Abstract
Cognitive rehabilitation is an effective intervention for addressing cognitive impairments in patients with schizophrenia. Previous research has shown that the early application of Cognitive Enhancement Therapy (CET) can improve neurocognitive and-social cognitive deficits in the early course of the disorder, and ultimately reduce the substantial functional disability that these patients experience. However, the lasting effects of CET on functional outcome in early course schizophrenia patients remain unknown. In this study, 58 patients in the early course of schizophrenia or schizoaffective disorder treated with two years of either CET or an Enriched Supportive Therapy (EST) control were followed-up one year after the completion of treatment to examine the durability of CET effects on functional outcome. At one-year post-treatment, a high (72%) retention rate was observed in both treatments. Results from intent-to-treat analyses employing linear mixed-effects models indicated that CET effects on functional outcome were broadly maintained one-year post-treatment, and that patients receiving CET continued to demonstrate highly significant differential functional benefits compared to patients treated with EST. These findings support the durability of CET effects on functional outcome in the early course of schizophrenia, and point to the potential of cognitive rehabilitation to have a lasting impact on the early trajectory of the disorder.
1. Introduction
Schizophrenia is characterized by marked impairments in neurocognition and social cognition (Heinrichs & Zakzanis, 1998; Penn, Corrigan, Bentall, Racenstein, & Newman, 1997), which have been consistently linked to poor functional outcomes in the disorder (Couture, Penn, & Roberts, 2006; Green, Kern, Braff, & Mintz, 2000). Cognitive rehabilitation has emerged as an effective strategy for addressing these cognitive impairments, and has produced meaningful gains in social adjustment, vocational functioning, and other functional domains in many studies (Hogarty et al., 2004; McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007; McGurk, Mueser, Feldman, Wolfe, & Pascaris, 2007). The early emergence of cognitive impairments in the course of schizophrenia (Saykin et al., 1994), their persistence after the remission of psychosis (Hoff et al., 1999), and the benefits of cognitive rehabilitation approaches for chronic patients (e.g., Hogarty et al., 2004), have generated enthusiasm that the early application of these approaches might significantly alter the frequently deleterious functional course of the disorder (Keshavan & Hogarty, 1999).
To date, while numerous studies have been conducted on cognitive rehabilitation for schizophrenia (McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007), few have examined the effectiveness of these approaches when applied to early course patients, who may experience less cognitive impairment in some domains (e.g., Braw et al., 2008). Recently, we completed a two-year randomized-controlled trial of Cognitive Enhancement Therapy (CET; Hogarty & Greenwald, 2006), an integrated approach to the remediation of social and non-social cognitive impairments, with 58 individuals in the early course of schizophrenia or schizoaffective disorder. Results indicated that not only was CET effective at improving both social cognition and neurocognition in early schizophrenia, but that the treatment was also effective at improving broad domains of functional outcome, including social functioning, major role adjustment, and competitive employment (Eack et al., 2009; Eack, Hogarty, Greenwald, Hogarty, & Keshavan, in press).
While CET has shown promise in early schizophrenia, the degree to which the beneficial effects of cognitive rehabilitation, particularly with regard to functional outcomes, are maintained after the completion of treatment remains unclear. In general, few studies have examined the durability of the effects of cognitive rehabilitation. We previously observed that the effects of CET on both cognition and social adjustment were durable one year post-treatment in a two-year trial of CET with chronic schizophrenia patients (Hogarty, Greenwald, & Eack, 2006). Wykes and colleagues (2007) also found that 3 months of neurocognitive remediation produced durable effects on memory functioning in a sample of chronic patients at a 6-month follow-up; however, effects on symptoms and self-esteem in this study were not maintained. In the only durability study of the effects of cognitive rehabilitation in early schizophrenia, Ueland and Rund (2005) observed favorable post-treatment effects on early visual information processing at one year following neurocognitive training, which were not present at treatment completion, in a sample of early-onset patients with schizophrenia. To date, no studies have examined the most critical question: whether the effects of cognitive rehabilitation on functional outcome in early course patients can be maintained after the completion of treatment.
In this investigation, we conducted a one-year follow-up study of functional outcome in early course schizophrenia patients treated with either CET or an Enriched Supportive Therapy (EST) control to examine the degree to which the previously reported significant effects of CET on functional outcome in this sample were maintained post-treatment (Eack et al., 2009). In addition, we examined the effects of baseline cognitive functioning and cognitive improvement during the trial on the probability that treatment gains in functional outcome would be durable in the year following treatment completion.
2. Methods
2.1. Participants
Participants consisted of 58 patients in the early course of schizophrenia (n = 38) or schizoaffective disorder (n = 20), confirmed by the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002). Eligibility criteria for participation included a diagnosis of schizophrenia, schizoaffective, or schizophreniform disorder; stabilization on antipsychotic medications; a time-span of no greater than 8 years since the onset of first psychotic symptoms; IQ ≥ 80; the absence of significant substance use problems for at least 2 months prior to study enrollment; and the presence of significant social and cognitive disability, as assessed using the Cognitive Style and Social Cognition Eligibility Interview (Hogarty et al., 2004). On average, participants included in this trial were young, with an age of 25.92 (SD = 6.31) years, and had been ill for 3.19 (SD = 2.24) years. The majority of participants were male (n = 40), Caucasian (n = 40), and not employed at study baseline (n = 43). Most participants had completed at least some college education (n = 39).
2.2. Measures
2.2.1. Functional outcome
Functional outcome was measured using the Social Adjustment Scale-II (SAS-II; Schooler, Weissman, & Hogarty, 1979) and the Major Role Adjustment Inventory (MRAI; Hogarty et al., 1974b), two standard measures of social adjustment in patients with schizophrenia. The SAS-II is an interview-based measure of social adjustment that consists of 44 items rated on a scale of 0 (good adjustment) to 4 or 5 (poor adjustment), depending on the item, that cover the domains of work affinity (e.g., work performance, time lost at work due to illness), primary/family relations in the household (e.g., friction with parents/spouse), social functioning outside the home (e.g., friction with extended relatives or parents not living with the patient), interpersonal anguish (e.g., distress at work, loneliness, satisfaction with social adjustment), sexual relations (e.g., frequency, interest in sexual relations), social leisure (e.g., frequency of social contacts, participation in social leisure activities, interpersonal effectiveness), and self-care (e.g., personal hygiene). The MRAI is a 32-item measure of major role adjustment that assesses vocational, social, and household role functioning. To complement data gathered from the SAS-II and maintain consistency with previous reports of CET (Hogarty et al., 2004; Eack et al., 2009), MRAI global functioning ratings concerning employment status (e.g., working, involved in volunteer work, involved in vocational rehabilitation), role functioning (e.g., meeting social and occupational obligations), functioning outside the home (e.g., interpersonal relations with friends), and overall functioning were included in this research. Previous studies have found the SAS-II and MRAI to have good interrater and test-retest reliability, to converge with other measures of functional outcome, and to be sensitive to treatment effects on functional outcome in schizophrenia (Hogarty et al., 2004; Hogarty et al., 1997b; Eack et al., 2009; Schooler, Weissman, & Hogarty, 1979). To summarize effects on overall functional outcome, SAS-II and MRAI items were scaled to a common metric and averaged to form a social adjustment composite index, which displayed adequate internal consistency (α = .73).
2.2.2. Cognition
Neurocognitive and social-cognitive data were collected during the active phases of this treatment trial to examine CET effects on these cognitive domains. These data are included in this durability study to examine the degree to which cognitive functioning at baseline, as well as cognitive improvement during treatment was associated with greater maintenance of effects on functional outcomes. Neurocognitive data reflected the relevant domains outlined by the NIMH MATRICS committee, and included measures from the Revised Wechsler Memory Scale (Wechsler, 1987), California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 1987), Revised Wechsler Adult Intelligence Scale (Wechsler, 1981), Trails B (Reitan & Waltson, 1985), Wisconsin Card Sorting Test (Heaton, Chelune, Talley, Kay, & Curtiss, 1993), Tower of London (Culbertson & Zillmer, 1996), and Neurological Evaluation Scale (Buchanan & Heinrichs, 1989). Social-cognitive data assessed the domains of social and emotional processing and were gleaned from the MATRICS recommended Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer, Salovey, Caruso, & Sitarenios, 2003), the Social Cognition Profile (Hogarty et al., 2004), and the Cognitive Styles and Social Cognition Eligibility Interview (Hogarty et al., 2004). The MSCEIT is a performance-based measure of emotional intelligence that has been shown to be a reliable and valid assessment of some emotional components of social cognition in schizophrenia (Nuechterlein et al., 2008; Eack et al., 2008). The Social Cognition Profile and the Cognitive Styles and Social Cognition Eligibility Interview are behavioral measures of social cognition based on clinical interviews, that cover broad social-cognitive domains, such as foresightfulness, reciprocity, and gistful abstraction, and have been described in detail elsewhere (Hogarty et al., 2004; Eack et al., in press; Eack, Hogarty, Greenwald, Hogarty, & Keshavan, in press). Given the large number of neurocognitive and social-cognitive measures employed, composite indexes of neurocognition and social cognition were constructed by averaging across individual test/rating items after placing them on a common metric. Both the neurocognitive (α = .87) and social-cognitive (α = .70) composites demonstrated adequate internal consistency.
2.3. Treatments
Participants in this research were randomized to receive either Cognitive Enhancement Therapy (CET) or an Enriched Supportive Therapy (EST) control. The details of these treatments have been extensively described in previous reports (Hogarty et al., 2004; Eack et al., 2009) and their components are outlined in Table 1. Briefly, CET is an integrated approach to the remediation of social and non-social cognitive impairments in schizophrenia. The treatment consists of 60 hours of computer-based training in attention, memory, and problem-solving; integrated with 45 1.5-hour social-cognitive group therapy sessions designed to facilitate the development of higher-order social-cognitive abilities, such as perspective-taking, social context appraisal, and foresightfulness. CET begins with approximately 3 months of computer-based training in attention in patient pairs, after which 3 to 4 pairs join together to form a social-cognitive group. Subsequently, neurocognitive and social-cognitive training continues concurrently throughout the remaining two years of treatment.
Table 1.
Overview of Some Therapeutic Elements of Cognitive Enhancement Therapy and Enriched Supportive Therapy.
| Example Intervention Strategies | CETa | ESTb |
|---|---|---|
| Individual supportive therapy | X | X |
| Psychoeducation | X | X |
| Relapse prevention | X | X |
| Stress and emotion management | X | X |
| Computer-based neurocognitive training | X | |
| Attention training | X | |
| Memory training | X | |
| Executive function training | X | |
| Group-based social cognition training | X | |
| Improving perspective-taking and social context appraisal | X | |
| Developing gistful thinking and abstracting abilities | X | |
| Enhancing cognitive flexibility and social problem-solving | X | |
| Reading non-verbal cues | X | |
| Being foresightful | X | |
| Giving support and feedback | X | |
| Negotiating and working with a partner | X | |
| Strengthening working memory | X | |
| Giving and receiving criticism | X |
Note. CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy
Complete details on the methods of CET are available in Hogarty and Greenwald (2006)
Complete details on the methods of EST are available in Hogarty (2004)
EST is a personalized, individual approach involving illness management and psychoeducation based on the demonstrably effective Personal Therapy (Hogarty, 2002). In this treatment, participants meet individually with a study clinician to learn more about schizophrenia, the effects of stress on the disorder, and how to develop and apply healthy coping strategies to prevent relapse and enhance adjustment. EST consists of two phases, the first of which is provided on a weekly basis and focuses on basic psychoeducation about schizophrenia and stress, and basic methods for avoiding/minimizing stress. The second phase is provided biweekly, and involves the personal identification of early warning signs of distress and application of coping strategies (e.g., diaphragmatic breathing, passive and active relaxation strategies) to manage stressors that pose particular challenges to each individual’s social and role adjustment.
Finally, all participants were maintained by a study psychiatrist on antipsychotic medication approved by the Food and Drug Administration for the treatment of schizophrenia or schizoaffective disorder. Medication changes throughout the study were allowed, although every effort was made to stabilize patients on an acceptable medication regimen prior to the initiation of psychosocial treatment. No significant differences in medication dose, type, or clinician estimated compliance were observed between CET and EST patients during treatment (Eack et al., 2009).
2.4. Procedures
Participants were recruited from inpatient and outpatient clinics at the Western Psychiatric Institute and Clinic, Pittsburgh, PA and several nearby community clinics and hospitals. Upon recruitment, participants were screened for eligibility in consensus conferences utilizing videotaped interviews and then randomized to two years of CET or EST treatment by a project statistician. Participants were then assessed using the aforementioned measures of functional outcome by highly trained clinical raters not blind to treatment assignment. Functional outcome assessments were conducted annually during the two years of CET or EST treatment, and one year after treatment completion to assess the durability of functional gains. Cognitive assessments were administered via computer-based tests or by trained neuropsychologists not involved in the patient’s treatment, and were conducted annually during the two years of active CET or EST treatment. In total, 58 patients were randomized and treated with either CET (n = 31) or EST (n = 27) in this trial. Of those randomized and treated, 49 (85%) and 46 (79%) patients completed the full two years of treatment, and 42 (72%) of patients were available for follow-up at 1-year post-treatment. There were no significant demographic or symptom differences between treatment groups at baseline (Eack et al., 2009), and no differences emerged between treatment groups with regard to attrition at 1-year post-treatment follow-up, χ2(1, N = 58) = .001, p = .976.
2.5. Data Analysis
Analysis of the durability of functional outcome effects of CET was conducted using an intent-to-treat approach with all 58 patients who were randomized and received any treatment in the trial. The primary test of treatment durability relied upon linear mixed-effects trend models to examine the degree to which CET maintained its functional advantage over EST at 1-year post-treatment on the composite index of social adjustment. These analyses were followed by within-group trend models that characterized the degree of post-treatment gain or loss in social adjustment for each treatment group. Primary analyses on the social adjustment composite were followed by exploratory analyses of within-composite components of adjustment. Finally, post-hoc moderator analyses using piecewise linear mixed-effects models (separating the active and maintenance treatment phases of the trial) were employed to examine the degree to which demographic, illness, and cognitive characteristics at baseline, as well as degree of neurocognitive and social-cognitive improvement during treatment, predicted differential rates in the maintenance of functional gains. All mixed-effects models used random intercept and slope parameters, were estimated using restricted maximum-likelihood, employed autoregressive error structures where appropriate, and controlled for the confounding effects of age, gender, IQ, and illness duration at baseline. Missing data were estimated using the maximum-likelihood expectation maximization approach (Dempster, Laird, & Rubin, 1977)
3. Results
3.1. One-Year Durability of Social Adjustment Effects
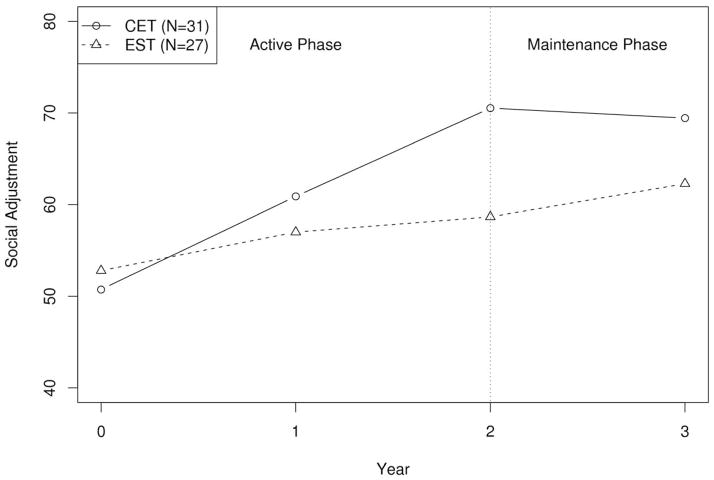
We began our analysis of the durability of CET effects on functional outcome by examining the persistence of differential effects between CET and EST on the overall multivariate composite of social adjustment, as well as its individual components, one year after treatment completion. Highly significant differential effects favoring CET on overall social adjustment continued to persist at 1-year follow-up (see Table 2). In addition, no significant decreases in adjustment were observed in CET patients during the follow-up period (see Figure 1). Interestingly, patients treated with EST showed a slight, but significant (p = .035) level of continued improvement in overall adjustment at 1-year post-treatment.
Table 2.
One-Year Durability Effects of Cognitive Enhancement Therapy on Social Adjustment in Early Schizophrenia.
| CET (N = 31) |
EST (N = 27) |
Between-Group Effect |
Within-Group Effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Year 3 | Baseline | Year 1 | Year 2 | Year 3 | Active Phase | Overall Trend | CET | EST | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | p | p | t | t | |
| Overall Social Adjustmenta | 50.73 (9.58) | 60.89 (11.22) | 70.53 (13.50) | 69.44 (14.19) | 52.79 (10.62) | 57.00 (9.45) | 58.66 (9.44) | 62.28 (13.01) | < .001 | .003 | −.68 | 2.13* |
| Employment | ||||||||||||
| Employmentb | 2.95 (1.30) | 2.32 (1.26) | 1.92 (1.21) | 1.92 (1.17) | 2.86 (1.36) | 2.20 (1.34) | 2.59 (1.20) | 2.23 (1.27) | .068 | .164 | .36 | −.81 |
| Work affinityc | 1.12 (.72) | .73 (.43) | .28 (.65) | .34 (.39) | 1.06 (.93) | 1.33 (.92) | .99 (.72) | .91 (.78) | .102 | .250 | −.82 | .01 |
| Social Functioning | ||||||||||||
| Relationships outside the homed | 2.71 (.89) | 2.00 (.91) | 1.50 (.66) | 1.78 (.85) | 2.51 (.89) | 2.28 (.92) | 2.25 (1.04) | 2.13 (1.03) | .002 | .034 | 1.44 | −.67 |
| Interpersonal anguishc | 1.09 (.64) | .79 (.50) | .64 (.49) | .68 (.54) | .86 (.64) | .77 (.60) | .77 (.62) | .60 (.55) | .019 | .247 | .34 | −1.60 |
| Sexual relationse | 3.29 (1.31) | 3.19 (1.51) | 2.69 (1.65) | 2.25 (1.71) | 3.69 (1.03) | 3.71 (1.00) | 3.44 (1.25) | 3.23 (1.33) | .315 | .209 | −1.36 | −.75 |
| Primary relationsc | .98 (.74) | .72 (.65) | .50 (.60) | .65 (.82) | 1.05 (.78) | .94 (.73) | .66 (.49) | .67 (.69) | .610 | .891 | .75 | −.03 |
| Social leisurec | 1.51 (.84) | .98 (.87) | .66 (.70) | .94 (.73) | 1.09 (.47) | 1.09 (.80) | 1.15 (.68) | 1.10 (.83) | < .001 | .004 | 1.85 | −.61 |
| Global Functioning | ||||||||||||
| Major role adjustmentf | 6.06 (.97) | 5.26 (1.57) | 3.83 (1.91) | 3.81 (1.85) | 6.11 (1.23) | 5.39 (1.49) | 5.17 (1.52) | 4.80 (1.72) | .012 | .036 | .11 | −1.34 |
| Overall functioningd | 4.83 (.35) | 4.38 (.71) | 3.78 (1.18) | 3.84 (1.14) | 4.54 (.75) | 4.39 (.72) | 4.30 (.74) | 4.17 (.93) | .002 | .017 | .36 | −1.07 |
| Self carec | 1.05 (.65) | .64 (.44) | .57 (.43) | .54 (.41) | 1.14 (.78) | 1.01 (.93) | .96 (.73) | .82 (.76) | .153 | .320 | −.26 | −.86 |
Note. CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy
Composite indexes were scaled with a baseline mean (SD) of 50 (10), with higher scores indicating better adjustment.
Possible scores range from 1 to 4, with higher scores indicating worse adjustment.
Possible scores range from 0 to 4, with higher scores indicating worse adjustment.
Possible scores range from 1 to 5, with higher scores indicating worse adjustment.
Possible scores range from 0 to 5, with higher scores indicating worse adjustment.
Possible scores range from 1 to 7, with higher scores indicating worse adjustment.
Figure 1.
One-Year Durability of the Effects of Cognitive Enhancement Therapy or Enriched Supportive Therapy on Social Adjustment
With regard to effects on individual components of the social adjustment composite, differential improvement favoring CET continued to be observed at 1-year post-treatment on 4 of the 5 measures of social adjustment that demonstrated significant effects during the active phases of treatment (see Table 2). In particular, significant maintenance of active CET treatment effects was found on social functioning in relationships outside the household and participation in social leisure activities, as well as on major role adjustment and overall ratings of global functioning. Previously observed differential positive effects on SAS-II interpersonal anguish scores were not maintained, primarily due to increased improvement on these scores in EST patients during post-treatment follow-up. In addition, trend-level employment effects were not significant at follow-up. In our previous report on competitive employment among patients treated in this trial, we observed a greater proportion of CET patients (54%) competitively employed compared to EST (18%) upon treatment completion (Eack et al., 2009; Eack, Hogarty, Greenwald, Hogarty, & Keshavan, in press). At 1-year follow-up, employment rates for CET patients were similar to those observed during active treatment (48%), whereas EST patients demonstrated a two-fold increase in competitive employment at follow-up (37%). Taken together, these findings indicate that CET effects on functional outcome among patients with early course schizophrenia are broadly maintained over the course of one year following the completion of treatment, and that EST may continue to promote some gains on social functioning and employment domains after treatment ends.
3.2. Predictors of One-Year Treatment Durability
Having found that the effects of CET on functional outcomes were broadly maintained at 1-year post-treatment, we proceeded to conduct a series of post-hoc analyses to examine the degree to which demographic and illness-related factors at baseline were predictive of treatment durability. In addition, we examined whether the magnitude of neurocognitive and social-cognitive improvement during the active phase of the trial was associated with a greater maintenance of treatment gains in social adjustment. Overall, no significant effects were observed for baseline age, gender, IQ, or illness duration on the durability of CET or EST effects on the social adjustment composite, all F(1, 121) < 1.33, all p > .252, and no interactions pointing to differential prediction of these factors between-treatment groups was observed, F(1, 121) < .25, all p > .619.
When examining the effects of cognitive factors on treatment durability, neither neurocognitive, F(1, 123) = .12, p = .730, nor social-cognitive, F(1, 123) = .12, p = .730 functioning at baseline was predictive of who would maintain treatment gains on the social adjustment composite. However, 2-year improvement in neurocognitive ability was significantly predictive of CET effects on social adjustment at 1-year follow-up, such that individuals who demonstrated greater neurocognitive improvement during the active phases of treatment were significantly more likely to maintain gains in social adjustment during post-treatment follow-up, F(1, 123) = 7.56, p = .007. Concerning social cognition, people maintained their adjustment gains regardless of their degree of social-cognitive improvement during the active phases of treatment, F(1, 123) = 1.56, p = .214.
4. Discussion
Cognitive rehabilitation is emerging as an effective treatment for addressing cognitive impairments in patients with schizophrenia (McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007). Targeting cognitive impairments early in the course of the disorder can result in significant functional benefits in such critical domains as employment, social functioning, and major role functioning (Eack et al., 2009). As with many studies of cognitive rehabilitation with chronic patients with schizophrenia, important questions remain about the lasting effects of these interventions, particularly their continued generalizability to broad functional improvements. In this study, we examined the effects of CET one year after treatment completion on functional outcomes in early course schizophrenia patients. Findings indicated that the benefits of CET on functional outcome were maintained in the year following treatment completion, particularly among those demonstrating earlier neurocognitive improvement, suggesting a lasting positive effect in the reduction of functional disability typically associated with the early course of the illness.
It is important to note that functional improvement was observed in both CET and EST. Improvements in EST likely reflect the benefits of psychoeducation and relapse prevention strategies, including stress reduction techniques, based on the effective Personal Therapy (Hogarty et al., 1997b), which are also provided in CET. Although there is evidence of functional improvement with antipsychotic medication alone in the early course of schizophrenia (Robinson, Woerner, Mcmeniman, Mendelowitz, & Bilder, 2004), that patients treated with CET continued to demonstrate large and significant improvements in social adjustment compared to EST suggests that CET confers greater benefits than those that can be achieved with combined antipsychotic treatment and effective psychoeducation and relapse prevention strategies. The social functioning domains that demonstrated the largest differential improvements favoring CET are to be expected, given the central focus of social cognition in the intervention. Other studies employing neurocognitive remediation strategies alone have had difficulty yielding broader functional benefits (Dickinson et al., 2010), which is consistent with the findings of a recent meta-analysis indicating that the effects of cognitive remediation programs on functional outcome are greater when combined with broader psychosocial interventions (McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007). CET accomplishes this by integrating neurocognitive and social-cognitive rehabilitation, which appears to confer significant functional benefits to both early course and chronic schizophrenia patients (Hogarty et al., 2004; Eack et al., 2009).
It is also interesting to note that the previously observed significant differential benefits of CET on competitive employment at the end of treatment were not maintained at 1-year post-treatment. The absence of differential employment effects for CET was not due to a loss of employment gains among CET patients, who continued to demonstrate relatively high rates of employment (48%), but rather was a result of improved post-treatment employment outcomes among EST patients. The continued improvement of EST patients at 1-year follow-up is likely reflective of the lasting benefits of stress regulation on functioning, which we have observed previously (Hogarty, Greenwald, & Eack, 2006). Such findings also suggest that CET may produce more rapid effects on work outcomes, which are eventually possible for patients treated with a disorder-relevant, enriched supportive intervention. CET did, however, continue to maintain a large advantage over EST in other domains of functional outcome. The necessity of providing the full two years of CET is supported by previous reports indicating that the strongest effects from the intervention accrued only after two years of treatment (Hogarty et al., 2004; Eack et al., 2009). It will be important, however, for future studies to examine the durability of these effects over longer time periods, as well as whether they result in significant cost savings for society.
Although the results of this research point to the durability of CET effects on functional outcome in early schizophrenia, they should be interpreted in the context of a number of limitations. Raters were not blind to treatment assignment, and although structured interviews and highly trained clinical raters were used to avoid substantial rater bias, the absence of blind raters remains a limitation in this follow-up study. In addition, due to budget constraints, we were not able to collect data on neurocognitive and social-cognitive measures post-treatment, and thus the durability of previously reported effects on these domains is not known (Eack et al., 2009). Further, CET patients did receive more hours of treatment compared to those treated with EST. However, evidence from mediator analyses currently underway points to cognitive improvements as active mechanisms of functional gains in CET, as was found with chronic schizophrenia patients (Hogarty, Greenwald, & Eack, 2006), supporting the presence of specific treatment effects on functional outcome. Finally, the modest sample size may have precluded the detection of smaller effects on functioning, particularly in the context of moderator analyses.
In summary, the results of this study indicate that the beneficial effects of CET on functional outcome in early schizophrenia can be maintained a year after the completion of treatment. While this investigation is limited by the absence of durability data on cognition and the use of non-blind raters, these findings highlight the potential lasting benefits of early cognitive rehabilitation in schizophrenia for reducing functional disability in the disorder. Given its potential lasting impact on the early trajectory of the illness, cognitive rehabilitation should be a key component of early intervention programs seeking to produce durable functional changes in the lives of early course schizophrenia patients.
Acknowledgments
This work was supported by NIMH grants MH 79537 (SME) and MH 60902 (MSK). We thank the late Gerard E. Hogarty, M.S.W. for his leadership and direction as Co-Principal Investigator of this work, and Susan Cooley M.N.Ed., Anne Louise DiBarry, M.S.N., Konasale Prasad, M.D., Haranath Parepally, M.D., Debra Montrose, Ph.D., Diana Dworakowski, M.S., Mary Carter, Ph.D., and Sara Fleet, M.S. for their help in various aspects of the study.
Role of Funding Source
The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Contributors
This durability study was designed by the late Prof. Hogarty, and Drs. Keshavan and Eack. Dr. Eack conducted the analyses. Dr. Eack wrote the initial draft of the manuscript, and Drs. Keshavan, Greenwald, and Mrs. Hogarty provided critical revisions and feedback on both the manuscript and analyses. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Braw Y, Bloch Y, Mendelovich S, Ratzoni G, Gal G, Harari H, Tripto A, Levkovitz Y. Cognition in young schizophrenia outpatients: comparison of first-episode with multiepisode patients. Schizophrenia Bulletin. 2008;34(3):544–554. doi: 10.1093/schbul/sbm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The Functional Significance of Social Cognition in Schizophrenia: A Review. Schizophrenia Bulletin. 2006;32(Suppl1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. Tower of London-DX manual. Unpublished manuscript 1996 [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society. Series B (Methodological) 1977;39(1):1–38. [Google Scholar]
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, Li L, Gold JM, Bellack AS. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. American Journal of Psychiatry. 2010;167(2):170–180. doi: 10.1176/appi.ajp.2009.09020264. [DOI] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophrenia Bulletin. doi: 10.1093/schbul/sbn091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive Enhancement Therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60(11):1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS. Effects of Cognitive Enhancement Therapy on employment outcomes in early schizophrenia: Results from a two-year randomized trial. Research on Social Work Practice. doi: 10.1177/1049731509355812. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Prasad KMR, Montrose DM, Goradia DD, Dworakowski D, Miewald J, Keshavan MS. An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(8):1873–1878. doi: 10.1016/j.pnpbp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the right stuff. Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal Neuropsychological Follow-Up Study of Patients With First-Episode Schizophrenia. American Journal of Psychiatry. 1999;156(9):1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- Hogarty GE. Personal Therapy for schizophrenia and related disorders: A guide to individualized treatment. New York: Guilford; 2002. [Google Scholar]
- Hogarty GE, Greenwald DP. Cognitive Enhancement Therapy: The Training Manual. University of Pittsburgh Medical Center: Authors; 2006. Available through www.CognitiveEnhancementTherapy.com. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, et al. Cognitive enhancement therapy for schizophrenia. Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61(9):866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR the Collaborative Study Group. Drug and sociotherapy in the aftercare of schizophrenic patients: III. Adjustment of nonrelapsed patients. Archives of General Psychiatry. 1974b;31(5):609–618. doi: 10.1001/archpsyc.1974.01760170011002. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of Cognitive Enhancement Therapy. Psychiatric Services. 2006;57(12):1751–1757. doi: 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald D, Ulrich RF, Kornblith SJ, Dibarry AL, Cooley S, Carter M, Flesher S. Three-year trials of personal therapy among schizophrenic patients living with or independent of family: II. Effects of adjustment of patients. American Journal of Psychiatry. 1997b;154(11):1514–1524. doi: 10.1176/ajp.154.11.1514. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Development and Psychopathology. 1999;11(3):525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3(1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. American Journal of Psychiatry. 2007;164(3):437–441. doi: 10.1176/ajp.2007.164.3.437. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A Meta-Analysis of Cognitive Remediation in Schizophrenia. American Journal of Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, et al. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein J, Newman L. Social cognition in schizophrenia. Psychological Bulletin. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Waltson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Robinson DG, Woerner MG, Mcmeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2004;161(3):473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schooler N, Weissman M, Hogarty G. Social adjustment scale for schizophrenics. In: Hargreaves WA, Attkisson CC, Sorenson J, editors. Resource material for community mental health program evaluators. Rockville, MD: National Institute of Mental Health; 1979. pp. 290–303. DHHS Pub. No. (ADM) 79328. [Google Scholar]
- Ueland T, Rund BR. Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatrica Scandinavica. 2005;111(3):193–201. doi: 10.1111/j.1600-0447.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A, Romeo R. Cognitive remediation therapy in schizophrenia. British Journal of Psychiatry. 2007;190(5):421–427. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]