Abstract
Background
Schizophrenia is hypothesized to involve disordered connectivity between brain regions. Currently, there are no direct measures of brain connectivity; functional and structural connectivity used separately provide only limited insight. Simultaneous measure of anatomical and functional connectivity and its interactions allow for better understanding of schizophrenia-related alternations in brain connectivity.
Methods
27 schizophrenia patients and 27 healthy controls undergone MRI imaging using resting state fMRI and Diffusion Tensor Imaging. Separate functional and anatomical connectivity maps were calculated and combined for each subject. Global, regional and voxel measures and K-means network analysis were employed to identify group differences and correlation with clinical symptoms.
Results
A global connectivity analysis indicated that patients had lower anatomical connectivity and lower coherence between the two imaging modalities. In schizophrenia these group differences correlated with clinical symptom severity. While anatomical connectivity nearly uniformly decreased, functional connectivity in schizophrenia was lower for some connections (e.g. middle temporal gyrus) and higher for others (e.g. cingulate and thalamus). Within the Default Mode Network (DMN) two separate subsystems can be identified. Schizophrenia patients showed decoupling between structural and functional connectivity that can be localized to networks originating in Posterior Cingulate Cortex as well as in the Task Positive Network and one of DMN components.
Conclusions
Combining two measures of brain connectivity provides more comprehensive descriptions of altered brain connectivity underlying schizophrenia. Patients show deficits in white matter anatomy but functional connectivity alterations are more complex. Fusion of both methods allows identification of subsystems showing both increased and decreased functional connectivity.
Keywords: imaging, schizophrenia, resting state, connectivity, Diffusion Tensor Imaging
INTRODUCTION
The hypothesis that schizophrenia is more associated with disordered connectivity between various brain regions than with deficits within any particular structure dates back over 100 years to Bleuler (1). It has been revisited with the advent of modern neuroimaging methods by Volkow et al. (2), and Friston (3). This conjecture has garnered interest and led subsequently to multiple imaging studies using various techniques that offer some confirmatory evidence (4). Two major approaches to measuring brain connectivity in schizophrenia include diffusion tensor imaging (DTI), a measure of structural connectivity, and resting state temporal correlations (RSTC), a measure of neural activity functional connectivity. The current study combined both techniques, as recently used in a study of healthy controls (5) to investigate differences in brain connectivity between schizophrenia patients and healthy controls.
Anatomical Connectivity in white matter in schizophrenia
DTI provides an in vivo measure of anatomical connectivity through white matter (WM) tracts. It uses the spatial measure of local water diffusion (diffusion tensor), mainly anisotropy, to detect the presence and structural coherence of WM tracts through measurement of altered water diffusion. Local measures of anisotropy quantify WM integrity, while directionality of the diffusion tensor allows for detection of WM fibers. The commonest reported finding in schizophrenia is decreased FA (6, 7). Reviews show a large diversity of affected regions that mostly converge upon frontal and temporal areas (8, 9). Several studies additionally report correlations between regional FA and behavioral measures in schizophrenia (10) (11). WM tractography-based studies go beyond identifying disturbances within tracts to detect compromised connections between specific regions. Such approaches may be limited to segmentation, e.g. Kubicki (12), or used to investigate fiber deficits directly, e.g. reporting connectivity reductions in the uncinate fasciculus and anterior thalamic radiation (13). A comprehensive review can be found in (14).
Resting State Temporal Correlations in schizophrenia
Resting state temporal correlations between fMRI time courses of distant brain regions were first observed by Biswal (15). Their underlying physiological mechanism is still not comletely understood, but it is widely believed that RSTC represents coordinated spontaneous oscillations in the rate of local neuronal activity (i.e., ‘functional connectivity’) in interconnected distant brain regions (16, 17). Resting state connectivity has been used to investigate connectivity deficits in schizophrenia with varied results. There are reports of both increased and decreased connectivity strength between various region pairs (18–25). A frequently examined circuit is the “default mode network” (DMN), which several papers (25–29) have shown is abnormal in schizophrenia. Another important network frequently detected by resting state correlation is Task Positive Network (TPN) system characterized by presence of activations in multiple cognitive tasks and by its negative correlation to DMN in resting state spontaneous fluctuations(21, 30), regions of TPN has also been implicated as affected by schizophrenia(21, 31).
Combination Resting State and DTI connectivity measures
Since the above methods both measure connectivity indirectly, the similarities in the connectivity matrices obtained from each approach have been used to validate the other (5, 32). DTI measures anatomical integrity of WM tracts, the material backbone for communication between brain regions, while resting connectivity represents the strength of the functional connectivity between regions. Weakened neuronal connections may, (but do not necessarily) interfere with information flow in the brain. On the other hand the breakdown in communication (and thus functional connectivity) between brain regions may result in compensatory changes other than physical disruption of neuronal fibers, e.g. reorganization of brain networks occurring after anatomical connections have been established, thus representing their over- or under-utilization.
Both structural and functional connectivity measures exhibit large variance in mean global connectivity. This lowers the power to detect localized connectivity differences. Combining both techniques allows one to use structural connectivity patterns to allow for more sensitive detection of altered functional connectivity from its anatomical backbone.
We hypothesized that spatial correlations between structural and functional connectivity measures would prove a useful measure to detect abnormal brain connectivity patterns. It should be noted that pathology is not necessarily always represented as lowered coherence between spatial and functional connectivity. For example, in normal aging, deterioration of white matter may cause natural reorganization of functional connectivity and thus cause the disconnection between functional connectivity from decaying structural while the lack of the functional reorganization manifesting itself as closer similarity between functional and structural connectivity may be a marker of dementia(33).
Therefore, the premise of this study is that spatial coherence between structural and functional connectivity can be an important tool for investigating brain connectivity. Therefore, our goal to further demonstrate the validity of this premise was realized by testing the primary hypothesis that schizophrenia patients would exhibit decoupling between these two aspects of brain connectivity. The specific neural networks involved were predicted based on relevant earlier work showing abnormality in schizophrenia. Earlier results (25, 31) suggest that DMN connectivity patterns is be affected in schizophrenia, while (34) suggest disruptions in limbic system networks especially in cingulate gyrus.
MATERIALS AND METHODS
All the imaging and data processing approaches follow methods we used previously in healthy individuals (5).
Participants
We analyzed data from 27 schizophrenia patients and 27 age and gender matched healthy control subjects. Patient ages (mean±SD) were 38±10, ranging between 25–56 years, 17 males. Schizophrenia subjects underwent behavioral testing that included evaluation with the PANSS (35) and Thought Disorder Index (TDI) (36). PANSS scores averaged: Positive 17.5±6.3, Negative 17.5±16.8 General 28.0±7.7, the average TDI score was 15 ±14. Medication information is provided in the Supplementary Material. Control subjects ages were 39±11, range 22–61 years, 17 males. They were free of psychiatric disorders, as assessed by the Structured Clinical Interview for DSM-IV (35), were neurologically normal, non substance abusers. All participants had negative urine screens for abused drugs. All participants underwent MRI scanning including structural anatomical scans, resting state fMRI and DTI imaging that were utilized for the current study. All were participating in protocols approved by the Hartford Hospital IRB and had signed informed consent.
MR imaging
MR imaging was performed using a 3T Siemens Allegra dedicated head scanner (Erlangen, Germany) equipped with 40 mT/m gradients and a standard quadrature head coil. Resting state data were collected during one run of 210 images at TR/TE 1500/28 msec, flip angle 65°, FOV 24×24 cm, 64×64 matrix, 3.75×3.75 mm2 in-plane resolution, 5 mm slice thickness, 30 slices, with slice timing correction. DTI was performed using a twice- refocused standard EPI sequence with TR/TE=5800/87 msec, FOV=20 cm, acquisition and reconstruction matrices = 128×96 and 128×128, diffusion sensitizing orientations in 12 directions with one b0 and 8 averages for each direction, b=1000 s/mm2, 45 contiguous axial slices with 3 mm section thickness.
Data analysis
Data analysis followed precisely methods presented in (5) and is only briefly presented here with full details in the Supplementary Material. Data processing was performed using SPM2, DtiStudio (Johns Hopkins University, Baltimore, MD; http://cmrm.med.jhmi.edu) (37) and in-house software written using MATLAB (http://www.mathworks.com/). To quantify the strength of anatomical connectivity between voxels we counted all possible tract paths connecting them that can be built from individual fibers identified during the tractography step with weighting that favored more directs paths.
Region of interest (ROI) definition
We defined ROIs for local connectivity maps and to quantify the strength of connectivity between regions. We chose a priori 28 ROIs to cover most gray matter using the Wake Forest atlas querying tool (38) (http://www.ansir.wfubmc.edu). The sizes and stereotactic (39) coordinates of ROIs are presented in the Supplementary Table.
Network analysis
Network identification was performed using a K-means clustering algorithm using 10 repetitions. The initial analysis was performed for individual 6000×6000 voxel resting state correlation maps with the number of clusters fixed at 2. The resulting delineation was combined between subjects to identify standardized subsystems . The two resulting components were identified with those identified previously as DMN and TPN (30). Both components were again analyzed and further subdivided using the K-means clustering algorithm this time using two average anatomical connectivity matrices (one each for controls and patients). For DMN this led to subdivision into two subcomponents that varied significantly in between-group comparisons and in correlation to clinical symptoms. The TPN subdivisions produced components that did not differ significantly between groups and were not further utilized. The internal connectivity within components was calculated by averaging connectivity for all voxels originating and ending in this component, but separated by a distance of more than 24 mm, as in (5).
Group differences and correlations to clinical symptoms
Throughout the paper anatomical and functional connectivity measures are used to detect differences related to schizophrenia. We predicted that group difference measures would significantly differ between controls. Furthermore, we anticipated that supplemental analyses would show a linear relationship between these measures and patients’ clinical symptoms.
Age and motion
Previous studies (40–42) report that brain alterations in schizophrenia are related to age/illness duration. Here, we decided not to explore such age related effects and their possible influence on between-group differences, but instead to use carefully age-matched groups. All between-subject correlations were calculated accounting for age dependence. None of the 6 motion parameters differed significantly between groups.
RESULTS
a) Global effects
We first analyzed global connectivity measures using group t test and Pearson correlation with clinical measures for the patient group. For mean fractional anisotropy (FA) values, the between-group difference was significant at p<0.0001, the number of fibers detected by fiber tracking algorithm differed at p<0.03, but the total length of detected fibers was not different between groups. The mean value of DTI connectivity (averaged over whole 6000×6000 matrix) was lower in patients than controls (p<0.005). For patients this measure also significantly correlated with the positive symptom score of the Positive and Negative Symptom Scale (PANSS) (p<0.002), and with Thought Disorder Index (TDI) (36) scores (p<0.02), but not with general or negative PANSS scores. More specific analysis of positive PANSS subscores showed that global DTI measures correlated negatively with indices of social avoidance (r= −0.57, p<0.001) and hallucinations (r = −0.45, p<0.01).
The global average of resting connectivity showed no significant difference between groups, but was positively correlated with positive PANSS (p<0.01) and TDI (p<0.005) scores and negatively correlated with the negative PANSS score (p<0.01).
In agreement with prior results (5), the spatial correlation between global arrays of resting and DTI connection was significant in the control group, mean r = 0.16±0.02 (p<0.0001), and weaker but still significant in patients r = 0.13±0.04 (p<0.002).
In a between-group comparison, the correlation between anatomical and functional connectivity matrices was significantly lower in patients than controls (p<0.02). The correlation of this measure with symptom scores in patients revealed a negative correlation with positive (p<0.05) and general (p<0.02) PANSS scores, but a positive correlation (in contrast to trend suggested by the group difference) with positive PANSS (p<0.05) and TDI scores at (p<0.05).
b) Regional Correlation maps
To further analyze and localize the spatial coherence between resting and DTI connectivity we calculated, for each of 28 predefined ROIs, resting and DTI connectivity map showing for every voxel the strength of its connectivity to this given ROI. Thus for each ROI we were able to quantify the spatial coherence between spatial and functional connectivity of network originating in it. All regions showed significant coherence between both connectivity measures in control subjects, and albeit less significantly for patients. These regional values were then compared between groups using t tests. For one region, posterior cingulate, the between-group difference in this between-modality coherence value was significant (p<0.02), with patients showing less coherence. Thus, in the patient group the functional connectivity pattern originating from posterior cingulate was less similar to this region’s pattern of anatomical connections in controls, suggesting that this network may be the most affected by functional reorganization related to schizophrenia.
c) Interregional correlations
For every subject and each of 28×27/2 region pairs, we analyzed the mean resting and DTI connectivity averaged for all voxel pairs connecting chosen regions and compared values between groups. For each connection between to ROIs and for each connectivity measure (anatomical and functional) we calculated two statistical maps, one of the significance of the between group difference , and the other of the correlation between connectivity measure and positive PANSS score in the patient group.
To account for multiple comparisons we used logical combination of those maps. The connection was considered to be affected when it showed a significant group difference and a correlation between connectivity measure and clinical symptoms. The differences in anatomical connectivity were more robust and we used threshold of p<0.01 for such comparisons, leading to p<0.04 after correction for multiple comparisons. No changes in functional connectivity survived at this significance level; thus we defined as affected all connection pairs that were significant at p<0.02 for the group difference and correlation comparison, and were also characterized by significantly lower anatomical connectivity at p<0.01.
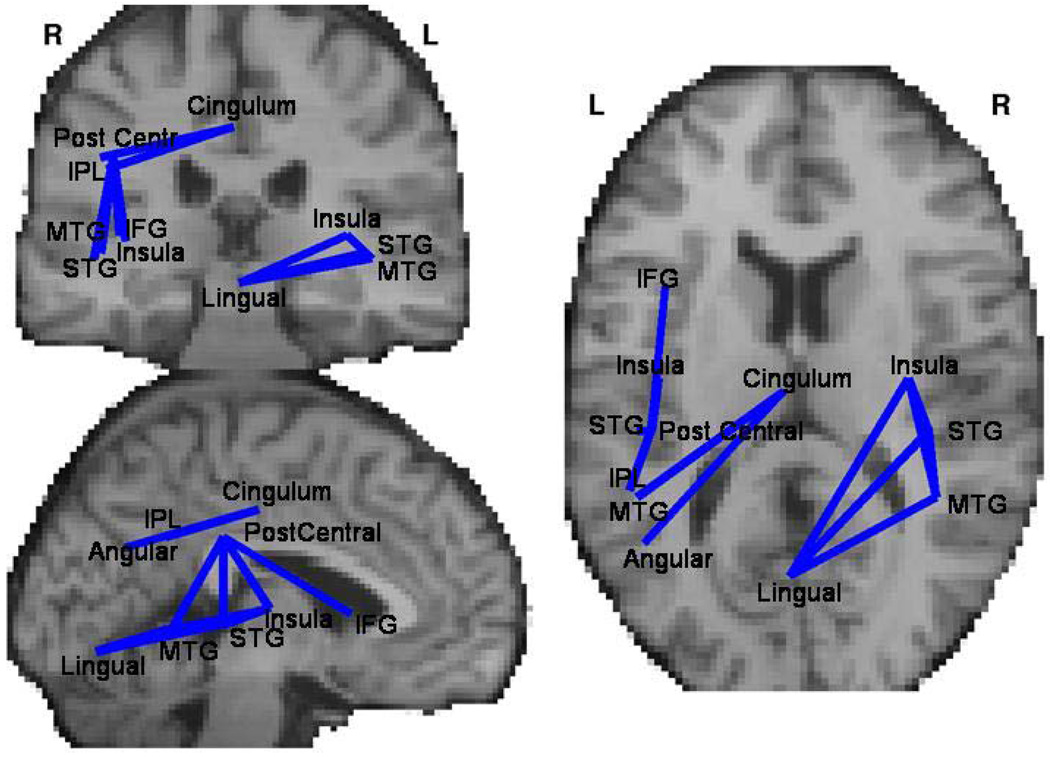
Anatomical connectivity strength among 12 region pairs differed significantly between groups. For all such pairs the anatomical (DTI) connectivity was lower for schizophrenia patients and correlated negatively with intensity of positive PANSS measures. These connections (Figure 1 and Table 1) originated in lingual and cingulate gyri and included connections with left inferior parietal lobule, middle and superior temporal gyri and inferior frontal gyrus.
Figure 1.
Connections between brain regions that differ between schizophrenia patients and controls in measure of anatomical (measured with DTI) connectivity (Table 2). Only connections for which anatomical connectivity was lower in patients than control were significant at p<0.01 for both the group difference and for correlation to clinical symptoms in clinical group.
Table 1.
Region pairs for which there is significant difference in anatomical connectivity. Anatomical connectivity is lower in patients and negatively correlated with positive PANSS subscores for all region pairs. The anatomical position of those connections is presented on figure 1.
| ROI 1 | ROI 2 | Functional Correlation Group Difference (t-value) |
Functional Connectiv ity PANSS correlation (r-value) |
Function al Connecti vity TDI correlatio n (r- value) |
Anatomic al Connectiv ity Group Difference t-value |
Anatomical Connectivity PANSS correlation (r- value) |
Anatomic al Connecti vity TDI correlatio n (r- value) |
|---|---|---|---|---|---|---|---|
| Angular Left | Cingulate | −0.9 | −0.39 | −0.10 | *−2.9 | *−0.50 | −0.22 |
| inferior parietal lobule Left |
Cingulate | 0.8 | −0.09 | −0.09 | *−3.5 | *−0.52 | −0.24 |
| Insula Right | Lingual | −2.0 | −0.39 | −0.18 | *−3.3 | *−0.49 | −0.08 |
| Insula Right | Middle Temporal Gyrus Right |
0.12 | −0.17 | −0.29 | *−3.5 | *−0.51 | −0.11 |
| Lingual | Middle Temporal Gyrus Right |
2.1 | −0.08 | 0.03 | −*3.2 | *−0.50 | −0.14 |
| Inferior Frontal Gyrus Left |
Postcentral Left |
0.6 | −0.35 | 0.21 | **−4.0 | *−0.48 | −0.18 |
| Insula Left | Postcentral Left |
0.7 | 0.01 | 0.18 | **−4.0 | *−0.48 | −0.18 |
| Middle Temporal Gyrus Left |
Postcentral Left |
*−2.9 | *−0.48 | −0.03 | **−3.8 | **−0.51 | −0.17 |
| Postcentral L | Superior Temporal Gyrus Left |
−0.3 | 0.00 | 0.17 | **−3.9 | *−0.49 | −0.18 |
| Insula Right | Superior Temporal Gyrus Right |
−0.7 | 0.02 | −0.19 | *−3.4 | *−0.49 | −0.02 |
| Lingual | Superior Temporal Gyrus Right |
−1.5 | 0.15 | −0.09 | *−3.2 | *−0.55 | −0.06 |
| Middle Temporal Gyrus Right |
Superior Temporal Gyrus Right |
1.3 | −0.48 | −0.35 | *−3.4 | *−0.49 | −0.10 |
denotes p<0.02,
p<0.01.
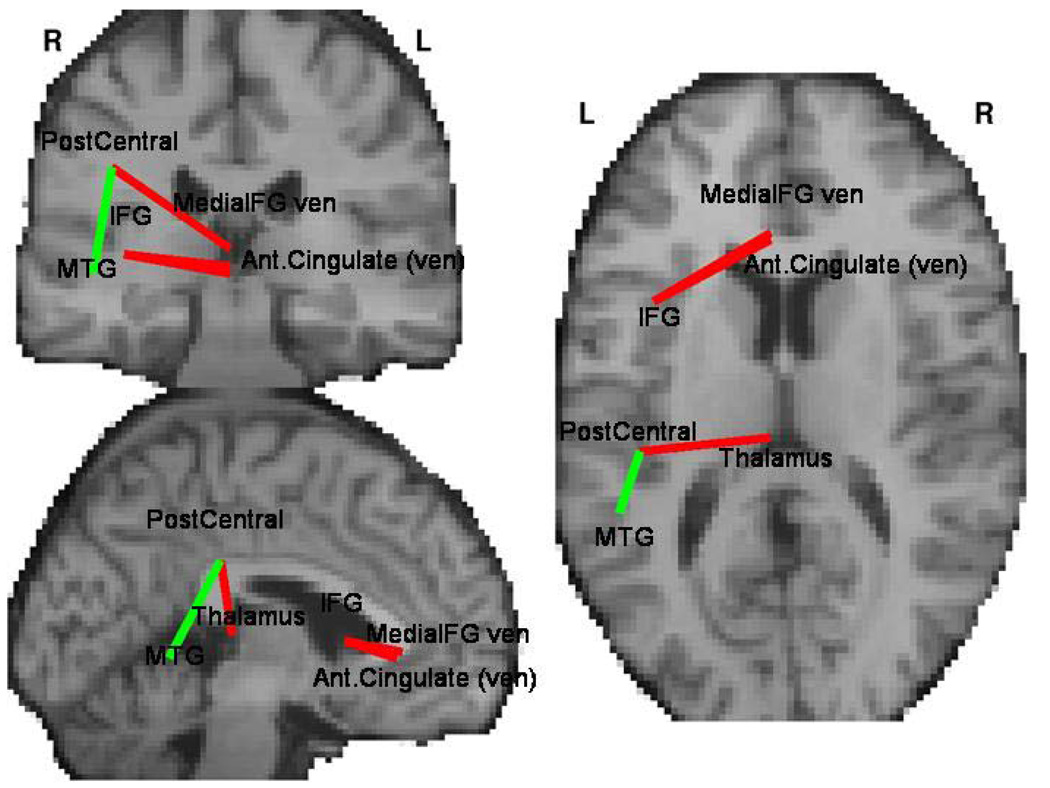
Four of the region pairs showed a between-group difference in strength of functional connectivity (Figure 2 and Table 2). In schizophrenia, all such connections showed significantly lower anatomical connectivity but three showed higher functional connectivity. These latter linked ventral anterior cingulate gyrus and ventral medial frontal gyrus with left inferior frontal gyrus, as well as thalamus with left postcentral cortex. Only one connection, linking left middle temporal gyrus with postcentral gyrus, showed lesser functional connectivity in schizophrenia.
Figure 2.
Connections between brain regions that differ between schizophrenia patients and controls in measure of functional connectivity (Table 2). Red lines represent connections for which the functional connectivity was higher in patients, while for green line connectivity was higher in controls. All shown connections showed significance of p<0.02 for both the group difference and for correlation to clinical symptoms in clinical group.
Table 2.
Region pairs showing significant difference in functional connectivity (measured by resting correlations). Anatomical connectivity is lower in patients and negatively correlated with positive PANSS subscores for all region pairs. The anatomical position of those connections is presented on figure 1.
| ROI 1 | ROI 2 | Functional Correlation Group Difference (t-value) |
Functional Connectivit y PANSS correlation (r-value) |
Functional Connectivit y TDI correlation (r-value) |
Anatomical Connectivit y Group difference (t-value) |
Anatomical Connectivit y PANSS correlation (r-value) |
Anatomical Connectivit y TDI correlation (r-value) |
|---|---|---|---|---|---|---|---|
| Ant.Cing. (Ventral) |
IFG Left | *2.96 | *0.46 | 0.18 | −**4.0 | −0.28 | −0.16 |
| Medial Frontal Gyrus (Ventral) |
IFG Left | *2.65 | **0.48 | 0.25 | **−3.9 | −0.29 | −0.18 |
| Thalamus | Postcentral Left |
*2.75 | *0.45 | *0.44 | **−3.9 | −0.29 | −0.06 |
| Medial Temporal Gyrus Left |
Postcentral Left |
*−3.0 | **−0.48 | −0.26 | **−3.64 | **−0.49 | −0.07 |
denotes p<0.02,
p<0.01.
d) Network analysis
K-means clustering algorithm led to delineation of the Task Positive Network (TPN) and default Mode Network (DMN) with DMN later subdivided into two components that exhibited different group differences. The between-group analysis mean connectivity averaged over all connections within those components showed both significantly less anatomical connectivity within each component separately and also between component connections in schizophrenia. Mean functional connectivity differed significantly only in TPN and was lower in patients.
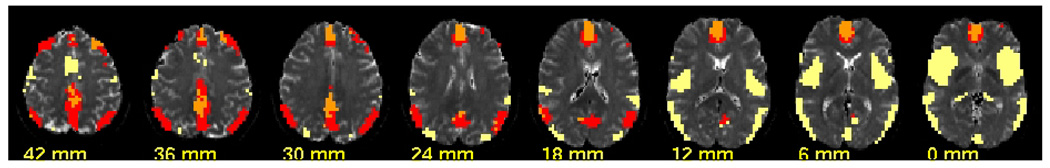
An additional K-means analysis was performed on each component defined above using the anatomical connectivity data averaged separately for control and patient groups. This led to a subdivision of DMN two subcomponents as shown in Figure 3.
Figure 3.
Components detected by k-means cluster analysis of connectivity. The functional resting connectivity led to identification of the default mode network (DMN), depicted in red and orange and its counter part – the task-positive network shown in yellow. The anatomical connectivity analysis further subdivided the DMN into two components : DMN-1 includes anterior cingulate and portions of posterior cingulate, shown in orange, is characterized by higher functional connectivity in schizophrenia and no change in anatomical connectivity between groups. DMN-2 includes parts of bilateral parietal cortices and bilateral dorsolateral prefrontal cortex (DLPFC) is characterized anatomical connectivity lower in schizophrenia.
Table 3 shows the group comparison and correlation to clinical scores in schizophrenia patients for the internal connectivity within each component and between DMN and task positive network as well as between positive and negative subcomponent of DMN.
Table 3.
This shows differences between control and patients in the internal connectivity for each of K-means defined components. Correlations with clinical measures of positive PANSS subscores and Thought Disorder Index also presented. Same data presented for average connectivity between DMN and task-posotive network as well as between subcomponents within DMN.
| Functional Connectivity group difference (t-test) |
Functional Connectivity PANNS correlation (r-value) |
Functional Connectivity TDI correlation (r-value) |
Anatomical Connectivity group difference (t-test) |
Anatomical Conenctivity PANNS correlation (r-value) |
Anatomical Conecttivity TDI correlation (r-value) |
|
|---|---|---|---|---|---|---|
| Within-component, internal connectivity | ||||||
| Task Positive |
−1.1* | −0.27 | 0.12 | −4.6** | −0.48 ** | −0.20 |
| DMN | −1.77 | 0.41 * | 0.03 | −3.4 ** | −0.34 | −0.11 |
| DMN1 | 2.36 * | 0.43 * | −0.25 | −0.2 | −0.0 | −0.08 |
| DMN2 | −1.25 | 0.28 | −0.13 | −5.5 ** | −0.44 ** | −0.16 |
| Between-component connectivity | ||||||
| DMN to Task Positive |
0.65 | −0.23 | −0.24 | −4.49** | −0.42* | −0.15 |
| DMN1 to DMN2 |
−0.87 | 0.53** | 0.18 | −3.6** | −0.31 | −0.09 |
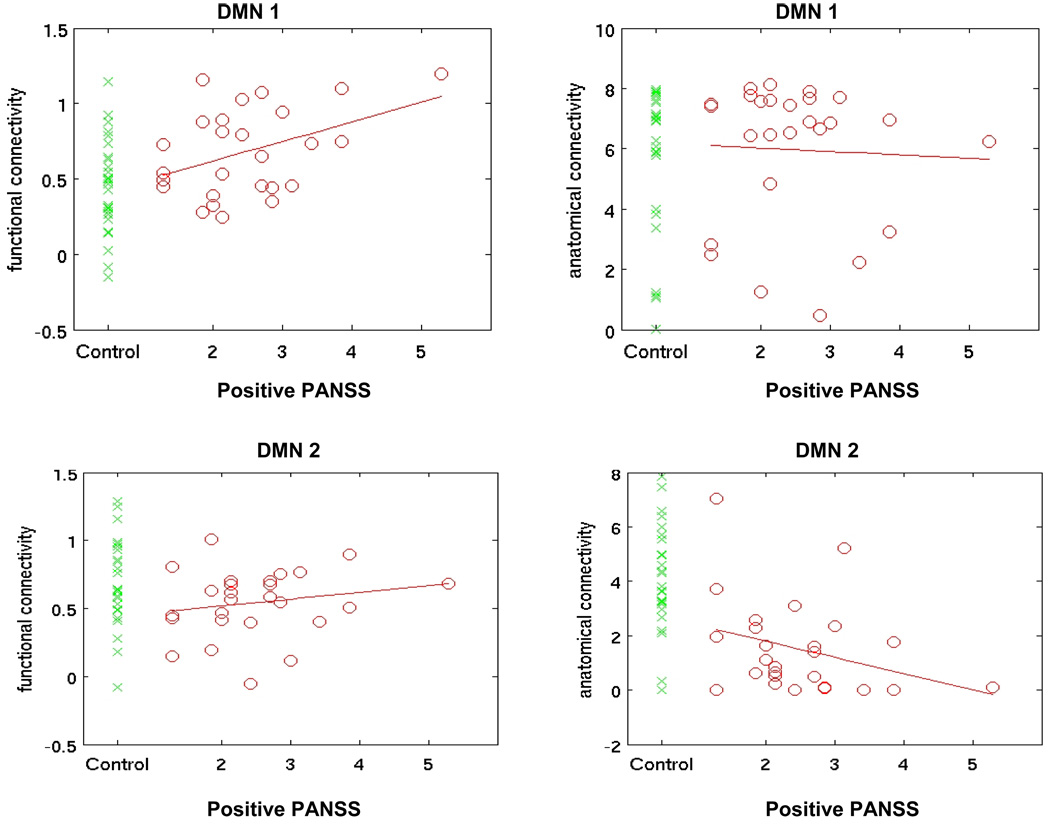
Two DMN subcomponents showed striking differences in between-group comparisons. DMN-1, that included anterior cingulate and portions of posterior cingulate cortex (PCC), showed higher functional connectivity in patients and commensurate positive correlation of functional connectivity with clinical symptoms, although no between-group differences in strength of anatomical connectivity. DMN-2 included parts of bilateral parietal cortices and bilateral dorsolateral prefrontal cortex (DLPFC) and was characterized by lower anatomical connectivity in schizophrenia. The values for subcomponents of DMN in patients and controls are presented in Figure 4.
Figure 4.
Mean functional (left column) and anatomical (right column) connectivity averaged over all voxel pairs within the DMN-1 (top row) and DMN-2 (bottom row) subcomponent of Default Mode Network. Green crosses represent values for healthy controls. Only voxel pairs separated by distance of more than 24 mm are included in component averaging.
The analysis of spatial coherence between functional and structural connectivity presented earlier for global connectivity matrices was extended to subnetworks DMN-1, DMN-2 and TPN. Only TPN (p<0.001) and DMN-1 (p<0.01) showed significant between-group differences, with lower coherence in schizophrenia patients. For those two subnetworks, the correlation with positive and general PANSS measures were significant at p<0.05, consistent with between-group differences (i.e. coherence decreased with increasing symptom severity). The correlation with TDI was significant for both TPN (p<0.02) and DMN-1 (p<0.01), but surprisingly for TPN the direction of difference in TDI was opposite to that of the PANSS, with the between group comparison for coherence increasing with increased TDI.
e) Results summary
The global tests of mean connectivity showed that schizophrenia patients had decreased anatomical connectivity and caused decoupling between anatomical and functional connectivity. Those findings were confirmed by inter-regional connectivity analysis and regional maps, localizing the decoupling to networks originating in PCC. Further network analysis showed that schizophrenia-related alterations affected portions of the DMN.
DISCUSSION
The combination of simultaneously measured anatomical and functional connectivity presented here reveals that patients display not only lowered anatomical connectivity but also complex differences in functional connectivity. This results in the relative decoupling of functional connectivity from its anatomical background that manifests in a lower correlation between these brain connectivity measures. Combining both measures allows for a more detailed examination of this process, identifies posterior cingulate cortex as the strongest focus of this decoupling and demonstrates internal differences within the DMN.
Estimates of brain connectivity
The global comparison results confirm earlier results of lower anatomical connectivity in schizophrenia shown using DTI (6, 8, 43–45), voxel-based morphometry (46, 47) as well as neuropathological studies (48, 49). The global average of functional values showed no group differences, consistent both with previous results (6, 18–25) and our more detailed local functional connectivity analysis that revealed both increases and decreases for different connections. It should be noted that our estimation method of functional connectivity based on resting state correlations may not allow detection of global connectivity changes (50) due to difficulties in accounting for global noise, but those problems do not affect the validity of estimated coherence between resting and DTI connectivity. The fact that this coherence (positive for both groups) was significantly lower for patients, reinforces the idea that the measure can be used to assess connectivity strength. Using this approach, one can treat anatomical connectivity as an individually defined pattern that can then be used to gauge functional connectivity strength.
Spatial coherence between functional and anatomical estimates
The analysis of spatial coherence between functional and anatomical connectivity is a useful measure of global connectivity patterns, but has limited power to detect the spatial localization of regions showing such coherence. Smaller networks can be addressed by analyzing the similarity of connectivity maps obtained from a full connectivity matrix by using individual voxels or regions as seeds. Only for one region – the posterior cingulate cortex (PCC) – did the spatial coherence between connectivity maps differ significantly between patients and controls, with patients showing lower coherence. This indicates that the integrity of connections originating in these regions is disrupted in schizophrenia, as suggested previously (25, 31). Furthermore, since coherence between anatomical and functional connectivity maps of PCC was lower in schizophrenia, it may suggest that in PCC-related networks patients could develop new patterns of functional connectivity that are not directly related to the underlying anatomical connection network. This interpretation agrees with the previous reports of abnormalities in cingulate cortex (51) as well as findings showing that PCC is situated between regions that show abnormal connectivity in schizophrenia (26) in ICA-based analyses of task-induced activations and resting connectivity. It complements previous findings that PCC plays important role in functional integration(52).
The global approach can be also applied to subnetworks. Instead of calculating spatial coherence between whole connectivity matrices, it can be limited to connectivity within smaller subnetworks. This can localize the effect of decoupling of functional and structural connectivity in schizophrenia to the Task Positive Network and to one of the two components of Default Mode Network (i.e. DMN-1 the one containing anterior and part of posterior cingulate).
The finding of directions of correlation of this global measure to the positive PANSS score and Thought Disorder Index that was opposite to correlations to the negative and general PANSS scores and to between-group differences was surprising. It may support a view that the spatial coherence between structural and functional connectivity may represent not just a decline in brain connectivity but also its reorganization in response to declining structural connections. The increase of coherence with increasing TDI and positive PANSS scores could suggest that it is a lack of functional reorganization, that represents further decline of functional connectivity that in turn leads to increased TDI and positive PANSS scores. Prospective studies of first-break schizophrenia patients might be able to confirm this possibility using similar analyses.
Differences in interregional connectivity
The possibility of decoupling of functional and anatomical connectivity is further informed by the analysis of interregional connections. These analyses showed that combining the two methods significantly increases the power to detect differences, even in a global analysis performed on a relatively small, non-homogeneous sample such as that studied here. More interesting is that this approach allows one to detect and to differentiate between regions characterized by different inter-relations between anatomical and resting connectivity. As reported previously, most affected regions showed lower anatomical connectivity values in schizophrenia; we found no regions pairs showing higher anatomical connectivity in patients. Also, we determined that although functional connectivity is also lower in schizophrenia for some connections, it can also be decoupled from anatomical connectivity, such that several connections originating in left IFG, ventral anterior cingulate and thalamus had lower anatomical but higher functional connectivity in schizophrenia. Interestingly, all three such regions are frequently implicated as functionally abnormal in schizophrenia (53). An alternative interpretation is to view anatomical and functional connectivity deficits as having separate etiologies – one affecting WM structure and another affecting neuronal firing rates via disrupted afferent/efferent signaling mechanisms. However, when anatomical and functional abnormalities occur in the same brain regions, it is plausible to first consider that they may be linked in a systematic and biologically plausible manner. Such overlap was seen particularly in left temporal lobe inter-regional connectivity analyses, consistent with numerous previous studies that strongly implicate abnormalities of this region in the pathophysiology of schizophrenia (23, 54, 55).
Default mode network
Our observations for DMN agree with earlier reports that schizophrenia patients (31) and their healthy relatives (25) display higher resting connectivity within the DMN. We now clarify that such increases are limited to one part of DMN (DMN-1). The DMN-2 (though still showing a strong functional connection to the remainder of the network) showed no between-group differences in functional connectivity, but a trend to lower connectivity in patients. Those subcomponents also differed significantly in their anatomical connectivity. DMN-1 showed no difference in anatomical connectivity between groups, while DMN-2 had significantly lower anatomical connectivity in patients. Hypothetically, anatomical connectivity deficits in schizophrenia may lead to functional reorganization, resulting in a hyperconnectivity between anatomically intact regions. The finding that DMN subcomponents exhibit differential behavior in schizophrenia further validates earlier conclusions (56), that the DMN should be seen not as a single unit, but as composed of substructures that all contribute to resting state activation but vary substantially in connectivity patterns. Here such a distinction is shown to be clinically relevant.
Limitations and future directions
The whole brain approach that was required to perform the first omnibus analysis of effect of schizophrenia on the relations between functional and anatomical connectivity required propagation of DTI based anatomical connectivity from white matter to its neighboring gray matter and thus resulted in the loss of spatial resolution that is usually derived from DTI analysis of individual white matter tracts. This also made it statistically difficult to detect more subtle changes that were not sufficiently marked to stand out from noise present in the global analysis. The propagation of DTI connectivity values from white to gray matter necessary for direct comparisons between both measures and the use of regions of interest based on averaged templates, further lowered spatial precision. Future studies focused on specific connections may be more successful in delineating more precisely anatomical tracts involved and functional components affected. Both anatomical and functional estimates of brain connectivity should be further optimized. The use of 12 gradient directions for DTI may be a limitation, but our method of including paths composed of several consecutive fibers allows one to minimize problems with crossing fibers. It should be noted that the two methods of estimating strength of connectivity may substantially differ in sensitivity, but this alone cannot explain the differences in effects observed here in comparison between control and clinical population. Networks detected via the above connectivity analysis should be combined with those identified in activation studies of cognitive deficits in schizophrenia. Other network analysis tools such as Independent Component Analysis should be applied in future to better delineate subsystems that capture most of the changes in the coherence between spatial and functional correlation and thus possibly better localize areas where the decoupling occurs.
Supplementary Material
Acknowledgments
The work was supported by NIMH grant R01 MH043775 (GDP) and NIBIB grant R01 EB006841 (VDC).
Footnotes
Financial Disclosure:
The authors report no biomedical financial interests or potential conflicts of interests.
References
- 1.Bleuler E. Dementia Praecox or the Group of Schizophrenias 9. International Universities Press; 1911. [Google Scholar]
- 2.Volkow ND, Brodie JD, Wolf AP, Gomezmont F, Cancro R, Vangelder P, et al. BRAIN ORGANIZATION IN SCHIZOPHRENIA. Journal of Cerebral Blood Flow and Metabolism. 1986;6:441–446. doi: 10.1038/jcbfm.1986.77. [DOI] [PubMed] [Google Scholar]
- 3.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatrica Scandinavica. 1999;99:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 4.Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: Search for a pathophysiological mechanism. International Review of Psychiatry. 2007;19:419–429. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: Diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43:554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, et al. White matter fractional anisotropy and outcome in schizophrenia. Schizophrenia Research. 2006;87:138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, Walterfang M, et al. Abnormal white matter microstructure in schizophrenia: A voxelwise analysis of axial and radial diffusivity. Schizophrenia Research. 2008;101:106–110. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- 8.Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. European Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Kanaan RAA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biological Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: Relationship to symptoms. Schizophrenia Research. 2008;98:157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, et al. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophrenia Research. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED, et al. White Matter Tractography in Bipolar Disorder and Schizophrenia. Biological Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity In The Motor Cortex Of Resting Human Brain Using Echo-Planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whalley HC, Simonotto E, Marshall I, Owens DGC, Goddard NH, Johnstone EC, et al. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Liang M, Tian LX, Wang K, Hao YH, Liu HL, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Liang M, Jiang TZ, Tian LX, Liu Y, Liu ZN, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 21.Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophrenia Bulletin. 2007;33:994–1003. doi: 10.1093/schbul/sbm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das P, Kemp AH, Flynn G, Harris AWF, Liddell BJ, Whitford TJ, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, et al. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Research-Neuroimaging. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang M, Zhou Y, Jiang TZ, Liu ZN, Tian LX, Liu HH, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal of Neuroscience. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DEJ. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, et al. Treatment with Olanzapine is Associated with Modulation of the Default Mode Network in Patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant "default mode" functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 32.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skudlarski P, Skudlarska BA, Pearlson G. Age Related Changes in the Brain Connectivity Revealed and Characterized by Fusion of Two Neuroimaging Modalities : Diffusion Tensor Imaging and Resting State Functional Connectivity. Annual Meeting of the American-Geriatrics-Society; Chicago, IL. 2009. pp. S34–S35. [Google Scholar]
- 34.Fujiwara H, Hirao K, Namiki C, Yamada M, Shimizu M, Fukuyama H, et al. Anterior cingulate pathology and social cognition in schizophrenia: A study of gray matter, white matter and sulcal morphometry. Neuroimage. 2007;36:1236–1245. doi: 10.1016/j.neuroimage.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview For Dsm-Iii-R Personality-Disorders (SCID-II) .1. DESCRIPTION. Journal of Personality Disorders. 1995;9:83–91. [Google Scholar]
- 36.Solovay MR, Shenton ME, Gasperetti C, Coleman M, Kestnbaum E, Carpenter JT, et al. Scoring Manual For The Thought-Disorder Index. Schizophrenia Bulletin. 1986;12:483–496. doi: 10.1093/schbul/12.3.483. [DOI] [PubMed] [Google Scholar]
- 37.Jiang HY, van Zijl PCM, Kim J, Pearlson GD, Mori S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : an approach to medical cerebral imaging. Stuttgart ; New York New York: G. Thieme ; Thieme Medical Publishers; 1988. [Google Scholar]
- 40.Carpenter DM, Tang CY, Friedman JI, Hof PR, Stewart DG, Buchsbaum MS, et al. Temporal characteristics of tract-specific anisotropy abnormalities in schizophrenia. Neuroreport. 2008;19:1369–1372. doi: 10.1097/WNR.0b013e32830abc35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DK, Catani M, Pierpaoli C, Reeves SJC, Shergill SS, O'Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Human Brain Mapping. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voineskos AN, Lobaugh NJ, Bouix S, Kennedy JL, Mulsant BH, Pollock BG, et al. Changes In Three Major Frontotemporal White Matter Tracts In Schizophrenia Across The Adult Lifespan: A Diffusion Tensor Tractography Study. 12th International Congress on Schizophrenia Research; San Diego, CA. 2009. pp. 206–207. [Google Scholar]
- 43.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakopoulos M, Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Current Opinion in Psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- 45.Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychological Medicine. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 46.Miyata J, Hirao K, Namiki C, Fujiwara H, Shimizu M, Fukuyama H, et al. Reduced white matter integrity correlated with cortico-subcortical gray matter deficits in schizophrenia. Schizophrenia Research. 2009;111:78–85. doi: 10.1016/j.schres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 48.Harrison PJ. The neuropathology of schizophrenia - A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 49.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. 2nd Conference of the International-Early-Psychosis-Association; New York, New York. 2000. pp. 637–648. [DOI] [PubMed] [Google Scholar]
- 50.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal D, Haznedar MM, Hazlett EA, Entis JJ, Newmark RE, Torosjan Y, et al. Diffusion tensor anisotropy in the cingulate gyrus in schizophrenia. Neuroimage. 2010;50:357–365. doi: 10.1016/j.neuroimage.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 52.Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 53.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levitt JG, Blanton RE, Caplan R, Asarnow R, Guthrie D, Toga AW, et al. Medial temporal lobe in childhood-onset schizophrenia. Psychiatry Research-Neuroimaging. 2001;108:17–27. doi: 10.1016/s0925-4927(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 55.Taylor JL, Blanton RE, Levitt JG, Caplan R, Nobel D, Toga AW. Superior temporal gyrus differences in childhood-onset schizophrenia. Schizophrenia Research. 2005;73:235–241. doi: 10.1016/j.schres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional Connectivity of Default Mode Network Components: Correlation, Anticorrelation, and Causality. Human Brain Mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.