Abstract
The cognitive impairment in Alzheimer's disease (AD) is associated with synaptic loss, neuritic sprouting and altered neuroplasticity. Compensatory neuritic sprouting might be beneficial, while aberrant sprouting could contribute to the neurodegenerative process. Nogo (or Rtn4) is a major myelin-derived inhibitor of axonal sprouting in adult CNS. Recent evidence has implicated both the Reticulon family of proteins and a receptor for Nogo, NgR, in reducing amyloid-β production, a key step in AD pathogenesis. To test the hypothesis that Nogo, as an inhibitor of axonal sprouting, modulates disease progression in a mouse model of AD, we introduced an APP transgene (a human APP minigene carrying the Swedish and Indiana mutations under the PDGFB promoter) into a Nogo null background and characterized the behavioral and neuropathological consequences. We found that deleting Nogo ameliorates learning and memory deficits of APP transgenic mice in the Morris water maze at an early/intermediate stage of the disease. Furthermore, deleting Nogo restored the expression levels of markers for synapto-dendritic complexity and axonal sprouting including synaptophysin, MAP2, GAP43 and neurofilament that are otherwise reduced in APP transgenic mice. Other aspects of disease progression including neuronal loss, astrogliosis, microgliosis and, importantly, Aβ levels and amyloid deposits were not significantly altered by Nogo deletion. These data support the hypothesis that Nogo-mediated inhibition of neuritic sprouting contributes to the disease progression in an APP transgenic model of AD in a way that is mechanistically distinct from what has been proposed for Rtn3 or NgR.
Keywords: Alzheimer's disease, central nervous system, myelin-derived axon growth inhibitors, neuritic sprouting, amyloid deposition, Reticulon
Alzheimer's disease (AD) is characterized by widespread neurodegeneration throughout the association cortex and limbic system, with formation of the Amyloid-β (Aβ) plaques and neurofibrillary tangles being the neuropathological hallmarks of the disease (Selkoe, 2005). One interesting aspect of disease progression is the cycles of synapse loss and aberrant sprouting (Troy et al., 1997; Hashimoto and Masliah, 2003). During the early stages of AD, the synapse loss in the limbic system appears to be partially compensated by sprouting of the cholinergic system in the neocortex and inner molecular layer of the hippocampus. However, as the disease progresses and synaptic damage is accentuated, aberrant sprouting of axons and dendrites may eventually contribute to the neurodegenerative process (Geddes and Cotman, 1991; Hashimoto and Masliah, 2003). Therefore, neuritic sprouting may be either beneficial or detrimental in disease progression. However, how neuritic sprouting modulates disease progression in AD remains poorly understood.
Axonal sprouting in the adult mammalian CNS has been extensively studied in the context of CNS injuries (Gonzenbach and Schwab, 2008). There is evidence that CNS myelin contains inhibitory molecules that restrict axonal sprouting following injury, thus limiting functional recovery. Nogo is a major myelin-derived inhibitor of axonal growth. The Nogo gene encodes three isoforms, Nogo-A, B and C, via alternative splicing and alternative promoter usage (Chen et al., 2000; GrandPre et al., 2000). The three isoforms share a C-terminal segment that contains two transmembrane domains separated by a 66 amino acid loop region (Nogo-66) that possesses potent inhibitory activity on neurite growth in vitro (GrandPre et al., 2000). The same C-terminal segment shares homology with a family of proteins called Reticulons (or Rtns), with Nogo (or Rtn4) being the 4th and last member of the family. Although Nogo alone cannot explain the very limited regeneration of injured axons in the CNS (Lee and Zheng, 2008; Lee et al., 2009), there is consensus for a role of Nogo in inhibiting compensatory sprouting of uninjured axons and in local axonal plasticity (Buffo et al., 2000; Raineteau et al., 2001; McGee et al., 2005; Cafferty and Strittmatter, 2006) Lee et al., in press.
The presence of neuritic sprouting in AD pathology raised the possibility that inhibitors of CNS axonal sprouting such as Nogo may influence the disease outcome. Interestingly, recent studies indicate that both Reticulons (especially Rtn3) and NgR (also known as NgR1), a receptor for the Nogo-66 inhibitory domain (Fournier et al., 2001), can reduce the production of Aβ by interacting with BACE1 and APP respectively (He et al., 2004; Park et al., 2006). However, the proposed mechanisms by which Reticulons or NgR influences neurodegeneration appear to be independent of the role of Nogo/Rtn4 as an inhibitor of axonal sprouting. Indeed, whether Nogo/Rtn4 plays a role in mouse models of AD remains unknown. We explored this possibility by generating and characterizing mice that express a mutated human APP transgene in a Nogo null background. We found that deleting Nogo ameliorates disease progression in this APP transgenic model of AD as assessed by behavioral and neuropathological measures. In particular, Nogo deletion restored the reduced expression of markers for axonal sprouting and synapto-dendritic complexity in APP transgenic mice. These date support the hypothesis that Nogo modulates disease progression in AD as an inhibitor of neuritic sprouting.
EXPERIMENTAL PROCEDURES
Experimental mice
The PDGFB-hAPP transgene with Swedish (K670N/M671L) and Indiana (V717F) mutations (Line J9) (Mucke et al., 2000) was crossed with homozygous Nogo null mice (Lee et al., 2009). APP tg, Nogo+/− mice were then crossed to Nogo−/− mice to obtain the experimental mice of four different genotypes (see Results) as littermates in a C57BL/6, 129S7 and DBA mixed genetic background. All experimental procedures were approved by the Institutional Animals Care and Use Committee at UCSD.
Behavioral studies
Mice were tested in the Morris water maze as described (Rockenstein et al., 2006) at 8–10 months of age. For this purpose, a pool was filled with opaque water and mice were first trained to locate a visible platform (days 1–3) and then a submerged hidden platform (days 4–7) in three daily trials 2–3 min apart. Mice that failed to find the hidden platform within 90 seconds were placed on it for 30 seconds. The platform location was fixed. The entry point into the water for each mouse was alternated randomly between two points at an equal distance from the platform. On day 8, another visible platform trial was performed to exclude differences in motivation and fatigue, and a probe trial was performed when there was no platform present to calculate percent time spent in the target quadrant. Time to reach the platform and path length were recorded with a Noldus Instruments EthoVision video-tracking system (San Diego Instruments).
Tissue processing
Mice were euthanized by deep anesthesia and brains removed and divided sagittally. One hemibrain was post-fixed in 4% paraformaldehyde at 4°C for 48 hrs and sectioned at 40 μm with a Vibratome 2000 (Leica), while the other hemibrain was snap frozen and stored at −70°C for protein analysis.
Immunohistochemistry
As described (Rockenstein et al., 2006), blind-coded Vibratome sections were immunostained with monoclonal antibodies against synaptophysin (1:20, Chemicon), MAP2, (1:40, Chemicon), NeuN (1:1000, Chemicon), GFAP (1:500, Chemicon), Iba-1 (1:1000, Wako Chemicals), GAP43 (1:100, Chemicon), neurofilament (SMI312, 1:1000, Abcam), APP (Mucke et al., 2000), and rabbit anti-Nogo (1:500) (Zheng et al., 2003). After overnight incubation with the primary antibodies, sections were incubated with fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibodies, transferred to SuperFrost slides and mounted under glass coverslips with anti-fading media. Alternatively, sections were detected with peroxidase reaction with standard techniques. All sections were processed under the same standardized conditions. The immunostained blind-coded sections were serially imaged with the laser scanning confocal microscope (MRC1024, BioRad) and analyzed with the Image 1.43 program (NIH). For each mouse, a total of three sections were analyzed and for each section, four fields in the frontal cortex and hippocampus were examined. The average of individual measurements was used to calculate group means. For GAP43, MAP2 and synaptophysin, results were expressed as percent area of the neuropil occupied by immunoreactive axons/dendrites/terminals; for neurofilament, GFAP and Iba-I, levels were expressed as optical density. For NeuN, the mean neuronal density was estimated using the optical disector method (Chana et al., 2003); briefly, for stereology, immunostained sections were counterstained with 1% cresyl violet and four 100-μm-wide fields from at least three sections (180-μm interval) per animal were analyzed and results averaged and expressed as total number per mm3. The extent of the Aβ deposits were detected using the mouse monoclonal antibody 4G8 (1:600, Senetek) as described (Rockenstein et al., 2006) and percent area occupied by Aβ deposits plotted.
Western blot
Antibodies used: Rtn3 (R458, 1:1000) a generous gift from Dr. R. Yan, Cleveland, OH, USA (He et al., 2004), NgR (1:1000, a generous gift from Dr. B. L. Tang, Singapore), LC3 (1:1000, MBL), Beclin 1 (1:1000, Novus Biologicals), Caspase 3 (1:1000 Cell Signaling), Aβ (6E10, 1:1000, Covance).
Statistical Analysis
Values in the figures were expressed as means ± SEM. To determine the statistical significance, values were compared by using the one-way ANOVA with post-hoc Dunnet when comparing the Non tg to the Nogo null or App tg mice. Additional comparisons were done using Tukey-Krammer or Fisher post-hoc tests.
RESULTS
In order to evaluate the functional effects of deleting Nogo on disease progression in an APP transgenic model of AD, an APP transgenic line (PDGFB-hAPP with Swedish and Indiana mutations) (Mucke et al., 2000) were crossed with Nogo null mice. Unlike other Nogo mutants reported in the literature, the Nogo null mutant used in this study is fully viable and lacks the expression of all known Nogo isoforms including Nogo-A,B,C (Lee et al., 2009). The PDGFB-hAPP transgenic line (referred to as APP tg here) was chosen because this line has been extensively characterized for neuritic sprouting (Chin et al., 2004). Four groups of mice were generated as littermates: 1) non-transgenic Nogo+/− controls (referred to as Non tg), 2) Nogo null mutants (Nogo−/−), 3) APP transgenic, Nogo+/− (APP tg); 4) APP transgenic, Nogo−/− (APP tg, Nogo−/−).
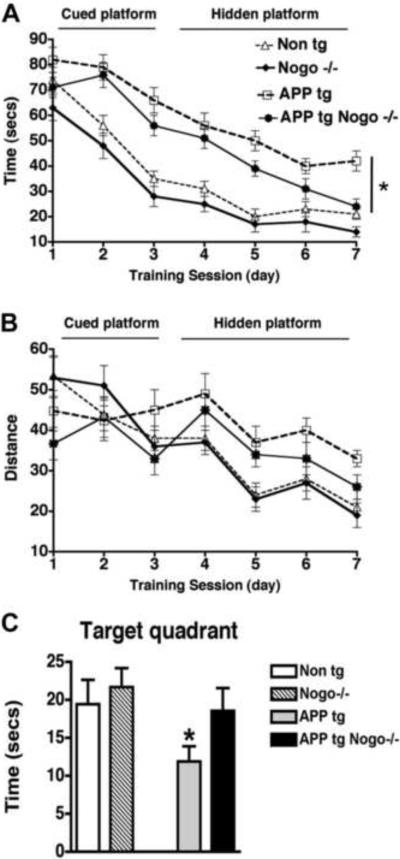
To determine whether Nogo deletion alters the disease progression in APP tg mice, we first tested the behavior of all four groups of mice in a Morris water maze at 8–10 months of age. This time line was chosen because it has been shown that at this age behavioral deficits and axonal pathology become apparent in the PDGFB-hAPP transgenic line (Chin et al., 2004). During the training portion of the test with the visible platform, APP tg mice and APP tg, Nogo−/− mice spent a longer time to reach the platform when compared with Non tg or Nogo−/− mice. (Fig. 1A, B). During the spatial learning portion with the platform submerged, as expected, the APP tg mice had significant deficits compared to the Non tg controls. Nogo−/− mice did not differ significantly from the Non tg controls. In contrast, APP tg, Nogo−/− mice showed significant improvement over time as compared with APP tg mice, with their time (or latency) to reach the platform approaching that of Non tg or Nogo−/− mice towards the end of the 7-day training/testing period (Fig. 1A, B). Consistent with this finding, in the final probe portion of the test, APP tg, Nogo−/− mice expended more time in the target quadrant than APP tg mice (Fig. 1C). Thus, deleting Nogo ameliorated a learning and memory deficit in the APP tg mice at an early/intermediate stage of the disease as assessed by the Morris water maze test.
Figure 1.
Effects of Nogo deletion on the behavioral performance of APP tg mice in the Morris water maze. A, B, Average time taken and distance traveled for mice of various genotypes to reach the platform during the cued and hidden platform phases of the test. C, Percentage of time spent in the target quadrant by mice of various genotypes on day 8. *P < 0.05 compared with Non tg, n = 8/group, Error bar = SEM.
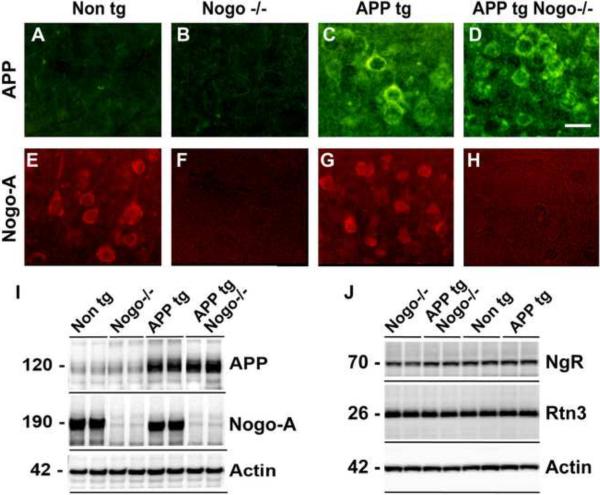
To verify APP transgene expression and Nogo deletion, we examined the expression of APP and Nogo-A in the brain of various genotypes by immunohistochemistry. In Non tg mice or Nogo−/− mice, APP immunoreactivity was detected at background levels (Fig. 2A, B). In contrast, a high level of APP immunoreactivity was detected in both APP tg mice and APP tg, Nogo−/− mice (Fig. 2C, D). The expression pattern is consistent with transgenic human APP protein being primarily expressed by neurons. Nogo-A is also predominantly expressed by neurons in the hippocampus in Non tg and APP tg mice and absent in Nogo−/− or APP tg, Nogo−/− mice (Fig. 2E–H). There does not appear to be any cross regulation between APP transgene and endogenous Nogo-A expression. These results were corroborated with Western blot analysis on brain protein extracts (Fig. 2I). Because NgR and Rtn3 have been implicated for a role in mouse models of AD (Park et al., 2006; Shi et al., 2009), we also examined the expression levels of these two proteins by Western blot analysis and found no appreciable differences among the genotypes (Fig. 2J).
Figure 2.
Expression of APP tg, Nogo-A, NgR and Rtn3 in crosses of APP tg and Nogo null mice. A–H, APP and Nogo-A immunoreactivity in the hippocampus from representative mice of various genotypes. Genotype is indicated on the top; antigen is indicated on the left. APP, green; Nogo-A, red. Scale bar = 15 μm. I, Western blot analysis of transgenic APP protein and Nogo-A along with Actin control on representative mice of various genotypes. J, Western blot analysis of NgR and Rtn3 on representative mice of various genotypes.
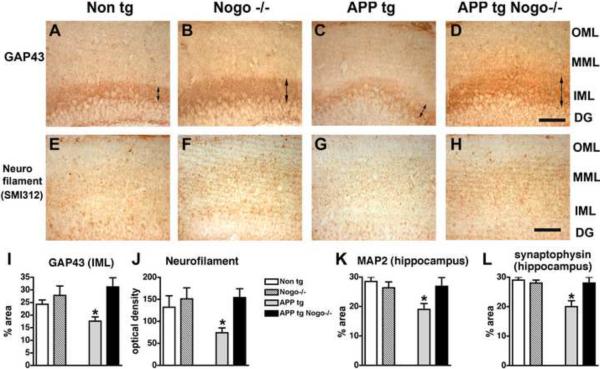
The behavioral deficits in the APP tg mice have been associated with synaptic deficits (Mucke et al., 2000). Since Nogo deletion ameliorated the behavioral deficits in the APP tg mice, we investigated the effects of deleting Nogo on axonal, dendritic and synaptic markers in the hippocampus of these mice. GAP43, a marker for new axonal sprouts, were reduced in the inner molecular layer of the dentate gyrus in APP tg mice (Fig. 3A, C, I), as expected (Chin et al., 2004). Deleting Nogo restored the level of GAP43 expression in the inner molecular layer (Fig. 3D, I). As a control, deleting Nogo alone did not significantly alter GAP43 expression in the inner molecular layer (Fig. 3B, I) (A trend for a slight increase in GAP43 immunoreactivity in Nogo mutants as compared with Non tg controls did not reach statistical significance). Similarly, the expression of neurofilament (a pan-axonal marker) in the molecular layer was reduced in APP tg mice but restored in APP tg, Nogo−/− mice (Fig. 3E–H, J). Likewise, a modest but significant reduction in the expression of MAP2 (a dendritic marker) and synaptophysin (a synaptic marker) in the molecular layer of the hippocampal dentate gyrus of APP tg mice were restored to control levels by Nogo deletion (Fig. 3K, L). Thus, Nogo deletion restored the expression level of axonal, dendritic and synaptic markers that was reduced in the dentate gyrus region of the APP tg mice, including a marker for new axonal sprouts.
Figure 3.
Effects of Nogo deletion on patterns of axonal, dendritic and synaptic markers in the hippocampus of APP tg mice. Representative images of GAP43 (A–D) and neurofilament (E–H) immunoreactivity in hippocampal regions from mice of various genotypes are shown. OML, outer molecular layer; MML, middle molecular layer; IML, inner molecular layer; DG, dentate gyrus. Scale bar = 50 μm. I–L, Quantification of GAP43 (I), neurofilament (J), MAP2 (K) and synaptophysin (L) immunoreactivity. *P < 0.05 compared with Non tg, n = 8/group.
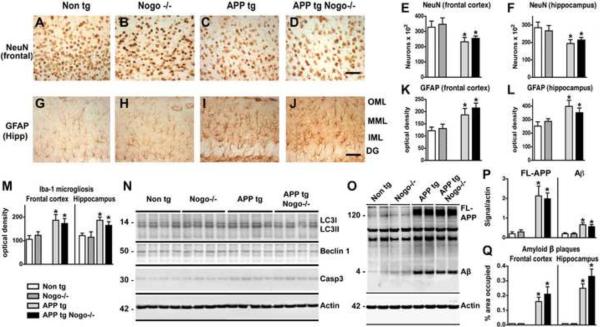
To determine whether the effect of Nogo deletion is specific to neuronal processes or more general in reversing APP tg-associated neuropathology, we examined the expression of markers for neurons, astrogliosis, microgliosis, autophagy, apoptosis, amyloid β levels and plaque deposition. APP tg mice between 8–10 months of age displayed a significant loss of neurons in both the frontal cortex and hippocampus as assessed with NeuN immunohistochemistry (Fig. 4A, C, E, F). Nogo deletion did not significantly alter the loss of neurons in APP tg mice (Fig. 4D, E, F), suggesting that the effect of Nogo deletion on disease progression is not due to its effect on neuronal loss. GFAP immunoreactivity, which indicates levels of reactive astrogliosis, was upregulated in the frontal cortex and hippocampus of APP tg mice (Fig. 4G, I, K, L), as expected (Mucke et al., 2000). This astroglial response was not significantly altered by Nogo deletion (Fig. 4J, K, L). Similarly, microgliosis in APP tg mice, as assessed by the elevated expression of the Iba-1 microglia marker, was not significantly altered by Nogo deletion (Fig. 4M). As a control, Nogo deletion alone did not significantly alter the expression of NeuN, GFAP or Iba-1 (Fig. 4B, E, F, H, K, L). Western blot analysis on brain protein extracts indicated that there were no appreciable differences in the ratio of LC3II/LC3I or the level of Beclin 1, both markers of autophagy, among the different genotypes (Fig. 4N). APP tg was associated with a modest increase in the level of active Caspase 3, which was not further altered by Nogo deletion (Fig. 4N).
Figure 4.
Lack of effect for Nogo deletion on other aspects of APP tg-associated neuropathology. A–D, G–J, Representative images of NeuN (A–D) and GFAP (G–J) immunoreactivity in the frontal cortex and hippocampus respectively from mice of various genotypes. OML, outer molecular layer; MML, middle molecular layer; IML, inner molecular layer; DG, dentate gyrus. Scale bar = 50 μm. E, F, K, L, M, Quantification of NeuN (E, F), GFAP (K, L) and Iba-1 (M) immunoreactivity. N, O, Western blot analysis on LC3I, LC3II, Beclin 1, Caspase 3 (Casp3) (N), Full-length APP (FL-APP) and Aβ (O) in brain protein extracts of various genotypes. P, Q, Quantification of FL-APP and Aβ levels (P) and amyloid plaque formation in the frontal cortex and hippocampus (Q). *P < 0.05 compared with Non tg, n = 8/group.
Finally, to determine whether Nogo modulates disease progression by altering Aβ metabolism or amyloid deposition, we assessed Aβ levels and amyloid plaque formation in the neocortex and hippocampus of these mice by Western blot and immunohistochemistry respectively. APP tg was associated with high levels of full-length APP and Aβ, which was not significantly altered by Nogo deletion (Fig. 4O, P). Likewise, APP tg mice displayed a substantial amount of amyloid plaques in the frontal cortex and hippocampus, which was not significantly altered by Nogo deletion (Fig. 4Q). Take together, these data indicate that Nogo deletion did not significantly alter Aβ metabolism or amyloid deposition.
DISCUSSION
It has long been hypothesized that neuritic sprouting plays a role in disease progression of AD (Geddes and Cotman, 1991; Masliah et al., 1992). Neuritic sprouting may be beneficial, compensating for the lost synaptic contacts, or detrimental if it becomes increasingly aberrant in nature. However, to our knowledge the hypothesis that neuritic sprouting modifies AD-like phenotypes in a mouse model of AD has never been directly tested. The availability of mice lacking inhibitors of CNS axon sprouting offers an opportunity to examine the effect of altered axonal sprouting in the disease progression of AD-like phenotypes. To test this hypothesis, we introduced a PDGFB-hAPP transgene carrying human mutations (abbreviated as APP tg here), which causes AD-like phenotypes, into a Nogo null background that lacks the expression of all known Nogo isoforms. We found that deleting Nogo ameliorates behavioral and neuropathological outcome at an early/intermediate stage of the disease in the APP tg line. These results support the hypothesis that Nogo modulates disease progression in an APP transgenic model of AD as an inhibitor of axonal sprouting through a mechanism that is distinct from what has been proposed for Rtn3 or NgR, the Nogo-66 receptor.
Nogo is a member of the Reticulon family of proteins and has three isoforms: Nogo-A,B,C (or Rtn4-A,B,C). Previous studies indicate that Reticulons, and in particular Rtn3 and Rtn4-B (or Nogo-B), biochemically interact with BACE1 (the enzyme responsible for β-secretase activity) and inhibits Aβ production in vitro (He et al., 2004; Murayama et al., 2006). From these studies it was anticipated that an excess of Reticulons (e.g. Rtn3) would lead to reduced Aβ levels and consequently reduced amyloid deposition and decelerated disease progression. However, the in vivo validation of this hypothesis has been complicated by the surprising finding that Rtn3 overexpression leads to Rtn3 aggregation and the formation of Rtn3 immunoreactive dystrophic neurites in the hippocampus, which was associated with impairments in spatial learning and memory (Hu et al., 2007). Because such Rtn3-immunoreactive dystrophic neurites also exist in human AD patients and in mice expressing mutant APP, it was proposed that Rtn3 aggregation contributes to AD pathogenesis by inducing neuritic dystrophy. Crossing the Rtn3 overexpression transgene into an APP/PS1 (Presenilin 1) double transgenic line reduced amyloid deposition in the cortex, the hippocampus CA3 region and dentate gyrus, as expected if Rtn3 were to inhibit Aβ production by interacting with BACE1; however, in the CA1 region preformed Rtn3 immunoreactive dystrophic neurites appeared to counteract with the beneficial effect of Rtn3 overexpression (Shi et al., 2009). Thus, Rtn3 may simultaneously have two opposite effects on neurodegenerative phenotypes in AD: beneficial as an antagonist of BACE1 activity and promoting the formation of dystrophic neurites that contribute to neurodegeneration when overexpressed. Whether endogenous Rtn3 plays a role in an animal model of AD awaits loss-of-function analysis. Furthermore, it is not known whether the aggregate-promoting effect of Rtn3 overexpression is shared with other Reticulons such as Nogo/Rtn4.
Interestingly, NgR, a receptor for the Nogo-66 inhibitory domain, has been found to physically interact with APP and overexpressing NgR in culture leads to reduced Aβ production (Park et al., 2006). As predicted from these in vitro data, genetically deleting NgR leads to increased Aβ levels, amyloid deposition and dystrophic neurites in an APPswe/PSEN-1(ΔE9) double transgenic model of AD (Park et al., 2006). Conversely, administration of a soluble NgR ectodomain reduces Aβ levels, amyloid deposition and dystrophic neurites in the same animal model. Thus, it appears that NgR can bind to APP, blocking it from being processed by β-secretase (Park et al., 2006). Such a mechanism would presumably not involve the Nogo-NgR inhibitory signaling pathway, since such an involvement would predict that soluble NgR ectodomain treatment and NgR deletion would elicit a similar effect, while the opposite was observed (Park et al., 2006). In our study, Aβ levels and amyloid deposition were not significantly altered in APP tg, Nogo−/− mice as compared with APP tg mice, further arguing against a mechanism where Nogo modulates disease progression through the effect of NgR on Aβ production.
Consistent with our hypothesis that Nogo modulates disease progression as an inhibitor of neurite growth, the reduced expression of several markers indicative of synaptic and dendritic complexity and axonal sprouting is restored by Nogo deletion in the APP tg mice. In contrast, other neurodegeneration-associated phenotypes such as astrogliosis, microgliosis and neuronal loss was not significantly affected by Nogo deletion. Nor was the expression of markers for autophagy and apoptosis examined. Most notably, Aβ levels and amyloid plaque formation in APP tg mice were not reduced by Nogo deletion. Thus, it appears that Nogo modulates disease progression without directly affecting Aβ metabolism or amyloid deposition, but rather, through its inhibitory effect on compensatory neuritic sprouting that occurs during disease progression, especially at an early/intermediate stage of the disease. Such a mechanism would be distinct from what has been proposed for Rtn3 or NgR on Aβ production, or Rtn3 overexpression on dystrophic neurites. Finally, both NgR and its ligands Nogo-66 and OMgp have been shown to regulate activity-dependent synaptic plasticity in the hippocampus (Lee et al., 2008) (R. J. Giger, University of Rochester, personal communication). It remains to be seen whether this functional aspect of the myelin inhibitory pathway may also contribute to – perhaps even provide an alternative explanation for – the improved behavioral outcome in the Nogo-deleted, APP transgenic mouse model of AD described here.
CONCLUSION
Our data provide evidence that axonal/neuritic sprouting is functionally relevant to disease progression in AD-like phenotypes, thus linking a major neurodegenerative disease and a major mechanism for axonal plasticity and central nervous system repair, two important areas of clinical neuroscience. Together with published reports, our study indicates that Nogo/Rtn4, other Reticulons (e.g. Rtn3) and the Nogo-66 Receptor have independent and complex roles in modulating AD pathogenesis.
Acknowledgements
Supported by research grants from NIH/NINDS to E.M. (AG018440, AG022074, NS0507096 and AG10435) and B.Z. (NS054734 and a pilot grant as part of P50AG005131 to the Alzheimer's Disease Research Center at UCSD and the late Dr. Leon Thal). F.X. was supported by a Christopher and Dana Reeve Foundation Postdoctoral Fellowship. We thank Bor Luen Tang and Riqiang Yan for the generous gift of NgR antibody and Rtn3 antibody respectively; Yuhong Zhu for technical assistance.
Abbreviations
- Rtn
Reticulon
- Rtn3
Reticulon 3
- Rtn4
Reticulon 4 (also known as Nogo)
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- APP tg
APP transgenic
- Non tg
Non transgenic
- Aβ
Amyloid-β
- CNS
central nervous system
- PDGFB
platelet-derived growth factor subunit B
- GAP43
Growth Associated Protein 43
- MAP2
microtubule-associated protein 2
- GFAP
glial fibrillary acidic protein
- BACE1
beta-site APP cleaving enzyme 1
- NgR
Nogo Receptor (also known as NgR1)
- DG
dentate gyrus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F. Application of neutralizing antibodies against NI-35/250 myelin- associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Cotman CW. Plasticity in Alzheimer's disease: too much or not enough? Neurobiol Aging. 1991;12:330–333. doi: 10.1016/0197-4580(91)90011-8. discussion 352–335. [DOI] [PubMed] [Google Scholar]
- Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: focusing on Nogo. Cell Mol Life Sci. 2008;65:161–176. doi: 10.1007/s00018-007-7170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- Hu X, Shi Q, Zhou X, He W, Yi H, Yin X, Gearing M, Levey A, Yan R. Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 2007;26:2755–2767. doi: 10.1038/sj.emboj.7601707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Zheng B. Axon regeneration after spinal cord injury: insight from genetically modified mouse models. Restor Neurol Neurosci. 2008;26:175–182. [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Chan AF, Luu SM, Zhu Y, Ho C, Tessier-Lavigne M, Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo, MAG and OMgp deficient mice. Neuron. doi: 10.1016/j.neuron.2010.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, Alford M, DeTeresa R, Terry R, Baudier J, Saitoh T. Localization of amyloid precursor protein in GAP43-immunoreactive aberrant sprouting neurites in Alzheimer's disease. Brain Res. 1992;574:312–316. doi: 10.1016/0006-8993(92)90831-s. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid beta-protein. Eur J Neurosci. 2006;24:1237–1244. doi: 10.1111/j.1460-9568.2006.05005.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Noth P, Thallmair M, Schwab ME. Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc Natl Acad Sci U S A. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J Neurosci Res. 2006;83:1252–1261. doi: 10.1002/jnr.20818. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Shi Q, Prior M, He W, Tang X, Hu X, Yan R. Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites. J Neurosci. 2009;29:9163–9173. doi: 10.1523/JNEUROSCI.5741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Stefanis L, Greene LA, Shelanski ML. Mechanisms of neuronal degeneration: a final common pathway? Adv Neurol. 1997;72:103–111. [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]