Abstract
Infection prevention guidelines do not endorse chlorhexidine gluconate (CHG) use in neonates less than two months old. A survey of U.S. neonatology program directors revealed that most NICUs utilize CHG, often with some restrictions. Prospective studies are needed to further address concerns regarding the safety of CHG in NICU patients.
Introduction
Chlorhexidine gluconate (CHG) is a broad-spectrum antiseptic which is effective against a host of neonatal pathogens. CHG-based products are used frequently in the healthcare setting for peripheral and central venous catheter (CVC) site skin preparation, daily bathing of intensive care unit patients, full-body newborn skin cleansing, umbilical cord care, and Staphylococcus aureus decolonization.1
In neonates, CHG antisepsis for CVC insertion site preparation and maintenance decreases CVC tip microbial colonization.2 Trials of full-body skin cleansing and umbilical cord care with CHG in the developing world, which included infants less than 34 weeks gestational age, have demonstrated reduced risk of neonatal mortality.3–4 Despite proven efficacy of CHG in neonates, current guidelines acknowledge that no recommendations with regards to CHG antisepsis can be made for infants less than 2 months of age due to incomplete safety data in this population.5 Existing evidence does not demonstrate systemic toxicity of CHG, but concerns remain especially regarding its use in preterm infants. CHG has been used in well-designed large clinical trials on tens of thousands of neonates without severe reported adverse events.3–4
The current extent to which CHG is being used as an antiseptic in neonatal intensive care units (NICUs) is unknown. Our objective was to assess the current practice of CHG use in United States NICUs and to understand real and perceived safety concerns of CHG in neonates.
Methods
In July of 2009, a survey was distributed via e-mail to all 100 neonatology training program directors in the United States. Non-responding members received a second survey one week later, followed by a third survey after three weeks. When there was no response from an institution, the survey was sent to the neonatology division chief of the program.
Study participants completed an on-line survey regarding their experiences with CHG including any associated adverse events and concerns they had with its use in neonates. (Table 1)
Table 1.
Survey questions regarding chlorhexidine use in the neonatal intensive Care unit
| With which institution are you primarily affiliated? |
|---|
| Please select the level that best describes the functional capacity of the NICU you primarily work at |
| How many very low birth weight infants (less than or equal to 1500 grams) does your primary NICU admit each year? |
| How long have you been in the neonatology field? |
| Have you ever used hexachlorophene (Phisohex) in the neonatal population in the past? |
| Who primarily determines if chlorhexidine is used for select patients and for select indications in the NICU? |
| For what purposes do you use chlorhexidine in your NICU? |
| If you use chlorhexidine for any indication, do you use it on neonates of a minimum chronological age? |
| Do you limit which neonates are exposed to chlorhexidine by birth weight or gestational age? |
| Which antiseptic agent do you primarily use to prepare the skin for central vascular catheters inserted in your NICU? |
| Have you experienced any adverse events using chlorhexidine in the NICU? |
| Do you have concerns about using chlorhexidine in the NICU? |
Data was analyzed using Stata version 11.0 (Stata Corp., College Station, TX). Categorical variables were compared using Pearson χ2 test or Fisher exact test. A two-tailed P value of ≤.05 was considered significant for all tests. The study was approved by The Johns Hopkins Institutional Review Board.
Results
There were 96 respondents (96%). Ninety (90%) respondents completed the survey in its entirety and only these respondents were included in the analysis. All respondents practiced at level 3b or higher NICUs in the United States. Compared with participants in the field for ≤30 years, those in the field for >30 years were less likely to report using CHG (77% and 23%, p=.05) and trended to have had a greater past experience with hexachlorophene (62% and 38%, p=.08). There was no significant association between involvement of infection control staff and use of CHG (56% and 44%, p=0.42).
Of those 55 NICU’s that utilized CHG, the most commonly reported use was CVC maintenance (78%), including dressing changes and catheter hub cleaning. Other uses of CHG included CVC insertion site preparation (70%), peripheral venous catheter insertion (60%), and skin preparation for umbilical catheter insertion (51%). One institution reported routinely bathing neonates with CHG and four institutions used CHG for MRSA decolonization.
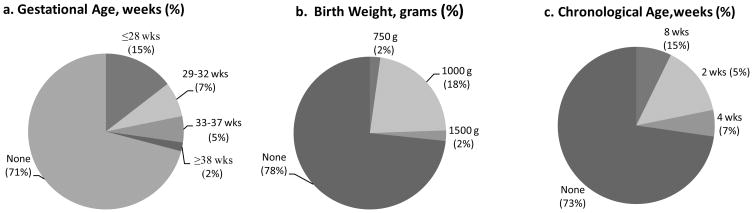
Of respondents that use CHG, 28 (51%) NICUs limit CHG use based on birth weight, gestational age, or chronological age [Figure 1]. Twenty- three (42%) restricted use by gestational age (median 28 weeks), 22 (40%) restricted use by birth weight (median 1000 grams), and 14 (26%) restricted use by chronological age (median 2 weeks).
Figure 1.
Restrictions in the use of chlorhexidine gluconate in United States neonatal intensive care units by (a) gestational age, (b) birth weight, and (c) chronological age based on responses from a national survey of neonatology program directors
Twenty-eight participants (51%) who used CHG in their NICU reported adverse reactions. All were skin reactions and included erythema (32%), erosions (7%), or burns (61%). Of the reported skin burns, 13 of 17 (76%) commented that the burns occurred in neonates with a birth weight of less than 1500 grams; the other four did not comment on the infant’s birth weights. Of the 37 respondents that were aware of CHG formulations used in their NICUs, 21 (57%) employed alcohol-based CHG products. There was no difference in reported adverse effects comparing NICUs utilizing different CHG formulations (p=.50). No neurologic toxicities were reported by any respondents.
Of all participants, 59 (65%) reported concerns regarding the use of CHG in the neonatal population. Common themes from open-ended questions included concerns about off-label, unapproved use of CHG in neonates with immature skin and limited safety data in premature infants.
Discussion
The use of CHG in the NICU setting is complicated by its potential to reduce healthcare-associated infections, yet real and perceived safety concerns of its use for off-label indications. As a result, national infection prevention guidelines, such as the Society for Healthcare Epidemiology of America compendium on strategies to prevent central line associated bloodstream infection state “chlorhexidine products are not approved by the US Food and Drug Administration for children younger than 2 months of age.”5 Surprisingly, despite these recommendations, our findings suggest that the majority of US NICUs with fellowship training programs are using CHG, mostly for CVC site preparation and subsequent CVC maintenance.
All adverse events reported by respondents were related to skin compromise. The most significant reactions (i.e. burns) were generally reported in infants with birth weights of less than 1500 grams. The epidermis has been shown to mature during the last quarter of gestation.6 Poor skin integrity of extremely low birth weight infants may render them susceptible to skin toxicities from CHG, which may necessitate its use with caution in this population. No systemic toxicities were reported in our survey.
Concerns regarding skin breakdown were the most common reservations neonatologists cited for their hesitation to use CHG. Although poor skin integrity may be a significant factor in the most immature infants, other reasons for skin toxicities exist. External pressure from an adhesive CHG impregnated dressing has been suggested to restrict capillary perfusion to the skin, leading to local skin breakdown.7 Contact dermatitis has not been reported in infants receiving full-body CHG skin cleansing where occlusive dressings are not necessary, even for very low birth weight infants and neonates as young as 28 weeks gestational age.8 Alcohol-based CHG preparations have been reported to cause burns in infants 24 to 26 weeks gestational age.9 Our survey was likely underpowered to detect a difference in adverse skin reactions between alcohol and non-alcohol based products.
Neonatologists practicing for more than 30 years were less likely to report using CHG. This may in part be due to their experiences with hexachlorophene, an antiseptic found to be percutaneously absorbed and cause neurotoxicity in preterm babies.10 Although trace absorption of CHG has been demonstrated in premature infants, there is no evidence that low levels of CHG detected in the bloodstream have any harmful consequences, but further investigation is needed.
The main strength of this study was a high response rate. Limitations include the sample size and the potential introduction of sampling bias by surveying NICUs only with neonatology training programs. All NICUs were affiliated with tertiary care medical centers with high patient acuity and the responses may not be representative of non-tertiary care NICUs.
Overall, our data suggests that current practices of CHG utilization in US NICUs are very heterogenous. Although the majority of surveyed NICUs report utilizing CHG, there are wide variations in limiting its use with regards to birth weight, gestational age, and chronological age. Prospective studies are needed to determine sub-populations in the NICU for which CHG is both safe and effective.
Acknowledgments
Financial support: PDT supported by NIH Training Grant Award # 5 T32 AI052071-07; AMM supported by NIH/NIAID 1 K23 AI081752-01; SWA no financial disclosures
Footnotes
Potential Conflicts of interest: AMM receives grant support from Sage Products, Inc. PDT and SWQ have no potential conflicts of interest
Contributor Information
Pranita D. Tamma, Email: ptamma1@jhmi.edu, Johns Hopkins Medical Institution, Department of Pediatrics, Division of Pediatric Infectious Diseases, The David M. Rubinstein Child Health Building, 200 N. Wolfe Street, Suite 3095, Baltimore, Maryland 21287, (t) 410-614-3917, (f) 410-614-1491.
Susan W. Aucott, Johns Hopkins Medical Institution, Department of Pediatrics, Division of Neonatology, Baltimore, Maryland.
Aaron M. Milstone, Johns Hopkins Medical Institution, Department of Pediatrics, Division of Pediatric Infectious Diseases, Baltimore, Maryland.
References
- 1.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the Armamentarium for Infection Control and Prevention. Clin Infect Dis. 2008;46:274–81. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 2.Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107:1431–37. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 3.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367:910–18. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tielsch JM, Darmstadt GL, Mullany LC, et al. Impact of Newborn Skin-Cleansing With Chlorhexidine on Neonatal Mortality in Southern Nepal: A Community-Based, Cluster-Randomized Trial. Pediatrics. 2007 Feb;119(2):e330–40. doi: 10.1542/peds.2006-1192. Epub 2007 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marschall J, Mermel LA, Classen D, et al. SHEA/IDSA practice recommendation: Strategies to Prevent Central Line Associated Bloodstream Infections in Acute Care Hospitals. Infect Control Hosp Epidemiol. 2008;29:S22–S306. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 6.Nachman RL, Esterly NB. Increased skin permeability in preterm infants. J Pediatr. 1971;79:628–32. doi: 10.1016/s0022-3476(71)80311-6. [DOI] [PubMed] [Google Scholar]
- 7.Visscher M, deCastro MV, Combs L, et al. Effect of chlorhexidine gluconate on the skin integrity at PICC line sites. J Perinatol. 2009 Dec;29(12):802–7. doi: 10.1038/jp.2009.116. Epub 2009 Aug 20. [DOI] [PubMed] [Google Scholar]
- 8.Cowen J, Ellis SH, McAinsh J. Absorption of chlorhexidine from the intact skin of newborn infants. Arch Dis Child. 1979 May;54(5):379–383. doi: 10.1136/adc.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins AM, Keogh EJ. Alcohol burns in the neonate. J Paediatr Child Health. 1992;28:306–308. doi: 10.1111/j.1440-1754.1992.tb02673.x. [DOI] [PubMed] [Google Scholar]
- 10.Powell H, Swarner O, Gluck L, Lampert P. Hexachlorophene myelinopathy in premature infants. J Pediatr. 1973 Jun;82(6):976–81. doi: 10.1016/s0022-3476(73)80428-7. [DOI] [PubMed] [Google Scholar]