Abstract
The complement system protects the host against invading organisms, initiates inflammation and dispose of immune complexes and the products of inflammatory injury. The complement system provides an important link between the innate and adaptive immune systems. Experimental observations suggest that increased complement activation causes and/or perpetuates inflammation during pregnancy. Recent studies suggest a link between complement activation and preeclampsia. Excessive activation or insufficient regulation of complement recruits leukocytes and unleashes potent inflammatory and anti-angiogenic mediators associated with placental insufficiency and maternal endothelial dysfunction characteristic of preeclampsia. We review the animal and human studies that link complement activation and pathogenic events in preeclampsia, present evidence that activation of the complement system is associated with the development of preeclampsia and provides new targets to prevent its complications.
Angiogenic and Immune Dysregulation in Preeclampsia
Preeclampsia is a complex multisystem disease that contributes significantly to maternal and neonatal mortality and morbidity. 1-3 The classical clinical manifestations, de novo hypertension and proteinuria, occur late in pregnancy, in the setting of maternal endothelial cell activation 4 and excessive systemic inflammation.5
Preeclampsia has been called the “disease of theories” as its cause is unclear. 2 The pathologic process originates in the placenta, with inadequate cytotrophoblast invasion in early pregnancy6, 7 leading to an oxidatively stressed placenta. 8, 9 It is suggested that placental oxidative stress and inflammation10 results in the release of anti-angiogenic factors11, 12, syncytiotrophoblast debris 13 and other placental factors 14, 15 into the maternal circulation where they contribute to widespread endothelial cell dysfunction4 and the clinical syndrome of preeclampsia.14, 16
Normal placental development requires coordinated expression of vascular endothelial growth factor (VEGF), angiogenic growth factors and placenta growth factor (PlGF), as well as expression of their respective receptors on invasive trophoblasts. 17 VEGF promotes placental development and invasiveness primarily through interaction with VEGF receptor-1 [VEGFR-1; also known as fms-like tyrosine kinase-1, (Flt-1)] and VEGFR-2. 18 Alternative splicing of VEGFR-1 results in production of the secreted protein, soluble VEGFR-1 (sVEGFR-1, also known as sFlt-1), which lacks the cytoplasmic and transmembrane domains but retains the ligand-binding domain. 19 Placental trophoblasts exposed to stress, such as hypoxia and inflammation, release large amounts of sVEGFR-1, a potent anti-angiogenic molecule that sequesters circulating VEGF and PlGF and prevents their interaction with endogenous receptors 20 on placental and maternal cells 12 leading to abnormal placentation associated with preeclampsia and IUGR 11, 17, 21, as well as the maternal syndrome. Along with soluble endoglin (sEng), another anti-angiogenic protein of placental origin, sVEGFR-1 to PlGF ratios have been shown to be elevated not only during clinical preeclampsia but also to anticipate the onset of symptoms by several weeks22, 23, suggesting that they contribute to disease pathogenesis. Our studies in experimental pregnancy models suggest that inflammation is a potent trigger of angiogenic dysregulation. 10
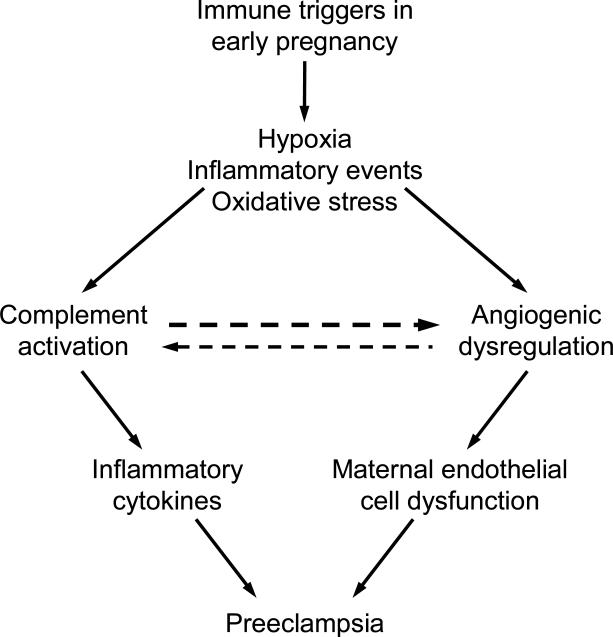
The immune system, a potent initiator of inflammatory pathways, is thought to play an important role in the etiology of preeclampsia. 24, 25 The immune maladaptation hypothesis of preeclampsia explains why preeclampsia is more frequent in women who have inadequate time to develop immune tolerance to paternally-derived antigens, such as nulliparous women (reviewed in 25, 26). Indeed, maternal inflammatory responses have been shown by many authors to be enhanced in preeclampsia. 5, 27 It is also recognized that women with risk factors associated with chronic inflammation (obesity, pre-pregnancy hypertension, diabetes mellitus and dyslipidemia) are at an increased risk of developing preeclampsia. 16, 28, 29. A striking example of the association of preeclampsia and inflammatory-mediated injury is its increased incidence in patients with autoimmune diseases, particularly systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS). APS is an autoimmune disease characterized by venous and/or arterial thrombosis and pregnancy complications that occur in the presence of antiphospholipid antibodies (aPL). 30, 31 The obstetric criteria for APS are: (1) one or more otherwise unexplained deaths of the fetus beyond the 10th week of gestation or (2) one or more premature births before the 34th week of gestation because of eclampsia or severe preeclampsia or placental insufficiency or (3) three or more unexplained spontaneous abortions before the 10th week of gestation 32). Pregnant women with SLE have significantly higher rates of hypertensive disorders compared to the general obstetric population (23% vs 8% 33 and preeclampsia has been described in 15-37% of patients with lupus nephritis. 34-39 Similarly, preeclampsia has been reported in 18%-48% of pregnant women with well-characterized APS 40, 41 Conversely, aPL antibodies were newly identified in 12%-17% of women with preeclampsia 42-44. In this review, we focus on the link between inflammation related to activation of the complement system and the pathogenesis of preeclampsia in women, particularly angiogenic dysregulation (Figure 1). Because some animal models of miscarriage and growth restriction are associated with angiogenic dysregulation characteristic of preeclampsia, studies in such models also will be described to elucidate define triggers of placental dysfunction.
Figure 1.
Immune triggers in early pregnancy leading to preeclampsia: Complement activation fragments and angiogenesis-related factors
The Complement System and Tissue Injury
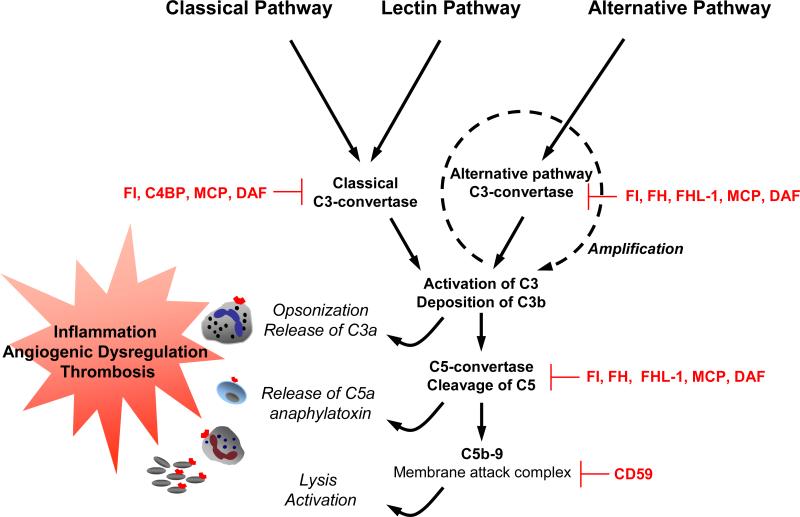
The complement system, composed of over 30 proteins that act in concert to protect the host against invading organisms, initiates inflammation and tissue injury (Figure 2) (described in 45-47). Like the clotting cascade, the complement cascade contains a potent amplification mechanism based on sequential cleavage of inactive zymogen forms of proteins by serine protease mechanisms. Complement activation promotes chemotaxis of inflammatory cells and generates proteolytic fragments that enhance phagocytosis by neutrophils and monocytes. The classical pathway is activated when natural or elicited antibodies bind to antigen, the mannose-binding lectin pathway by carbohydrates, and the alternative pathway is initiated spontaneously. The 3 pathways converge to generate enzymes called C3 convertases which cleave C3 into C3b and C3a. C3b tags pathogens/foreign surfaces for opsonization and is a major effector molecule of the complement system. C3a is an anaphylatoxin. Factors B, D and Properdin initiate activation of C3b directly through the alternative pathway initiation complex or through the amplification loop where C3b is formed (Figure 2). The production of C3b, triggered from engagement of the classical or lectin pathways, is augmented through the alternative pathway amplification. 45 The convergence of three pathways on C3 results in a common path to effector functions: generation of the fragments C3a, an anaphylatoxin that activates inflammatory cells, and C3b, that attaches covalently to targets and leads to the assembly of C5 convertase and subsequent cleavage of C5 to C5a and C5b. C5a is a potent chemotactic molecule that recruits and stimulates leukocytes and endothelial cells, triggering release of cytokines and chemokines and expression of adhesion molecules. Binding of C5b to cell surface initiates assembly of the C5b-9 membrane attack complex (MAC) which inserts into membranes, damages cells and activates proinflammatory pathways (Figure 2).
Figure 2. The human complement system: effectors and regulators.
Three pathways are activated by immune complexes and apoptotic cells (classical); microbes and stressors (lectin); spontaneously (alternative). The effect of complement: clearance of apoptotic cells, opsonisation of pathogens and immune complexes for phagocytosis, release of anaphylatoxins and lysis (shown in italics) and activation of effectors cells that express receptors for C5a and/or C3a (neutrophils, monocytes and platelets) are shown on the left. Complement inhibitors are indicated in red. Soluble inhibitors are factor I (FI), C4b-binding protein (C4BP), factor H (FH), and FH-like protein (FHL-1). Membrane bound inhibitors include: MCP (CD46), DAF (CD55) and CD59.
Adapted from Sjöberg AP et al. Trends in Immunology. 2009;30:83-9047
By means of these recognition and activation mechanisms, the complement system identifies and responds to “dangerous” situations presented by foreign antigens, pathogens, apoptotic cells, ischemia and necrosis and can mediate fetal injury initiated by cellular or humoral immune mechanisms.
Experimental observations suggest that increased complement activation causes and/or perpetuates inflammation during pregnancy. 48, 49 As fetal tissues are semi-allogeneic, the placenta is subject to classical and alternative pathway-mediated immune attack. 48 Because activated complement fragments have the capacity to bind and damage self-tissues, it is imperative that bystander cells be protected from the deleterious effects of complement. 47, 50, 51 Thus, although complement split products are present in normal placentas 52, 53, excessive complement activation is prevented in successful pregnancy by regulatory proteins that are highly expressed on trophoblast membranes 54, 55, strategically positioned to protect the fetus. The three membrane proteins are decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46), and CD59. DAF and MCP regulate the activation of C3 56, and CD59 prevents assembly of C5b-9 MAC, blocking the terminal effector functions of complement 57. The role of the soluble regulators of complement, factors H, FHL-1 58, factor I and factor H-related protein 1, in pregnancy is uncertain. The importance of complement regulation has been reviewed recently59 and is underscored by recent reports of the strong association of mutations in membrane bound as well as surface attached soluble complement regulatory proteins (causing ineffective C3 inactivation) with hemolytic uremic syndrome 60, glomerular injury and age-related macular degeneration. 61
Models of the Antiphospholipid Antibody Syndrome and Other Adverse Pregnancy Outcomes
That appropriate complement inhibition is an absolute requirement for normal pregnancy has been demonstrated by the finding that deficiency of Crry (a membrane-bound intrinsic complement regulatory protein in mice, like DAF and MCP, that blocks classical and alternative pathway activation of C3 62) leads to embryonic lethality in mice; Crry-/- embryos are surrounded by activated C3 fragments and PMN. 63 Importantly, embryos are rescued when C3 deficiency or factor B deficiency is introduced to Crry-/- embryos. 63 Defective placental development was associated with pregnancy loss and solely dependent on alternative pathway activation. 64
Inhibition of complement also protects pregnancies from aPL antibody-induced fetal death or growth restriction. Using a murine model of APS induced by passive transfer of human aPL antibodies, we have shown that complement activation plays an essential and causative role in pregnancy loss and fetal growth restriction. 65, 66 Passive transfer of IgG from women with recurrent miscarriage and high titer aPL antibodies results in increased fetal resorptions compared to mice treated with IgG from healthy individuals, and reduction in average weight of surviving fetuses.65 Using mice deficient in complement elements and inhibitors of complement activation in our model of APS, we identified C5, and particularly, its cleavage product C5a as a key mediator of fetal injury and showed that antibodies or peptides that block C5a-C5a receptor interactions prevent pregnancy complications. Our results indicate that both classical and alternative pathway activation contribute to damage. Mice deficient in alternative and classical pathway components (factor B, C4, C3, C5 and C5a receptor) or treated with inhibitors (anti-factor B mAb, anti-C5 mAb or C5a receptor antagonist peptide) were resistant to aPL-induced fetal injury. 65, 66 Based on the results of these mouse studies, we proposed a mechanism for pregnancy complications associated with aPL antibodies: aPL preferentially targets placental tissue and activate complement via the classical (Fc- and C4-dependent) pathway, leading to generation of potent anaphylatoxins (C3a and C5a), and the release of proinflammatory (TNF-α and tissue factor) 66, 67, 68 (and anti-angiogenic factors (sVEGFR-1, see below). The result is an influx of inflammatory cells into the placenta leading to intrauterine fetal demise or intrauterine growth restriction.
Given that anticoagulation with heparin reduced the incidence of pregnancy loss among women with APA-related pregnancy loss in a number of studies 30, 69, we considered the possibility that heparin inhibits complement activation on trophoblasts and that anticoagulation, in and of itself, is not sufficient to prevent pregnancy complications in APS. We found that treatment of mice with unfractionated or low molecular weight heparin protected pregnancies from aPL-induced damage even at doses that did not prolong coagulation parameters. In contrast, treatment with hirudin or fondaparinux (anticoagulants without anti-complement effects) was not protective; demonstrating that anticoagulation is insufficient therapy for APS-associated miscarriage. 70 Furthermore, heparins inhibited both aPL-induced elevations in circulating C3a and increased C3b deposition in decidua (neither was altered by hirudin or fondaparinux) and blocked C3 cleavage in vitro. These studies provide a framework for understanding how sub-anticoagulant doses of heparin exert beneficial effects in antibody-mediated tissue injury, and suggest that heparin ameliorates pregnancy complications by limiting complement activation and the ensuing inflammatory response at the maternal-fetal interface, rather than by inhibiting thrombosis. 70
Studies in mouse models indicate that even antibody-independent fetal allograft rejection is associated with complement activation and influx of inflammatory cells, perhaps mediated through the alternative pathway. 71 CBA/J-mated female DBA/2 mice have been extensively studied as a model of immune-mediated pregnancy loss. Embryos derived from mating CBA/J females with DBA/2 males show an increased frequency of resorption and surviving CBA/J x DBA/2. Fetuses show consistent and significant growth restriction (IUGR). Factor B, C3, C5 and C5a receptor are required for pregnancy loss and growth restriction in this antibody-independent model of spontaneous miscarriage 10fetuses from DBA/2-mated CBA/J pregnant mice are rescued by inhibiting complement. Importantly, in experimental mouse models complement activation triggers dysregulation of the angiogenic factors required for normal placental development, creating an angiogenic imbalance similar to that of preeclampsia. CBA/J x DBA/2 matings complicated by miscarriage or growth restriction were characterized by inflammatory infiltrates in placentas, functional deficiency of free VEGF, elevated levels of soluble VEGF receptor-1; a potent anti-angiogenic molecule) and defective placental development. Inhibition of complement activation in vivo blocked the increase in sVEGFR-1 and rescued pregnancies. In vitro stimulation of human neutrophils and monocytes with products of the complement cascade directly triggered release of sVEGFR-1, which sequesters VEGF. C5a-induced expression of tissue factor on neutrophils and monocytes potentiates inflammation and release of anti-angiogenic factors. 72 These studies provided the first evidence linking complement to the angiogenic factor imbalance associated with placental dysfunction, and identified a new effector of immune-triggered pregnancy complications. 10
Overall, mouse models support the hypothesis that appropriate complement regulation is necessary to control placental inflammation and that increased complement activation fragments are highly deleterious to the developing fetal-placental unit. Although the mouse models described focus on placental inflammation and pregnancy loss, ongoing studies suggest that similar mechanisms contribute to systemic features of preeclampsia in these experimental models.
Complement and Human Pregnancy
Little information is available about complement activation in normal and abnormal human pregnancy. 73 During normal gestation, serum levels of C3, C4, and total hemolytic complement (CH50) gradually increase 10%-50% 74, likely reflecting increased synthesis, and levels of complement split products increase, suggesting low grade classical pathway activation75. Studies performed nearly 20 years ago showed marked elevations in levels of Bb, C3a, C4d, and soluble C5b-9 in preeclampsia, indicating excess classical and alternative pathway activation.76, 77 Complement activation with release of anaphylatoxins and terminal C5b-9 complement complexes was also noted in women with the HELLP syndrome.78 Recently, Richani et al. confirmed these findings and also noted higher concentrations of complement activation fragments (C3a, C4a and C5a) in the maternal circulation in normal human pregnancy compared with non-pregnant women. 79 Complement activation products have been found in deciduas, chorionic villi, and as subendothelial deposits in vessel walls in normal and preeclampsia placentas. 77, 80, 81 Recently, Rampersad et al reported that the presence in C5b-9 MAC on trophoblasts was associated with fibrin deposits at sites of villous injury in vivo in normal placentas, but especially in placentas from pregnancies complicated by IUGR or preeclampsia.82 Again, these findings implicate complement in tissue injury, but do not address the question of whether complement activation is a cause or consequence of placental damage nor do they indicate whether specific patterns of complement activation detected in the circulation will predict fetal loss or injury.
As described above, pregnancy in women with SLE or APS, two systemic autoimmune diseases characterized by complement-mediated injury, is associated with an increased risk for preeclampsia, placental insufficiency, fetal growth restriction and miscarriage. 83 In these patients, autoantibodies targeted to the placenta initiate local complement activation which, if unabated, leads to abnormal placental development. Indeed, complement activation products have been demonstrated in deciduas, chorionic villi and vessel walls in placentas of patients with aPL and in patients with preeclampsia. 77, 81, 84 Studies performed over a decade ago found evidence of complement activation in pregnant patients with SLE. 75, 76, 85-87 It may be difficult to identify decreased complement synthesis or increased consumption due to lupus because changes induced by gestation can either falsely normalize (if synthesis increases) or exaggerate (if synthesis does not increase) disease-related hypocomplementemia.75, 76, 88 Buyon et al. have suggested that certain patterns of complement activation are associated with either disease flare or preeclampsia, but it is particularly difficult to differentiate these conditions in patients with nephritis. In normal pregnancies, excessive complement activation is prevented by complement regulatory proteins that are highly expressed on trophoblast membranes 54, 55 (MCP, DAF, and CD59) as well as circulating complement regulatory proteins [complement factor H (CFH), C4b binding protein and complement factor I (CFI)]. It has been suggested that abnormal function of complement regulators may predispose to preeclampsia. 89 (see below).
In 2003, we initiated the The PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkers In antiphospholipid antibody Syndrome and Systemic lupus Erythematosus), a prospective, multi-center observational study to translate findings in mice to humans and evaluate the role of complement in SLE and aPL antibody-associated pregnancy complications. The study is ongoing and will test the hypotheses that (1) classical, alternative and terminal complement pathway activation and (2) dysregulation of angiogenic factors will be detected in the circulation of patients destined for complications, defined by the composite outcome of unexplained fetal death, early neonatal death due to complications of prematurity, and medically indicated preterm birth because of gestational hypertension, preeclampsia or IUGR. Our goal is to study 700 pregnant patients, and we have already enrolled over 500. The PROMISE study has the potential to identify new biomarkers for adverse pregnancy outcomes that in addition to being good predictors of these outcomes are also part of the mechanistic process of these pregnancy complications. In the future, it may be possible to perhaps direct attention to the use of complement inhibitors to prevent or modify the inflammatory-related sequelae associated with adverse pregnancy outcomes.
Complement Activation in Preeclampsia Patients
In 2005, inspired by novel research in the animal model of APS 65, 90, 91, Lynch et al conducted a prospective study in human pregnancy (n = 701) to investigate whether elevated levels of complement activation fragment Bb (reflecting alternative complement pathway activation) at a single point in early pregnancy (less than 20 weeks gestation) were predictive of preeclampsia later in pregnancy. 92 This was the first large prospective study to examine the relationship between Bb levels in the early part of pregnancy with preeclampsia in humans. Adjusted for other risk factors, women with levels of Bb in the top decile in early pregnancy were almost four times more likely to develop preeclampsia later in pregnancy compared with women with levels less than the top decile in early pregnancy.
As discussed above, it has been demonstrated that preeclampsia has pathogenic links to an imbalance in placental-derived angiogenesis-related factors, notably placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng). 11, 12, 23 The inter-relationship between complement activation and angiogenesis-related factors in humans is unknown, although a causal link has been suggested in an animal model of pregnancy loss.10 The primary objective of a secondary analysis of this Denver cohort was to determine if altered levels of complement activation fragments (Bb, C3a and sC5b-9) and angiogenesis-related factors (PlGF, sFlt-1 and sEng) at 10 to 15 weeks gestation independently predict the development of preeclampsia, and if these biomarkers were related to one another. 93 Univariate analysis showed that women who developed preeclampsia later in pregnancy had significantly higher levels of complement fragment Bb in early pregnancy as reported previously.92 No meaningful relationships were found between the complement-activation fragments and the angiogenesis-related factors examined as continuous variables. The study by Lynch et al. suggests that the innate immune system is triggered in early pregnancy to activate complement, particularly the alternative pathway, and that elevated concentrations of circulating complement-activated fragment Bb are associated with subsequent development of preeclampsia 92, which may not be absolutely dependent on angiogenesis-related factors. 93 The study was relatively small (only 32 patients had preeclampsia) and preeclampsia is a heterogeneous disease with many triggers. Future studies will determine the relative importance of complement activation in its pathogenesis. That complement activation fragments are elevated in early pregnancy in women who have a preterm birth (<34 weeks’ gestation) and in women who develop gestational hypertension94, 95 underscores the importance of complement in pathological pregnancies.
We also considered the role of complement in pregnancy complications in women with impaired complement regulatory function. Loss-of-function mutations in CFH, MCP and CFI that lead to undesirable complement activation, and gain-of-function mutations in complement components factor B and C3, occur in patients with atypical hemolytic uremic syndrome (aHUS), a microangiopathy characterized by microvascular endothelial activation, cell injury and thrombosis 96-101. Complement dysregulation (specifically, enhanced function of the alternative pathway) contributes to the pathology of aHUS, a disease associated with glomerular endothelial cell injury and microthrombi not unlike that seen in preeclampsia. In some of these patients, aHUS presents during pregnancy.96 Indeed, pregnancy precipitated microthrombotic angiopathy in the form of atypical HELLP with severe renal involvement in four patients with genetic defects in complement regulatory protein function89, 102
Finally, placental malaria is potentially informative as a model for inflammation-driven placental dysfunction leading to preeclampsia. A relationship between preeclampsia and malaria has long been suspected, but its basis was unclear. First-time mothers have the highest rates of Plasmodium falciparum placental malaria (related to the absence of protective antibodies). These patients develop severe placental inflammation and go on to poor pregnancy outcomes. Rather than causing a decrease in blood pressure typical of malaria, placental malaria in first-time pregnancies is associated with hypertension, elevated levels of sVEGFR-1 and histological features of placental inflammation. 103 Recent in vitro studies have shown that parasitized erythrocytes, as well as parasite products, activate C5, induce C5a receptor expression, and synergistically increase production of inflammatory cytokines, chemokines, and the anti-angiogenic factor sVEGFR-1. Similar to our findings in mouse models, C5a-C5a receptor interactions were crucial triggers of inflammatory and anti-angiogenic activities. Studies extended to patients showed increased levels of C5a in both peripheral and placental plasma in primigravid women with placental malaria. 104 Although it is not clear whether elevated products of complement activation are a cause or consequence of placental dysfunction, taken together, these data provide further evidence that excessive activation of complement, specifically generation of C5a, contributes to poor pregnancy outcomes by inducing dysregulated inflammatory and angiogenic responses that impair placental function.
Conclusion
The complement system provides a link between the innate and adaptive immune systems, recognizing and responding to danger. In preeclampsia, perhaps related to immune maladaptation or oxidative stress, there is increased complement activation at the maternal-fetal interface. Excessive activation or insufficient regulation of complement recruits leukocytes and unleashes potent inflammatory and anti-angiogenic mediators associated with placental insufficiency and maternal endothelial dysfunction characteristic of preeclampsia. The link between complement activation and pathogenic events in preeclampsia identifies potential biomarkers to predict patients at risk for preeclampsia and new targets to prevent its complications.
Acknowledgements
This research was supported in part by NIH AR49772 (JES), AR38889 (JES) and K23 HD049684 (AML), the Mary Kirkland Center for Lupus Resarch (JES), American Heart Association (AML), the Center for Women's Health Research and the List Family Foundation at University of Colorado Denver (AML) and Newborn Hope Colorado (AML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22(2):203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 5.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(Suppl A):S55–65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med. 2004;200(8):951–5. doi: 10.1084/jem.20041783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;21:21. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–8. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 12.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–7. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 17.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 19.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90(22):10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol. 1999;13(4):537–45. doi: 10.1210/mend.13.4.0265. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 23.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 24.Moffett A, Hiby SE. How Does the maternal immune system contribute to the development of pre-eclampsia? Placenta. 2007;28(Suppl A):S51–6. doi: 10.1016/j.placenta.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Dekker G, Robillard PY. Pre-eclampsia: Is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76(1-2):8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Dekker G. The partner's role in the etiology of preeclampsia. J Reprod Immunol. 2002;57(1-2):203–15. doi: 10.1016/s0165-0378(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 27.Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):21–6. doi: 10.1016/j.ejogrb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177(5):1003–10. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 29.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166(11):1312–9. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 30.Giannakopoulos B, Passam F, Ioannou Y, Krilis SA. How we diagnose the antiphospholipid syndrome. Blood. 2009;113(5):985–94. doi: 10.1182/blood-2007-12-129627. [DOI] [PubMed] [Google Scholar]
- 31.Hanly JG. Antiphospholipid syndrome: an overview. Cmaj. 2003;168(13):1675–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(3):899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- 34.Moroni G, Quaglini S, Banfi G, Caloni M, Finazzi S, Ambroso G, et al. Pregnancy in lupus nephritis. Am J Kidney Dis. 2002;40(4):713–20. doi: 10.1053/ajkd.2002.35678. [DOI] [PubMed] [Google Scholar]
- 35.Nicklin JL. Systemic lupus erythematosus and pregnancy at the Royal Women's Hospital, Brisbane 1979-1989. Aust N Z J Obstet Gynaecol. 1991;31(2):128–33. doi: 10.1111/j.1479-828x.1991.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 36.Lockshin MD, Qamar T, Druzin ML, Goei S. Antibody to cardiolipin, lupus anticoagulant, and fetal death. J Rheumatol. 1987;14(2):259–62. [PubMed] [Google Scholar]
- 37.Julkunen H, Kaaja R, Palosuo T, Gronhagen-Riska C, Teramo K. Pregnancy in lupus nephropathy. Acta Obstet Gynecol Scand. 1993;72(4):258–63. doi: 10.3109/00016349309068034. [DOI] [PubMed] [Google Scholar]
- 38.Kleinman D, Katz VL, Kuller JA. Perinatal outcomes in women with systemic lupus erythematosus. J Perinatol. 1998;18(3):178–82. [PubMed] [Google Scholar]
- 39.Yasmeen S, Wilkins EE, Field NT, Sheikh RA, Gilbert WM. Pregnancy outcomes in women with systemic lupus erythematosus. J Matern Fetal Med. 2001;10(2):91–6. doi: 10.1080/714904302. [DOI] [PubMed] [Google Scholar]
- 40.Branch DW, Silver RM, Blackwell JL, Reading JC, Scott JR. Outcome of treated pregnancies in women with antiphospholipid syndrome: an update of the Utah experience. Obstet Gynecol. 1992;80(4):614–20. [PubMed] [Google Scholar]
- 41.Lima F, Khamashta MA, Buchanan NM, Kerslake S, Hunt BJ, Hughes GR. A study of sixty pregnancies in patients with the antiphospholipid syndrome. Clin Exp Rheumatol. 1996;14(2):131–6. [PubMed] [Google Scholar]
- 42.Sletnes KE, Wisloff F, Moe N, Dale PO. Antiphospholipid antibodies in pre-eclamptic women: relation to growth retardation and neonatal outcome. Acta Obstet Gynecol Scand. 1992;71(2):112–7. doi: 10.3109/00016349209007966. [DOI] [PubMed] [Google Scholar]
- 43.Branch DW, Andres R, Digre KB, Rote NS, Scott JR. The association of antiphospholipid antibodies with severe preeclampsia. Obstet Gynecol. 1989;73(4):541–5. [PubMed] [Google Scholar]
- 44.Milliez J, Lelong F, Bayani N, Jannet D, el Medjadji M, Latrous H, et al. The prevalence of autoantibodies during third-trimester pregnancy complicated by hypertension or idiopathic fetal growth retardation. Am J Obstet Gynecol. 1991;165(1):51–6. doi: 10.1016/0002-9378(91)90222-d. [DOI] [PubMed] [Google Scholar]
- 45.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–16. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 46.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 47.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30(2):83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin Obstet Gynaecol. 1992;6(3):439–60. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 49.Morgan BP, Holmes CH. Immunology of reproduction: protecting the placenta. Curr Biol. 2000;10(10):R381–3. doi: 10.1016/s0960-9822(00)00476-0. [DOI] [PubMed] [Google Scholar]
- 50.Abbas AK, Janeway CA., Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100(1):129–38. doi: 10.1016/s0092-8674(00)81689-x. [DOI] [PubMed] [Google Scholar]
- 51.Song WC. Membrane complement regulatory proteins in autoimmune and inflammatory tissue injury. Curr Dir Autoimmun. 2004;7:181–99. doi: 10.1159/000075693. [DOI] [PubMed] [Google Scholar]
- 52.Weir PE. Immunofluorescent studies of the uteroplacental arteries in normal pregnancy. Br J Obstet Gynaecol. 1981;88(3):301–7. doi: 10.1111/j.1471-0528.1981.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 53.Wells M, Bennett J, Bulmer JN, Jackson P, Holgate CS. Complement component deposition in uteroplacental (spiral) arteries in normal human pregnancy. J Reprod Immunol. 1987;12(2):125–35. doi: 10.1016/0165-0378(87)90040-4. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham DS, Tichenor JR., Jr. Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clin Immunol Immunopathol. 1995;74(2):156–61. doi: 10.1006/clin.1995.1023. [DOI] [PubMed] [Google Scholar]
- 55.Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J Immunol. 1993;151(3):1562–70. [PubMed] [Google Scholar]
- 56.Holers VM, Kinoshita T, Molina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today. 1992;13(6):231–6. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 57.Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15(4):369–96. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 58.Zipfel PF, Skerka C. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol Today. 1999;20(3):135–40. doi: 10.1016/s0167-5699(98)01432-7. [DOI] [PubMed] [Google Scholar]
- 59.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 60.Stahl AL, Vaziri-Sani F, Heinen S, Kristoffersson AC, Gydell KH, Raafat R, et al. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111(11):5307–15. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- 61.Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory states the examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv Immunol. 2007;96:141–77. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- 62.Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, et al. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181(1):151–9. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287(5452):498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 64.Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, et al. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19(6):813–22. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- 65.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174(1):485–90. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 68.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110(7):2423–31. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol. 2002;99(1):135–44. doi: 10.1016/s0029-7844(01)01646-5. [DOI] [PubMed] [Google Scholar]
- 70.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 71.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2(1):64–8. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 72.Girardi G, Mackman N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: a pro-inflammatory molecule. Lupus. 2008;17(10):931–6. doi: 10.1177/0961203308094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imrie HJ, McGonigle TP, Liu DT, Jones DR. Reduction in erythrocyte complement receptor 1 (CR1, CD35) and decay accelerating factor (DAF, CD55) during normal pregnancy. J Reprod Immunol. 1996;31(3):221–7. doi: 10.1016/0165-0378(96)00977-1. [DOI] [PubMed] [Google Scholar]
- 74.Abramson SB, Buyon JP. Activation of the complement pathway: comparison of normal pregnancy, preeclampsia, and systemic lupus erythematosus during pregnancy. Am J Reprod Immunol. 1992;28(3-4):183–7. doi: 10.1111/j.1600-0897.1992.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 75.Lockshin MD, Qamar T, Redecha P, Harpel PC. Hypocomplementemia with low C1s-C1 inhibitor complex in systemic lupus erythematosus. Arthritis Rheum. 1986;29(12):1467–72. doi: 10.1002/art.1780291207. [DOI] [PubMed] [Google Scholar]
- 76.Buyon JP, Tamerius J, Belmont HM, Abramson SB. Assessment of disease activity and impending flare in patients with systemic lupus erythematosus. Comparison of the use of complement split products and conventional measurements of complement. Arthritis Rheum. 1992;35(9):1028–37. doi: 10.1002/art.1780350907. [DOI] [PubMed] [Google Scholar]
- 77.Sinha D, Wells M, Faulk WP. Immunological studies of human placentae: complement components in pre-eclamptic chorionic villi. Clin Exp Immunol. 1984;56(1):175–84. [PMC free article] [PubMed] [Google Scholar]
- 78.Haeger M, Unander M, Andersson B, Tarkowski A, Arnestad JP, Bengtsson A. Increased release of tumor necrosis factor-alpha and interleukin-6 in women with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Acta Obstet Gynecol Scand. 1996;75(8):695–701. doi: 10.3109/00016349609065729. [DOI] [PubMed] [Google Scholar]
- 79.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17(4):239–45. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Labarrere C, Mullen E. Fibrinoid and trophoblastic necrosis with massive chronic intervillositis: an extreme variant of villitis of unknown etiology. Am J Reprod Immunol Microbiol. 1987;15(3):85–91. doi: 10.1111/j.1600-0897.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 81.Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE, Pecorari D. Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clin Exp Immunol. 1990;80(2):236–40. doi: 10.1111/j.1365-2249.1990.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29(10):855–61. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tincani A, Bazzani C, Zingarelli S, Lojacono A. Lupus and the antiphospholipid syndrome in pregnancy and obstetrics: clinical characteristics, diagnosis, pathogenesis, and treatment. Semin Thromb Hemost. 2008;34(3):267–73. doi: 10.1055/s-0028-1082270. [DOI] [PubMed] [Google Scholar]
- 84.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007;196(2):167, e1–5. doi: 10.1016/j.ajog.2006.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hopkins P, Belmont HM, Buyon J, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum. 1988;31(5):632–41. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- 86.Lockshin MD, Harpel PC, Druzin ML, Becker CG, Klein RF, Watson RM, et al. Lupus pregnancy. II. Unusual pattern of hypocomplementemia and thrombocytopenia in the pregnant patient. Arthritis Rheum. 1985;28(1):58–66. doi: 10.1002/art.1780280110. [DOI] [PubMed] [Google Scholar]
- 87.Buyon JP, Cronstein BN, Morris M, Tanner M, Weissmann G. Serum complement values (C3 and C4) to differentiate between systemic lupus activity and pre-eclampsia. Am J Med. 1986;81(2):194–200. doi: 10.1016/0002-9343(86)90251-2. [DOI] [PubMed] [Google Scholar]
- 88.Levy RA, Qamar T, Lockshin MD. Alternative complement pathway in hypocomplementemic/normal C1s-C1 inhibitor complex patients with SLE. Clin Exp Rheumatol. 1990;8(1):11–5. [PubMed] [Google Scholar]
- 89.Fakhouri F, Jablonski M, Lepercq J, Blouin J, Benachi A, Hourmant M, et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112(12):4542–5. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- 90.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(2):ii46–50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42(1):87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 92.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198(4):385, e1–9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lynch A, Murphy J, Gibbs R, Levine R, Giclas P, Salmon J, Holers V. The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG. 2010;117:456–462. doi: 10.1111/j.1471-0528.2009.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynch A, Gibbs R, Murphy J, Giclas P, Salmon J, Byers T, et al. Early complement activation and subsequent risk for gestational hypertension. Society for Maternal-Fetal Medicine, 29th Annual Meeting, San Diego, California January 26-312009 (poster 246). American Journal Obstetrics and Gynecology. 2008;199(6A):S80. [Google Scholar]
- 95.Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, et al. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199(4):354, e1–8. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108(4):1267–79. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, Carreras Berges L, Lopez-Trascasa M, Sanchez-Corral P, et al. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet. 2005;14(5):703–12. doi: 10.1093/hmg/ddi066. [DOI] [PubMed] [Google Scholar]
- 98.Fang CJ, Fremeaux-Bacchi V, Liszewski MK, Pianetti G, Noris M, Goodship TH, et al. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood. 2008;111(2):624–32. doi: 10.1182/blood-2007-04-084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112(13):4948–52. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104(1):240–5. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 102.Fang CJ, Richards A, Liszewski MK, Kavanagh D, Atkinson JP. Advances in understanding of pathogenesis of aHUS and HELLP. Br J Haematol. 2008;143(3):336–48. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- 103.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med. 2006;3(11):e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conroy A, Serghides L, Finney C, Owino SO, Kumar S, Gowda DC, et al. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS ONE. 2009;4(3):e4953. doi: 10.1371/journal.pone.0004953. [DOI] [PMC free article] [PubMed] [Google Scholar]