Abstract
Despite intense study in mammals, the different roles played by the immune system in detecting (immunosurveillance), controlling and remodeling (immunoediting) neoplasia, and perhaps in metastasis are not fully understood. In this review, I will present evidence of neoplasia and invasive malignancy, as well as tumor immunity in invertebrates and nonmammalian vertebrates. I will also present a comparative and evolutionary view of the complex interactions between neoplasia and the host immune system. Overall, I wish to go beyond the too simplistic dichotomy between invertebrates with innate immunity that are only affected with benign neoplasia and vertebrates with adaptive immunity that are affected by metastatic malignancies or cancer.
Keywords: Evolution, immune surveillance, neoplasia, oncovirus, Xenopus, metazoan
Introduction
The consensual definition of cancer or neoplasia is a class of diseases that results from abnormal, uncontrolled proliferation of genetically altered cells that invade and destroy adjacent tissues. The genetic abnormalities (e.g., mutation, translocation, etc) of transformed or tumor cells can be caused by physical (e.g., radiation), chemical (e.g., carcinogens) or infectious (e.g., oncoviral) agents. Neoplasia can be benign, which means that cells abnormally proliferating or tumors remain localized and do not invade other tissues. For the purpose of this review, I will call neoplasia any abnormal growth or tumors for which there is no evidence of metastasis. In contrast, neoplasia can spread or metastasize to other parts of the body of an organism; in this review, this will be designated as malignancy. In mammals, it is now well accepted that malignancy is a multistep process that requires six essential alterations in cell physiology: (i) self-sufficiency in growth signals; (ii) insensitivity to antigrowth signals; (iii) evasion of apoptosis; (iv) unlimited replicative potential; (v) sustained angiogenesis; and (vi) tissue invasion and metastasis (reviewed in [1]). Many genes and pathways critically involved in neoplastic transformation and metastasis are evolutionarily conserved. Indeed, some forms of cancers are relatively widespread in all multicellular animals or metazoan, whose evolutionary origin is estimated to be more than 900 million years ago (MYA). In fact, non-mammalian model organisms such as Xenopus [2], Drosophila [3, 4] and Caenorhabditis elegans [5, 6] are contributing significantly to understanding tumorigenesis.
In the early 20th century, Ehrlich first proposed the existence of immune surveillance for eradicating nascent transformed cells before they are clinically detected. Fifty years later, Burnet and Thomas postulated that the control of nascent transformed cells may represent an ancient immune system that played a critical role in surveillance against malignant transformation (reviewed in [7]). Besides earlier experiments using drugs or irradiation to impair the immune system (reviewed in [8]), more direct evidence of the crucial role of the immune system in preventing malignancies has been obtained using mouse strains deficient for specific genes controlling adaptive immunity such as Rag 2, Interferon γ or Baft3 [8, 9]. Studies in mice also suggest a role for elements of the innate immune system such as type I interferon and NK cells in immune surveillance (reviewed in [8, 10]). In human, analyses of clinical data show strong correlations between immunosupression and cancer incidence (reviewed [8]). In addition, the type, density, and location of immune cells recorded in an extensive study of human colorectal tumors were found predictive of clinical outcome [11]. While, evidence consistent with the concept of cancer immune surveillance is relatively well documented in mammals, especially in the mouse model, the importance of this role of the immune system in survival and natural selection is usually overlooked. In contrast to information concerning tumor occurrence and tumorigenesis, little is known about the role of the immune system in detecting and controlling neoplastic diseases in animals other than selected mammalian and avian species. This is of relevance since recent studies in mice and humans have stressed the multiple and complex interactions that occur between malignant cancers and their hosts (reviewed in [8, 10]). While the immune system maintains a surveillance that is crucial for detecting and preventing tumors, it may also establish selective pressures that shape and even may generate new variants that escape the immune system and display increased tumorigenicity and metastatic potential (i.e., immunoediting). So far, evidence in support of the process of immunoediting comes mainly from mouse studies comparing the properties of tumors induced by chemical carcinogen in animals that are either immunocompetent or immunodeficient (e.g., Rag2- or perforin-deficient). Tumors obtained from immunocompetent mice are more tumorigenic and, therefore, more modified than those that have developed under low or absent immune pressure. As in immunesurveillance, the innate immune system appears also involved in shaping tumor. For example, NKp46, a specific NK killer receptor recognizing unidentified tumor ligands, can mediate mechanisms of tumor editing [12].
Although evidence in mouse and human collectively support immune surveillance and immunoediting concepts, there are still many unanswered questions. For example the respective contributions of the adaptive (e.g., T cell responses) and innate (e.g., pro-inflammatory responses) immune systems is unclear [13]. How early during tumorigenesis the immune system can detect transformed cells and how efficient it is at these early stages to eliminate tumor cells is still an active area of research. In addition, the generally accepted assumption that malignancies with metastasis or true cancers are tightly linked to the adaptive immune system of jawed vertebrates, and the corollary that associates the lack of adaptive immunity in invertebrates with the rarity and benignity of neoplasia are perhaps a bit too premature and simplistic.
Therefore, it could be useful to reevaluate the available evidence of tumor occurrence and severity in invertebrates and jawless vertebrates whose immune systems are fundamentally different from those of mammals. In fact, even ectothermic vertebrates that share a similar adaptive immune system with mammals may provide important clues on the importance of cancer immune surveillance. The objectives of this review are first to critically evaluate the evidence for neoplasia and malignancy in the animal kingdom from invertebrates to vertebrates, then to examine the information available about anti-tumor immunity in the different classes of animals, with a special focus on the frog Xenopus, and finally, to try to draw a synthesis based on comparative and evolutionary views of the intricate interactions between immune systems and cancer.
1. Evidence of neoplasia in metazoan
1.1. Invertebrates
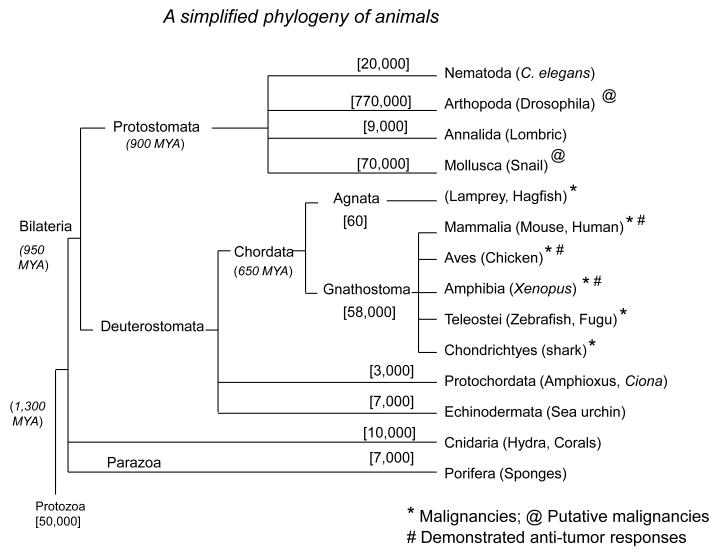
The metazoan or multicellular animal kingdom is estimated to have emerged on earth more than 1,300 million years ago (MYA), and the separation of organisms without symmetry from those with radial symmetry, or with bilateral symmetry (bilaterian) occurred about 900 MYA (Fig. 1).
Figure 1. A simplified phylogeny of animals.
Adapted from [101]. Approximate time in million of years for the emergence of the main group of organisms in parenthesis, and approximate number of species of main taxa are indicated in brackets. An example of organism of each class is also given. *Malignancies; @Putative malignancies; # Demonstrated anti-tumor responses
Remarkably, and despite some confusion in the literature about the diagnostic distinction between pseudoneoplasia and real neoplasia and other types of deregulated proliferation resulting from wound repair or infection (e.g., granuloma caused by mycobacterial or mucosal infection), diseases with the characteristics of neoplasia have been reported in several invertebrate species including species evolutionary very distant from vertebrates (reviewed in [14, 15]). Furthermore, increasing evidence suggests that genes and pathways critically involved in tumorigenesis are all conserved in the entire metazoan kingdom. We succinctly review here some salient examples.
Freshwater and marine sponges (phylum: Porifera) are among the most distant relatives of vertebrates (chordata). These are diploblastic invertebrate organisms (e.g., formed by only two primary germ layers: the ectoderm and endoderm) without clear body symmetry (asymmetric). Although, to date, no well defined neoplastic disease has been reported in this phylum, the response of their cells to mitogens such as phorbol miristate acetate, and the presence of pro-oncogenes including ras, src and ets [16, 17], suggest that oncogenic transformation is possible.
Corals are part of another major phylum distant from chordata, the cnidarians. These diploblastic organisms have a radial symmetry and are also mostly marine. A relatively well described disease known as calicoblastic neoplasm affects several species of corals of the Acroporidae family [18]. These apparent tumors form slightly hemispherical protuberances that are characterized by fewer numbers of polyps per surface area, fewer zooxanthellae per polyp, and finer skeletal structures than normal. Tumor-like formation appears localized, stable and non-epidemic, but tumorous tissue appears to have suffered a higher mortality than normal tissue. The mechanism triggering tumor formation remains unknown [19], and some have interpreted the lack of evidence of anaplasia or mitotic figures (common but not necessarily required morphologic indicators of neoplasia) as growth anomalies rather than neoplasia [20]. Like the Porifera, several genes and pathways involved in malignancy have been characterized in cnidarians. This includes the identification and molecular function of the p53 tumor suppressor-like protein nvp63 in a non-bilaterian animal, the starlet sea anemone Nematostella vectensis [21].
Nematodes or roundworms are a diverse phylum of triploblastic pseudoceolomate animals with more than 80,000 different species. The nematode C. elegans is a well-known model organism for which a tumor from the germinal layer induced by a member of the Notch family has been described [22]. More recently, a homologue of the retinoblastoma susceptibility gene (Rb; also known as Rb1) that is functionally inactivated in most human solid tumors, has been characterized in C. elegans and shown to play a role in cell proliferation and differentiation [23]. It is noteworthy that C. elegans has become a useful model organism to dissect critical genes and pathways affected during tumorigenesis such as apoptosis and Ras signaling [5].
Mollusca (e.g., mollusks) is one of the two major phyla of triploblastic protostome animals. Many different tumors have been described in mollusks with either single or double shells. A number of examples of tumors with some malignant features exist, for example, oyster epithelioma, neurofibromas, sarcomas, and leukocytic neoplasms (leukemia-like disorders). A type of cancer similar to leukemia is well described in the mollusk Mya arenaria, a bivalve. These putative malignant leukemia cells are polyploid, transplantable, and can be grown in vitro [24]. Although it is a matter of debate whether this type of tumor can be considered metastatic it is clearly invasive. Hemic neoplasia characterized by the presence of single anaplastic cells with enlarged nuclei and frequent mitosis, in hemolymph vessels and sinuses in the connective tissue are also found with increasing prevalence in the blue mussel Mytilus trossulus from polluted areas of Nakhodka Bay in the Sea of Japan [25] and the Black sea [26]. Besides the role of environmental “stressor”, evidence suggests the involvement of mutations of homologs of the ras oncogene and deregulated expression of p53 tumor suppressor [27, 28].
Arthropoda (e.g., insects, crustaceans) is the second major triploblastic protostome phyla of which insects represent by far the largest number of animal species (more than 500,000). In insects, several types of tumors have been reported, especially in the fly Drosophila melanogaster, including neuroblastoma, ovarian, and imaginal disk tumors [29, 30]. Moreover, the incidence of tumors of the testis and gut in D. melanogaster increases with age [31]. Mutations affecting asymmetric division and the centrosome function of stem cells promote tumorigenesis [32]. In addition, larval brain cells from transgenic lines of D. melanogaster with extra centrosomes can generate metastatic tumors when they are transplanted into the abdomen of wild-type hosts [33]. The metastatic potential of tumors in D. melanogaster is also supported by the conservation of molecular control of cell migration (e.g., SAP97, TGF pathway) involved in mammalian metastasis [34, 35]. Finally, a large number of mammalian homologs of tumor suppressor genes and other genes controlling proliferation and migration (e.g., Serine/threonine-protein kinase LATS1, Ras signaling, Raf/MAPK pathway) have been identified and characterized in D. melanogaster [36]; this further supports that the tumorigenesis process is conserved in this species, and that spontaneous neoplasia and even malignancies are at least possible.
Neoplasia in other arthropods such as crustacean are less well documented, and are claimed to occur less frequently than in insects [15]. The best described cases are: a lymphoma-like neoplasm in the white shrimp, a decapod (Penaeus vannamei; [37]) that may result from viral infection; and a putative carcinoma-like neoplasm in the hindgut of the red king crab, Paralithodes camtschatica [38]. It is noteworthy that decapod crustaceans living in polluted areas postulated to induce neoplasia in mollusks, don’t show similar diseases, and that experimental exposure to water-born carcinogens at doses known to induce tumors in fish and mollusks are ineffective in decapods such as the white shrimp [15].
In the group of deuterostomes, evidence of neoplasia in echinoderms is weak. Indeed, review of the early literature by Wellings [39, 40] does not reveal any definite case of neoplasia among echinoderms. According to Sparks [41] the only possible neoplasms recorded in echinoderms are the tumor-like epidermal lesions consisting of densely packed cells (mainly melanocytes and spherulocytes) that were originally reported by Fontaine [42] in the ophiuroid Ophiocoma nigra. However, similar tumor-like mass of cells with brownish pigmented granules frequently occur within echinoderm tissues and can also correspond to unwanted material, mostly degenerating ceolomocytes, in the process of being eliminated [40]. Another putative tumor observed in Holothuria leucopilota is more likely to be an unusual outgrowth of the ventral hemal vessel of the holothuroid gut [40].
In summary, multiple examples of neoplastic diseases in diverse invertebrates exist, but definitive evidence in this group of spontaneous metastasis such as a clear invasion of multiple tissues by tumor cells is rare. Possible exceptions may be found in mollusks and drosophila where the molecular control of cell migration present in mammals is conserved, and experimental metastasis have been obtained by transfer of mutated cells with asymmetric division [34, 35]. Another peculiarity of neoplastic diseases reported in aquatic invertebrates is that they are often associated with environmental stressors and pollutants (i.e., viral etiology is rarely found). Finally, the incidence of neoplasia varies greatly among different taxa (from relatively frequent in insects and mollusks to rare or very rare in crustaceans and echinoderms). This should be interpreted as a warning for making generalizations too hastily.
1.2. Vertebrates
In Agnathans or jawless vertebrates, an extensive study has revealed a high incidence of hepatomas and other tumors in the gut, pancreas and kidneys of the Atlantic hagfish collected from the Gullmar Fjord (Myxine glutinosa); these are possibly related to the high content of the pollutant DDT in this location [43, 44]. Cysts and tumor-like lesions in the endocrine pancreas have also been observed in the river lamprey (L. fluviatilis) [45].
In jawed vertebrates, elasmobranchs (i.e., rays and sharks) have been mistakenly claimed by some to be tumor-free [46]. In fact, they appear to be as susceptible as any other vertebrates to tumors, and a large variety of neoplasms and malignancies have been reported, including neuroblastomas, osteomas, ranal and heptic carcinomas, cutaneous fibrosarcomas and fiborma, metastatic, melanomas, adenocarcinomas, and lymphomas [47].
In bony fish, presumably as the result of a greater scrutiny owing to their economical importance, there is a large body of information concerning neoplastic diseases (reviewed in [14] [48, 49]). Indeed, a wide diversity of neoplasia and malignancies matching those found in humans has been described in more than 200 different species. The causes for many of these tumors are often unclear. Environmental factors, especially in aquaculture may play a role in the genesis of some of them, whereas oncoviruses appear to be involved in several cases. Only a few extensively studied examples are considered here. Skin neoplasms in bony fish include tumors in pigmented skin cells such as melanoma but also erythrophoromas and iridophoromas that are not found in mammals [50]. Interestingly, several retroviruses have been found associated with neoplasia in bony fish [51]. The best known is the Walleye dermal sarcoma virus (WDSV) that has been etiologically linked to the development of Walleye dermal sarcoma, a common skin neoplastic disease of walleye fish (Sander vitreus) in the United States and Canada [52]. Another retrovirus has also been identified in association with an outbreak of leiomyosarcoma in the swim bladder of the Atlantic salmon [53]. Additionally, a less well characterized virus-like agent is the possible cause of transmissible neurofibromatosis and neurofibrosarcomas (tumors of neural tissues) in the bicolor damselfish Stegastes partitus [54, 55]. A platyfish-swordtail (Xiphophorus) tumor system has even been used to study the etiology of cancer and reports have demonstrated that neoplasia in these fishes is due to aberrant regulation of certain genes during the multistep process of cancer formation [56, 57]. In addition, model animals such as zebrafish display conserved genes and pathways involved in tumorigenesis and metastasis [58, 59].
As is the case for most animals other than mammals, literature pertaining to amphibian neoplasia is scattered, often incomplete, and difficult to interpret owing to inconsistencies in diagnosis, nomenclature and misdiagnosis of infectious and inflammatory conditions [60]. Among interesting cases to report is a spontaneous epithelioma of the Japanese newt, Cynops pyrrhogaster, that is of possible viral origin [61]. Other neoplasia in different Urodelean (salamanders) and Anuran (frogs and toads) species have been reported [60, 62]. These include pancreatic and renal carcinoma [63] [64]. From a large study of more than 1,200 animals, various spontaneous skin tumors have been described in Rana temporiaria and R. ridibunda [65]. Mast cell tumors (mastocytomas) have also been found in a number of A. mexicanum and A. trigrinum (tiger salamander; [66]). These leukocytic tumors were located in the skin and invaded underneath tissues such as muscles of relatively aged inbred animals. Melanomas have been described in axolotls [67], and interestingly, can be induced by regeneration [68]. One notable type of adenocarcinoma, the Lucké tumor, found in Rana pipiens is among the first documented case of a virally-induced tumor in vertebrates [69-71].
Despite the wide use of the South African clawed frog Xenopus laevis as an experimental animal model, there are only a few reports of spontaneous tumors in this species. In addition, the prevalence, etiology and pathogenesis of these diseases are not well established. To highlight some of the confusion in the literature pertaining to the true diagnosis of neoplasia in amphibians, it’s worth mentioning a pseudotumor, namely an infectious granuloma that was originally reported as a spontaneous malignant lymphosarcoma of viral origin [72]. Subsequent to that report, however, no virus particles could be detected by electron microscopy, and thanks to a series of transplantation studies, the X. laevis tumor-like growth was found to be a transmissible, but not transplantable, bacteria-induced infectious granuloma [73, 74].
This case notwithstanding, there is no doubt that like other vertebrates Xenopus is affected by a whole variety of true neoplastic diseases including tumors that metastasize. Adenoma of the liver appears relatively frequently. It is usually detected during dissection as a markedly enlarged liver lobe that can reach twice its normal size [75]. In most cases, further diagnostics are missing. In our large X. laevis colony, we estimate that such hepatomas affect about 5% of our animals per year. Notably, spontaneous teratomas containing significant thyroid carcinoma components have been described in X. laevis [76]. These teratomas were associated with developmental defects in multiple sibling frogs suggesting a genetic basis for this disease. Other spontaneous neoplasias in X. laevis include tumors of the digestive track [77], melanophoromas, and more rarely, renal carcinoma and nephroblastoma [75, 78]. We have recently described a type of ovarian tumor in X. laevis [2]; a similar tumor was also described in another anuran species (Leptodactylus fallax; [79]).
A particularly interesting type of malignancy studied in X. laevis is a lymphoid tumor. Over the course of about a year, a series of spontaneous thymic lymphoid tumors occurred in the Xenopus colony of the Basel Institute of Immunology. Several stable tumor lymphoid cell lines (named B3B7, 15/0, 15/40 and ff-2) were derived from these frogs [80] [81]. The availability of these tumor lines as well as genetically defined MHC compatible inbred strains and clones of X. laevis have provided the unique opportunity to study both tumorigenesis and anti-tumor immunity in an amphibian (reviewed in [82]). Metastasis in the original animals as well as in some compatible recipients in which tumor cells were transplanted was unequivocally observed. A similar type of thymic tumor was described in another laboratory [83], and another type of spontaneous leukocytic, possibly monocytic, tumor very different from the thymic tumors mentioned, was recently reported by Du Pasquier et al. [84]. Finally, although virus-induced neoplasia has not been documented so far in X. laevis, an endogenous retrovirus has been found that is closely related to the epsilon-retroviruses WDSV and WEHV types 1 and 2, that are associated with neoplasia in walleye fish [85].
In reptiles, among the large variety of neoplasias reported, one can mention a metastatic oviduct adenocarcinoma in snakes (e.g., Boa [86]), and a fibropapillomatosis likely caused by a herpes virus in sea turtles [87, 88]. Herpesvirus-associated papillomatosis has just recently been described in the green lizard, Lacerta viridis, ([89]). Lymphosarcoma and renal neoplasia have also been observed. Interestingly, evidence of tumors has been found in dinosaur fossil records from the Cretaceous [90, 91]. These neoplasia included hemangiomas and metastatic cancer, desmoplastic fibroma, and osteoblastoma.
The literature on malignancies in endothermic (warm blooded) vertebrates (e.g., birds and mammals) is obviously far more abundant and these diseases are better characterized than in invertebrate and ectothermic (cold blooded) vertebrate species. Almost 100 years have passed since Peyton Rous found that a sarcoma in chickens could be transmitted by cell-free extracts of tumor tissue (reviewed in [92]). The identification of the Rous sarcoma virus (RSV) that induces tumors of the connective tissues (sarcomas) initiated a new field of research leading to the discovery of oncogenes. In birds, various other oncogenic retroviruses cause neoplasia and leukemia [93, 94]. For example, Marek’s disease (MD) is caused by a ubiquitous lymphotropic alphaherpesvirus, MD virus (MDV). This disease is characterized by a variety of clinical signs among which are neurological symptoms, chronic wasting and, most notably, the development of multiple lymphomas that manifest as solid tumors in the viscera and musculature [95, 96]. The first successful anti-tumor vaccine has been developed against MD, although vaccine efficacy has decreased concomitantly with the increase in virulence of the virus [97]. Other retrovirus-caused neoplasias include avian leukosis virus (ALV), reticuloendotheliosis virus (REV) and lymphoproliferative disease virus (LPDV).
Given the high incidence of tumors and the volumes of research reported in mammals, only two interesting types of neoplasia will be mentioned here, because of their mode of transmission and apparent lack of immunogenicity. The Tasmanian devil facial tumor disease (DFTD) and the canine transmissible venereal tumor (CTVT) are the only known naturally occurring clonally transmissible cancers [98]. In dogs, DFTV is a fatal monophyletic clonally transmissible tumor that is basically an allograft transmitted between devils by biting [99]. Phylogenetic analyses indicate that CTVT most likely originated from a wolf or an East Asian breed of dog between 200 and 2500 years ago [100]. An interesting common feature of these two types of tumors is the apparent lack or poor immune response of the mammalian host.
In summary, in the entire vertebrate subphylum there is abundant evidence of a large variety of malignancies associated with metastasis. In addition, many of these malignancies are virally-induced.
2. Tumor immunity
The characterization of immunity in the whole animal kingdom has recently made marked progress in parallel with the progress in expressed sequence tag (ESTs) data banks, and whole genome sequencing and analysis for an increasing number of organisms (http://www.ncbi.nlm.nih.gov/genomes/leuks.cgi). In particular, the use of short-range synteny relationships, or gene neighboring, has provided a powerful way to identify many immune genes that were hard to find by molecular techniques because of their sequence divergence. This information has led to new insights about the diversity and complexity of immune systems in various animals. This is particularly true for invertebrate immunity, usually referred to as innate immunity, which involves a wide variety of cells and pathways that globally target pathogens (reviewed in [101]).
However, in contrast to molecular, phylogenetic and to some extent expression patterns, comparative functional analysis of immune genes is still mainly under development. As such, investigation of the role of the immune system in detecting and controlling neoplasia is still poorly investigated in species other than X. laevis, chickens and mammals. A general presentation of the immune systems of invertebrates and vertebrates is beyond the scope of this review and can be found elsewhere [102, 103]. This discussion will focus on aspects related to tumor immunity, and only in invertebrates and nonmammalian vertebrates.
2.1. Invertebrates
A large body of data indicates that invertebrate marine organisms produce a multitude of active substances that, in humans, exhibit potent anti-tumor activity; these include alkaloids and terpenoids (reviewed in [104, 105]). Peptides named alloferons isolated from diptera (insects) also have anti-tumor capacities when tested on mammalian cells [106]. However, it is not clear whether these different substances are produced in response to neoplastic transformation and if they can effectively control self-tumor development in the organisms that produce them. In drosophila, hemocytes have been shown to adhere to tumors upon detection of basement membrane disruption and subsequently counter their growth [107]. Furthermore, these anti-tumor responses involve JAK/STAT-activating cytokines.
Concerning potential cell-mediated anti-tumor immune responses in invertebrates, NK-like cell mediated cytotoxicity has been described for some invertebrate species including sponges [108], annelids [109, 110] mollusks [111], insects and sea urchins [112, 113]. Although immune recognition in invertebrates is not based on MHC molecules, C-type lectin-binding molecules closely similar to vertebrate CD94 and NKR-P1 (typical NK cell markers in mammals) have been identified in urochordates [114]. A CD94 homolog was also identified in hemocytes of the tunicate Ciona intestinalis [115]. In fact, in addition to receptors specific for MHC class I molecules, mice and human NK cells also possess inhibitory receptors specific for non-MHC ligands such as E-Cadherins, lectins or collagen [116-120]. Since these families of molecules are present in all metazoans, their interaction with NK cells and their involvement in anti-tumor NK cell responses in invertebrates is possible [121-123]. It is important to note, however, that the cytotoxic activity in all the studies reported so far in invertebrates has been characterized in the context of either alloreaction or using as cell targets mammalian (mouse, human) tumor cell lines that are especially sensitive to NK killing in vitro. Therefore, the ability of invertebrate NK-like cells to kill invertebrate cognate tumor cells, as well as the active involvement of these effector cells in in vivo host responses against tumor still remain to be demonstrated. Other immune defenses in invertebrates include phagocytosis, encapsulation, and in arthropods, melanization. Although these effector functions can play a critical role in anti-tumor responses, their involvement has not been formally studied.
A last point to consider is that although the immune systems of invertebrates are usually considered to consist of immune receptors and effector molecules that are of limited diversity and germ-line encoded specificities, “adaptive somatic diversification” of molecules involved in immune defense has arisen as well, apparently by convergence, in drosophila [124], snails [125] and sea urchins [126]. Increasing evidence suggests that these surprisingly diversified repertoires of immune molecules are involved in non-self recognition. Whether these immune elements play a role in immune responses to neoplasia remains to be determined.
2.2. Nonmammalian vertebrates
A review of mammalian tumor immunity, a very active area of research especially in mice and humans, is beyond the scope of this paper; moreover, many reviews are already available ([10, 120, 127]). Rather, this review will focus on evidence of tumor immunity in ectothermic vertebrates and birds.
Unfortunately, in jawless fish (Agnatha), virtually nothing is known about tumor immunity despite the substantial record of malignancy occurring in lamprey and hagfish. NK or other types of cell-mediated cytotoxicity has not been reported so far in this taxon. However, this may soon change. The capacity to generate a clonally diverse anticipatory repertoire of lymphocyte receptors was, for a long time, thought to be the prerogative of jawed vertebrates. Recently, however, a remarkable convergent, adaptive-like immune system that is based on somatic recombinatorial assembly of leucine-rich-repeat genetic modules encoding variable lymphocyte receptors has been discovered both in lamprey and hagfish [128, 129]. In addition, the cells producing these immune molecules have the morphology of lymphocytes with characteristics analogous to the B and T cells of jawed vertebrates [130]. Given the postulated complex interactions between tumors and the adaptive immune system of jawed vertebrates, it would be highly relevant to explore the immune response to tumors in these agnathan species.
In contrast to jawless vertebrates, the innate and adaptive immune systems of jawed vertebrates are fundamentally conserved from elasmobranchs to mammals. In particular, genes involved in antigen processing and presentation (e.g., MHC class I, class II, immunoproteasome) and generation of somatic repertoires of lymphocyte receptors (e.g., Rag, Ig, TCRα,β,γ,δ) are all present. However, other than Xenopus that will be examined in the next section, investigation of anti-tumor immune responses has not yet been formally investigated in any ectothermic vertebrate.
In elasmobranchs, both cell-mediated spontaneous and antibody-dependent killing has been characterized in the nurse shark [131]. However, the anti-tumor potential of these types of killing, even against mammalian targets, has not been evaluated. More generally, evidence for the role of the adaptive immune system either direct (e.g., characterization of anti-tumor T or B cell responses) or indirect (e.g., increased prevalence of tumors with age) is completely lacking in this taxon.
In bony fish (teleosts), NK-like and other less well defined types of cell-mediated cytotoxicity has been reported in several species including carp [132, 133] and zebrafish [134]. One of the more promising models for functional studies of cell-mediated cytotoxicity is the catfish where a unique in vitro culture system has been designed to generate stable leukocyte cell lines, and in which both NK and CD8 T cells have been characterized molecularly and functionally albeit not in anti-tumor responses [135-137]. Investigations of anti-tumor immunity in this animal model would likely provide important information. Although genes encoding CD8 and CD4 as well as many relevant cytokines have been characterized in several fish species (reviewed in [138, 139, 140], nothing is known about anti-tumor T cells or immune responses to virally-induced neoplasia in bony fish. Similarly, type I interferon and other related pro-inflammatory responses against pathogens is an active area of study in several model and economically important fish species [138, 141]. However, the possible involvement of these systems in immune surveillance and anti-tumor responses awaits investigation.
In conclusion, despite the large and increasing number of comparative immunological studies in cartilaginous and bony fish, investigations directly addressing the type and potency of immune responses to tumors are lacking.
2.2.1. Xenopus
To date, the amphibian Xenopus is the only ectothermic vertebrate species where the study of anti-tumor responses has been formally investigated in vitro and in vivo thanks to the unique collection of thymic lymphoid tumors described in section 1.2 (reviewed in [2]). Earlier studies with the ff-2 lymphoid tumor that is transplantable and tumorigenic in tadpoles and young post-metamorphic froglets with a relatively poor T cell effector system, but not transplantable in fully mature adults unless they are T cell deficient (i.e., thymectomized at early developmental stage, or sublethaly γ-irradiated) provided the first clear evidence of the conserved critical involvement of T cells in tumor immunity [142, 143]. Further studies, using the 15/0 tumor that is transplantable and very tumorigenic both in larvae and adults that share the same MHC alleles (a/c) with the tumor, have demonstrated that NK cells, CD8 T cells, and other less well-characterized cell types are responsible for generating anti-tumor immune responses in Xenopus (reviewed in [2]).
The active role of NK cells in vivo against transplanted tumors was demonstrated by pre-treatment, one day prior to tumor challenge, with the Xenopus-specific anti-NK cell monoclonal antibody (mAb) 1F8. This resulted in accelerated appearance of tumors as well as an increase in the rate of tumor growth [144]. In addition, potent NK cytotoxic activity has also been demonstrated in vitro against both 15/0 and B3B7 thymic lymphoid tumor cells that do not express classical MHC class Ia proteins [145-147]. The phenotypic and functional characterization of these anti-tumor NK cells in spleens reveals that they are large granulated cells that express the NK surface marker 1F8, but not surface Ig and CD8 molecules, and that they also differentiate in frogs that had been thymectomized at an early developmental stage and rendered T-cell deficient. Their cytotoxic activity requires stimulation by T-cell derived growth factors thereby highlighting the importance of T cells and their secretory products for the development of a potent anti-tumor immune response [145]. Mammalian NK cells are activated through the cumulative signals integrated by their cell surface activating and inhibitory receptors (reviewed in [148]). Ligands for NK cell receptors include MHC class I molecules, and either their absence or their deregulated expression on the surface of cells, can lead to the activation of NK cells [149]. As in mammals, Xenopus NK cells do not kill cognate cell targets expressing MHC class I molecules (i.e., they kill class Ia negative 15/0 tumor cells derived from the LG-15 Xenopus clone but not class I+ splenic lymphoblasts). Interestingly, 15/0 tumors express several nonclassical MHC class Ib (class Ib) gene products that require association with β2-microglobulin (β2-m) for efficient surface expression [150]. Notably, 15/0 tumor cells whose class Ib surface expression is impaired by RNA interference targeting β2-m or class Ib, are more susceptible to NK cell killing in vitro. These data suggest that Xenopus NK cells express inhibitory receptors that can interact with class Ib molecules. Although Xenopus has only a few genes encoding killer inhibitory (KIR) molecules, there is a large family of Fc-like receptors most of which are of the inhibitory type with an immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytoplasmic tail [151, 152].
Similar to NK cells, the role of T cells in anti-tumor responses was first evidenced by immunization with the heat shock proteins (hsps) gp96 or hsp70 purified from 15/0 tumor. As in mammals, Xenopus hsps can chaperone antigens from 15/0 tumors and elicit an anti-tumor cellular response with potency, specificity and kinetics typical of a T cell response [153, 154]. Particular involvement of CD8 T cells in tumor immunity was revealed in vivo by antibody depletion that accelerated the development of transplanted 15/0 tumors [144]. Further evidence was obtained by adoptive transfer of CFSE-stained splenocytes from hsp-immunized Xenopus clones into naïve recipients of the same clone that were subsequently challenged with 15/0 tumors [155]. Significant proliferation of adoptively transferred primed CD8 T cells, but not naïve control ones, was detected by the dilution of the CFSE dye using two-color flow cytometry.
Although, in Xenopus hsps are able to elicit conventional class Ia-restricted CTL responses against minor histocompatibility (H) antigens, hsp-mediated CD8 T cell responses against the 15/0 tumor cannot be class Ia-restricted since this tumor does not express class Ia molecules. We have further characterized these anti-tumor class Ia-unrestricted CD8 T cells (CCU-CTLs) and shown that they interact with class Ib molecules [150]. This was achieved by RNAi silencing of either β2-m or directly class Ib expression, which results in a knockdown of class Ib surface protein on the 15/0 tumor [150]. This treatment resulted both in an impairment of the CD8 T cell’s ability to recognize and kill this tumor in vitro and in the increased tumorigenicity of the class Ib-silenced tumor clones in vivo. These experiments point to the critical role of CD8 T cells as anti-tumor effector cells in Xenopus and also demonstrate that at least a subset of these effector cells can interact with class Ib molecules to mediate anti-tumor immune responses.
Last but not least, some indirect evidence suggests that in Xenopus, NKT-like cells may also be involved in anti-tumor immune responses. In mouse and human, despite their small number relative to T and NK cells, NKT and so called CTL-NK cells appears to contribute significantly in anti-tumor responses either directly or through regulation of other anti-tumor effectors [156, 157]. In Xenopus as stated earlier, in vivo treatment with the anti-NK cell mAb 1F8 impairs rejection of transplanted tumor cells. Importantly, this NK cell-associated molecule is also expressed by a minor population of CD8 T cells in which fully rearranged TCRβ mRNA can be identified [144]. Approximately 4% of adult splenocytes co-express CD8 and the NK cell-associated molecule 1F8. Therefore, in Xenopus, a population of cells exists that expresses both NK- and T cell-associated markers. In addition to inhibiting NK cells, in vivo treatment with the 1F8 mAb likely also impairs NKT-like cells in Xenopus.
Finally, as in other ectothermic vertebrates antibody responses against tumors are sketchy. The only piece of data concerns the detection of antibody following immunization of frogs from the MHC homozygous partially inbred F line with the thymic lymphoid tumor ff-2 against a large surface molecule (180–200 kDa) that is also recognized by anti-F alloserum.
2.3. Endothermic vertebrates
A review of mammalian tumor immunity, a very active area of research especially in mice and humans, is beyond the scope of this paper; moreover, many reviews are already available ([10, 120, 127]). Rather, this review will focus on evidence of tumor immunity in chicken.
2.3.1 Chicken
The chicken is perhaps the best nonmammalian vertebrate model that has been characterized in terms of the immune response, the genetics of disease resistance and vaccine response, and the genomic structure and function of the MHC. With respect to anti-tumor immunity, studies with chickens have concentrated mainly on immune responses against oncoviruses; this focus has resulted in the development of successful vaccines against tumors [97].
Particularly interesting is the association of certain MHC haplotypes with resistance to virus-induced malignancies. The first of such association to be described, and still among the strongest known, is the association of the MHC haplotype B with resistance to Marek’s disease [158-160]. However, particular haplotypes are also associated with resistance of other oncogenic viruses including ALV [161] and RSV [162, 163]. The strong association of MHC haplotypes and resistance to virus-induced malignancies is presumably related to the peculiarity of chicken MHC, which compared to the MHC of typical mammals, is much smaller and simpler, with a different genomic organization (reviewed in [164]). Recombination is rare within the chicken MHC, and this property has been proposed to allow coevolution between interacting genes, such as the class I, TAP1, TAP2 and tapasin genes. A consequence of this coevolution is the expression of a single dominantly expressed MHC class I molecule due to peptide-binding specificity and cell-surface expression level, which determines the immune response to certain infectious pathogens and explain the striking associations with resistance to these pathogens. However, recent genetic studies have revealed that allelic difference of BG1 an IgSF receptor-like protein located in the MHC locus that contains an immunoreceptor tyrosine-based inhibition motif (ITIM) plays a major role in Marek disease and perhaps other oncogene-induced lymphomas [158]. Interestingly, the association of MHC haplotypes with resistance to oncoviruses is not only due to antiviral responses but also to a better ability to control tumors growth and metastasis [97, 162]. Indeed, vaccination with low oncogenic RSV strains induces not only protective immunity but also results in regression of visceral tumors and erythroblastosis induced by RSV [165]. Finally, contribution of non-MHC genes in resistance to viral replication and tumor growth is also documented [162, 166].
3. Conclusions and speculations
Given both case reports and experimental data, there is little doubt that neoplasia is widespread in the whole animal kingdom from sponges to man. The risk of uncontrolled proliferation appears to be a condition of multicellularity and differentiation. The evolutionary emergence of genes controlling differentiation programs and cell proliferation has provided the way for their disruption and deregulation through mutations and escape by cell transformation. The increase in complexity of animals should have offered more possibility for neoplastic transformation. Considering the potential danger of neoplastic transformation for survival, it is reasonable to postulate that any ability to detect and control such tumor cells would provide a selective advantage, and therefore that neoplasia may have played an important role in the evolution of the immune system as much as pathogens. In fact, like host-pathogen interactions, the intricacy of interactions between tumors and host immune systems can be viewed as the result of their “arms race” during evolution that increased in complexity and degree of specialization in parallel with the diversification of organisms.
It is generally considered that neoplasia is less frequent in invertebrates than vertebrates. Certainly, the information in the literature about neoplasia in invertebrates is scant. However, in many instances, reports are more than 20 years old, and recent surveys of neoplasia are limited to organisms of economical importance. Therefore, one has to remain cautious about any conclusion that can be drawn from such a small sample of data. The frequency of tumors in any invertebrate species may depend on the extent to which monitoring is performed. In addition, it is difficult to estimate the possible fraction of animals in the wild that die from a neoplastic disease and therefore disappear from the environment. These caveats notwithstanding, it still appears that both the frequency and the diversity of types of neoplasia in invertebrates are less than in vertebrates, and in some invertebrate taxa such as echinoderms tumor are virtually absent. Furthermore, when we look over the data available in marine and fresh water invertebrates, the major factors for neoplastic disease appear to be environmental stressors (e.g., pollutants), whereas there is little to no evidence of virally-induced tumors. It is possible that the aquatic environment is more favorable for long term exposure to chemicals acting as mutagens or directly affecting regulation of differentiation and proliferation. Owing to the identification of several oncoviruses in several fish species, it is more difficult to explain why similar viruses are not as frequent in invertebrates other than that they have not yet been identified.
As already mentioned, neoplasia has been reported far more frequently in vertebrates than in invertebrates, and the diversity of tumor types is also greatly increased. However, a potentially more fundamentally important difference in neoplastic diseases between invertebrates and vertebrates is metastasis or real malignancy. With the possible exception of bivalves and drosophila, evidence of metastasis is absent in invertebrates, whereas malignancies have been frequently described in all vertebrates including elasmobranchs and agnathans. The particular interest in considering neoplasia in terms of differences in severity and its capacity to metastasize concerns the possible role of the immune system. It has been proposed that the adaptive immune system of jawed vertebrates is playing an active role in the malignancy process. According to this view, neoplasia in invertebrates, with a few exceptions being due to pollution, remain mostly benign and without differentiation of invasive tumor variants because the innate immune system is relatively inefficient in eradicating tumors and, therefore, does not establish a strong enough selective pressure. In contrast, the increased capacity of the vertebrate immune system to generate strong and specific anti-tumor responses ultimately results, over time, in the selection of tumor variants that escape immune surveillance and are more invasive.
A presumptive relationship between development of metastasis and pressure of adaptive immunity could also be drawn in jawless fish. Although the anti-tumor capacity of the agnathan’s immune system is not known, it is noteworthy that its adaptive immune system is distinct and convergent to that of jawed vertebrates and that malignancies have been described. Lampreys and hagfish have an adaptive immune system that uses somatic diversification analogous to, but completely independent of, the adaptive immune system of jawed vertebrates. In particular, T-like cells express surface receptors generated by gene conversion. It is tempting to speculate that these T-like cell effectors can be involved in tumor immune surveillance, and as such, are involved in selection of tumor variants that ultimately escape the control of the immune system and invade other tissues, as is the case for jawed vertebrates.
In this context, it is also tempting to speculate about the evidence of malignancy in the fruit fly. Although spontaneous malignancies have not been reported in drosophila, the invasive nature of tumors resulting from the mutation of genes regulating asymmetric division constitutes clear evidence that metastasis could occur in this invertebrate. On the other hand, the immune system of drosophila and other dipterians, has been revealed to be more complex than previously thought (reviewed in [167]). Not only do they have a conventional innate immune system that is armed by a large variety of receptors suggesting a refined capacity to detect antigens, but they also enjoy a huge potential for somatic diversification of DSCAM molecules generated by alternative splicing that show some features analogous to an adaptive immune system. Although not much is known about anti-tumor immunity in bivalves, rapid progress has been made in characterizing immune systems in mollusks including the large and diversified repertoire of molecules such as the family of fibrinogen-related proteins (FREPS) of the freshwater snail Biomphalaria glabrata [125]. It is, therefore, not impossible that different groups of invertebrates are capable of more complex immune surveillance and responses than usually assumed. Rather than a simplistic dichotomy between invertebrates and vertebrates, one should perhaps consider in more detail for any given taxon or group of organisms, the potentiality of their immune systems in relation to the kind of neoplastic diseases that can occur. This consideration begs for obtaining more information about neoplasia in the animal kingdom.
As a last remark, it will also be very relevant in the future to explore and investigate the possible involvement of viruses in tumorigenesis of ectothermic vertebrates (including the Agnathan) and invertebrates with a putative adaptive-like immunes system such as in the diptera. One can easily envision a strong evolutionary pressure for developing an adaptive capacity of host immune defense in response to emerging viral pathogens.
Table 1.
Summary of available information about the types of tumor and anti-tumor defense found in different classes of animals
| Tumors | Innate immunity | Adaptive immunity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Classes of animals |
Neoplasia | Malignancy Metastasis |
Anti- tumor mol. |
NK-like cells |
Adaptive -like |
T cells | CTLs | B cell (Ab) |
|
| Invertebrates | Porifera | + | NF | + | + | − | − | − | − |
| Cnidaria | + | NF | + | + | + | − | − | − | |
| Nematoda | + | NF | ? | ? | − | − | − | − | |
| Annalida | + | NF | ? | + | − | − | − | − | |
| Mollusca | + | (+/−) | + | + | + | − | − | − | |
| Arthropoda | + | (+/−) | + | + | + | − | − | − | |
| Ascidiacea | ? | NF | ? | + | + | − | − | − | |
| Protochordata | ? | ? | ? | (+) | + | − | − | − | |
| Vertebrates | Agnata | + | + | ? | ? | ? | T-like | ? | B-like |
| Chondrichtyes | + | + | + | + | + | + | + | + | |
| Teleostei | + | + | + | + | + | + | + | + | |
| Amphibia | + | + | + | + (+) | +* | +* | +* | + | |
| Aves | + | + | + | + (+) | + * | + * | + * | +* | |
| Mammalia | + | + | + | + (+) | + * | + * | + * | +* | |
?: unknown; NF: Not found; +: Found or present; (+/−) Some evidence; +*Cognate anti-tumor activity demonstrated.
Acknowledgements
Research cited from the author’s laboratory was supported by 1R03-HD061671-01 and 2R24-AI-059830-06 from NIH. I would like to thank Hristina Nedelkovska as well as Drs. Edith Lord, Ana Goyos, and Nicholas Cohen, for their critical reading of the manuscript.
Abbreviations
- MHC
(major histocompatibility complex)
- mAb
(monoclonal antibody)
- T-cell receptor
(TCR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [2].Goyos A, Robert J. Tumorigenesis and anti-tumor immune response in Xenopus laevis. Frontiers in Bioscience. The frog Xenopus as a model to study evolutionary, developmental and biomedical immunology. 2009. pp. 167–76. [DOI] [PMC free article] [PubMed]
- [3].Vidal M, Cagan RL. Drosophila models for cancer research. Curr Opin Genet Dev. 2006;16(1):10–6. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C, et al. Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science. 2009;323(5918):1218–22. doi: 10.1126/science.1157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poulin G, Nandakumar R, Ahringer J. Genome-wide RNAi screens in Caenorhabditis elegans: impact on cancer research. Oncogene. 2004;23(51):8340–5. doi: 10.1038/sj.onc.1208010. [DOI] [PubMed] [Google Scholar]
- [6].Hyenne V, Chartier NT, Labbe JC. Understanding the role of asymmetric cell division in cancer using C. elegans. Dev Dyn. [DOI] [PubMed]
- [7].Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004:22329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- [9].Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19(2):203–8. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [11].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- [12].Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O. Tumor Immunoediting by NKp46. J Immunol. doi: 10.4049/jimmunol.0901644. [DOI] [PubMed] [Google Scholar]
- [13].Hayakawa S. No cancer in cancers: evolutionary trade-off between successful viviparity and tumor escape from the adaptive immune system. Med Hypotheses. 2006;66(5):888–97. doi: 10.1016/j.mehy.2005.12.010. [DOI] [PubMed] [Google Scholar]
- [14].Smith AC. Comparative pathology: human disease counterparts in marine animals. Arch Pathol Lab Med. 2000;124(3):348–52. doi: 10.5858/2000-124-0348-CPHDCI. [DOI] [PubMed] [Google Scholar]
- [15].Vogt G. How to minimize formation and growth of tumours: potential benefits of decapod crustaceans for cancer research. Int J Cancer. 2008;123(12):2727–34. doi: 10.1002/ijc.23947. [DOI] [PubMed] [Google Scholar]
- [16].Suga H, Katoh K, Miyata T. Sponge homologs of vertebrate protein tyrosine kinases and frequent domain shufflings in the early evolution of animals before the parazoan-eumetazoan split. Gene. 2001;280(1-2):195–201. doi: 10.1016/s0378-1119(01)00784-3. [DOI] [PubMed] [Google Scholar]
- [17].Cetkovic H, Grebenjuk VA, Muller WE, Gamulin V. Src proteins/src genes: from sponges to mammals. Gene. 2004;342(2):251–61. doi: 10.1016/j.gene.2004.07.044. [DOI] [PubMed] [Google Scholar]
- [18].Peters EC, Halas JC, McCarty HB. Calicoblastic neoplasms in Acropora palmata, with a review of reports on anomalies of growth and form in corals. J Natl Cancer Inst. 1986;76(5):895–912. [PubMed] [Google Scholar]
- [19].Yamashiro H, Yamamoto M, van Woesik R. Tumor formation on the coral Montipora informis. Dis Aquat Organ. 2000;41(3):211–7. doi: 10.3354/dao041211. [DOI] [PubMed] [Google Scholar]
- [20].Work TM, Aeby GS, Coles SL. Distribution and morphology of growth anomalies in Acropora from the Indo-Pacific. Dis Aquat Organ. 2008;78(3):255–64. doi: 10.3354/dao01881. [DOI] [PubMed] [Google Scholar]
- [21].Pankow S, Bamberger C. The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE. 2007;2(9):e782. doi: 10.1371/journal.pone.0000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berry LW, Westlund B, Schedl T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 1997;124(4):925–36. doi: 10.1242/dev.124.4.925. [DOI] [PubMed] [Google Scholar]
- [23].Schertel C, Conradt B. C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development. 2007;134(20):3691–701. doi: 10.1242/dev.004606. [DOI] [PubMed] [Google Scholar]
- [24].Walker C, Bottger SA, Mulkern J, Jerszyk E, Litvaitis M, Lesser M. Mass culture and characterization of tumor cells from a naturally occurring invertebrate cancer model: applications for human and animal disease and environmental health. Biol Bull. 2009;216(1):23–39. doi: 10.1086/BBLv216n1p23. [DOI] [PubMed] [Google Scholar]
- [25].Usheva LN, Frolova LT. A connective tissue tumor in the mussel Mytilus trossulus from a polluted region of Nakhodka Bay, the Sea of Japan. Ontogenez. 2000;31(1):63–70. [PubMed] [Google Scholar]
- [26].Ciocan C, Sunila I. Disseminated neoplasia in blue mussels, Mytilus galloprovincialis, from the Black Sea, Romania. Mar Pollut Bull. 2005;50(11):1335–9. doi: 10.1016/j.marpolbul.2005.04.042. [DOI] [PubMed] [Google Scholar]
- [27].Ciocan CM, Moore JD, Rotchell JM. The role of ras gene in the development of haemic neoplasia in Mytilus trossulus. Mar Environ Res. 2006;62(SupplS):147–50. doi: 10.1016/j.marenvres.2006.04.020. [DOI] [PubMed] [Google Scholar]
- [28].Muttray AF, Schulte PM, Baldwin SA. Invertebrate p53-like mRNA isoforms are differentially expressed in mussel haemic neoplasia. Mar Environ Res. 2008;66(4):412–21. doi: 10.1016/j.marenvres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- [29].Scharrer B, Lochhead MS. Tumors in the invertebrates: a review. Cancer Res. 1950;10(7):403–19. [PubMed] [Google Scholar]
- [30].Sparks AK. Review of tumors and tumor-like conditions in protozoa, coelenterata, platyhelminthes, annelida, sipunculida, and arthropoda, excluding insects. Natl Cancer Inst Monogr. 1969:31671–82. [PubMed] [Google Scholar]
- [31].Salomon RN, Jackson FR. Tumors of testis and midgut in aging flies. Fly (Austin) 2008;2(6):265–8. doi: 10.4161/fly.7396. [DOI] [PubMed] [Google Scholar]
- [32].Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37(10):1125–9. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- [33].Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, et al. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133(6):1032–42. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jang AC, Starz-Gaiano M, Montell DJ. Modeling migration and metastasis in Drosophila. J Mammary Gland Biol Neoplasia. 2007;12(2-3):103–14. doi: 10.1007/s10911-007-9042-8. [DOI] [PubMed] [Google Scholar]
- [35].Froldi F, Ziosi M, Tomba G, Parisi F, Garoia F, Pession A, et al. Drosophila lethal giant larvae neoplastic mutant as a genetic tool for cancer modeling. Curr Genomics. 2008;9(3):147–54. doi: 10.2174/138920208784340786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Woodhouse EC, Liotta LA. Drosophila invasive tumors: a model for understanding metastasis. Cell Cycle. 2004;3(1):38–40. [PubMed] [Google Scholar]
- [37].Lightner DV, Brock JA. A lymphoma-like neoplasm arising from hematopoietic tissue in the white shrimp, Penaeus vannamei Boone (Crustacea: Decapoda) J Invertebr Pathol. 1987;49(2):188–93. doi: 10.1016/0022-2011(87)90159-5. [DOI] [PubMed] [Google Scholar]
- [38].Sparks AK, Morado JF. A putative carcinoma-like neoplasm in the hindgut of a red king crab, Paralithodes camtschatica. J Invertebr Pathol. 1987;50(1):45–52. doi: 10.1016/0022-2011(87)90144-3. [DOI] [PubMed] [Google Scholar]
- [39].Wellings SR. Neoplasia and primitive vertebrate phylogeny: echinoderms, prevertebrates, and fishes--A review. Natl Cancer Inst Monogr. 1969:3159–128. [PubMed] [Google Scholar]
- [40].Jangoux M. Diseases of Echinodermata. IV. Structural abnormalities and general considerations on biotic diseases. Dis aquat Org. 1987:3221–9. [Google Scholar]
- [41].Sparks AK. Noncommunicable Diseases. Academic Press; New York: 1972. Invertebrate Pathology. [Google Scholar]
- [42].Fontaine AR. Pigmented tumor-like lesions in an ophluroid echinoderm. Natl Cancer Inst Monogr. 1969:31255–61. [PubMed] [Google Scholar]
- [43].Falkmer S, Marklund S, Mattsson PE, Rappe C. Hepatomas and other neoplasms in the atlantic hagfish (Myxine glutinosa): a histopathologic and chemical study. Ann N Y Acad Sci. 1978:298342–55. doi: 10.1111/j.1749-6632.1977.tb19277.x. [DOI] [PubMed] [Google Scholar]
- [44].Falkmer S. The tumor pathology of Myxine glutinosa. In: Jorgensen JM, Lombolt JP, Weber RE, Malte H, editors. The biology of hagfishes. Chapman and Hall; London: 1998. pp. 101–7. [Google Scholar]
- [45].Hardisty MW. Cysts and tumour-like lesions in the endocrine pancreas of the lamprey (Lampetra fluviatilis) J Zool. 1975:178305–17. [Google Scholar]
- [46].Lane IW, Comac L. Sharks Don’t Get Cancer: How Shark Cartilage Could Save Your Life. Avery Pub Group; Garden City Park, NY: 1992. [Google Scholar]
- [47].Ostrander GK, Cheng KC, Wolf JC, Wolfe MJ. Shark cartilage, cancer and the growing threat of pseudoscience. Cancer Res. 2004;64(23):8485–91. doi: 10.1158/0008-5472.CAN-04-2260. [DOI] [PubMed] [Google Scholar]
- [48].Brown ER, Hazdra JJ, Keith L, Greenspan I, Kwapinski JBG, Beamer P. Frequency of Fish Tumors Found in a Polluted Watershed as Compared to Nonpolluted Canadian Waters. Cancer Res. 1973;33(2):189–98. [PubMed] [Google Scholar]
- [49].Groff JM. Neoplasia in fishes. Vet Clin North Am Exot Anim Pract. 2004;7(3):705–56. vii. doi: 10.1016/j.cvex.2004.04.012. [DOI] [PubMed] [Google Scholar]
- [50].Masahito P, Ishikawa T, Sugano H. Pigment cells and pigment cell tumors in fish. J Invest Dermatol. 1989;92(5 Suppl):266S–70S. doi: 10.1111/1523-1747.ep13076602. [DOI] [PubMed] [Google Scholar]
- [51].Holzschu D, Lapierre LA, Lairmore MD. Comparative pathogenesis of epsilonretroviruses. J Virol. 2003;77(23):12385–91. doi: 10.1128/JVI.77.23.12385-12391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].LaPierre LA, Casey JW, Holzschu DL. Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs. J Virol. 1998;72(11):8765–71. doi: 10.1128/jvi.72.11.8765-8771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Paul TA, Quackenbush SL, Sutton C, Casey RN, Bowser PR, Casey JW. Identification and characterization of an exogenous retrovirus from atlantic salmon swim bladder sarcomas. J Virol. 2006;80(6):2941–8. doi: 10.1128/JVI.80.6.2941-2948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rahn JJ, Gibbs PD, Schmale MC. Patterns of transcription of a virus-like agent in tumor and non-tumor tissues in bicolor damselfish. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138(3):401–9. doi: 10.1016/j.cca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [55].Campbell CE, Gibbs PD, Schmale MC. Progression of infection and tumor development in damselfish. Mar Biotechnol (NY) 2001;3(Supplement 1):S107–14. doi: 10.1007/s10126-001-00323. [DOI] [PubMed] [Google Scholar]
- [56].Chatterjee K, Kollinger G, Schmidt CR, Anders A, Anders F. Cytogenetics of neoplasia of Xiphophorus. Cancer Genet Cytogenet. 1981;3(3):195–209. doi: 10.1016/0165-4608(81)90085-6. [DOI] [PubMed] [Google Scholar]
- [57].Ozato K, Wakamatsu Y. Multi-step genetic regulation of oncogene expression in fish hereditary melanoma. Differentiation. 1983;24(3):181–90. doi: 10.1111/j.1432-0436.1983.tb01318.x. [DOI] [PubMed] [Google Scholar]
- [58].Lam SH, Wu YL, Vega VB, Miller LD, Spitsbergen J, Tong Y, et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24(1):73–5. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- [59].Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res. 2008;6(5):685–94. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- [60].Stacy BA, Parker JM. Amphibian oncology. Vet Clin North Am Exot Anim Pract. 2004;7(3):673–95. vi–vii. doi: 10.1016/j.cvex.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [61].Pfeiffer CJ, Asashima M, Hirayasu K. Ultrastructural characterization of the spontaneous papilloma of Japanese newts. J Submicrosc Cytol Pathol. 1989;21(4):659–68. [PubMed] [Google Scholar]
- [62].Anver MR. Amphibian tumors: a comparison of anurans and urodeles. In Vivo. 1992;6(4):435–7. [PubMed] [Google Scholar]
- [63].Masahito P, Nishioka M, Ueda H, Kato Y, Yamazaki I, Nomura K, et al. Frequent development of pancreatic carcinomas in the Rana nigromaculata group. Cancer Res. 1995;55(17):3781–4. [PubMed] [Google Scholar]
- [64].Masahito P, Nishioka M, Kondo Y, Yamazaki I, Nomura K, Kato Y, et al. Polycystic kidney and renal cell carcinoma in Japanese and Chinese toad hybrids. Int J Cancer. 2003;103(1):1–4. doi: 10.1002/ijc.10774. [DOI] [PubMed] [Google Scholar]
- [65].Khudoley VV, Mizgireuv IV. On spontaneous skin tumours in amphibia. Neoplasma. 1980;27(3):289–93. [PubMed] [Google Scholar]
- [66].Harshbarger JC, Chang SC, DeLanney LE, Rose FL, Green DE. Cutaneous mastocytomas in the neotenic caudate amphibians Ambystoma mexicanum (axolotl) and Ambystoma tigrinum (tiger salamander) J Cancer Res Clin Oncol. 1999;125(3-4):187–92. doi: 10.1007/s004320050262. [DOI] [PubMed] [Google Scholar]
- [67].Khudoley VV, Eliseiv VV. Multiple melanomas in the axolotl Ambystoma mexicanum. J Natl Cancer Inst. 1979;63(1):101–3. [PubMed] [Google Scholar]
- [68].Sheremetieva EA. Spontaneous melanoma in regenerating tails of axolotls. Journal of Experimental Zoology. 1965;158(1):101–21. doi: 10.1002/jez.1401580110. [DOI] [PubMed] [Google Scholar]
- [69].Naegele RF, Granoff A, Darlington RW. The presence of the Lucke herpesvirus genome in induced tadpole tumors and its oncogenicity: Koch-Henle postulates fulfilled. Proc Natl Acad Sci U S A. 1974;71(3):830–4. doi: 10.1073/pnas.71.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Granoff A. Herpesvirus and the Lucke tumor. Cancer Res. 1973;33(6):1431–3. [PubMed] [Google Scholar]
- [71].McKinnell RG, Carlson DL. Lucke renal adenocarcinoma, an anuran neoplasm: studies at the interface of pathology, virology, and differentiation competence. J Cell Physiol. 1997;173(2):115–8. doi: 10.1002/(SICI)1097-4652(199711)173:2<115::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [72].Balls M. Lymphosarcoma in the South African clawed toad, Xenopus laevis: a virus tumor. Ann N Y Acad Sci. 1965;126(1):256–73. doi: 10.1111/j.1749-6632.1965.tb14279.x. [DOI] [PubMed] [Google Scholar]
- [73].Asfari M. Mycobacterium-induced infectious granuloma in Xenopus: histopathology and transmissibility. Cancer Res. 1988;48(4):958–63. [PubMed] [Google Scholar]
- [74].Asfari M, Thiebaud CH. Transplantation studies of a putative lymphosarcoma of Xenopus. Cancer Res. 1988;48(4):954–7. [PubMed] [Google Scholar]
- [75].Reichenbach-Klinke H, Elkan E. The Principal Diseases of Lower Vertebrates. Academic Press; New York: 1965. New York. [Google Scholar]
- [76].Cheong SW, Fukui A, Asashima M, Pfeiffer CJ. Spontaneous thyroid-containing teratoma associated with impaired development in the African clawed frog, Xenopus laevis. J Comp Pathol. 2000;123(2-3):110–8. doi: 10.1053/jcpa.2000.0400. [DOI] [PubMed] [Google Scholar]
- [77].Elkan E. A spontaneous anaplastic intestinal metastasising carcinoma in a South African clawed toad (Xenopus laevis Daudin) J Pathol. 1970;100(3):205–7. doi: 10.1002/path.1711000309. [DOI] [PubMed] [Google Scholar]
- [78].Meyer-Rochow VB, Asashima M, Moro SD. Nephroblastoma in the clawed frog Xenopus laevis. J Exp Anim Sci. 1991;34(5-6):225–8. [PubMed] [Google Scholar]
- [79].Fitzgerald SD, Duncan AE, Tabaka C, Garner MM, Dieter A, Kiupel M. Ovarian dysgerminomas in two mountain chicken frogs (Leptodactylus fallax) J Zoo Wildl Med. 2007;38(1):150–3. doi: 10.1638/06-015.1. [DOI] [PubMed] [Google Scholar]
- [80].Du Pasquier L, Robert J. In vitro growth of thymic tumor cell lines from Xenopus. Dev Immunol. 1992;2(4):295–307. doi: 10.1155/1992/41823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Robert J, Guiet C, Du Pasquier L. Lymphoid tumors of Xenopus laevis with different capacities for growth in larvae and adults. Dev Immunol. 1994;3(4):297–307. doi: 10.1155/1994/37392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Robert J, Cohen N. Evolution of immune surveillance and tumor immunity: studies in Xenopus. Immunol Rev. 1998:166231–43. doi: 10.1111/j.1600-065x.1998.tb01266.x. [DOI] [PubMed] [Google Scholar]
- [83].Earley EM, Reinschmidt DC, Tompkins R, Gebhardt BM. Tissue culture of a mixed cell thymic tumor from Xenopus laevis. In Vitro Cell Dev Biol Anim. 1995;31(4):255–7. doi: 10.1007/BF02633995. [DOI] [PubMed] [Google Scholar]
- [84].Du Pasquier L, Wilson M, Sammut B. The fate of duplicated immunity genes in the dodecaploid Xenopus ruwenzoriensis. Front Biosci. 2009:14177–91. doi: 10.2741/3239. [DOI] [PubMed] [Google Scholar]
- [85].Kambol R, Kabat P, Tristem M. Complete nucleotide sequence of an endogenous retrovirus from the amphibian, Xenopus laevis. Virology. 2003;311(1):1–6. doi: 10.1016/s0042-6822(03)00263-0. [DOI] [PubMed] [Google Scholar]
- [86].Pereira ME, Viner TC. Oviduct adenocarcinoma in some species of captive snakes. Vet Pathol. 2008;45(5):693–7. doi: 10.1354/vp.45-5-693. [DOI] [PubMed] [Google Scholar]
- [87].Quackenbush SL, Work TM, Balazs GH, Casey RN, Rovnak J, Chaves A, et al. Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virology. 1998;246(2):392–9. doi: 10.1006/viro.1998.9207. [DOI] [PubMed] [Google Scholar]
- [88].Jones AG. Sea turtles: old viruses and new tricks. Curr Biol. 2004;14(19):R842–3. doi: 10.1016/j.cub.2004.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Literak I, Robesova B, Majlathova V, Majlath I, Kulich P, Fabian P, et al. Herpesvirus-associated papillomatosis in a green lizard. J Wildl Dis. 46(1):257–61. doi: 10.7589/0090-3558-46.1.257. [DOI] [PubMed] [Google Scholar]
- [90].Rothschild BM, Tanke DH, Helbling M, 2nd, Martin LD. Epidemiologic study of tumors in dinosaurs. Naturwissenschaften. 2003;90(11):495–500. doi: 10.1007/s00114-003-0473-9. [DOI] [PubMed] [Google Scholar]
- [91].Rothschild BM, Witzke BJ, Hershkovitz I. Metastatic cancer in the Jurassic. Lancet. 1999;354(9176):398. doi: 10.1016/S0140-6736(99)01019-3. [DOI] [PubMed] [Google Scholar]
- [92].Vogt PK. A humble chicken virus that changed biology and medicine. Lancet Oncol. 2009;10(1):96. doi: 10.1016/S1470-2045(08)70338-3. [DOI] [PubMed] [Google Scholar]
- [93].Payne LN. Retrovirus-induced disease in poultry. Poult Sci. 1998;77(8):1204–12. doi: 10.1093/ps/77.8.1204. [DOI] [PubMed] [Google Scholar]
- [94].Hatai H, Ochiai K, Nagakura K, Imanishi S, Ochi A, Kozakura R, et al. A recombinant avian leukosis virus associated with fowl glioma in layer chickens in Japan. Avian Pathol. 2008;37(2):127–37. doi: 10.1080/03079450801898815. [DOI] [PubMed] [Google Scholar]
- [95].Jarosinski KW, Tischer BK, Trapp S, Osterrieder N. Marek’s disease virus: lytic replication, oncogenesis and control. Expert Rev Vaccines. 2006;5(6):761–72. doi: 10.1586/14760584.5.6.761. [DOI] [PubMed] [Google Scholar]
- [96].Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek’s disease virus: from miasma to model. Nat Rev Microbiol. 2006;4(4):283–94. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- [97].Gimeno IM. Marek’s disease vaccines: a solution for today but a worry for tomorrow? Vaccine. 2008;26(Suppl):3C31–41. doi: 10.1016/j.vaccine.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [98].Murchison EP. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene. 2008;27(Suppl):2S19–30. doi: 10.1038/onc.2009.350. [DOI] [PubMed] [Google Scholar]
- [99].Murchison EP, Tovar C, Hsu A, Bender HS, Kheradpour P, Rebbeck CA, et al. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science. 327(5961):84–7. doi: 10.1126/science.1180616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126(3):477–87. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Flajnik MF, Du Pasquier L. Evolution of the immune system. In: Paul WE, editor. Fundamental Immunology. 6 th Edition Lippincott Williams & Wilkins; 2008s. pp. 57–122. [Google Scholar]
- [102].Loker ES, Adema CM, Zhang SM, Kepler TB. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol Rev. 2004:19810–24. doi: 10.1111/j.0105-2896.2004.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238(6):1249–70. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].O’Hanlon LH. Scientists are searching the seas for cancer drugs. J Natl Cancer Inst. 2006;98(10):662–3. doi: 10.1093/jnci/djj218. [DOI] [PubMed] [Google Scholar]
- [105].Lebar MD, Heimbegner JL, Baker BJ. Cold-water marine natural products. Nat Prod Rep. 2007;24(4):774–97. doi: 10.1039/b516240h. [DOI] [PubMed] [Google Scholar]
- [106].Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, et al. Antiviral and antitumor peptides from insects. Proc Natl Acad Sci U S A. 2002;99(20):12628–32. doi: 10.1073/pnas.192301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1(2-3):144–54. doi: 10.1242/dmm.000950. discussion 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nappi AJ, Ottaviani E. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays. 2000;22(5):469–80. doi: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [109].Porchet-Hennere E, Dugimont T, Fischer A. Natural killer cells in a lower invertebrate, Nereis diversicolor. Eur J Cell Biol. 1992;58(1):99–107. [PubMed] [Google Scholar]
- [110].Cooper EL, Cossarizza A, Suzuki MM, Salvioli S, Capri M, Quaglino D, et al. Autogeneic but not allogeneic earthworm effector coelomocytes kill the mammalian tumor cell target K562. Cell Immunol. 1995;166(1):113–22. doi: 10.1006/cimm.1995.0013. [DOI] [PubMed] [Google Scholar]
- [111].Franceschi C, Cossarizza A, Monti D, Ottaviani E. Cytotoxicity and immunocyte markers in cells from the freshwater snail Planorbarius corneus (L.) (Gastropoda pulmonata): implications for the evolution of natural killer cells. Eur J Immunol. 1991;21(2):489–93. doi: 10.1002/eji.1830210235. [DOI] [PubMed] [Google Scholar]
- [112].Lin W, Zhang H, Beck G. Phylogeny of natural cytotoxicity: cytotoxic activity of coelomocytes of the purple sea urchin, Arbacia punctulata. J Exp Zool. 2001;290(7):741–50. doi: 10.1002/jez.1124. [DOI] [PubMed] [Google Scholar]
- [113].Arizza V, Giaramita FT, Parrinello D, Cammarata M, Parrinello N. Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):389–94. doi: 10.1016/j.cbpa.2007.01.022. [DOI] [PubMed] [Google Scholar]
- [114].Khalturin K, Becker M, Rinkevich B, Bosch TC. Urochordates and the origin of natural killer cells: identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. Proc Natl Acad Sci U S A. 2003;100(2):622–7. doi: 10.1073/pnas.0234104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zucchetti I, Marino R, Pinto MR, Lambris JD, Du Pasquier L, De Santis R. ciCD94-1, an ascidian multipurpose C-type lectin-like receptor expressed in Ciona intestinalis hemocytes and larval neural structures. Differentiation. 2008;76(3):267–82. doi: 10.1111/j.1432-0436.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- [116].Schwartzkopff S, Grundemann C, Schweier O, Rosshart S, Karjalainen KE, Becker KF, et al. Tumor-associated E-cadherin mutations affect binding to the killer cell lectin-like receptor G1 in humans. J Immunol. 2007;179(2):1022–9. doi: 10.4049/jimmunol.179.2.1022. [DOI] [PubMed] [Google Scholar]
- [117].Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203(2):289–95. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lebbink RJ, Meyaard L. Non-MHC ligands for inhibitory immune receptors: novel insights and implications for immune regulation. Mol Immunol. 2007;44(9):2153–64. doi: 10.1016/j.molimm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- [119].Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20(3):344–52. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9(8):568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cox EA, Hardin J. Sticky worms: adhesion complexes in C. elegans. J Cell Sci. 2004;117(Pt 10):1885–97. doi: 10.1242/jcs.01176. [DOI] [PubMed] [Google Scholar]
- [122].Heino J, Huhtala M, Kapyla J, Johnson MS. Evolution of collagen-based adhesion systems. Int J Biochem Cell Biol. 2009;41(2):341–8. doi: 10.1016/j.biocel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- [123].Wood-Charlson EM, Weis VM. The diversity of C-type lectins in the genome of a basal metazoan, Nematostella vectensis. Dev Comp Immunol. 2009;33(8):881–9. doi: 10.1016/j.dci.2009.01.008. [DOI] [PubMed] [Google Scholar]
- [124].Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309(5742):1874–8. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- [125].Zhang SM, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305(5681):251–4. doi: 10.1126/science.1088069. [DOI] [PubMed] [Google Scholar]
- [126].Ghosh J, Buckley KM, Nair SV, Raftos DA, Miller C, Majeske AJ, et al. Sp185/333: a novel family of genes and proteins involved in the purple sea urchin immune response. Dev Comp Immunol. 34(3):235–45. doi: 10.1016/j.dci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [127].Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–8. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124(4):815–22. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [129].Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310(5756):1970–3. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- [130].Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9(3):319–27. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- [131].McKinney EC, Flajnik MF. IgM-mediated opsonization and cytotoxicity in the shark. J Leukoc Biol. 1997;61(2):141–6. doi: 10.1002/jlb.61.2.141. [DOI] [PubMed] [Google Scholar]
- [132].Nakayasu C, Somamoto T, Hasegawa S, Yoshitomi T, Okamoto N. Differential spontaneous killing of human and murine tumour cell lines by leucocyte subpopulations from carp peripheral blood leucocytes. Fish Shellfish Immunol. 2005;19(2):115–26. doi: 10.1016/j.fsi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [133].Sato A, Okamoto N. Characterization of the cell-mediated cytotoxic responses of isogeneic ginbuna crucian carp induced by oral immunisation with hapten-modified cellular antigens. Fish Shellfish Immunol. 2008;24(6):684–92. doi: 10.1016/j.fsi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- [134].Moss LD, Monette MM, Jaso-Friedmann L, Leary JH, 3rd, Dougan ST, Krunkosky T, et al. Identification of phagocytic cells, NK-like cytotoxic cell activity and the production of cellular exudates in the coelomic cavity of adult zebrafish. Dev Comp Immunol. 2009;33(10):1077–87. doi: 10.1016/j.dci.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [135].Stuge TB, Wilson MR, Zhou H, Barker KS, Bengten E, Chinchar G, et al. Development and analysis of various clonal alloantigen-dependent cytotoxic cell lines from channel catfish. J Immunol. 2000;164(6):2971–7. doi: 10.4049/jimmunol.164.6.2971. [DOI] [PubMed] [Google Scholar]
- [136].Zhou H, Stuge TB, Miller NW, Bengten E, Naftel JP, Bernanke JM, et al. Heterogeneity of channel catfish CTL with respect to target recognition and cytotoxic mechanisms employed. J Immunol. 2001;167(3):1325–32. doi: 10.4049/jimmunol.167.3.1325. [DOI] [PubMed] [Google Scholar]
- [137].Praveen K, Leary JH, 3rd, Evans DL, Jaso-Friedmann L. Nonspecific cytotoxic cells of teleosts are armed with multiple granzymes and other components of the granule exocytosis pathway. Mol Immunol. 2006;43(8):1152–62. doi: 10.1016/j.molimm.2005.07.027. [DOI] [PubMed] [Google Scholar]
- [138].Whyte SK. The innate immune response of finfish--a review of current knowledge. Fish Shellfish Immunol. 2007;23(6):1127–51. doi: 10.1016/j.fsi.2007.06.005. [DOI] [PubMed] [Google Scholar]
- [139].Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19(16):R678–82. doi: 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- [141].Sun B, Robertsen B, Wang Z, Liu B. Identification of an Atlantic salmon IFN multigene cluster encoding three IFN subtypes with very different expression properties. Dev Comp Immunol. 2009;33(4):547–58. doi: 10.1016/j.dci.2008.10.001. [DOI] [PubMed] [Google Scholar]
- [142].Robert J, Guiet C, Du Pasquier L. Ontogeny of the alloimmune response against a transplanted tumor in Xenopus laevis. Differentiation. 1995;59(3):135–44. doi: 10.1046/j.1432-0436.1995.5930135.x. [DOI] [PubMed] [Google Scholar]
- [143].Robert J, Guiet C, Cohen N, Du Pasquier L. Effects of thymectomy and tolerance induction on tumor immunity in adult Xenopus laevis. Int J Cancer. 1997;70(3):330–4. doi: 10.1002/(sici)1097-0215(19970127)70:3<330::aid-ijc14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [144].Rau L, Gantress J, Bell A, Stewart R, Horton T, Cohen N, et al. Identification and characterization of Xenopus CD8+ T cells expressing an NK cell-associated molecule. Eur J Immunol. 2002;32(6):1574–83. doi: 10.1002/1521-4141(200206)32:6<1574::AID-IMMU1574>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [145].Horton TL, Minter R, Stewart R, Ritchie P, Watson MD, Horton JD. Xenopus NK cells identified by novel monoclonal antibodies. Eur J Immunol. 2000;30(2):604–13. doi: 10.1002/1521-4141(200002)30:2<604::AID-IMMU604>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [146].Horton TL, Stewart R, Cohen N, Rau L, Ritchie P, Watson MD, et al. Ontogeny of Xenopus NK cells in the absence of MHC class I antigens. Dev Comp Immunol. 2003;27(8):715–26. doi: 10.1016/s0145-305x(03)00040-5. [DOI] [PubMed] [Google Scholar]
- [147].Goyos A, Cohen N, Gantress J, Robert J. Anti-tumor MHC class Ia-unrestricted CD8 T cell cytotoxicity elicited by the heat shock protein gp96. Eur J Immunol. 2004;34(9):2449–58. doi: 10.1002/eji.200425105. [DOI] [PubMed] [Google Scholar]
- [148].Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Gleimer M, Parham P. Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity. 2003;19(4):469–77. doi: 10.1016/s1074-7613(03)00272-3. [DOI] [PubMed] [Google Scholar]
- [150].Goyos A, Guselnikov S, Chida AS, Sniderhan LF, Maggirwar SB, Nedelkovska H, et al. Involvement of nonclassical MHC class Ib molecules in heat shock protein-mediated anti-tumor responses. Eur J Immunol. 2007;37(6):1494–501. doi: 10.1002/eji.200636570. [DOI] [PubMed] [Google Scholar]
- [151].Guselnikov SV, Ramanayake T, Erilova AY, Mechetina LV, Najakshin AM, Robert J, et al. The Xenopus FcR family demonstrates continually high diversification of paired receptors in vertebrate evolution. BMC Evol Biol. 2008:8148. doi: 10.1186/1471-2148-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Guselnikov SV, Reshetnikova ES, Najakshin AM, Mechetina LV, Robert J, Taranin AV. The amphibians Xenopus laevis and Silurana tropicalis possess a family of activating KIR-related Immunoglobulin-like receptors. Dev Comp Immunol. 34(3):308–15. doi: 10.1016/j.dci.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Robert J, Menoret A, Basu S, Cohen N, Srivastava PR. Phylogenetic conservation of the molecular and immunological properties of the chaperones gp96 and hsp70. Eur J Immunol. 2001;31(1):186–95. doi: 10.1002/1521-4141(200101)31:1<186::AID-IMMU186>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [154].Robert J, Gantress J, Rau L, Bell A, Cohen N. Minor histocompatibility antigen-specific MHC-restricted CD8 T cell responses elicited by heat shock proteins. J Immunol. 2002;168(4):1697–703. doi: 10.4049/jimmunol.168.4.1697. [DOI] [PubMed] [Google Scholar]
- [155].Maniero GD, Robert J. Phylogenetic conservation of gp96-mediated antigen-specific cellular immunity: new evidence from adoptive cell transfer in xenopus. Transplantation. 2004;78(10):1415–21. doi: 10.1097/01.tp.0000140846.73210.91. [DOI] [PubMed] [Google Scholar]
- [156].Moretta L, Mingari MC, Bottino C, Pende D, Biassoni R, Moretta A. Cellular and molecular basis of natural killer and natural killer-like activity. Immunol Lett. 2003;88(2):89–93. doi: 10.1016/s0165-2478(03)00074-9. [DOI] [PubMed] [Google Scholar]
- [157].Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008:101277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Goto RM, Wang Y, Taylor RL, Jr., Wakenell PS, Hosomichi K, Shiina T, et al. BG1 has a major role in MHC-linked resistance to malignant lymphoma in the chicken. Proc Natl Acad Sci U S A. 2009;106(39):16740–5. doi: 10.1073/pnas.0906776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Koch M, Camp S, Collen T, Avila D, Salomonsen J, Wallny HJ, et al. Structures of an MHC class I molecule from B21 chickens illustrate promiscuous peptide binding. Immunity. 2007;27(6):885–99. doi: 10.1016/j.immuni.2007.11.007. [DOI] [PubMed] [Google Scholar]
- [160].Briles WE, Briles RW, Taffs RE, Stone HA. Resistance to a malignant lymphoma in chickens is mapped to subregion of major histocompatibility (B) complex. Science. 1983;219(4587):977–9. doi: 10.1126/science.6823560. [DOI] [PubMed] [Google Scholar]
- [161].Mays JK, Bacon LD, Pandiri AR, Fadly AM. Response of white leghorn chickens of various B haplotypes to infection at hatch with subgroup J avian leukosis virus. Avian Dis. 2005;49(2):214–9. doi: 10.1637/7315-120104R. [DOI] [PubMed] [Google Scholar]
- [162].Taylor RL., Jr. Major histocompatibility (B) complex control of responses against Rous sarcomas. Poult Sci. 2004;83(4):638–49. doi: 10.1093/ps/83.4.638. [DOI] [PubMed] [Google Scholar]
- [163].Suzuki K, Matsumoto T, Kobayashi E, Uenishi H, Churkina I, Plastow G, et al. Genotypes of chicken major histocompatibility complex B locus associated with regression of Rous sarcoma virus J-strain tumors. Poult Sci. 89(4):651–7. doi: 10.3382/ps.2009-00513. [DOI] [PubMed] [Google Scholar]
- [164].Kaufman J, Jacob J, Shaw I, Walker B, Milne S, Beck S, et al. Gene organisation determines evolution of function in the chicken MHC. Immunol Rev. 1999:167101–17. doi: 10.1111/j.1600-065x.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- [165].Plachy J, Karakoz I, Geryk J, Hala K. Vaccination with a low-oncogenic strain of Rous sarcoma virus prevents visceral tumors in chickens. Exp Clin Immunogenet. 1995;12(4):272–82. [PubMed] [Google Scholar]
- [166].Heifetz EM, Fulton JE, O’Sullivan NP, Arthur JA, Cheng H, Wang J, et al. Mapping QTL affecting resistance to Marek’s disease in an F6 advanced intercross population of commercial layer chickens. BMC Genomics. 2009:1020. doi: 10.1186/1471-2164-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Schmucker D, Chen B. Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23(2):147–56. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]