Abstract
Cognitive processing inefficiency, often measured using digit symbol coding tasks, is a putative vulnerability marker for schizophrenia and a reliable indicator of illness severity and functional outcome. Indeed, performance on the digit symbol-coding task may be the most severe neuropsychological deficit patients with schizophrenia display at the group level. Yet, little is known about the contributions of simpler cognitive processes to coding performance in schizophrenia (e.g. decision making, visual scanning, relational memory, motor ability). We developed an experimental behavioral task, based on a computerized digit symbol coding task, which allows the manipulation of demands placed on visual scanning efficiency and relational memory while holding decisional and motor requirements constant. Although patients (n=85) were impaired on all aspects of the task when compared to demographically matched healthy comparison subjects (n=30), they showed a particularly striking failure to benefit from the presence of predictable target information. These findings are consistent with predicted impairments in cognitive processing speed due to schizophrenia patients’ well-known memory impairment, suggesting that this mnemonic deficit may have consequences for critical aspects of information processing that are traditionally considered quite separate from the memory domain. Future investigation into the mechanisms underlying the wide-ranging consequences of mnemonic deficits in schizophrenia should provide additional insight.
Keywords: schizophrenia, processing speed, digit symbol coding, relational memory, cognitive efficiency, slowing
1.1 Introduction
Evidence for reduced information processing speed has been consistently observed in individuals diagnosed with schizophrenia (e.g., [Chapman and Chapman 1973]; King, H.E., 1991; [Mohamed and others 1999]; [Nuechterlein 1977]. Recently, the evaluation of potential illness biomarkers has brought renewed attention to information processing inefficiency in schizophrenia, with particular focus on timed digit-symbol coding tasks, such as the Wechsler Digit Symbol-Coding Test (DSC; Wechsler D., 1997). A recent meta-analysis concluded that patients with schizophrenia have the most significant impairment on the DSC relative to all common neuropsychological measures, a finding that was not sensitive to medication exposure [Dickinson and others 2007]. Furthermore, illness-related slowing predicted diagnostic status even after patients’ substantial generalized cognitive deficit was taken into account [Dickinson and others 2008]. Reduced processing speed is observed in patients with schizophrenia prior to the onset of illness [Niendam and others 2003] and is associated with clinical [Leeson and others 2008] and functional outcomes [Brekke and others 1997]; [Gold and others 2002]. Finally, processing speed deficits are present among patients’ non-schizophrenic first- and second-degree relatives, suggesting that there is a vulnerability-related component to slowed processing [Glahn and others 2007]. Together, these data suggest that processing speed inefficiencies, as measured by the digit symbol coding, represent an important behavioral marker of the pathophysiology of schizophrenia.
Despite growing interest in processing speed inefficiencies [Dickinson 2008]; [Morrens and others 2007]; [Salthouse 1996b], the cognitive roots of this deficit remain unclear. Two cognitive components are traditionally considered critical for determining good performance on the DSC: motor speed and relational memory [Joy and others 2004]; [Salthouse 1996b], the latter thought to be most critical when test takers do not consult the DSC code key for each item. After reviewing reports of DSC performance among healthy individuals, Joy and colleagues (2003) suggested that visual scanning efficiency is also critical for good performance, emphasizing its role when test takers consult the code key frequently during test administration. Additionally, they raised the possibility that among cognitively impaired individuals, these abilities may contribute differently to overall processing speed than they do among unimpaired study participants. Currently, the relative contributions of these cognitive constructs to the speed of processing deficit observed in schizophrenia are unknown. Indeed, patients with schizophrenia show impairments in motor coordination and speed (e.g., [Cannon and others 2000]; [Saykin and others 1994], relational memory performance (e.g., [van Erp and others 2008], and visual scanning efficiency (e.g., [Mahurin and others 1998].
To date, published attempts to deconstruct the processing speed impairment in schizophrenia have focused almost exclusively on regression-based approaches in which performance on neuropsychological tests thought to share particular cognitive components with DSC (e.g., graphomotor speed as measured by the Symbol Copy Test; Wechsler D., 1997) is used to predict digit symbol coding performance (e.g., [Joy and others 2004]. Several of these quite clever studies converge to suggest that patients’ motor slowing cannot account entirely for their general reduction in processing speed ([Jogems-Kosterman and others 2001], [Morrens and others 2006]). Although informative with respect to links with published work, this approach has limitations, and may be somewhat misleading in regard to unique variance attributable to a given neuropsychological test [Dickinson and others 2008].
A complementary approach involves the development of “refined behavioral tasks” [Jonides and Nee 2005] that work experimentally to isolate, control, and/or exaggerate the role of particular information sub-processes to identify their roles in influencing the production of complex, overt behavior. Unfortunately, to our knowledge there are no published manuscripts that attempt to deconstruct the processing speed deficits in schizophrenia. To this end, we adapted a computerized digit symbol-coding task [Glahn and others 2007] in order to independently manipulate the demands placed on visual scanning efficiency and relational memory while holding decisional and motoric requirements constant. More specifically, this novel digit symbol coding task includes conditions where the set size and presentation consistency are varied over 60-second blocks of trials. Increasing the number of digit-symbol pairs or set size from 3 to 6 to 9 pairs augments the demands placed upon both scanning efficiency and relational memory. In contrast, presentation consistency, designed to localize relational memory, was assessed by maintaining the digit-symbol pairing throughout the block of trials (Fixed Condition) or by randomly assigning these pairing for each trial in a 60-second block (Random Condition).
In the current study, we applied this task to a large, clinically well characterized group of patients with schizophrenia and community control subjects. We predicted that despite the apparently minor influence of relational memory on cognitive processing speed when measured in a healthy sample (e.g., [Joy and others 2003], increasing subjects’ capacity to boost processing efficiency by utilizing relational memory would greatly enhance the disparity between non-ill controls and a sample of schizophrenia patients.
1.2 Methods and Materials
1.2.1 Participants
This study was approved by the UTHSCSA IRB, and written informed consent was obtained from all subjects prior to participation. The sample included 85 outpatients with schizophrenia and 30 healthy comparison subjects matched for age (mean age in years [s.d.]: patients, 45.83 [10.06], controls, 43.82 [10.13]), education level (mean number of years completed [s.d.]: patients, 11.83 [4.15], controls, 12.57 [2.53]), sex (number female: patients, 45/83, and controls, 16/3), and handedness. The ethnic and racial makeup of the groups did not differ from each other (χ2[4,115]=2.3, p=0.6) and was representative of the South Texas community from which they were recruited. Patients were recruited through UTHSCSA outpatient clinics and community mental health facilities. Inclusion criteria for patients were: 1) a diagnosis of schizophrenia/schizoaffective disorder as determined by the Structured Clinical Interview for DSM-IV [First and others 1998a; First and others 1998b]; 2) no current concomitant Axis I disorder; 3) no history of medical or neurologic condition that might affect cognitive function. Individuals with history of substance abuse were included in the study, provided that they had not met criteria for a DSM-IV substance abuse or dependence diagnosis in the preceding 6 months.
Healthy comparison subjects were recruited through advertisements placed in local newspapers and on bulletin boards on the UTHSCSA campus, according to the same exclusion criteria used for patients. In addition, control participants had no history of Axis I disorder based on MINI-Plus interview, and no history of schizophrenia in first-degree relatives.
Severity of clinical symptomatology at the time of assessment was rated with the Brief Psychiatric Rating Scale Expanded version (BPRS [Ventura and others 1993]) for patients with schizophrenia. To dissociate severity of psychotic and mood symptoms, items from the expanded version of the BPRS were grouped into empirically defined indices of psychotic, depressive and activation symptoms [Velligan and others 2005].
All 85 patients with schizophrenia were clinically stable outpatients with treatment histories of varying lengths (19.2±9; [1-38 years]). Patients were rated on average as moderately ill with mean BPRS total scores of 45.8±13 [28-87]. Forty-two percent of the sample (n=36) had histories of alcohol or substance abuse. Two patients with schizophrenia previously received treatment for an anxiety disorder. At the time of study, 72 of patients were receiving atypical antipsychotic medications and 13 were taking conventional antipsychotics. In addition to antipsychotics, 43 patients were taking antidepressants and 32 patients were taking benzodiazepines. Forty-four patients lived in board and care facilities, 32 lived with family members and 9 lived independently.
1.2.2 Parametric Digit-Symbol Coding Task
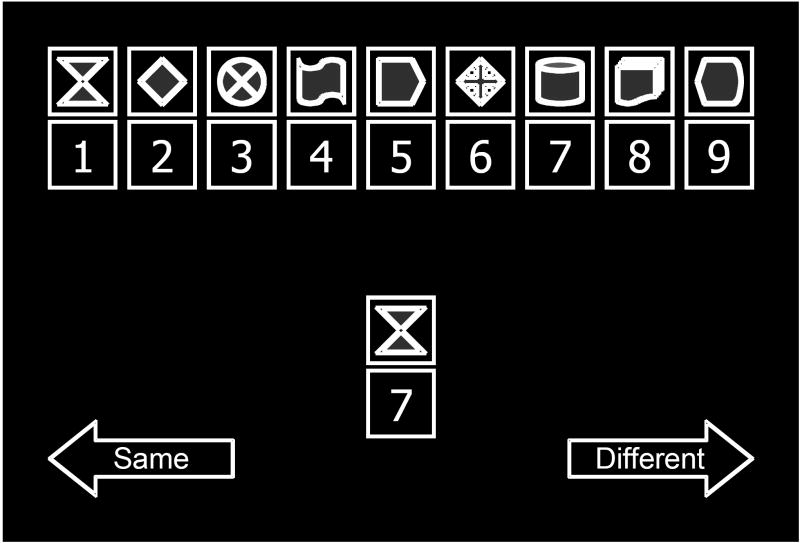
Each item of the parametric digit-symbol coding task included the presentation of a reference set of digit-symbol pairs and a single target digit-symbol pair. Subjects were instructed to indicate, via button press, if the target pair was identical to one the digit-symbol pairs in the reference set (see Figure 1). Trials were self-paced and feedback was not provided.
Figure 1. Schematic Illustration of the Parametric Digit Symbol Coding Task.
In this illustration of a trial of the computerized digit symbol coding task, the single digit-symbol pair in the middle of the figure represents the target pair, which the participant must check against the reference set, represented by the row of digit-symbol pairings along the top of the figure. For publication, bright blue features of symbols were transformed to gray scale. Reference set size was varied parametrically (3, 6, or 9 pairs) between blocks of trials. Additionally, blocks included either a fixed reference set, in which the digit-symbol pairings remained constant from trial to trial, or a random reference set, in which digits were randomly reassigned to the symbols at the beginning of each new trial. Performance during the fixed condition is enhanced by memory of the reference set. Conversely, performance during the random condition is particularly dependent upon visual scanning, because the target pair must be compared to the reconfigured reference set on each trial. In all trials, subjects indicate via button press, if the target pair was identical to one the digit-symbol pairs in the reference set. The arrows reminding the subject which button to press to indicate that the target is the same as or different than a pairing in the reference set remained visible throughout task performance.
The task was administered under two conditions (fixed or random), each with three reference set sizes (3, 6, or 9 pairs), making 6 total blocks of trials. Each block lasted 60 seconds and the number of correct responses achieved during that interval was recorded. Block order was assigned randomly for each individual.
During the fixed condition, the digit-symbol pairings in the reference set were held constant thought the trial. Hence, performance during this condition is enhanced if one is able to keep the reference set in mind. Conversely, poor immediate or relational memory would hamper good performance, particularly when the reference set included 6 or 9 digit-symbol pairs.
During the random condition, the digit-symbol pairings in the reference set were changed for each item. Hence, performance during this condition is particularly dependent upon visual scanning because the target digit-symbol pair must be compared to the reconfigured reference set on each trial.
Prior to performing the task, participants were administered 5 practice items. These example items allowed subjects to become familiar with the test format and provided feedback. The task was self-paced and took between 7-9 minutes to complete.
1.2.3 Hypothesis Tests
Statistical analyses address the following predictions: 1) Patients with schizophrenia will perform more poorly than healthy comparison subjects. 2) All subjects will perform worse on the random compared to the fixed condition. 3) All subjects will perform worse as the number of digit-symbol pairs increases in the reference set. 4) Consistent with poor relational memory, patients with schizophrenia will benefit less from fixing the reference set than healthy subjects (significant Diagnostic Group × Condition interaction).
Statistical analyses were performed with SAS 9.1. To assess the first four predictions, a 2 × 2 × 3 mixed-effects ANCOVA, testing main and interactive effects of Diagnostic Group (schizophrenia, control), Condition (fixed, random) and reference Set Size (3, 6, 9 pairs) was applied. Condition and set size were used as a within-participant (repeated measures) factors and age was covaried. All variables were found to conform to the assumptions of normalcy (Shapiro-Wilk test, p > 0.01). The significance criterion for all hypothesis testing was set at alpha=0.05, two-tailed. F-values are Greenhouse-Geisser corrected when appropriate; however, df values listed are not corrected.
1.3 Results
The 2 × 2 × 3 mixed-effects ANCOVA resulted in a main effect of Diagnostic Group (F[1,112]=58.95, p<0.01), in which control participants displayed a greater number of correct responses than patients did, supporting Hypothesis 1. Consistent with Hypothesis 2, subjects performed better in the fixed condition than they did in the random condition (F[1,112]=21.21, p<0.01). A main effect of Set Size (F[2,224]=8.90, p<0.01), best described by a linear contrast (F[1,112]=11.98, p<0.01), supported Hypothesis 3. A main effect of Age was also evident (F[1,112]=11.92, p<0.01), although it did not test any a priori predictions.
Hypothesis 4, that patients with schizophrenia will benefit less from fixing the reference set than healthy subjects, was supported by a significant Diagnostic Group × Condition interaction (F[1,112]=20.13, p<0.01), whereby control participants displayed a greater difference in number correct when the fixed and random conditions are compared than patients did, regardless of set size. The Diagnostic Group × Set Size interaction approached significance (F[2,224]=2.74, p=0.070), suggestive of a possible group-wise difference in the influence of increasing reference set size. The Age × Set Size condition also reached a trend level of significance (F[2,224]=2.74, p=0.070), but Condition did not interact significantly with Age (F[1,112]=1.453, p=0.231) or with Set Size (F[2,224]=1.49, p=0.228). The three-way interaction of Diagnostic Group, Condition, and Set Size also failed to reach significance (F[2,224]=0.62, p=0.535). Repeating analyses without Age as a covariate did not affect the significance level of any effects.
Finally, when performance on the random condition was entered as a covariate for the fixed condition, the group effect remained significant at each set size (set size 3: F[1,110]=7.168, p<0.01; set size 6: F[1,110]=6.374, p=0.01; set size 9: F[1,110]=15.623, p<0.01). Conversely, when performance on the fixed condition was entered as a covariate for the random condition, the group effect was attenuated at each set size (set size 3: F[1,110]=3.557, p=0.06; set size 6: F[1,110]=3.030, p=0.09; set size 9: F[1,110]=0.028, p=0.87). This pattern of results is consistent with the notion that variance associated with the fixed condition is driving the group effect.
1.4 Discussion
The present findings are consistent with our prediction that schizophrenia patients’ failure to utilize relational memory to enhance cognitive processing efficiency may account for a substantial extent of their processing speed impairment. The group difference in processing efficiency in the fixed pair, memory-intensive condition was much greater than the difference observed in the random pair condition, when heavy demands were placed on visual scanning ability – a result that may be somewhat surprising, given that visual scanning is widely accepted to be abnormal in patients with schizophrenia (e.g., [Lee and Williams 2000]; [Thaker 2008]. Although previous work had suggested that memory deficits might play some role in patients’ slowed / inefficient information processing (e.g., [Morrens and others 2008], the current experimental design was required to display how dramatic the influence of memory may be when constrained by illness-related impairment. These results provide and informative first step in our understanding of patients’ slowed information processing, and illustrate the truly multifactorial nature not only of efficient cognitive processing but also of relative impairments in processing efficiency.
An alternative interpretation is that the multiplex nature of efficient cognitive processing requires a greater level of neurocognitive coordination than schizophrenia patients can support. That is, patients may suffer from a general neural coordination impairment or reduced cognitive reserve (e.g. [Dawson and Nuechterlein 1984], rather than a memory impairment per se. By this formulation, the successful coordination of multiple cognitive components (e.g., visual scanning and matching, memory encoding and retrieval, response selection and execution) may take longer on average if particular operations must be prolonged or repeated so they run in a particular temporal relationship with other processes. A growing body of neuroscientific evidence suggests that the neural mechanisms likely responsible for instantiating the coordination of these constituent cognitive operations is uniquely disrupted in schizophrenia [Glahn and others 2005]; [Minzenberg and others 2009]; [Stephan and others 2009]. For instance, a number of recent electrophysiological studies in schizophrenia patients have produced results suggestive of abnormal coordination among large-scale neural assemblies subserving a wide range of cognitive tasks (e.g., [Roach and Mathalon 2008]. The relational memory-intensive condition would therefore exhibit the greatest relative deficit because optimal performance in that circumstance would depend on the most complex, most temporally precise coordination of constituent sub-components (cognitive and/or neural), similar to phenomena suggested in the cognitive aging literature [Salthouse 1996b]. The question of whether memory impairment or coordination inefficiency is the ultimate cause of patients’ processing slowdown must wait for future study.
It is worth noting that, at present, we cannot determine definitively whether patients lack the relational memory capacity to support speeded cognitive processing, or whether they simply fail to engage this mnemonic strategy. Whichever is the case, the end result is an enhanced gap between patients’ and controls’ performance, a disparity that persists even when group differences in non-mnemonic cognitive functions are controlled. The present findings therefore highlight the role of memory in boosting cognitive processing efficiency, and offer yet another example of how the pervasive learning and memory deficits associated with schizophrenia may affect patients’ behavior in a wide array of challenging situations not typically thought of as memory-intensive. Indeed they suggest that the definitive separation of cognitive factors may prove quite complicated.
One notable consequence of our employing an experimental task design is the degree of uncertainty regarding the extent to which inferences based on performance on the experimental digit symbol coding task generalize to performance on the traditional, paper-and-pencil version. The present set of results are consistent with those from published clinical studies utilizing the traditional version of the test. Nevertheless, future work incorporating both the experimental and traditional measure will be necessary to address the issue definitively.
These results also provide clues as to how investigators should proceed in attempting to understand the mechanisms associating digit symbol coding performance with vulnerability to developing schizophrenia, and with the illness-specific factors such as clinical and functional outcome. For instance, multivariate models of illness vulnerability using a neuropsychological measure of processing speed as a predictor might incorporate variance related to memory functioning as well (e.g., [Salthouse 1996a]. From an interventional perspective, the present findings suggest that a cognitive remediation-based or pharmacological treatment strategy focused on improving memory functioning might also enhance processing speed, depending on specific task and treatment parameters. These possibilities take for granted that schizophrenia patients not only exhibit a range of cognitive deficits, but also that these deficits may influence each other’s expression in ways that require greater levels of complexity in our neurocognitive models of psychiatric illness.
Figure 2. Digit Symbol Coding Performance.
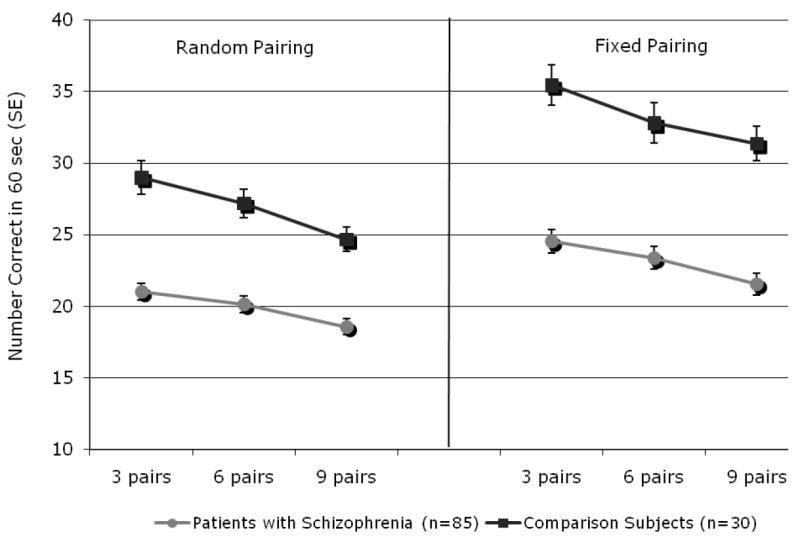
Performance is plotted in number of correct responses provided within a 60 second block of trials, separately for each set size and condition. Patient (N=85) averages are depicted as filled circles and control (N=30) averages are filled squares. Main effects of Condition, Set Size, and Diagnostic Group are apparent, whereby performance was better in the fixed condition, and at lower set sizes. Controls also performed better than patients, a difference that was greater in the fixed condition than in the random condition.
Acknowledgments
The authors would like to thank the research subjects for their generous participation in this study.
Role of the Funding Source. Funding for this study was provided by a National Alliance for Research on Schizophrenia and Depression, NARSAD, young investigator award to Dr. Glahn, and by a National Institute of Mental Health, NIMH, grant R01MH074047 to Dr. Velligan. The funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors. Dr. Glahn designed the experimental task. Drs. Glahn and Velligan supported subject recruitment, which was carried out by Mr. Rice and Ms. Woolsey, Chaves, and Maples, all of whom also participated in data collection and clinical rating. Dr. Reichenberg provided statistical consultation, and Drs. Glahn and Bachman conducted statistical analyses and interpreted the results. Drs. Glahn and Bachman wrote the first draft of the paper. All authors contributed to and have approved the final manuscript.
Conflicts of Interest. The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter Bachman, Email: bachman@psych.ucla.edu.
Abraham Reichenberg, Email: Avi.Reichenberg@iop.kcl.ac.uk.
Patrick Rice, Email: ricep@uthscsa.edu.
Mary Woolsey, Email: woolseym@uthscsa.edu.
Olga Chaves, Email: chaves@uthscsa.edu.
David Martinez, Email: MartinezDM@uthscsa.edu.
Natalie Maples, Email: maplesn@uthscsa.edu.
Dawn I Velligan, Email: VELLIGAND@uthscsa.edu.
David C Glahn, Email: david.glahn@yale.edu.
Literature Cited
- Brekke JS, Raine A, Ansel M, Lencz T, Bird L. Neuropsychological and psychophysiological correlates of psychosocial functioning in schizophrenia. Schizophr Bull. 1997;23(1):19–28. doi: 10.1093/schbul/23.1.19. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67(2):369–82. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Disordered thought in schizophreni. xi. New York: Appleton-Century-Crofts; 1973. p. 359. [Google Scholar]
- Dawson ME, Nuechterlein KH. Psychophysiological dysfunctions in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10(2):204–32. doi: 10.1093/schbul/10.2.204. [DOI] [PubMed] [Google Scholar]
- Dickinson D. Digit symbol coding and general cognitive ability in schizophrenia: worth another look? Br J Psychiatry. 2008;193(5):354–6. doi: 10.1192/bjp.bp.108.049387. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823–7. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, Research Version, Non-Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998a. [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, Research Version, Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998b. [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, Meyenberg N, Castro MP, Barrett J, Nicolini H, et al. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(2):242–9. doi: 10.1002/ajmg.b.30446. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. American Journal of Psychiatry. 2002;159:1395–1402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Jogems-Kosterman BJ, Zitman FG, Van Hoof JJ, Hulstijn W. Psychomotor slowing and planning deficits in schizophrenia. Schizophr Res. 2001;48(2-3):317–33. doi: 10.1016/s0920-9964(00)00097-9. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Assessing dysfunction using refined cognitive methods. Schizophr Bull. 2005;31(4):823–9. doi: 10.1093/schbul/sbi053. [DOI] [PubMed] [Google Scholar]
- Joy S, Fein D, Kaplan E. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003;10(1):56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol--Coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19(6):759–67. doi: 10.1016/j.acn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM. Eye movement dysfunction as a biological marker of risk for schizophrenia. Aust N Z J Psychiatry. 2000;34(Suppl):S91–100. doi: 10.1080/000486700228. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Barnes TR, Harrison M, Matheson E, Harrison I, Mutsatsa SH, Ron MA, Joyce EM. The Relationship Between IQ, Memory, Executive Function, and Processing Speed in Recent-Onset Psychosis: 1-Year Stability and Clinical Outcome. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahurin RK, Velligan DI, Miller AL. Executive-frontal lobe cognitive dysfunction in schizophrenia: a symptom subtype analysis. Psychiatry Res. 1998;79(2):139–49. doi: 10.1016/s0165-1781(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56(8):749–54. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Matton C, Madani Y, van Bouwel L, Peuskens J, Sabbe BG. Delineating psychomotor slowing from reduced processing speed in schizophrenia. Cogn Neuropsychiatry. 2008;13(6):457–71. doi: 10.1080/13546800802439312. [DOI] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33(4):1038–53. doi: 10.1093/schbul/sbl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Van Hecke J, Peuskens J, Sabbe BG. Sensorimotor and cognitive slowing in schizophrenia as measured by the Symbol Digit Substitution Test. J Psychiatr Res. 2006;40(3):200–6. doi: 10.1016/j.jpsychires.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160(11):2060–2. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3(3):373–428. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34(5):907–26. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. General and specific speed mediation of adult age differences in memory. J Gerontol B Psychol Sci Soc Sci. 1996a;51(1):P30–42. doi: 10.1093/geronb/51b.1.p30. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996b;103(3):403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35(3):509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–73. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Lesh TA, Knowlton BJ, Bearden CE, Hardt M, Karlsgodt KH, Shirinyan D, Rao V, Green MF, Subotnik KL, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100(1-3):181–90. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan D, Prihoda T, Dennehy E, Biggs M, Shores-Wilson K, Crismon ML, Rush AJ, Miller A, Suppes T, Trivedi M, et al. Brief psychiatric rating scale expanded version: How do new items affect factor structure? Psychiatry Res. 2005;135(3):217–28. doi: 10.1016/j.psychres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the expanded Brief Psychiatric Rating Scale. International Journal of Methods in Psychiatry Research. 1993;3:227–244. [Google Scholar]