Abstract
Arsenic trioxide exhibits antiproliferative, antiangiogenic and proapoptotic activity in cancer cells, and many genes associated with these responses are regulated by specificity protein (Sp) transcription factors. Treatment of cancer cells derived from urologic (bladder and prostate) and gastrointestinal (pancreas and colon) tumors with arsenic trioxide demonstrated that these cells exhibited differential responsiveness to the antiproliferative effects of this agent and this paralleled their differential repression of Sp1, Sp3 and Sp4 proteins in the same cell lines. Using arsenic trioxide responsive KU7 and non-responsive 253JB-V bladder cancer cells as models, we show that in KU7 cells, ≤ 5 μM arsenic trioxide decreased Sp1, Sp3 and Sp4 and several Sp-dependent genes and responses including cyclin D1, epidermal growth factor receptor, bcl-2, survivin and vascular endothelial growth factor, whereas at concentrations up to 15 μM, minimal effects were observed in 253JB-V cells. Arsenic trioxide also inhibited tumor growth in athymic mice bearing KU7 cells as xenografts, and expression of Sp1, Sp3 and Sp4 was significantly decreased. Inhibitors of oxidative stress such as glutathione or dithiothreitol protected KU7 cells from arsenic trioxide-induced antiproliferative activity and Sp repression, whereas glutathione depletion sensitized 253JB-V cells to arsenic trioxide. Mechanistic studies suggested that arsenic trioxide-dependent downregulation of Sp and Sp-dependent genes was due to decreased mitochondrial membrane potential and induction of reactive oxygen species, and the role of peroxides in mediating these responses was confirmed using hydrogen peroxide.
Keywords: Arsenic trioxide, anticancer activity, Sp repression, ROS
INTRODUCTION
Arsenical compounds alone or in combination with other agents have been used widely in medicine to treat a variety of conditions and diseases including syphilis, psoriasis, trypanosomiasis, pernicious anemia and Hodgkin’s disease [1–3]. Moreover, until the mid-1990s, arsenic trioxide was used as the major drug for treatment of chronic myeloid leukemia and other leukemias [2,3]. Arsenic trioxide has been associated with adverse side-effects and studies on individuals occupationally or environmentally exposed to high levels of arsenicals exhibit increased incidence of skin cancer [4]. These observations contributed to a temporary decrease in the medicinal use of arsenicals until studies from China reported that arsenic trioxide was remarkably effective for treating patients with acute promyelocytic leukemia (APL) [5–7]. Subsequent clinical studies have confirmed the effectiveness of this compound which is now routinely used for treating APL [2,3]. Based on the success of arsenic trioxide for treating APL, the clinical applications of this drug alone or in combination with other agents for treatment of solid tumors is currently being investigated [2,8,9].
The chemotherapeutic effectiveness of arsenic trioxide is due to modulation of several pathways in cancer cells leading to increased apoptosis, enhanced differentiation, inhibition of cell proliferation, and angiogenesis [2,3]. Most patients with APL overexpress the PML-RARα fusion gene resulting from t(15;17) chromosome translocation and PML-RARα inhibits expression of genes involved in myeloid differentiation [10,11]. Arsenic trioxide induces inactivation or degradation of PML-RARα by multiple pathways resulting in the subsequent activation of myeloid differentiation pathways [12,13]. Arsenic trioxide also targets the mitochondria in cancer cell lines resulting in decreased mitochondrial membrane potential (MMP), induction of reactive oxygen species (ROS), release of cytochrome c, and activation of several cell death pathways [14–18].
Previous reports show that arsenic trioxide decreases expression of genes associated with angiogenesis (VEGF), survival (bcl-2 and NFκB), and cell proliferation (cyclin D1) in cancer cell lines [19–27]. Recent studies in this laboratory show that anticancer drugs such as curcumin, tolfenamic acid and betulinic acid also decrease expression of these same genes [28–32] and this was due, in part, to decreased expression of specificity protein transcription factors (Sp), Sp1, Sp3 and Sp4 which are overexpressed in many tumors [33–37] and bind to GC-rich promoter elements in Sp-regulated genes. These genes and others are also decreased in cancer cells transfected with a mixture (iSp) of small inhibitory RNAs for Sp1, Sp3 and Sp4 [28–32]. Since arsenic trioxide decreases expression of several Sp-dependent genes, we hypothesized that the anticancer activity of this compound may be due, in part, to repression of Sp1, Sp3 and Sp4 transcription factors. Results of this study show that arsenic trioxide does indeed decrease expression of Sp1, Sp3 and Sp4 proteins and Sp-dependent genes in several cancer cell lines derived from solid tumors and, using bladder cancer cells as models, we show that the mechanism of Sp downregulation is related to decreased MMP and induction of ROS. This demonstrates for the first time that mitochondrial-induced ROS leads to repression of Sp1, Sp3 and Sp4 and several Sp-dependent genes and these effects contribute to the anticancer activity of arsenic trioxide.
MATERIALS AND METHODS
Cell lines, reagents and antibodies
KU7 and 253JB-V human bladder cancer cells were provided by Dr. A. Kamat (M.D. Anderson Cancer Center, Houston, TX). LNCaP and PC3 human prostate carcinoma cells were obtained from American Type Culture Collection (Manassas, VA). RKO and SW480 human colon carcinoma cell lines were provided by Dr. Stanley Hamilton (University of Texas M.D. Anderson Cancer Center, Houston, TX). The Panc28 cell line was a generous gift from Dr. Paul Chiao (University of Texas M.D. Anderson Cancer Center, Houston, TX). L3.6pL cell line was developed at the M.D. Anderson Cancer Center (Houston, TX) and kindly provided by Dr. I.J. Fidler. SW480, L3.6pL, RKO and Panc28 cells were maintained in Dulbecco’s modified/Ham’s F-12 (Sigma-Aldrich, St. Louis, MO) with phenol red supplemented with 0.22% sodium bicarbonate, 5% fetal bovine serum and 10 ml/L 100x antibiotic anti-mycotic solution (Sigma). 253JB-V, KU7, LNCaP and PC3 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 0.15% sodium bicarbonate, 0.011% sodium pyruvate, 0.24% Hepes and 10 ml/L of antibiotic/antimycotic cocktail solution. The cells were grown in 150 cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C and passaged approximately every 3–5 days. With the exception of cleaved poly (ADP) ribose polymerase (PARP) (Cell Signaling Technology, Danvers, MA), Sp1 (Millipore, Temecula, CA), survivin (R&D Systems, Minneapolis, MN), p65 (Abcam,Cambridge, MA) and β-actin antibodies (Sigma-Aldrich), all remaining antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Diethyl maleate (DEM), glutathione, 98% (γ-glu-cys-Gly, GSH), butylated hydroxyanisole (BHA), proline, N-acetylcysteine (NAC), catalase, and arsenic trioxide (99.995% pure) were purchased from Sigma-Aldrich. Dithiothreitol (DTT, 98%) was obtained from Boehringer Mannheim Corp, (Indianpolis, IN).
Cell proliferation assays
All the above-mentioned cancer cell lines were plated (3 × 104 per well) using DMEM:Ham’s F-12 medium containing 2.5% charcoal-stripped FBS in 12-well plates and left to attach for 24 hr. Cells were then treated with either vehicle or the indicated concentrations of arsenic trioxide. After 24 hr of treatment, cells were counted using a Coulter Z1 particle counter. RNA interference studies using a small inhibitory RNA for Sp1 as a prototype was carried out essentially as described [28,30]. Each experiment was done in triplicate and results are expressed as means ± SE for each determination.
Western blot assays
All the above-mentioned cancer cells were seeded in DMEM:Ham’s F-12 medium containing 2.5% charcoal-stripped FBS. Twenty-four hours later, cells were treated with either vehicle or the indicated compounds for 24 hr and western blot analysis was determined. The tumor tissues from the KU7 bladder cancer xenograft study were also processed similarly and probed for proteins of interest and β-actin served as loading control. Pretreatment with diethyl maleate for 60 min was carried out to deplete the GSH levels, and 253JB-V cells were then treated with solvent control or arsenic trioxide. Cells were also co-treated with DTT and glutathione (GSH) in the presence or absence of arsenic trioxide for a time interval of 24 hr. Protein quantification used Image J software, and optical densities for each protein were normalized to β-actin and the solvent control group. For the effects of multiple inhibitors on arsenic trioxide-dependent downregulation of Sp proteins, we used concentrations used in comparable studies or the highest concentrations that were not cytotoxic. Nuclear extracts were obtained using the NE-PER kit (Pierce, Rockford, IL).
Terminal deoxyribonucleotide transferase mediated nick-end labeling (TUNEL) assay
253JB-V and KU7 cells (7 × 104) were seeded in four-chambered glass slides and left overnight to attach. After treatment with indicated compounds for 18 hr, the in situ cell death detection POD kit was used for the terminal deoxyribonucleotide transferase-mediated nick-end labeling (TUNEL) assay according to the instructions in the protocol manual for fixed cells. The percentage of apoptotic cells was calculated by counting the stained cells in eight fields, each containing 50 cells. The total number of apoptotic cells was plotted as a percentage in both cell lines.
Xenograft tumor study
Athymic female nude mice, age 3 to 5 weeks, were purchased from Harlan Laboratories (Indianapolis, IN). KU7 cells (1 × 106) in 1:1 ratio of Matrigel (BD Biosciences, San Jose, CA) were injected into both the sides of the flank area of nude mice. A week after the tumor cell inoculation, mice were divided into two groups of six animals each. The first group received 100 μL of vehicle (PBS/KOH) by i.p. injection, and the second group of animals received 5 mg/kg/d injection of arsenic trioxide in PBS/KOH every other day for 24 days (12 doses) by i.p. injection. Mice were weighed, and tumor areas were measured throughout the study. After 24 days, the animals were sacrificed; final body and tumor weights were determined and plotted. At the end of the experiment, major visceral organs were collected and analyzed for Sp protein expression levels using western blotting as described earlier.
GSH estimation
The Stallion Imaging workstation, equipped with a Zeiss Axiovert 200M microscope (Carl Zeiss Microimaging, Thornwood, NY) and slidebook software (Intelligent Imaging Innovations Inc., Denver, Co), was used with a 20X objective 0.75NA for acquiring images. Cellular GSH levels were evaluated with the cell permeant probe mBCl. mBCl is nonfluorescent, but forms a fluorescent adduct with GSH in a reaction catalyzed by glutathione S-transferase. Following 20–22 hr treatment, kinetic analysis of mBCl-GSH conjugation was performed at room temperature by exciting the cells at 365 nm wavelength and recording changes in fluorescence intensity with a BP 445/50 nm filter at 1-min intervals for up to 15 min. GSH level per cell was then determined by applying non linear regression analysis to acquired data. Two experiments were preformed on different days. At least 50 cells per treatment group were collected in these studies.
ROS estimation
Cellular ROS levels were evaluated with the cell permeable probe CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′ dichlorodihydrofluorescein diacetate acetyl ester). CM-H2DCFDA is nonfluorescent until removal of the acetate groups by intracellular esterases and oxidation occurs within the cell. Following 20–24 hr treatment, cells plated on 96 well cell culture plate were loaded with 10 μM CM-H2DCFDA for 30 min, washed once with serum free medium, and analyzed for ROS levels using the BioTek Synergy 4 plate reader (BioTek Instruments, Inc., Winooski, VT) set at 480 nm and 525 nm excitation and emission wavelengths respectively. Following reading of ROS, cultures were washed twice with PBS and fixed with methanol for 3 min at room temperature. Methanol was then completely removed and 1 mg/ml Janus green was added to the cultures for 3 min. Following removal of Janus green, cultures were washed twice with PBS and 100 μl of 50% methanol was added to each well. Cell counts were then determined with the plate reader set to an absorbance of 654 nm and ROS intensities were then corrected accordingly. Two experiments were preformed on different days. At least 16 wells per treatment were analyzed for each experiment.
Measurement of MMP
The MMP was measured with Mitochondrial Membrane Potential Detection Kit (Stratagene, Cedar Creek, TX). Briefly, cells were seeded on Lab-Tek Coverglass system (NUNC, NY) and treated with compounds alone or with inhibitors for 24 hr. They were then incubated with 1X JC-1 cationic dye at 37°C for 30 min according to the manufacturer’s instruction. After washing with 1X JC-1 assay buffer twice, cells were subjected to microscopic analysis using a confocal instrument (Zeiss LSM510, Germany). The mitochondrial potential shift was also measured by flow cytometry analysis (Beckman Coulter, Miami, FL). Cells were seeded in cell culture plates and treated with indicated compounds for 24 hr. They were then incubated with JC-1 dye for a further 30 min. After washing with JC-1 assay buffer twice, cells were trypsinized and suspended in cell culture medium. J-aggregates are detected as red fluorescence and J-monomers are detected as green fluorescence.
Quantitative real-time PCR of mRNA
cDNA was prepared from KU7 cell lines using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Each polymerase chain reaction (PCR) was carried out in triplicate in a 20-μl volume using SYBR GreenER (Invitrogen) at 95°C for 10 min, then 40 cycles of 95°C for 15 s and 60°C for 1 in the Applied Biosystems 7500 Fast Real-time PCR System. The primers for all genes have previously been described [37].
Statistical analysis
Statistical significance of differences was determined by an analysis of variance and student t-test, and the levels of probability were noted. IC50 values were calculated using non-linear regression analysis and expressed in μM, at 95% confidence intervals.
RESULTS
Arsenic trioxide inhibits cancer cell and tumor growth and decreases Sp1, Sp3 and Sp4 proteins
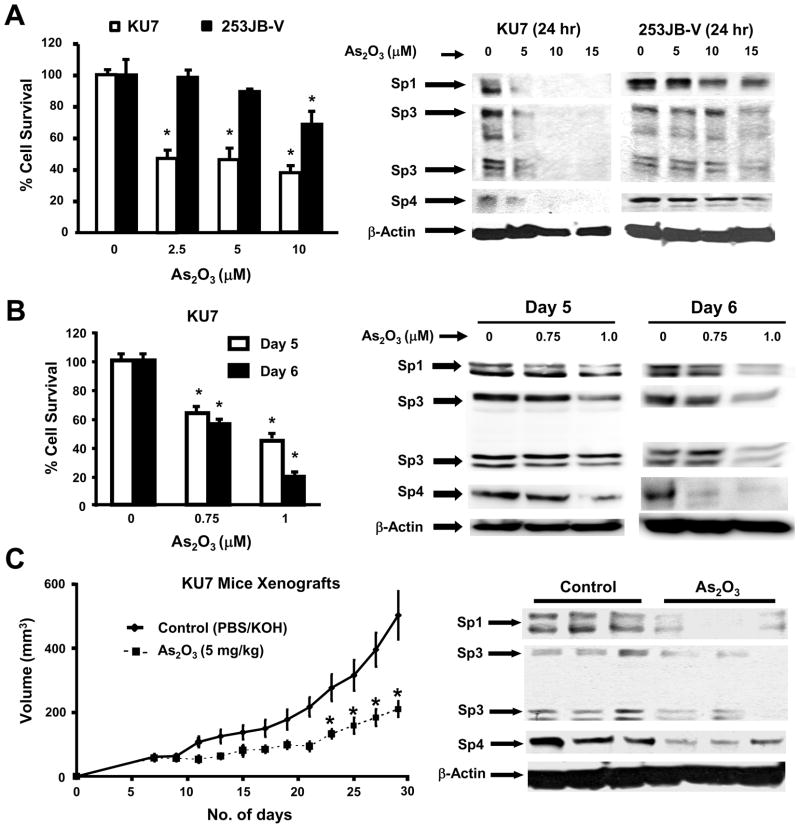
KU7 and 253JB-V bladder cancer cells were used as models for investigating the molecular mechanisms associated with the anticarcinogenic activity of arsenic trioxide and Figure 1A shows that treatment of KU7 cells with 2.5, 5.0 and 10 μM arsenic trioxide for 24 hr resulted in decreased proliferation with an IC50 value of 2.3 μM. In contrast, 253JB-V cells were more resistant to the growth inhibitory effects of arsenic trioxide with an IC50 value of 14.1 μM after treatment for 24 hr. IC50 values after treatment of KU7 and 253JB-V cells for 72 hr were 1.4 and 5.1 μM, respectively (data not shown). The anticancer activity of arsenic trioxide in different cancer cell lines is accompanied by decreased expression of genes/responses such as bcl-2, VEGF and angiogenesis, cyclin D1, and NFκB [19–27]. RNA interference studies in this laboratory show that basal expression of these genes/responses is dependent on Sp transcription factors [28–32,38–40], and we hypothesized that the anticancer activity of arsenic trioxide may be due, in part, to downregulation of Sp1, Sp3 and Sp4. Figure 1A shows that arsenic trioxide induces a concentration-dependent downregulation of Sp1, Sp3 and Sp4 proteins in KU7 cells which are also sensitive to the growth inhibitory effects of this compound. In contrast, arsenic trioxide only minimally decreased Sp1, Sp3 and Sp4 in 253JB-V cells after treatment for 24 hr and there was a correlation between resistance of this cell line to arsenic trioxide-induced Sp degradation and growth inhibition. Treatment of 253JB-V cells with arsenic trioxide for 72 hr resulted in more pronounced repression of Sp1, Sp3 and Sp4 (data not shown). Previous studies reported that treatment of NB4 leukemia cells for 10 days with 0.75 μM arsenic trioxide resulted in oxidation of Sp1 but changes in Sp1 protein levels were not observed [41]. Figure 1B shows that treatment of KU7 cells with 1.0 μM arsenic trioxide decreased both cell growth and expression of Sp1, Sp3 and Sp4 proteins. This corresponded with the 24 hr studies (Fig. 1A) which required higher concentrations of arsenic trioxide to inhibit growth and induce downregulation of Sp proteins. However, levels of Sp1, Sp3 and Sp4 were not affected by 0.75 μM arsenic trioxide after treatment for 5 days and, after 6 days, only Sp4 and Sp1 (slight decrease) but not Sp3 expression was changed. These results indicate that growth inhibition by arsenic trioxide was also Sp-independent.
Fig. 1.
Arsenic trioxide inhibits bladder cancer cell and tumor growth and downregulates Sp1, Sp3 and Sp4. [A] Inhibition of cell growth and Sp downregulation. Cells were treated with arsenic trioxide for 24 hr and cell growth and Sp proteins were determined as outlined in the Materials and Methods. [B] Prolonged treatment of KU7 cells. Cells were treated with 0.75 or 1.0 μM arsenic trioxide for 120 or 144 hr and cell proliferation and expression of Sp1, Sp3 and Sp4 proteins were determined by cell counting and western blots as described in the Materials and Methods. High and low molecular weight forms of Sp3 protein were detected as previously described in other cancer cell lines [28,34–37,39,40]. [C] Arsenic trioxide inhibits bladder tumor growth and downregulates Sp proteins. Athymic nude mice were treated with PBS/KOH (solvent control) or arsenic trioxide (5 mg/kg/day) in PBS/KOH for 24 days. Tumor volumes were determined every second day and tumor weights were determined at sacrifice as described in the Materials and Methods. Expression of Sp proteins was determined in triplicate (3 animals/group) by western blot analysis of tumor lysates as described in the Materials and Methods, and significant (p < 0.05) decreases in Sp1, Sp3 and Sp4 proteins were observed in the arsenic trioxide-treated tumors.
The effectiveness of arsenic trioxide as a tumor growth inhibitor was investigated in athymic nude mice bearing KU7 bladder cancer cells as xenografts. After the initial appearance of palpable tumors, mice were treated with buffered arsenic trioxide (5 mg/kg/d) or buffer alone, and tumor volumes were measured over the 24 day treatment period. The results (Fig. 1C) show that arsenic trioxide significantly inhibited tumor volume, and tumor weights were also significantly decreased in the arsenic trioxide-treated animals. Lysates from tumors of treated and control mice were analyzed by western blots and there was a significant (p < 0.05) decrease in expression of Sp1, Sp3 and Sp4 proteins in tumors from arsenic trioxide-treated animals (Fig. 1C) and this has previously been observed with other anticancer agents that also decrease Sp1, Sp3 and Sp4 expression in cancer cell culture and tumors in athymic nude mice [28,38,40]. We also examined Sp1, Sp3 and Sp4 protein expression in the gastrointestinal tract and liver by western blots and low to non-detectable levels were detected (data not shown).
The comparative effects of arsenic trioxide on growth inhibition and downregulation of Sp proteins was also examined in pancreatic (L3.6pL and Panc28), colon (SW480 and RKO), and prostate (LNCaP and PC3) cancer cell lines (Suppement Fig. 1). L3.6pL and Panc28 exhibited differential arsenic trioxide responsiveness as observed for KU7 and 253JB-V cells, whereas in the other four cell lines, there was a similar concentration-dependent decrease in growth inhibition and Sp1, Sp3 and Sp4 proteins. These results suggest that arsenic trioxide induces downregulation Sp transcription factors in multiple cancer cell lines and tumors and this effect may contribute to the anticancer activity of this compound.
Arsenic trioxide decreases Sp-dependent genes and responses
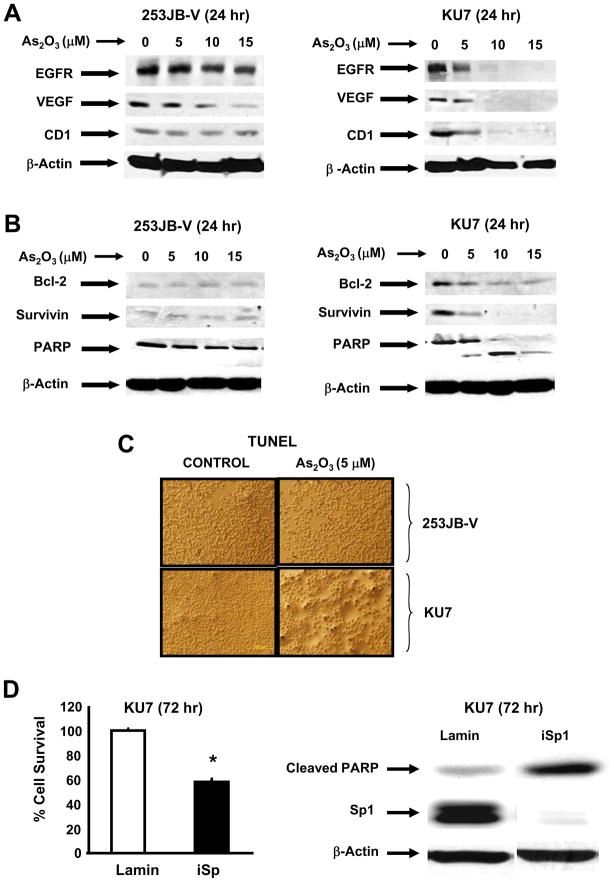
Previous RNA interference studies in bladder cancer cells demonstrated that several genes associated with cell proliferation (EGFR, CD1), survival (survivin and bcl-2), and angiogenesis (VEGF) were regulated by Sp1, Sp3 and Sp4 [28]. Results in Figures 2A and 2B confirm that arsenic trioxide decreased expression of these proteins in KU7 cells. In contrast, arsenic trioxide-dependent downregulation of these proteins was minimal in 253JB-V cells after treatment for 24 hr and there was a correlation between responsiveness to arsenic trioxide-induced downregulation of Sp1, Sp3 and Sp4 and the parallel decrease of Sp-dependent genes in the two bladder cancer cell lines. In addition, the relative responsiveness of KU7 vs. 253JB-V cells to arsenic trioxide was also observed with respect to induction of PARP cleavage (Fig. 2B) and in the apoptotic TUNEL assay where increased TUNEL staining was observed for KU7 but not 253JB-V cells (Fig. 2C). Confirmation that downregulation of Sp proteins by arsenic trioxide contributes to the growth inhibitory and proapoptotic effects of this compound was determined in KU7 cells transfected with a small inhibitory RNA for Sp1 (iSp1) as a prototype for the Sp proteins. Results in Figure 2D confirm that knockdown of Sp1 resulted in inhibition of KU7 cell growth and induction of caspase-dependent PARP cleavage. Thus, downregulation of Sp proteins resulted in growth inhibition and apoptosis which was comparable to the effects of arsenic trioxide in this cell line (Figs. 1B, 2B and 2C). Attempts to reverse the effects of arsenic trioxide on Sp proteins by overexpression of Sp1 (as a model) were unsuccessful (data not shown). This may be due to the sensitivity of cells to levels of Sp1 and this is supported, in part, by the proapoptotic effects observed after overexpression of Sp1 in cancer cells [42].
Fig. 2.
Arsenic trioxide differentially affects Sp-dependent responses in KU7 and 253JB-V cancer cells. Effects on [A] EGFR, cyclin D1 (CD1), and VEGF and [B] proapoptotic proteins/responses. Cells were treated with 5, 10 or 15 μM arsenic trioxide for 24 hr and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Similar results were observed in duplicate experiments. [C] TUNEL staining. KU7 and 253JB-V cells were treated with 5 μM arsenic trioxide for 24 hr and cells were examined for TUNEL staining as described in the Materials and Methods. Significant TUNEL staining was observed only in KU7 cells in replicate experiments. [D] RNA interference studies. KU7 cells were transfected with siRNA for Sp1 as indicated and the effects of Sp1 knockdown on cell proliferation and PARP cleavage were determined by cell counting and western blot analysis, respectively as described in the Materials and Methods. Significant (p < 0.05) effects on cell proliferation are indicated (*) from replicate (3) determinations.
Previous studies show that downregulation of Sp1, Sp3, Sp4 and Sp-regulated genes by various agents is either proteasome-dependent or -independent [28,29,38,39]. Treatment of KU7 cells with arsenic trioxide decreased Sp1, Sp3, Sp4 and Sp-regulated EGFR, VEGF, survivin, cyclin D1 and bcl-2 protein expression (Fig. 3A) and cotreatment with the proteasome inhibitor MG132 did not inhibit arsenic trioxide-dependent downregulation of these proteins. These results were in contrast to the proteasome-dependent downregulation of these proteins in KU7 cells treated with curcumin [28]. Since arsenic trioxide induces apoptosis in KU7 cells, we also examined the effects of the pan-caspase inhibitor Z-VAD-fmk on arsenic-induced downregulation of Sp and Sp-regulated proteins. The results show that the caspase inhibitor had minimal to non-detectable effects on arsenic trioxide-induced downregulation of Sp1, EGFR, VEGF, survivin, cyclin D1 or bcl-2 protein expression (Fig. 3B); however, there was some inhibition of Sp3 downregulation and the caspase inhibitor effectively blocked repression of Sp4. These results show for the first time that induction of caspases may contribute to downregulation of Sp4 and Sp3. Previous RNA interference studies showed that the p65 subunit of NFκB was an Sp-regulated gene in KU7 cells [28]. Results illustrated in Figure 3C show that arsenic trioxide-induced downregulation of p65 was not affected by the proteasome or caspase inhibitors MG132 and Z-VAD-fmk, respectively, and this was similar to effects observed for other Sp-regulated proteins (Figs. 3A and 3B). Figure 3D shows that arsenic trioxide decreases expression of Sp1, Sp3, Sp4, EGFR, survivin and VEGF mRNA levels, demonstrating that arsenic trioxide modulates the transcription of these genes. In contrast, only a minimal decrease was observed for cyclin D1 mRNA levels.
Fig. 3.
Arsenic trioxide-induced downregulation of Sp and Sp-regulated proteins in KU7 cells. Effects of MG132 [A] and Z-VAD-fmk [B]. KU7 cells were treated with 10 μM arsenic trioxide alone or in combination with 10 μM MG132 or 20 μM Z-VAD-fmk for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. [C] Effects of MG132 and Z-VAD-fmk. KU7 cells were treated as described above, and p65 and p50 proteins expressed in nuclear extracts were analyzed by western blots as described in the Materials and Methods. [D] Arsenic trioxide decreases mRNA levels of Sp and Sp-regulated genes. KU7 cells were treated with 10 μM arsenic trioxide for 24 hr and mRNA levels were determined by real time PCR as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group, and significantly (p < 0.05) decreased mRNA levels are indicated (*).
Arsenic trioxide induces ROS and ROS inhibitors block downregulation of Sp transcription factors
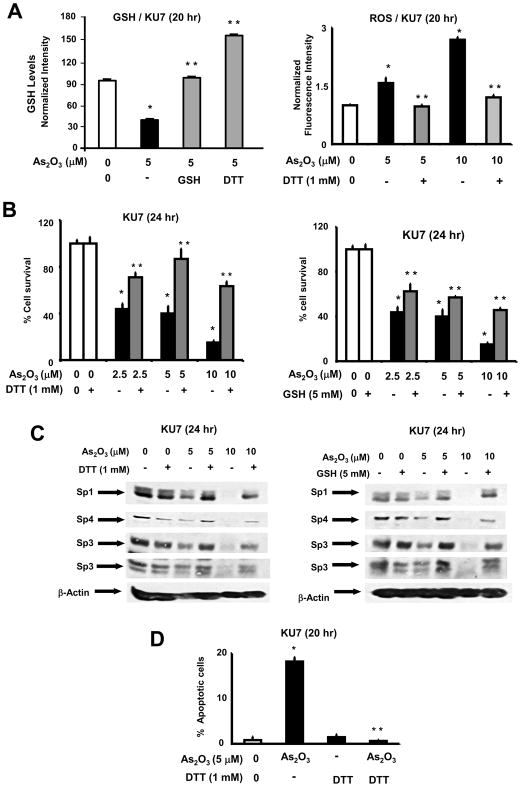
Several reports indicate that the proapoptotic and growth inhibitory effects of arsenic trioxide were associated with decreased MMP and induction of ROS [15–18]. Moreover, the susceptibility of several cancer cell lines to the cytotoxicity of arsenic trioxide correlated with the relative expression of the antioxidant GSH [43]. Constitutive GSH(253JB-V)/GSH(KU7) ratios were 1.47 and the corresponding ROS ratio was approximately 1.0 in the two cell lines indicating that enhanced expression of GSH in 253JB-V cells may contribute to the differences in the arsenic trioxide-responsive and -nonresponsive KU7 and 253JB-V bladder cancer cells, respectively. Treatment of KU7 cells with arsenic trioxide significantly decreased GSH and this response was blocked after treatment with the antioxidants GSH or DTT (Fig. 4A). Moreover, arsenic trioxide also induced ROS in KU7 cells and this effect was inhibited after cotreatment with DTT (Fig. 4A). RNA interference studies in which Sp1, Sp3 and Sp4 were decreased individually or in combination did not affect ROS levels (Supplement Figure 2). Since GSH and DTT inhibited arsenic trioxide-induced ROS, we investigated the role of ROS in mediating the effects of arsenic trioxide on KU7 cell proliferation and Sp protein expression. Arsenic trioxide alone inhibited KU7 cell proliferation and decreased Sp1, Sp3 and Sp4 protein expression (Figs. 4B and 4C, respectively); however, after cotreatment with GSH or DTT, there was significant reversal of the arsenic trioxide-induced responses. Moreover, in gel mobility shift assays, we also observed that nuclear extracts from KU7 cells treated with arsenic trioxide exhibited decreased binding to a GC-rich oligonucleotide that binds Sp proteins, and cotreatment of KU7 cells with DTT restored binding to this oligonucleotide (Supplement Figure 3). In addition, we also used the TUNEL assay and the quantitative results in Figure 4D show that the increased TUNEL staining observed in KU7 cells after treatment with arsenic trioxide was significantly inhibited after cotreatment with DTT.
Fig. 4.
Role of glutathione/oxidative stress in mediating the anticancer activity of arsenic trioxide in KU7 cells. [A] Effects of DTT on arsenic trioxide-induced effects on GSH and ROS levels. KU7 cells were treated with aqueous solvent, arsenic trioxide alone, or in combination with DTT. Cellular GSH levels and ROS were determined as described in the Materials and Methods. Results are expressed at means ± SE for 3 separate experiments for each treatment group and significant (p < 0.05) effects by arsenic trioxide (*) and reversal by DTT (**) are indicated. GSH values in control (100%), arsenic trioxide, arsenic trioxide + DTT groups were 100±0.7, 40.0±0.1, 101.5±1.9 and 157.6±1.2, respectively. ROS values in control (100%), arsenic trioxide alone (5 or 10 μM), and DTT + arsenic trioxide (5 or 10 μM) were 100±2, 156±13, 269±6, 97±4 and 120±6, respectively. [B] GSH and DTT inhibit the antiproliferative activity of arsenic trioxide. KU7 cells were treated with solvent control, 2.5, 5 or 10 μM arsenic trioxide alone, or in combination with DTT (left) or GSH (right). After 24 hr, cells were counted as described in the Materials and Methods. Results are means ± SE for 3 determinations for each treatment group and significant (p < 0.05) inhibition by arsenic trioxide (*) and reversal by DTT or GSH (**) are indicated. [C] Sp expression levels. KU7 cells were treated with arsenic trioxide alone or in the presence of DTT (left) or GSH (right) for 24 hr and whole cell lysates were examined by western blot analysis as described in the Materials and Methods. [D] TUNEL assay. KU7 cells were treated with solvent (control), 5 μM arsenic trioxide alone, or in combination with DTT and the TUNEL assay was determined and quantified as described in the Materials and Methods. The percent of apoptotic cells in control (100%), arsenic trioxide alone, DTT alone, and arsenic trioxide + DTT were 100±46, 2234±58, 182±35 and 77±14, respectively. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) induction of TUNEL staining (*) and inhibition of this response by DTT (**) are indicated.
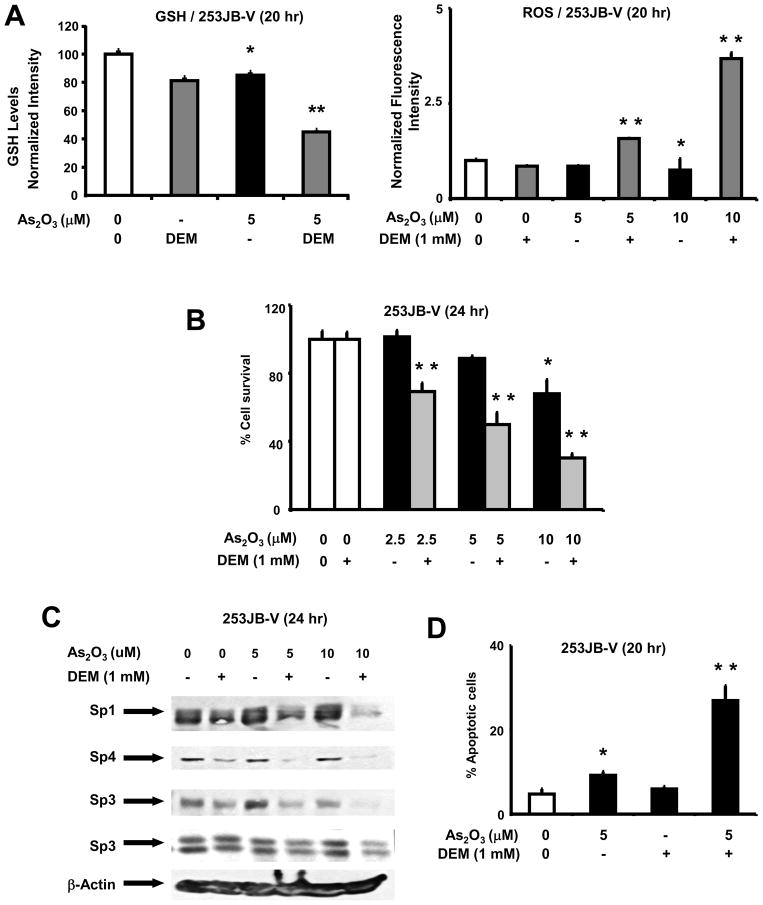
253JB-V cells were resistant to the cytotoxic effects of arsenic trioxide and the role of GSH and antioxidants thiols in regulating resistance was investigated using DEM, a reagent that depletes intracellular GSH. Figure 5A shows that treatment of 253JB-V cells with arsenic trioxide or DEM resulted in a 10.2% decrease in GSH levels; however, in cells cotreated with DEM plus arsenic trioxide, a 55.6% decrease in GSH was observed. Using the same treatment protocol, arsenic trioxide or DEM alone had minimal effects on ROS levels, whereas in 253JB-V cells treated with DEM plus arsenic trioxide, there was a 2.2- or 4.9-fold increase in ROS in cells treated with 5 or 10 μM arsenic trioxide (compared to untreated cells). Treatment of 253JB-V cells with arsenic trioxide alone or in combination with DEM showed that DEM-dependent depletion of GSH sensitized these cells to arsenic trioxide-induced inhibition of cell proliferation (Fig. 5B) and repression of Sp1, Sp3 and Sp4 proteins (Fig. 5C). Moreover, quantitation of the TUNEL staining results (Fig. 5D) show that minimal staining was observed in 253JB-V cells treated with arsenic trioxide (Fig. 3C); however, in cells cotreated with DEM plus arsenic trioxide, there was a significant increase in TUNEL staining. Thus, GSH expression and induction of ROS are critical factors that regulate arsenic trioxide-induced anticarcinogenic responses in bladder cancer cells and this includes downregulation of Sp proteins and Sp-dependent gene products and induction of apoptosis.
Fig. 5.
Role of glutathione/oxidative stress in mediating the anticancer activity of arsenic trioxide in 253JB-V cells. [A] Effects of arsenic trioxide and DEM on GSH and ROS. 253JB-V cells were treated with aqueous solvent, arsenic trioxide alone, or in combination with DEM. Cellular GSH levels and ROS were determined as described in the Materials and Methods. Results are expressed at means ± SE and significant (p < 0.05) effects by arsenic trioxide (*) and enhancement by DEM (**) are indicated. GSH values in control (100%), DEM, arsenic trioxide alone or in combination with DEM were 100±0.7, 79.8±0.5, 85.0±0.3 and 44.4±0.4, respectively. ROS values in control (100%), arsenic trioxide alone (5 or 10 μM), DEM alone, and DEM + arsenic trioxide (5 or 10 μM) were 100±2, 85±0.7, 75±1.2, 85±1.7, 163±30 and 368±15, respectively. [B] DEM enhances the antiproliferative activity of arsenic trioxide. 253JB-V cells were treated with solvent control, 2.5, 5 or 10 μM arsenic trioxide alone, or in combination with DEM. After 24 hr, cells were counted as described in the Materials and Methods. Results are means ± SE for 3 determinations for each treatment group and significant (p < 0.05) inhibition by arsenic trioxide (*) and enhancement by DEM (**) are indicated. [C] Sp expression levels. 253JB-V cells were treated with arsenic trioxide alone or in the presence of DEM for 24 hr and whole cell lysates were examined by western blot analysis as described in the Materials and Methods. [D] TUNEL assay. 253JB-V cells were treated with solvent (control), 5 μM arsenic trioxide alone, or in combination with DEM and the TUNEL assay was determined and quantitated as described in the Materials and Methods. The percent of apoptotic cells in control (100%), arsenic trioxide alone, DEM alone, and arsenic trioxide + DEM were 100±25, 193±18, 125±12 and 566±105, respectively. Results are expressed as means ± SE for 2 replicate determinations for each treatment group and significant (p < 0.05) induction of TUNEL staining (*) is indicated.
Antioxidants block hydrogen peroxide and arsenic trioxide-dependent growth inhibition and Sp downregulation
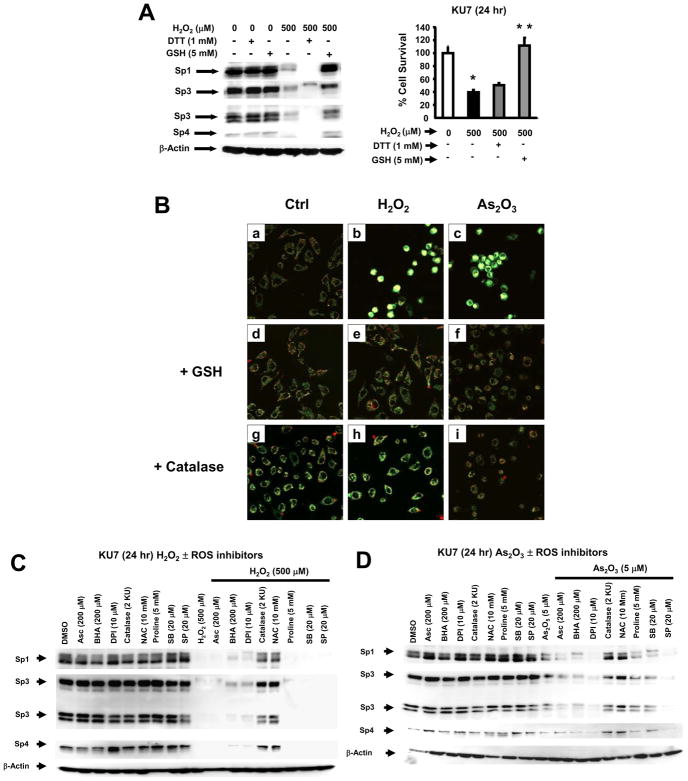
These results in bladder cancer cells suggest that induction of ROS by arsenic trioxide appears to be a critical common factor in mediating growth inhibition and Sp protein downregulation, and therefore, we investigated the effects of hydrogen peroxide alone on expression of Sp1, Sp3 and Sp4 in KU7 cells. The results show that treatment of KU7 cells with hydrogen peroxide (500 μM) for 24 hr decreased expression of Sp1, Sp3 and Sp4 proteins and cotreatment with hydrogen peroxide plus GSH blocked downregulation of Sp proteins, whereas only minimal effects were observed for DTT (Fig. 6A). In addition, hydrogen peroxide (500 μM) also inhibited KU7 cell growth and this response was also inhibited after cotreatment with GSH. Downregulation of Sp proteins and growth inhibition was also observed using t-butylhydroperoxide (data not shown). In some cancer cell lines, arsenic trioxide-induced ROS has been associated with mitochondriotoxicity and loss of MMP [14,15,44]. The comparative effects of arsenic trioxide and hydrogen peroxide on MMP was determined by confocal microscopy in KU7 cells (Fig. 6B) using JC-1, a red fluorescent dye that accumulates and forms oligomers in the mitochondrial matrix. In solvent-treated cells, the green (cytosol) and red (mitochondrial) staining was evident using confocal microscopy, whereas after treatment with 10 μM arsenic trioxide or 500 μM hydrogen peroxide, there was a decrease in red and an increase in green staining consistent with decreased MMP (Fig. 6B). Similar results were observed in FACS analysis where there was an increase in the ratio of green/red stained cells (Supplement Fig. 4). Moreover, the effects of arsenic trioxide and hydrogen peroxide on decreased MMP were partially reversed in KU7 cells cotreated with the thiol antioxidant GSH; catalase, which catalyzes the detoxication of peroxides, blocked the effects of hydrogen peroxide but was minimally effective against arsenic trioxide in this assay (Fig. 6B).
Fig. 6.
Comparative effects of hydrogen peroxide and arsenic trioxide on Sp proteins and MMP in KU7 cells. [A] Hydrogen peroxide downregulates Sp proteins and inhibits proliferation. KU7 cells were treated with 500 μM hydrogen peroxide alone or in combination with GSH or DTT, and Sp protein expression and cell growth were determined by western blot analysis and cell counting as described in the Materials and Methods. Cell proliferation results are given as means ± SE for replicate (3) determinations, and significant (p < 0.05) growth inhibition by hydrogen peroxide (*) and reversal of this effect by GSH (**) are indicated. [B] Effects of arsenic trioxide and hydrogen peroxide on MMP. KU7 cells were treated with the compounds or catalase, and MMP was determined by confocal microscopy as described in the Materials and Methods. Effects of various inhibitors on hydrogen peroxide [C]- or arsenic trioxide [D]-induced downregulation of Sp1, Sp3 and Sp4. KU7 cells were treated with the compounds alone or in combination with various inhibitors, and Sp proteins were examined by western blot analysis as described in the Materials and Methods. β-Actin served as a loading control for the western blots. The following concentrations of inhibitors were used: SB203580 (20 μM); SP6000125 (20 μM); NAC (10 mM), ascorbic acid (200 μM); BHA (200 μM); DPI (10 μM), and catalase (2 KU).
The effects of the antioxidant BHA on arsenic trioxide- and hydrogen peroxide-dependent decrease in MMP was investigated. BHA did not ameliorate the effects of these compounds on MMP and was minimally effective as an inhibitor of ROS in the bladder cancer cell lines (data not shown). The linkage between arsenic trioxide- and hydrogen peroxide-induced ROS and downregulation of Sp proteins was further investigated using several inhibitors including catalase, proline, an inhibitor of oxidative stress, NAC, BHA, diphenyliodonium (DPI), an inhibitor of NADPH oxidase, and inhibitors of p38 (SB203580) and JNK (SP6000125) kinase pathways. Catalase and NAC blocked hydrogen peroxide-induced downregulation of Sp1, Sp3 and Sp4 proteins (Fig. 6C), whereas minimal (DPI and BHA) or no inhibition was observed with the other compounds. The same set of inhibitors was used to investigate arsenic trioxide-dependent downregulation of Sp proteins (Fig. 6D) and similar results were observed except that minimal inhibition was observed for ascorbate, proline and SB203580. Thus, thiol reducing agents, such as NAC, GSH and DTT that block arsenic trioxide-dependent decrease in MMP and formation of ROS, or catalase, which decreases ROS, also inhibited downregulation of Sp proteins. This suggests that, in KU7 cells, the anticarcinogenic activity of arsenic trioxide is associated, in part, with Sp downregulation which is due to decreased MMP and induction of ROS.
DISCUSSION
Sp transcription factors are members of the Sp/Krüppel-like family (KLF) of 25 transcription factors that bind GC-rich promoter sequences and regulate basal expression of multiple mammalian and viral genes [45]. Although knockout of many of the Sp genes in mice is embryolethal or induces serious defects in the neonates, the expression of Sp1, the most widely distributed Sp/KLF member, is significantly decreased in rodent and human tissues with increasing age [46,47]. Studies in this laboratory show that in mouse xenograft studies, Sp1, Sp3 and Sp4 expression is low in liver [40] and also in more proliferative tissues such as the gastrointestinal tract, whereas expression of these proteins is high in breast, colon, pancreatic, prostate and bladder cancer cells [28–32,37–40] and in other cancer cell lines (data not shown). RNA interference studies which simultaneously decreased expression of Sp1, Sp3 and Sp4 indicate that these transcription factors regulate several genes critical for cancer cell survival, angiogenesis and proliferation [28–31,40]. For example, in bladder cancer cells, results of RNA interference studies show that Sp1, Sp3 and Sp4 regulate expression of EGFR, cyclin D1, survivin, bcl-2, VEGF and NFκB-dependent activity and this is only a partial list of Sp-regulated genes [28].
Therefore, based on the overexpression of Sp proteins in cancer cells and tumors [28–32,37–40] and the fact that Sp1 is a negative prognostic factor for survival of patients with some solid tumors [33,34], we have been investigating the mechanism of action of several anticancer agents that may act, in part, through decreasing expression of Sp transcription factors in tumors. Tolfenamic acid, betulinic acid, and curcumin induce degradation of Sp1, Sp3 and Sp4 in pancreatic, prostate and bladder cancer cells and tumors, respectively [18,38–40], and we have recently shown that the synthetic triterpenoid methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate (CDODA-Me) also decreases Sp1, Sp3 and Sp4 in colon cancer cells and tumors [39]. The remarkable anticancer activity of arsenic trioxide for treating leukemia and the potential of this drug for treatment of solid tumors coupled with the reported effects of arsenic trioxide on downregulation of several Sp-dependent genes and responses (VEGF, angiogenesis, survivin, bcl-2, NFκB activity) [20–27] prompted us to examine the effects of arsenic trioxide on Sp expression. A previous study with promyeolcytic leukemia cells treated with arsenic trioxide for 10 days showed that Sp1-DNA binding was decreased and this was correlated with increased Sp1 oxidation and not decreased expression of this transcription factor [41]. In contrast, we observed that, in KU7 bladder cancer cells, arsenic trioxide (1 μM) decreased expression of Sp1, Sp3 and Sp4 after treatment for 6 days indicating comparable sensitivity in leukemia vs. bladder cancer cells but clearly different effects on Sp1, Sp3 and Sp4 protein expression. The effects of arsenic trioxide on Sp1 (downregulation) observed in this study were also reported in gall bladder carcinoma [26]; however, the mechanism of this response and the effects on Sp3 and Sp4 were not determined.
Figure 1 and Supplemental Figure 1 demonstrate that in a series of bladder, prostate, pancreatic and colon cancer cell lines, there was a correspondence between their responsiveness to the antiproliferative effects of arsenic trioxide and their downregulation of Sp1, Sp3 and Sp4 proteins. Using KU7 and 253JB-V bladder cancer cells as prototypical models of arsenic trioxide responsive and non-responsive cells, respectively, it was apparent that relatively low concentrations (≤ 5 μM) inhibited KU7 cell proliferation (Fig. 1A) and KU7 tumor growth in athymic nude mice (Fig. 1C). Arsenic trioxide also downregulated Sp1, Sp3 and Sp4 in KU7 cells and tumors (Figs. 1A and 1C), whereas at comparable concentrations, only minimal effects on growth and Sp downregulation were observed in 253JB-V cells. Results in Supplemental Figure 1 confirm that arsenic trioxide inhibits growth and induces Sp downregulation at similar concentrations in pancreatic, colon and prostate cancer cell lines. A comparison of arsenic trioxide induced antiproliferative, antiangiogenic and proapoptotic genes/responses in KU7 and 253JB-V cells (Fig. 2) also demonstrated comparable cell context-dependent responsiveness and non-responsiveness to the effects of arsenic trioxide, respectively. Interestingly, unlike betulinic acid, tolfenamic acid and curcumin [28,38,40] (in the same bladder cancer cells), arsenic trioxide did not induce proteasome-dependent degradation of Sp1, Sp3 and Sp4 proteins or Sp-regulated gene products (Figs. 3A and 3C) but decreased mRNA levels of Sp and Sp-regulated genes (Fig. 3D). Previous studies showed that activation of caspases can result in cleavage of Sp1 [48,49], and we therefore investigated the effects of the pan-caspase inhibitor Z-VAD-fmk on arsenic trioxide-induced downregulation of Sp1, Sp3, Sp4 and Sp-regulated proteins. The pan-caspase inhibitor had minimal to non-detectable effects on expression of Sp1 or the Sp-regulated EGFR, VEGF, survivin, cyclin D1 and bcl-2 proteins; however, Z-VAD-fmk clearly inhibited arsenic trioxide-induced downregulation of Sp3 and Sp4 (Fig. 3B). These results are unique among the agents that target Sp transcription factors [28,29,38,39] and show for the first time that downregulation of Sp1, Sp3 and Sp4 by arsenic trioxide may involve different pathways for these transcription factors, and this is currently being investigated using other agents that also decrease expression of Sp and Sp-regulated genes. In summary, our results show that arsenic trioxide decreases Sp transcription factors and Sp-dependent genes and results of Sp protein knockdown studies in bladder cancer cells [28] (Fig. 4D) indicate that the effects of arsenic trioxide on Sp proteins contribute to the growth inhibitory, proapoptotic and antiangiogenic activity of this compound.
Previous studies in 19 different cancer cell lines (including bladder cancer cells) reported that their differential responsiveness to the antiproliferative effects of arsenic trioxide were related, in part, to their expression of GSH [43]. Moreover, since intracellular GSH is an important buffer against mitochondrial disruption and ROS which is rapidly induced by arsenic trioxide in cancer cell lines [2,3,11–16], we hypothesized that ROS/GSH levels may be responsible for the cytotoxic/proapoptotic effects of arsenic trioxide and be an important determinant for regulating Sp expression. Absolute levels of ROS were similar in KU7 and 253JB-V cells, but there was a 47% higher level of GSH in 253JB-V compared to KU7 cells and this may contribute to their responsiveness to arsenic trioxide. It was also apparent that arsenic trioxide induced ROS and decreased GSH in KU7 cells and these responses were ameliorated after cotreatment with the thiol antioxidants GSH or DTT (Fig. 4A). GSH and DTT also significantly protected against the antiproliferative effects of arsenic trioxide in KU7 cells (Fig. 4B) and inhibited downregulation of Sp1, Sp3 and Sp4 proteins in KU7 cells treated with arsenic trioxide (Fig. 4C). In contrast, the decrease in GSH levels in 253JB-V cells treated with arsenic trioxide (Fig. 5A) was much less than observed in KU7 cells and, in the former cell line, this was not accompanied by changes in ROS. However, enhanced depletion of GSH by diethyl maleate in 253JB-V cells sensitized this “non-responsive” cell line to arsenic trioxide-mediated antiproliferative and proapoptotic activity and downregulation of Sp proteins (Fig. 5). These results in KU7 and 253JB-V cells suggest that induction of ROS by arsenic trioxide is a key element in the subsequent downregulation of Sp proteins and ROS has been directly linked to the cytotoxicity of arsenic trioxide and other mitochondriotoxic anticancer drugs [3,14,15,44].
Hydrogen peroxide and other pro-oxidants are cytotoxic to various transformed cell lines [41]. The linkage between ROS and downregulation of Sp proteins was investigated in KU7 cells treated with 500 μM hydrogen peroxide for 24 hr (Fig. 6A). Like arsenic trioxide, hydrogen peroxide inhibited growth and decreased expression of Sp1, Sp3 and Sp4 proteins in KU7 cells and these responses were blocked after cotreatment with the antioxidant glutathione. We also observed that both DTT and GSH antioxidants were effective as inhibitors of arsenic trioxide-induced growth inhibition and Sp downregulation (Fig. 4), whereas only GSH blocked the effects of hydrogen peroxide (Fig. 6A). The reasons for these differences are unclear and are currently being investigated. Arsenic trioxide induces ROS by several pathways and this includes direct effects on mitochondria and thiol-containing mitochondrial proteins which leads to decreased MMP, release of proapoptotic factors such as cytochrome c, and induction of ROS [3,44]. Using confocal microscopy and FACS analysis, we showed that both arsenic trioxide and hydrogen peroxide decreased MMP in KU7 cells and this response was partially blocked after cotreatment with GSH (Fig. 6B). Catalase blocked hydrogen peroxide-dependent decrease in MMP in KU7 cells (Fig. 6B) and induction of ROS by hydrogen peroxide was also inhibited by catalase (data not shown). Catalase also inhibited induction of ROS by arsenic trioxide (data not shown) but had minimal effects on decreased MMP in KU7 cells treated with arsenic trioxide (Fig. 6B). These results suggest that induction of extramitochondrial ROS by arsenic trioxide in KU7 cells has a minimal effect on MMP, indicating that arsenic trioxide-dependent decrease in MMP and induction of ROS are due to direct effects on the mitochondria. This data is also consistent with the effectiveness of thiol reducing agents such as GSH in ameliorating the activity of arsenic trioxide in cancer cells since these agents act not only as antioxidants but also counteract interactions of arsenic trioxide on thiol-containing mitochondrial proteins [3,44].
Arsenic trioxide-mediated induction of ROS and downstream effects have also been linked to activation of the flavoprotein-dependent NADPH oxidase enzyme [18] or inhibition of thioredoxin reductase which can result in activation of downstream stress kinase pathways such as p38MAPK and JNK [50,51]. A recent report also showed that inhibition of arsenic trioxide-induced ROS by BHA in some leukemia cell lines did not affect induction of apoptosis, suggesting an ROS-independent pathway [52]. We therefore compared the effects of catalase and NAC, an additional thiol antioxidant, with BHA, stress kinase inhibitors of p38MAPK and JNK (SB203580 and SP600125), and the NADPH oxidase inhibitor DPI on hydrogen peroxide- and arsenic trioxide-mediated downregulation of Sp1, Sp3 and Sp4 in KU7 cells (Figs. 6C and 6D). The results showed that SB203580, SP6000125, DPI and BHA had minimal to non-detectable effects on arsenic trioxide-induced downregulation of Sp1, Sp3 and Sp4 proteins, suggesting that the major pathway targeting these transcription factors involves mitochondria and induction of ROS.
Low dose toxicity of arsenic trioxide in endothelial cells and increased growth of some tumors has been associated with increased angiogenesis [52–55]. In contrast, this study shows that 1.0 μM arsenic trioxide decreased KU7 cell growth and expression of Sp1, Sp3 and Sp4 and this corresponded to comparable effects of higher concentrations of arsenic trioxide in several different cancer cell lines (Figs. 1 and Supplement Fig 1). However, the overall contributions of downregulation of Sp transcription factors to the anticancer activity of arsenic trioxide will be variable and dependent on cancer cell and tumor type and other activities and/or pathways activated by this compound. For example, the effects of low concentrations of arsenic trioxide (0.75 and 1.0 μM) on proliferation and downregulation of Sp1, Sp3 and Sp4 proteins indicate that 0.75 μM arsenic trioxide significantly decreased KU7 cell proliferation after treatment for 5 days but did not appreciably affect levels of Sp proteins, suggesting an Sp-independent effect of arsenic trioxide on growth inhibition. Currently, we are investigating the role of drug-induced ROS and specific oxidative stress pathways that are important for downregulation of Sp transcription factors in cancer cells and the mechanism of oxidative stress-Sp1/Sp3/Sp4 interactions. In contrast to results of a recent study in colon cancer cells with the synthetic triterpenoid CDODA-Me [39], arsenic trioxide did not affect expression of microRNA-27a and ZBTB10, an Sp-repressor (data not shown) in KU7 cells; however, the role of other microRNAs as proximal regulators of other Sp repressor proteins is currently being investigated in bladder and other cancer cell lines and tumors.
Supplementary Material
Supplement Figure 1. Arsenic trioxide decreases cell growth and Sp1, Sp3 and Sp4 expression in cancer cell lines. Pancreatic [A], colon [B], and prostate [C] cancer cells were treated with solvent (control) and arsenic trioxide in PBS/KOH for 24 hr; cell growth was determined, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Western blot results illustrated in the Figure were typical of duplicate (or more) determinations. Results of cell proliferation studies are expressed as means ± SE for 3 replicate determinations for each treatment group, and significant (p < 0.05) inhibition of cell growth is indicated (*).
Supplement Figure 2. Effects of Sp knockdown on ROS. KU7 cells were transfected with siRNA against Sp1, Sp3, Sp4 and Sp1/Sp3/Sp4 (combined) as described [28, 31, 32]. ROS was determined as outlined in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations.
Supplement Figure 3. EMSA analysis. KU7 cells were treated with different concentrations of arsenic trioxide alone or 10 μM arsenic trioxide in combination with DTT (5 mM) and analyzed by electrophorectic mobility shift assay (EMSA) using a 32P-labeled consensus GC-rich oligonucleotide or unlabeled wild-type or mutant oligonucleotides (competitors) as described (28, 29). The Sp-DNA retarded band complex is indicated by an arrow.
Supplement Figure 4. Effects of arsenic trioxide, GSH and catalase on MMP. The changes in red and green fluorescence associated with effects on MMP were determined by FACS analysis as described in the Materials and Methods.
Acknowledgments
Funding: The financial assistance of the National Institutes of Health (5R01-CA136571) and Texas A&M AgriLife is gratefully acknowledged.
ABBREVIATIONS
- APL
acute promyelocytic leukemia
- BHA
butylated hydroxyanisole
- CDODA-Me
methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate
- DEM
diethyl maleate
- DPI
diphenyliodonium
- DTT
dithiothreitol
- FBS
fetal bovine serum
- GSH
glutathione
- iSp
small inhibitory RNAs for Sp1, Sp3 and Sp4
- KLF
Krüppel-like family
- MMP
mitochondrial membrane potential
- NAC
N-acetylcysteine
- PARP
poly (ADP) ribose polymerase
- ROS
reactive oxygen species
- Sp
specificity protein transcription factors
Footnotes
Conflict of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Indira Jutooru, Email: ijutooru@cvm.tamu.edu.
Gayathri Chadalapaka, Email: gchadalapaka@cvm.tamu.edu.
Sandeep Sreevalsan, Email: ssreevalsan@cvm.tamu.edu.
Ping Lei, Email: plei@ibt.tamhsc.edu.
Rola Barhoumi, Email: rmouneimne@cvm.tamu.edu.
Robert Burghardt, Email: rburghardt@cvm.tamu.edu.
References
- 1.Klaassen CD. Heavy metals and heavy-metal antagonists. In: Hardman JG, Gilman AG, Limbird LE, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1996. pp. 1649–1672. [Google Scholar]
- 2.Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33:542–564. doi: 10.1016/j.ctrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 4.Chan PC, Huff J. Arsenic carcinogenesis in animals and in humans: mechanistic, experimental, and epidmeiological evidence. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev. 1997;C15:83–122. [Google Scholar]
- 5.Zhang P, Wang SY, Hu XH. Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chin J Hematol. 1996;17:58–62. [Google Scholar]
- 6.Sun HD, Ma L, Hu XC, Zhang TD. Ai-Lin 1 treated 32 cases of acute promyelocytic leukemia. Chinese Journal of Integrated Chinese and Western Medicine. 1992;12:170–172. [PubMed] [Google Scholar]
- 7.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 8.Verstovsek S, Giles F, Quintas-Cardama A, Perez N, Ravandi-Kashani F, Beran M, Freireich E, Kantarjian H. Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol Oncol. 2006;24:181–188. doi: 10.1002/hon.787. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Fang J, Dong Y, Chen SJ, Chen Z. Arsenic in cancer therapy. Anticancer Drugs. 2005;16:119–127. doi: 10.1097/00001813-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 10.de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor a gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 11.Borrow J, Goddard AD, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990;249:1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 12.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de TH, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 13.Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo CF, Avvisati G, Testa U, Peschle C, Gambacorti-Passerini C, Nervi C, Miller WH., Jr Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR a protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 14.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 15.Shen ZY, Shen J, Cai WJ, Hong C, Zheng MH. The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int J Mol Med. 2000;5:155–158. doi: 10.3892/ijmm.5.2.155. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 17.Kang YH, Lee SJ. The role of p38 MAPK and JNK in arsenic trioxide-induced mitochondrial cell death in human cervical cancer cells. J Cell Physiol. 2008;217:23–33. doi: 10.1002/jcp.21470. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach PH, Polverini PJ. Arsenic trioxide enhances the therapeutic efficacy of radiation treatment of oral squamous carcinoma while protecting bone. Mol Cancer Ther. 2008;7:2060–2069. doi: 10.1158/1535-7163.MCT-08-0287. [DOI] [PubMed] [Google Scholar]
- 20.Xiao YF, Liu SX, Wu DD, Chen X, Ren LF. Inhibitory effect of arsenic trioxide on angiogenesis and expression of vascular endothelial growth factor in gastric cancer. World J Gastroenterol. 2006;12:5780–5786. doi: 10.3748/wjg.v12.i36.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen ZY, Shen J, Chen MH, Wu XY, Wu MH, Zeng Y. The inhibition of growth and angiogenesis in heterotransplanted esophageal carcinoma via intratumoral injection of arsenic trioxide. Oncol Rep. 2003;10:1869–1874. [PubMed] [Google Scholar]
- 22.Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP, Jr, Rafii S. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–1530. [PubMed] [Google Scholar]
- 23.Cheng Y, Chang LW, Tsou TC. Mitogen-activated protein kinases mediate arsenic-induced down-regulation of survivin in human lung adenocarcinoma cells. Arch Toxicol. 2006;80:310–318. doi: 10.1007/s00204-005-0045-1. [DOI] [PubMed] [Google Scholar]
- 24.Jin HO, Yoon SI, Seo SK, Lee HC, Woo SH, Yoo DH, Lee SJ, Choe TB, An S, Kwon TJ, Kim JI, Park MJ, Hong SI, Park IC, Rhee CH. Synergistic induction of apoptosis by sulindac and arsenic trioxide in human lung cancer A549 cells via reactive oxygen species-dependent down-regulation of survivin. Biochem Pharmacol. 2006;72:1228–1236. doi: 10.1016/j.bcp.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARa/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 26.Ai Z, Lu W, Ton S, Liu H, Sou T, Shen Z, Qin X. Arsenic trioxide-mediated growth inhibition in gallbladder carcinoma cells via down-regulation of Cyclin D1 transcription mediated by Sp1 transcription factor. Biochem Biophys Res Commun. 2007;360:684–689. doi: 10.1016/j.bbrc.2007.06.123. [DOI] [PubMed] [Google Scholar]
- 27.Han SS, Kim K, Hahm ER, Park CH, Kimler BF, Lee SJ, Lee SH, Kim WS, Jung CW, Park K, Kim J, Yoon SS, Lee JH, Park S. Arsenic trioxide represses constitutive activation of NF-B and COX-2 expression in human acute myeloid leukemia, HL-60. J Cell Biochem. 2005;94:695–707. doi: 10.1002/jcb.20337. [DOI] [PubMed] [Google Scholar]
- 28.Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, III, Li X, Safe S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expession in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68:317–329. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 30.Abdelrahim M, Samudio I, Smith R, Burghardt R, Safe S. Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J Biol Chem. 2002;277:28815–28822. doi: 10.1074/jbc.M203828200. [DOI] [PubMed] [Google Scholar]
- 31.Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–6749. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 32.Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345:292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 34.Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 35.Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, Schlumberger M, Filetti S. Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer. 2002;2:35. doi: 10.1186/1471-2407-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol. 2004;25:461–468. [PubMed] [Google Scholar]
- 37.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein (Sp) transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 38.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 39.Chintharlapalli S, Papineni S, Abdelrahim M, Abudayyeh A, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott SU, Vanderlaag K, Cho SD, Smith R, 3rd, Safe S. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18b-olean-1,12-dien-30-oate in colon cancer cells. Int J Cancer. 2009;125:1965–1974. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 41.Chou WC, Chen HY, Yu SL, Cheng L, Yang PC, Dang CV. Arsenic suppresses gene expression in promyelocytic leukemia cells partly through Sp1 oxidation. Blood. 2005;106:304–310. doi: 10.1182/blood-2005-01-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang JY, Wu CH, Lai MD, Chang WC, Hung JJ. Overexpression of Sp1 leads to p53-dependent apoptosis in cancer cells. Int J Cancer. 2009;125:2066–2076. doi: 10.1002/ijc.24563. [DOI] [PubMed] [Google Scholar]
- 43.Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81:796–799. doi: 10.1038/sj.bjc.6690766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroemer G, de TH. Arsenic trioxide, a novel mitochondriotoxic anticancer agent? J Natl Cancer Inst. 1999;91:743–745. doi: 10.1093/jnci/91.9.743. [DOI] [PubMed] [Google Scholar]
- 45.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 46.Ammendola R, Mesuraca M, Russo T, Cimino F. Sp1 DNA binding efficiency is highly reduced in nuclear extracts from aged rat tissues. J Biol Chem. 1992;267:17944–17948. [PubMed] [Google Scholar]
- 47.Oh JE, Han JA, Hwang ES. Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun. 2007;353:86–91. doi: 10.1016/j.bbrc.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 48.Rickers A, Peters N, Badock V, Beyaert R, Vandenabeele P, Dorken B, Bommert K. Cleavage of transcription factor SP1 by caspases during anti-IgM-induced B-cell apoptosis. Eur J Biochem. 1999;261:269–274. doi: 10.1046/j.1432-1327.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 49.Piedrafita FJ, Pfahl M. Retinoid-induced apoptosis and Sp1 cleavage occur independently of transcription and require caspase activation. Mol Cell Biol. 1997;17:6348–6358. doi: 10.1128/mcb.17.11.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci USA. 2007;104:12288–12293. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajiguchi T, Yamamoto K, Iida S, Ueda R, Emi N, Naoe T. Sustained activation of c-jun-N-terminal kinase plays a critical role in arsenic trioxide-induced cell apoptosis in multiple myeloma cell lines. Cancer Sci. 2006;97:540–545. doi: 10.1111/j.1349-7006.2006.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284:12886–12895. doi: 10.1074/jbc.M806546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soucy NV, Ihnat MA, Kamat CD, Hess L, Post MJ, Klei LR, Clark C, Barchowsky A. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol Sci. 2003;76:271–279. doi: 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- 54.Soucy NV, Mayka D, Klei LR, Nemec AA, Bauer JA, Barchowsky A. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc Toxicol. 2005;5:29–41. doi: 10.1385/ct:5:1:029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, Pagano PJ, Stolz DB, Barchowsky A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Arsenic trioxide decreases cell growth and Sp1, Sp3 and Sp4 expression in cancer cell lines. Pancreatic [A], colon [B], and prostate [C] cancer cells were treated with solvent (control) and arsenic trioxide in PBS/KOH for 24 hr; cell growth was determined, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. Western blot results illustrated in the Figure were typical of duplicate (or more) determinations. Results of cell proliferation studies are expressed as means ± SE for 3 replicate determinations for each treatment group, and significant (p < 0.05) inhibition of cell growth is indicated (*).
Supplement Figure 2. Effects of Sp knockdown on ROS. KU7 cells were transfected with siRNA against Sp1, Sp3, Sp4 and Sp1/Sp3/Sp4 (combined) as described [28, 31, 32]. ROS was determined as outlined in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations.
Supplement Figure 3. EMSA analysis. KU7 cells were treated with different concentrations of arsenic trioxide alone or 10 μM arsenic trioxide in combination with DTT (5 mM) and analyzed by electrophorectic mobility shift assay (EMSA) using a 32P-labeled consensus GC-rich oligonucleotide or unlabeled wild-type or mutant oligonucleotides (competitors) as described (28, 29). The Sp-DNA retarded band complex is indicated by an arrow.
Supplement Figure 4. Effects of arsenic trioxide, GSH and catalase on MMP. The changes in red and green fluorescence associated with effects on MMP were determined by FACS analysis as described in the Materials and Methods.