− background. Instead, our data show that expression of endogenous TCR chains on 8.8 T cells were required for wild-type vaccinia virus to induce disease in 8.8 mice. Consistent with the hypothesis that 8.8 T cells are activated via a viral antigen-specific TCR that is co-expressed with the 8.8 TCR, a population of T cells co-expressing the 8.8 TCR and Vβ6 expanded following vaccinia virus infection and this population was specifically enriched in T cells that respond to viral antigens. CD4+Vβ6+ T cells did not expand following infection of 8.8 mice (data not shown), indicating that the virus does not function as a superantigen that activates T cells expressing particular Vβ chains independent of antigen-specificity. T cells expressing Vα11, 13 and 14 were also enriched in activated compared to non-activated T cells following vaccinia infection. We conclude from these data that wild-type vaccinia virus breaks 8.8 T cell tolerance by triggering T cell activation via virus-specific TCRs that are co-expressed with the 8.8 MBP-specific TCR.
The ability to activate 8.8 T cells via a second TCR indicates that the effector functions of 8.8 T cells are intact despite their lack of response to endogenous MBP. Similarly, in mice engineered to express two transgenic TCRs, one of which induced strong anergy in vivo upon interaction with a neo-self antigen, stimulation via the second transgenic TCR activated the anergic T cells33. Our model differs from this in that the 8.8 T cells are specific for a bona fide self-antigen implicated in MS pathogenesis and are not subjected to clonal deletion or anergy in vivo11.
Although our model utilizes TCR transgenic mice, T cells expressing dual TCRs exist in both mice and humans. Dual TCR T cells usually express one TCR β chain and two α chains because allelic exclusion is less complete for the Tcra loci. In mice, the percentage of T cells reported to express two TCR α chains varies from 2–15%34–36, and a 33% frequency has been reported for humans37. The frequency of T cells expressing two TCR β chains was estimated to be ~1% in humans and 3% in mice, with the frequency increasing with age16, 38, 39. While our data do not distinguish the specific pairing of TCR chains comprising virus-specific TCRs in 8.8 mice, it is more likely that a second TCR is generated in 8.8 T cells by pairing an endogenous α-chain with the transgenic β-chain or an endogenous β-chain paired with the transgenic α-chain rather than via a failure of allelic exclusion at both the Tcra and Tcrb loci in individual transgenic T cells.
Peripheral T cells expressing dual TCRs have been shown to be beneficial by expanding the repertoire of T cells that respond to foreign antigens40. Since the first demonstration of T cells expressing two α-chains on the cell surface; however, most studies focused on the hypothesis that expression of dual TCRs may promote autoimmunity and alloreactivity18, 41. Support for a significant contribution to alloreactivity was recently provided by studies demonstrating that dual TCR T cells play a dominant role in graft-versus-host disease42. In contrast, studies using animal models of autoimmune disease including collagen-induced arthritis, EAE and diabetes failed to show any role for dual TCR T cells in autoimmunity35, 43, 44. We previously showed that the frequency of spontaneous EAE in CD4+ MBP-specific TCR transgenic mice increased when the level of microbial exposure in the environment increased12, 45; however, a role for dual TCRs responding to environmental antigens could not be established in this model because the loss of regulatory T cells on the Rag2−/− background strongly enhances the incidence of spontaneous EAE46. Thus, to our knowledge, the studies reported here are the first to reveal a mechanism for triggering autoimmune disease that depends on expression of dual TCRs.
These findings suggest a new perspective on the proposed virally induced etiology of MS that is consistent with the inability to detect infectious virus in the CNS. As the frequency of T cells co-expressing a myelin-specific and a virus-specific TCR in the peripheral T cell repertoire should be low, and will likely vary among individuals, this mechanism may represent one way by which a common infection can trigger autoimmunity in a small subset of genetically predisposed individuals. The cumulative data regarding a connection between EBV infection and MS is consistent with this hypothesis. Despite the association of MS with increasing serum titers of EBNA-1 antibodies22 and with increased frequency of EBV-specific CD4+ and CD8+ T cells21}, the evidence for association of lytic EBV replication with MS is controversial. A recent study reported an almost 100% incidence of EBV infection in CNS B cells in MS patients, as well as viral reactivation in B cells present in CNS follicles accompanied by accumulation of activated CD8+ T cells47, implicating reactivation of EBV as a key factor in MS pathogenesis. However, the detection of EBV DNA in the CNS of MS patients was not reproduced in another study using highly sensitive techniques48, suggesting that the function of EBV as a risk factor for MS is not dependent on reactivation of the virus during autoimmune disease. This notion is consistent with our conclusion that viral infection can activate T cells expressing dual TCRs, one specific for a viral epitope and one for a myelin epitope, that then drive the autoimmune process independent of an ongoing immune response against the pathogen. The low probability of this event may account not only for the low incidence of autoimmunity among individuals infected with one common pathogen, but for the elevated antibody titers in MS patients for several other common viruses21.
Methods
Mice
MBP79-87-specific TCR transgenic 8.8 and 8.6 mice on the Mbp+/+, Mbp−/− and Rag2−/− background have been previously described11. Thy1.1 C3HeB/FeJ mice were generated by backcrossing the Thy1.1 allele onto the C3HeB/FeJ background for twelve generations. All mice were bred and maintained in a specific pathogen-free facility at the University of Washington (Seattle, Washington). Mice used for EAE induction were female mice between 8–12 weeks old. All procedures have been approved by the Institutional Animal Care and Use Committee at the University of Washington.
EAE induction by vaccinia virus infection
Wild-type (New York City Board of Health (NYCBH)) and recombinant vaccinia virus encoding MBP (Vac-MBP) were obtained from Therion Biologics. Vaccinia viruses were grown in HeLa cells and titered in BSC-40 cells. Mice were injected i.p. with 1–5 × 106 PFU of wild-type or Vac-MBP virus. 8.8 Rag2−/− mice were injected i.v. with 2.5 × 106 naive splenocytes from wild-type mice two weeks prior to viral infection. Mice were weighed daily and sacrificed when they lost more than 20% of their original body weight. Neurological symptoms, such as ataxia, knuckling, hypersensivity or difficulty in walking, usually appeared six days post infection.
Flow cytometry
Splenocytes from naïve mice or mice infected seven days earlier with vaccinia virus, and CNS monocuclear cells were isolated from perfused mice as previously described49 and stained with combinations of antibodies specific for CD8 (clone 53–6.7), Thy1.2 (clone 30-H12), CD44 (clone IM7), CD62L (clone MEL-14), Vβ2 (clone B20.6), Vβ4 (clone KT4), Vβ5 (clone MR9-4), Vβ6 (clone RR4-7), Vβ7 (clone TR310), Vβ8 (clone MR5-2), Vβ9 (clone MR10-2), Vβ10 (clone B21.5), Vβ11 (clone RR3-15), Vβ14 (clone 14-2) (purchased from BD Biosciences) or PE-conjugated H-2Kk/MBP79-87 tetramer. The tetramer was generated in house using a construct encoding Kk provided by the National Institutes of Allergy and Infectious Diseases Tetramer Core Facility and conjugated to phycoerythrin. Cells were analyzed on a FACScan, FACScanto, or LSR II (BD Biosciences).
In vivo CTL killing assay
Splenocytes from wild-type mice were incubated with or without 20 μM MBP79-87 peptide for 1–2 h at 37 °C in complete RPMI 1640 media (HyClone). The splenocytes were then washed with PBS and incubated in 5 μM (peptide-pulsed) or 1.2 μM (non-pulsed) CFSE for 10 min at 37 °C. After washing three times with PBS, 1 × 107 peptide-pulsed and non-pulsed splenocytes were mixed together and i.v. injected into mice. Mice were sacrificed 20 ho later. Spleens were harvested and CFSE-labeled cells were analyzed by flow cytometry.
Intracellular IFN-γ staining
Splenocytes (1 × 106)from infected mice were incubated with 1 × 106 either naïve Thy1.1 splenocytes, naïve Thy1.1 splenocytes plus 5 μM MBP peptide, or Thy1.1 splenocytes infected with vaccinia virus in a 96-well U-shape plate. After incubation overnight at 37 °C, cells were further cultured with Golgi Plug (1 μl/ml, BD Biosciences) for 5 h. The cells were stained for CD8, Thy1.2, Vβ8, and Vβ6 (for Fig. 5 only). Cells were fixed and permeabilized (Cytofix/Cytoperm kit; BD Biosciences) and subsequently stained with anti-IFN-γ (clone XMG1.2) or Rat IgG1 isotype antibody (R3–34) (purchased from BD Biosciences). Samples were washed and fixed in PBS containing1% paraformaldehyde and analyzed on a FACS Calibur or FACS Canto (BD Biosciences).
Quantitative RT-PCR
Total RNA was extracted from sorted cells using the RNeasymini kit (Qiagen), and first-strand cDNA was synthesized using SuperScript II (Invitrogen) according to the manufacturer’s directions. Quantitative PCR was performed on an ABI 7300 Real Time PCR System (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems). Tcra Vα gene primers and a Cα primer have been previously described50. Reactions were run in duplicate, and samples were normalized to the internal β actin control.
Statistical analysis
All P values were calculated with a two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank N. Mausolf for animal husbandry and technical assistance, and S. Lee and E. Pierson for critical comments on the manuscript. This work was supported by the US National Institutes of Health (AI07272737 to J.M.G.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Q.J. conducted most of the experiments and analyzed the data; A.P. performed the initial disease induction experiments and critiqued the manuscript; Q.J. and J.M.G. designed the study and wrote the manuscript, J.M.G. secured the funding.
References
- 1.Goverman J, Perchellet A, Huseby ES. The role of CD8(+) T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy. 2005;4:239–245. doi: 10.2174/1568010053586264. [DOI] [PubMed] [Google Scholar]
- 2.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 3.Crawford MP, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 4.Coles AJ, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 5.De Jager PL, et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41:776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook SD, Dowling PC. Multiple sclerosis and viruses: an overview. Neurology. 1980;30:80–91. doi: 10.1212/wnl.30.7_part_2.80. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cermelli C, Jacobson S. Viruses and multiple sclerosis. Viral Immunol. 2000;13:255–267. doi: 10.1089/08828240050144590. [DOI] [PubMed] [Google Scholar]
- 9.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 11.Perchellet A, Stromnes I, Pang JM, Goverman J. CD8+ T cells maintain tolerance to myelin basic protein by ‘epitope theft’. Nat Immunol. 2004;5:606–614. doi: 10.1038/ni1073. [DOI] [PubMed] [Google Scholar]
- 12.Goverman J, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 13.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 14.Friese MA, et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat Med. 2008;14:1227–1235. doi: 10.1038/nm.1881. [DOI] [PubMed] [Google Scholar]
- 15.Borgulya P, Kishi H, Uematsu Y, von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 16.Balomenos D, et al. Incomplete T cell receptor V beta allelic exclusion and dual V beta-expressing cells. J Immunol. 1995;155:3308–3312. [PubMed] [Google Scholar]
- 17.Hurst SD, Sitterding SM, Ji S, Barrett TA. Functional differentiation of T cells in the intestine of T cell receptor transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3920–3925. doi: 10.1073/pnas.94.8.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath WR, Miller JF. Expression of two alpha chains on the surface of T cells in T cell receptor transgenic mice. J Exp Med. 1993;178:1807–1811. doi: 10.1084/jem.178.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009;22:201–206. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- 21.Pohl D. Epstein-Barr virus and multiple sclerosis. J Neurol Sci. 2009;286:62–64. doi: 10.1016/j.jns.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Ascherio A, Munger KL. Epstein-Barr Virus Infection and Multiple Sclerosis: A Review. J Neuroimmune Pharmacol. 2010 doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 23.De Jager PL, et al. Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology. 2008;70:1113–1118. doi: 10.1212/01.wnl.0000294325.63006.f8. [DOI] [PubMed] [Google Scholar]
- 24.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen TR, et al. Multiple sclerosis after infectious mononucleosis. Arch Neurol. 2007;64:72–75. doi: 10.1001/archneur.64.1.72. [DOI] [PubMed] [Google Scholar]
- 26.Hosking MP, Lane TE. The Biology of Persistent Infection: Inflammation and Demyelination following Murine Coronavirus Infection of the Central Nervous System. Curr Immunol Rev. 2009;5:267–276. doi: 10.2174/157339509789504005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SD, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 28.Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001;108:311–318. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croxford JL, Ercolini AM, Degutes M, Miller SD. Structural requirements for initiation of cross-reactivity and CNS autoimmunity with a PLP139-151 mimic peptide derived from murine hepatitis virus. Eur J Immunol. 2006;36:2671–2680. doi: 10.1002/eji.200635876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsunoda I, Kuang LQ, Kobayashi-Warren M, Fujinami RS. Central nervous system pathology caused by autoreactive CD8+ T-cell clones following virus infection. J Virol. 2005;79:14640–14646. doi: 10.1128/JVI.79.23.14640-14646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokhtarian F, Zhang Z, Shi Y, Gonzales E, Sobel RA. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J Neuroimmunol. 1999;95:43–54. doi: 10.1016/s0165-5728(98)00254-9. [DOI] [PubMed] [Google Scholar]
- 32.Cabbage SE, et al. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J Immunol. 2007;178:887–896. doi: 10.4049/jimmunol.178.2.887. [DOI] [PubMed] [Google Scholar]
- 33.Teague RM, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam SM, Gascoigne NR. Posttranslational regulation of TCR Valpha allelic exclusion during T cell differentiation. J Immunol. 1998;160:3883–3890. [PubMed] [Google Scholar]
- 35.Elliott JI, Altmann DM. Dual T cell receptor alpha chain T cells in autoimmunity. J Exp Med. 1995;182:953–959. doi: 10.1084/jem.182.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heath WR, et al. Expression of two T cell receptor alpha chains on the surface of normal murine T cells. Eur J Immunol. 1995;25:1617–1623. doi: 10.1002/eji.1830250622. [DOI] [PubMed] [Google Scholar]
- 37.Padovan E, et al. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 38.Padovan E, et al. Normal T lymphocytes can express two different T cell receptor beta chains: implications for the mechanism of allelic exclusion. J Exp Med. 1995;181:1587–1591. doi: 10.1084/jem.181.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davodeau F, et al. Dual T cell receptor beta chain expression on human T lymphocytes. J Exp Med. 1995;181:1391–1398. doi: 10.1084/jem.181.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He X, et al. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat Immunol. 2002;3:127–134. doi: 10.1038/ni751. [DOI] [PubMed] [Google Scholar]
- 41.Padovan E, Casorati G, Dellabona P, Giachino C, Lanzavecchia A. Dual receptor T-cells. Implications for alloreactivity and autoimmunity. Ann N Y Acad Sci. 1995;756:66–70. doi: 10.1111/j.1749-6632.1995.tb44482.x. [DOI] [PubMed] [Google Scholar]
- 42.Morris GP, Allen PM. Cutting edge: Highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. J Immunol. 2009;182:6639–6643. doi: 10.4049/jimmunol.0900638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott EA, et al. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J Clin Invest. 1996;98:1602–1612. doi: 10.1172/JCI118954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corthay A, Nandakumar KS, Holmdahl R. Evaluation of the percentage of peripheral T cells with two different T cell receptor alpha-chains and of their potential role in autoimmunity. J Autoimmun. 2001;16:423–429. doi: 10.1006/jaut.2001.0504. [DOI] [PubMed] [Google Scholar]
- 45.Brabb T, et al. Triggers of autoimmune disease in a murine T-cell receptor transgenic model for multiple sclerosis. J Immunol. 1997;159:497–507. [PubMed] [Google Scholar]
- 46.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serafini B, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willis SN, et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain. 2009;132:3318–3328. doi: 10.1093/brain/awp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington CJ, et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.