Abstract
Effective therapies for metastatic sarcomas remain elusive. Oncolytic viruses have shown promise as anticancer agents, but their access to metastatic sites following systemic delivery is low. Because systemic delivery of small molecule chemotherapy is enhanced by prior treatment with anti-angiogenic agents due to changes in intravascular-to-tumor interstitial pressure, we sought to determine if anti-angiogenic pretreatment increases antitumor efficacy of systemic virotherapy by increasing virus uptake into tumor. Virus biodistribution and anti-tumor effects were monitored in tumor-bearing mice given anti-human VEGF or anti-mouse VEGFR2 before or after an intravenous injection of virus. Without pretreatment, the average virus titers in the tumor samples amplified 1700-fold over 48 hours but were undetectable in other organs. Following anti-angiogenic treatment, average virus titers in the tumor samples were unchanged or in some cases decreased by up to 100-fold. Thus, anti-angiogenic pretreatment failed to improve tumor uptake of systemic oncolytic HSV, in contrast to previously reported enhanced uptake of small molecules. Superior tumor control due to the combined effects of virus and anti-VEGF was seen most dramatically when anti-VEGF was given after virus. Our data suggest intravenous oncolytic HSV can treat distant sites of disease and be enhanced by anti-angiogenic therapy, but only when given in the proper sequence.
Keywords: Oncolytic Virotherapy, HSV, VEGF, Anti-angiogenesis, Ewing sarcoma, Rhabdomyosarcoma
Introduction
Advanced stage sarcomas remain significant clinical challenges, as the use of chemotherapy has largely failed to improve overall survival for adult patients.1 While there have been improvements in survival of patients with certain localized childhood sarcomas such as rhabdomyosarcoma, osteosarcoma, and Ewing sarcoma,2, 3 most other sarcomas of childhood are chemoresistant.4 In tumor types where survival has improved with treatment, relapsed tumors are often resistant to standard therapies. In addition, therapy may be complicated in children by subsequent bone growth deformities, endocrinologic disturbances, and secondary cancers.5 Even for generally treatable sarcoma types, <30% of patients with metastatic disease survive beyond five years despite aggressive use of modern, combination therapies.6 Therefore, there remains a significant need for novel sarcoma therapies to treat metastatic disease.
Oncolytic viruses are being developed as anticancer agents, either using relatively non-pathogenic virus species or viruses attenuated by deletion of critical genes required for viral replication in normal cells but dispensable in cancer cells.7, 8 rRp450 is an oncolytic herpes simplex virus (oHSV) derived from wild type herpes simplex type 1, KOS strain, by replacement of the UL39 gene with rat CYP2B1 gene.9 The UL39 gene encodes for the large subunit of ribonucleotide reductase, normally required for the virus to replicate efficiently in quiescent cells, so its deletion leads to selective viral replication in rapidly growing cancers and other dividing cells with pre-existing elevated expression of cellular ribonucleotide reductase. The CYP2B1 gene encodes a cytochrome P450 enzyme that activates oxazaphosphorine pro-drug chemotherapies. We have previously documented safety and supra-additive efficacy of intratumoral rRp450 and cyclophosphamide in models of pediatric sarcomas.10
A barrier to the use of oncolytic viruses for systemic disease is a relatively poor understanding of parameters determining efficiency of delivery and uptake into distant tumor sites. Interstitial fluid pressure in tumors is elevated due to leakiness of tumor vessels,11-13 impairing the delivery of small molecules to the tumor interstitium. Jain et al. showed that blockade of VEGF signaling results in maturation of tumor neovasculature and leads to a transient decrease in interstitial pressure and a pronounced intravascular-interstitial pressure gradient, peaking 2-4 days following anti-angiogenic treatment.14, 15 As predicted from these findings, prior anti-angiogenic treatment has been shown to increase the systemic delivery of chemotherapeutic agents.16 We thus hypothesized that anti-VEGF pretreatment of mice bearing sarcomas would also improve the systemic delivery of oHSV.
We studied the effect of VEGF blockade on systemically administered virus bio-distribution and anti-tumor effects. In contrast to the findings with small molecule therapeutics, our results suggest anti-angiogenic agents do not enhance and in some cases diminish tumor uptake of systemically administered oHSV. We found that combination effects could be realized primarily when anti-angiogenic therapy follows virus administration.
Results
Systemic delivery of oHSV to distant tumors is impaired but selectively amplified over time
We first chose a syngeneic tumor model to study virus bio-distribution in order to capture any anti-viral immune effects. To determine the bio-distribution of systemically administered oHSV into tumors at different locations, FVBN mice were injected with syngeneic mouse rhabdomyosarcoma (MR366) cells into both subcutaneous (flank) and intramuscular (quadriceps) sites. When the subcutaneous tumors reached >100 mm3, animals were given rRp450 IV; tumors and organs were harvested at different timepoints and analyzed for presence of virus. In previous experiments, cardiac perfusion with saline prior to tumor and organ harvest did not affect levels of detectable virus (data not shown), so the contribution from intravascular virus in these experiments is considered to be minimal.
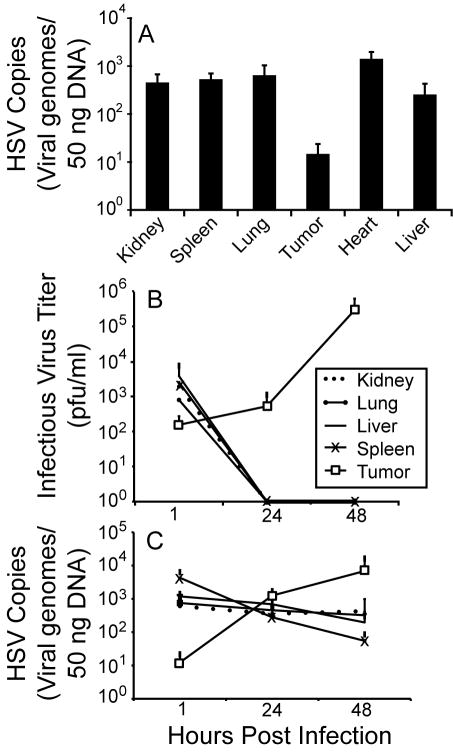
At the 1 hour baseline time point, which should represent initial uptake of virus, the amount of HSV genomes detected in the subcutaneous and intramuscular tumors by qPCR was up to 1-2-logs less than in normal organs (Fig. 1A for subcutaneous and Fig. 1C for intramuscular tumors). The phenomenon appeared similar between these different tumor sites as well as different tumor sizes; the subcutaneous tumor weights in this experiment at the 1 hour harvest were 236±167 mg and the intramuscular tumor weights were 2188±150 mg (tumors in the orthotopic location grow more rapidly than the subcutaneous location, though some of their higher weights may be due to non-tumor tissue because they are difficult to dissect apart from normal muscle and bone). This finding is consistent with high interstitial pressure impairing intratumoral delivery of oHSV. Detection of infectious virions was also consistent with lower initial delivery to tumor sites compared with normal organs (Fig. 1B). The amount of infectious virions recovered relative to the 107 pfu administered intravenously was ~0.01-0.1% in organs and 0.001% in tumors. These numbers are likely quite low because infectivity of virus is lost when it enters cells (until newly made infectious virus is shed). Consistent with absence of viral replication and innate immune-mediated viral clearance from normal organs, there were no infectious viruses detected at 24 and 48 hours post viral injection (Fig. 1B). In sharp contrast, there was significant virus replication in the tumors, leading to a 1700-fold increase in titer at 48h compared with 1h. These data correlated with the detection of HSV genomes, which amplified over time in the tumors but progressively decreased in normal organs at 24 and 48 hours post virus injection (Fig. 1C).
Figure 1.
oHSV is preferentially delivered to normal organs but selectively amplified in tumors over time. FVBN mice (n=4) bearing both (A) subcutaneous and (B,C) imtramuscular mouse rhabdomyosarcoma tumors (MR366) were given a single IV injection of rRp450. (A) Subcutaneous tumors and organs from animals sacrificed 1 hour virus Were analyzed by qPCR for HSV genomes and showed that normal organs had up to 2 logs more virus than the tumors. Intramuscular tumors and organs from animals sacrificed at different timepoints were analyzed by (B) viral plaque assay and (C) qPCR. The results showed that infectious virus was cleared from normal organs but replicated 1700-fold in tumors. Similarly, there was significant amplification of viral genomes in the tumors but a decrease in the normal organs by 48hrs.
Anti-angiogenesis fails to enhance the systemic delivery of oHSV to distant tumors
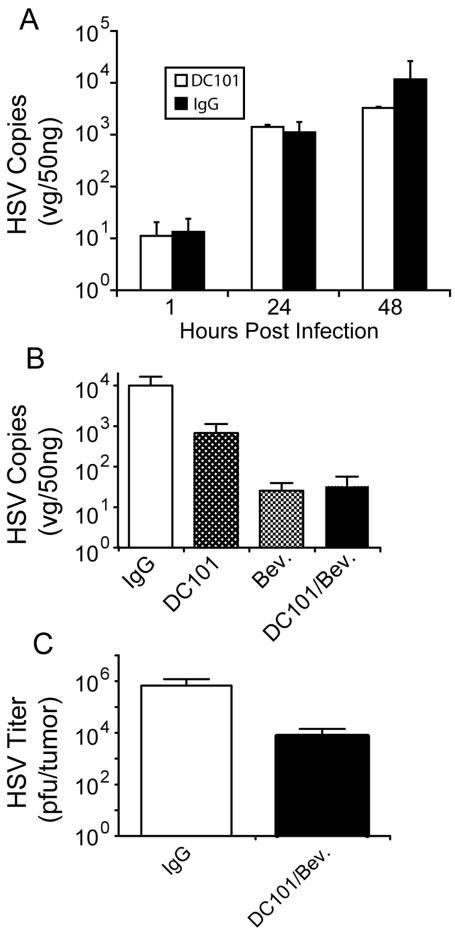
To determine the effect of anti-angiogenic pretreatment on systemic delivery of oHSV, tumor-bearing mice were first given anti-human VEGF and/or anti-mouse VEGFR2 antibodies using both a human xenograft model and a syngeneic mouse tumor model. The A673 human xenograft model was chosen for these studies because of its known dependence on VEGF expression: it exhibits high levels of VEGF secretion relative to other cell lines we tested (data not shown) and was the original model used in the assessment of activity of the anti-VEGF antibody, bevacizumab17. Nude mice harboring flank human A673 tumors were treated with the anti-mouse VEGFR2 antibody, DC101, bevacizumab, or both while FVBN mice bearing intramuscular MR366 tumors were treated with the anti-mouse VEGFR2 antibody, DC101. Both sets of mice received rRp450 IV 3 days later. They were then sacrificed at various time points and qPCR was performed on harvested organs.
There was no difference in the amount of HSV DNA in DC101-treated and IgG-treated MR366 tumors at 1, 24 and 48 hours (Fig. 2A) post-virus injection. In the A673 xenograft model, IgG-treated animals had 1-2 logs more virus detectable in tumors than in those harvested from animals pretreated with the anti-angiogenic antibodies (p=0.09 for IgG vs. DC101, p=0.014 for IgG vs. bevacizumab, p=0.07 for IgG vs DC101+bevacizumab; Fig. 2B). To ensure that we did not miss the “window” time-period during which decreased interstitial pressure occurs, a cohort of A673 xenografts were given daily injections of rRp450 for 4 days, beginning 24 hours following treatment with IgG or the DC101/bevacizumab combination. Again, antiangiogenic therapy did not enhance virus uptake into tumors; in fact, there was a trend for tumors from DC101/bevacizumab treated animals to show less oHSV virus by ~2 logs than those from IgG treated animals (p=0.11, Fig. 2C).
Figure 2.
Prior anti-angiogenic therapy does not improve the systemic delivery of oHSV. Nude mice bearing subcutaneous A673 flank tumors and FVBN mice with syngeneic orthotopic rhabdomyosarcoma tumors were pretreated with IP DC101, IP bevacizumab or both and then given IV rRp450. (A) There was no significant difference in viral genomes between DC101-treated rhabdomyosarcoma tumors and IgG-treated tumors at 1, 24 or 48h post viral injection (n=4). (B) IgG-treated A673 tumors at 3 days post-virus injection had more viral genomes than bevacizumab and/or DC101 treated tumors (n=4-8, see Methods). (C) The delivery of oHSV to anti-angiogenic-treated tumors was decreased compared with IgG-treated tumors when virus was given in daily divided doses over 4 days post-DC101/bevacizumab administration. Tumors were harvested for analysis 24 h after the last virus dose.
Subsequent anti-angiogenic therapy enables combined effects of antitumor efficacy with systemically administered oncolytic HSV
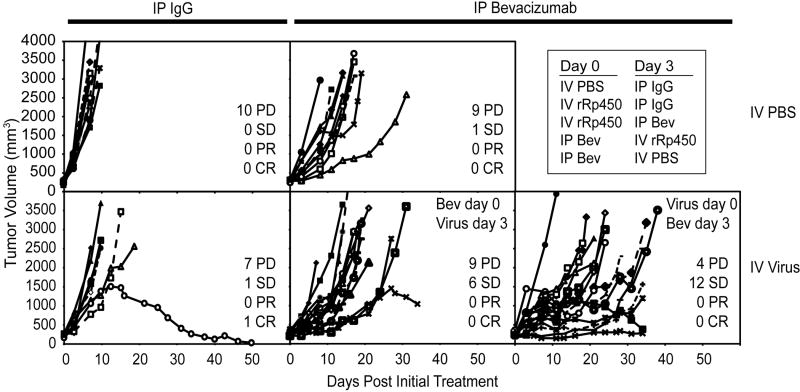
Based on our findings of decreased virus uptake following VEGF blockade in the A673 model, we postulated that a combination effect would be most evident if virus is administered before anti-VEGF antibody. To test this hypothesis, two independent experiments whose results were compiled to increase statistical power, mice bearing A673 xenografts 250-500 mm3 were given intravenous (IV) rRp450 or intraperitoneal (IP) bevacizumab. Three days later animals were given the other treatment (IP bevacizumab or IV rRp450, respectively) and tumor sizes were monitored. Animals given either treatment alone were given the active agent (rather then the control) at the initial timepoint to allow for maximal effects when tumors were smallest. Animals were euthanized when tumors reached >2500 mm3 or, in the first experiment, at day 34, whichever came first. The effects on tumor growth are shown in Fig. 3 and on animal survival (time to reach 2500 mm3) are shown in Fig. 4. Because of the variability in tumor growth, each individual tumor was plotted and the best response was catalogued as progressive disease (PD, >50% growth), stable disease (SD, <50% growth or shrinkage for at least two consecutive measurements during the experiment), partial response (PR, >50% shrinkage compared with pretreatment size), and complete response (CR, no palpable or visible evidence of tumor). All control tumors showed rapidly progressive disease (Fig. 3A). Those given single agent bevacizumab showed a delay in tumor growth as determined by survival (see below) but little effect on response, with an overall response (SD+PR+CR) of 10% (p=0.5 vs. control). Those given single agent virus also showed a delay in growth but little effect on response, with an overall response of 22% (p=0.2 vs. control). The combination with bevacizumab given first showed an increase in response compared to either agent alone, with an overall response of 40% (p=0.028 vs. control), whereas the reverse order (virus first) was more dramatic, with an overall response of 75% (p<0.0002 vs. control, p=0.044 vs. reverse order).
Figure 3.
Effect of Bevacizumab/oHSV combination therapy on tumor growth. Mice bearing A673 xenograft tumors 250-500 mm3 were given one or the other treatment (IP Bevacizumab or IV rRp450 virus) or control (IP IgG or IV PBS) on days 0 and 3. Tumor growth was measured twice weekly and is shown as days post the first injection. The data are from two combined experiments, and in the first animals were sacrificed at 34 days. In the second, animals were sacrificed only when tumors reached >2500 mm3. Best response rates were determined for each tumor (PD, SD, PR, CR); see text for details.
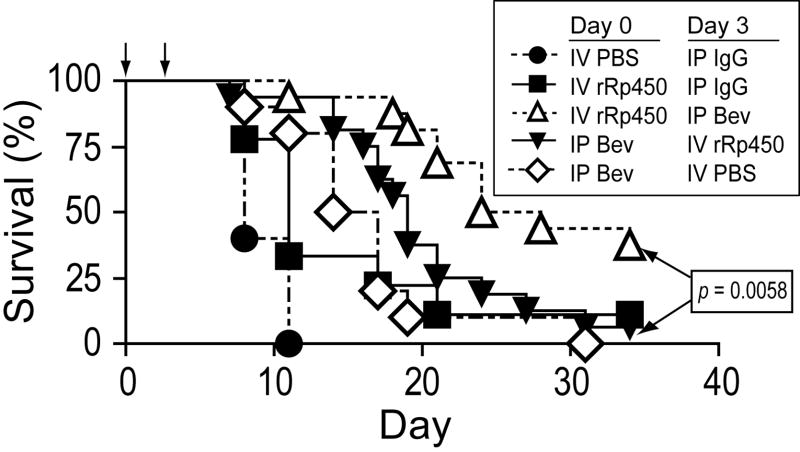
Figure 4.
Effect of Bevacizumab/oHSV combination therapy on animal survival. Mice bearing A673 xenograft tumors 250-500 mm3 were given one or the other treatment or control on days 0 and 3 (arrows). Survival of animals was monitored and is shown as days post the first injection.
Significant antitumor effects were also apparent when these data were analyzed for animal survival. IP bevacizumab alone prolonged survival compared to control mice given IP IgG, confirming the anti-tumor effect of anti-VEGF in this model (p=0.0002, Fig. 4). IV oHSV alone also had a significant antitumor effect (p=0.0306), which was not significantly different from the effect of IP bevacizumab alone (p=0.8299). The addition of IV oHSV following IP bevacizumab had no additional effect on survival compared with IP bevacizumab alone (p=0.1239). When IV oHSV was given prior to IP bevacizumab, however, there was a significantly prolonged survival compared with either single agent alone (p<0.006 for both) and compared with the same combination in reverse order (p=0.0058). One mouse in the virus-only group from the second experiment (which was not arbitrarily terminated) appeared to be cured of the tumor with no palpable mass at day 60. The marked difference in antitumor effect based on the sequence of administration is consistent with our finding that virus uptake in tumors is inhibited by prior VEGF blockade.
Discussion
We found that the initial delivery of systemically administered oHSV to distant tumors is impaired compared with normal organs, but that infectious virions are amplified rapidly in tumors and cleared from normal organs. Prior anti-angiogenic therapy did not enhance and in some cases was detrimental to tumor uptake of systemically administered oHSV, and combination therapy was more effective than single agent therapy only when virus was given first.
Systemic biodistribution has been a major stumbling block for virus vectors used in gene transfer and virotherapy. The best studied example is adenovirus, where the “first-pass effect” causes most particles given IV to be sequestered in the liver.18 Perhaps because HSV is a larger, enveloped particle there appears to be less of a first-pass effect, with only about one-third of detectable HSV in the liver at 12 hours post administration.19 In fact, antitumor efficacy has been demonstrated in animal models following intravenously administered HSV mutants for subcutaneous tumors20, 21 and pulmonary metastases,22-24 even in the presence of neutralizing antibodies.25 Uptake of oncolytic HSV following arterial infusion has also been demonstrated in human trials.26, 27 These data and studies of other viruses28, 29 suggest systemic intravenous virotherapy may be useful for the treatment of metastatic disease, though little is understood about the mechanisms mediating virus uptake in tumors.
Oncolytic HSV biodistribution has been examined in a genetically engineered spontaneous mouse model of prostate cancer using four virus injections over a 10 day period. HSV genomes were detected equally in prostate tumors and liver, with less in lymph node, lung, and blood.30 There was no evidence of virus amplification in tumor sites, but virus genomes were not quantified before day 11; importantly, virus genomes persisted in the prostate tumors whereas they were rapidly cleared from normal organs. The analyses were performed 11 days after the first virus injection and 1 day after the last, so the actual amount delivered to various sites, as opposed to that resulting from virus replication or migration, is unclear. Interestingly, oncolytic HSV given intraperitoneally was also found in distant tumor sites and induced an antitumor effect, though full biodistribution studies were not performed.
One limitation to systemic HSV delivery is innate immunity. Following infection with most strains of wild type HSV, mice mount vigorous innate and adaptive anti-viral immune responses. HSV has evolved mechanisms such as Fc receptor binding to subvert adaptive anti-viral immunity, but innate immune factors including complement have been shown in some cases to limit viral delivery to tumor sites when given regionally.31-33 One strategy to subvert inactivation of viruses given systemically that has been pioneered with other virus types is the use of tumor-homing cells, such as tumor cells or T cells, as delivery vehicles.34, 35 Induction of a transient vascular leak with vasoactive agents including IL-2, enhanced by inhibition of Tregs, has been shown to markedly enhance delivery of an oncolytic virus to distant tumor sites.36
It is well documented that small molecule drugs linked to or encapsulated in liposomes show preferential accumulation in tumors due to leaky neoangiogenic vessels (with intercellular gaps as large as 600-800 nm) and impaired lymphatic drainage, an effect known as enhanced permeability and retention.37 A similar phenomenon for HSV uptake was not borne out by our data, as we found decreased amounts of virus within the tumor relative to other organs shortly after virus administration. It may be that compared with small molecule drugs and their lipid conjugates, the larger size of HSV particles (~150nm diameter) prevents passive extravasation through inter-endothelial cell gaps regardless of a pressure differential. In addition, there may be fewer herpesvirus receptors in tumors compared with normal organs,38, 39 resulting in only low levels of active receptor-mediated uptake, which would be unaffected by pressure differentials. Whether or not tumors in different sites or organs other than the flanks and intramuscular tumors tested in this study differ in this regard is unknown.
VEGF blockade has been shown to decrease tumor interstitial pressure, transiently enabling increased delivery of systemic chemotherapeutic agents to xenograft tumors before the vasculature is fully remodeled.16, 40 In contrast, we found that VEGF blockade did not increase, and in fact in some cases decreased delivery of oHSV to tumor sites. The most likely explanation is the reduced tumor perfusion following antiangiogenic therapy, though we cannot rule out the formal possibility that HSV receptors were downregulated on endothelial or tumor cells by anti-VEGF antibody. This remains a possibility in light of data suggesting VEGF upregulates HSV receptors on endothelial cells,41 though we did not observe any effect of anti-VEGF on HSV-mediated cytotoxicity on tumor cells in culture (Supplementary Figure S1). The effect of VEGF blockade on virus replication and spread after it has already reached the tumor is also unknown.
Anti-VEGF given after systemic oHSV administration led to better tumor control and animal survival than when given prior to virus. In the latter case, there was a trend toward additive effects of combination therapy compared with bevacizumab alone (p=0.13) but not compared to virus alone, suggesting that the lower antitumor effect of having less virus was offset by the additional antitumor effect of the bevacizumab. The reason DC101 appeared to have less of an effect than bevacizumab is not known, but may be related to the fact that we did not optimize the doses of each antibody or to variables inherent to binding soluble ligands versus membrane bound receptors. Also, administration of DC101 in mice results in a compensatory increase in native systemic mouse VEGF levels,42 which may have partially counteracted the effects of DC101. Whether such an effect occurs with bevacizumab has not been tested.
Whether or not our findings are broadly applicable to oncolytic viruses in general is not known. We did not investigate whether tumor uptake of smaller viruses, such as adenovirus or Sindbis virus, or those of a different shape (e.g. filamentous), which also require receptor-mediated uptake but may better fit through endothelial gaps, are also impeded by anti-angiogenic agents. Similarly, we do not yet know if the effect will be less pronounced in other tumor models that are less dependent on VEGF. Regardless, our data suggest that tumor vascular remodeling by anti-angiogenic agents fails to enhance and in some cases decreases tumor uptake of systemically administered oncolytic HSV particles and should therefore be used after, but not before, systemic virus administration.
Materials and Methods
Cells and viruses
Human Ewing sarcoma (A673) cells were purchased from the American Type Culture Collection (Manassas, VA). Mouse rhabdomyosarcoma (MR366) cells were kindly donated by Glen Merlino (NCI, Bethesda, Maryland), after they were derived from a p53-/-, HGF-transgenic mouse.43 ICP6-deleted rRp450 HSV-1 (derived from strain KOS) was kindly provided by E. Antonio Chiocca (Ohio State University, Columbus, Ohio). oHSV-Luc (derived from strain F) is deleted for expression of both ICP6 and ICP34.5, and expresses firefly luciferase from the ICP6 early viral gene promoter as previously described.44
In vivo studies
Animal studies were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee. Mice were euthanized per institutional guidelines once tumor size reached 10% of body weight. Six to eight weeks old female mice were injected with 5 × 106 A673 human Ewing sarcoma cells (subcutaneously in athymic nude (nu/nu) mice) or MR366 mouse rhabdomyosarcoma cells (subcutaneously or intramuscularly in FVBN mice, Harlan Sprague Dawley, Indianapolis, IN) in 30% matrigel (BD Biosciences, San Diego, CA). Tail veins of mice were used for all intravenous (IV) injections.
VEGF ELISA
Athymic nude mice were treated with increasing doses of DC101. At 24 hr post administration, mice were anesthetized and phlebotomy performed at the retro-orbital venus plexus. Blood was collected in Microtainer plasma separating tubes (Becton Dickinson, San Jose CA) and allowed to clot at room temperature for 30 minutes. Clotted blood was centrifuged at 1,200 × g for 10 minutes at 4°C. Supernatants (plasma) were collected and analyzed for mouse VEGF levels using a mouse VEGF ELISA kit (R&D systems, Minneapolis, MN) according to the manufacturer’s instructions. Immunodepletion was performed on samples to remove any DC101 had that may be cross-reactive with the VEGF ELISA kit following as previously described 42.
Virus biodistribution
To determine baseline bio-distribution of systemically administered rRp450, 4 FVBN mice bearing both subcutaneous (SC) and intramuscular (IM) MR366 tumors were given 107 plaque forming units (PFU) of rRp450 IV. At various times post-injection they were euthanized and organs and tumors were harvested (Fig. 1). To determine the effect of anti-angiogenic therapy on systemic delivery of oHSV, another group of MR366 tumor-bearing FVBN mice (n=4) were treated with intraperitoneal injections (IP) of 40mg/kg of DC101 (ImClone Systems, Inc., New York, NY) or non-specific rat IgG. The activity of DC101 was verified by its stimulation of plasma VEGF levels in mice as per Bocci et al.42 (Supplementary Fig. S2). On day 3, each mouse was given 107 rRp450 IV, and sacrificed at 1, 24 and 48 hours post injection (Fig. 2A). A similar experiment was performed in A673 xenografts: 8 mice per group bearing A673 tumors were treated with 40mg/kg of DC101, 10mg/kg bevacizumab or bevacizumab and DC101 combination or non-specific rat IgG IP. On day 3 all mice were given 108 rRp450 IV and euthanized for organ and tumor harvest on day 6 (Fig. 2B). Three mice from the bevacizumab and four from the bevacizumab and DC101 combination group died before day 6 and were not included in the analysis. To ensure that virus was administered during the period of lowest interstitial fluid pressure, which in most tumor models has been determined to occur by day 4 post anti-angiogenic therapy,45 nude mice bearing SC A673 tumors (4 per group) were treated with DC101/bevacizumab (40mg/kg and 10mg/kg, respectively) or rat IgG IP. On days 1, 2, 3 and 4 post-antibody injection, each mouse was treated with 108 PFU rRp450 IV. On day 5, all the mice were euthanized, organs and tumors were then harvested for viral plaque assay (Fig. 2C).
Plaque assays
Harvested tumors and organs were snap-frozen in liquid nitrogen and stored at - 80°C until processed. The tumors and organs were homogenized with a Powergen tissue homogenizer (Fischer Scientific, Pittsburg, PA) in 1 ml of a protease inhibitor cocktail (Roche, Indianapolis, IN). Viral plaque assays for infectious virus as well as qPCR reactions to quantify HSV genomes were performed on the harvested organs as previously described.10
Antitumor efficacy
Tumor volume was determined by V= (L × W2) × π/6, where L is the length of the tumor and W is the width. When A673 tumor volumes reached 200-250 mm3, mice were treated with 10mg/kg bevacizumab IP or 108 PFU of rRp450 IV on day 1. On day 3 the treatments were reversed and the tumor sizes were followed. Control mice received IP rat IgG or IP bevacizumab alone. In the first experiment, when tumor volumes reached 2500mm3 or at day 34 (arbitrary cutoff) animals were euthanized. In the second experiment, animals were only sacrified when tumor volumes reached 2500mm3. Results from the two experiments were compiled in Figs. 3 and 4 (n=9-16).
Statistical Analysis
Comparisons between two means were performed with an unpaired student’s t test and more than two means by ANOVA. Survival was analyzed by log-rank. GraphPad Prism version 5 software (GraphPad Software, San Diego, California) was used for all statistical analyses except Fig. 2B, in which case we used SPSS. Because of the variability and heteroscedacity, data were transformed to log base 10 and analyzed by the Games-Howell nonparametric post-hoc test. Tumor growth response rates in Fig. 3 were analyzed by Fisher’s exact test.
Supplementary Material
Acknowledgments
We thank Daniel Demopoulos, Cindy Lambert, Steve Mayer, and Cindy Klotz (Hematology/Oncology Pharmacy at Cincinnati Children’s Hospital Medical Center) for providing Bevacizumab, Dan Hinklin (ImClone Systems) for DC101, E. Antonio Chiocca (Ohio State University) for providing rRp450, David Krisky and Bill Goins (University of Pittsburgh) for production and purification of rRp450, and Arturo Maldonado for help with biostatistic analyses. This work was supported by Cincinnati Children’s Hospital Medical Center Division of Hematology/Oncology, teeoffagainstcancer.org, the Katie Linz Foundation, The Limb Preservation Foundation, an American Cancer Society Fellowship Award (FKE) and NIH grant R01-CA114004 (TPC).
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: a meta-analysis and clinical practice guideline. Sarcoma. 2000;4(3):103–12. doi: 10.1080/13577140020008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappo AS, Shapiro DN, Crist WM, Maurer HM. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13:2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 3.Ries L, Smith M, Gurney J, Linet M, Tamra T, Young J, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995, NIH Pub No 99-4649. National Cancer Institute, SEER Program; Bethesda, MD: 1999. [Google Scholar]
- 4.Pratt C, Maurer H, Gieser P, Salzberg A, Rao B, Parham D, et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: A Pediatric Oncology Group study. Med Pediatr Oncol. 1998;30:201–209. doi: 10.1002/(sici)1096-911x(199804)30:4<201::aid-mpo1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Goldsby R, Burke C, Nagarajan R, Zhou T, Chen Z, Marina N, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer. 2008;113(9):2597–604. doi: 10.1002/cncr.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miser JS, Goldsby RE, Chen Z, Krailo MD, Tarbell NJ, Link MP, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy--a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49(7):894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- 7.Smith ER, Chiocca EA. Oncolytic viruses as novel anticancer agents: turning one scourge against another. Expert Opin Investig Drugs. 2000;9(2):311–27. doi: 10.1517/13543784.9.2.311. [DOI] [PubMed] [Google Scholar]
- 8.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99(23):1768–81. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 9.Aghi M, Chou TC, Suling K, Breakefield XO, Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59(16):3861–5. [PubMed] [Google Scholar]
- 10.Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16(5):879–85. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52(18):5110–4. [PubMed] [Google Scholar]
- 12.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60(15):4251–5. [PubMed] [Google Scholar]
- 14.Duda DG. Antiangiogenesis and drug delivery to tumors: bench to bedside and back. Cancer Res. 2006;66(8):3967–70. doi: 10.1158/0008-5472.CAN-05-4536. [DOI] [PubMed] [Google Scholar]
- 15.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 16.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–50. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 17.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281(2):951–61. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds P, Dmitriev I, Curiel D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Therapy. 1999;6:1336–1339. doi: 10.1038/sj.gt.3300941. [DOI] [PubMed] [Google Scholar]
- 19.Schellingerhout D, Bogdanov A, Jr, Marecos E, Spear M, Breakefield X, Weissleder R. Mapping the in vivo distribution of herpes simplex virions. Hum Gene Ther. 1998;9(11):1543–9. doi: 10.1089/hum.1998.9.11-1543. [DOI] [PubMed] [Google Scholar]
- 20.Oyama M, Ohigashi T, Hoshi M, Nakashima J, Tachibana M, Murai M, et al. Intravesical and intravenous therapy of human bladder cancer by the herpes vector G207. Hum Gene Ther. 2000;11(12):1683–93. doi: 10.1089/10430340050111331. [DOI] [PubMed] [Google Scholar]
- 21.Cinatl J, Jr, Cinatl J, Michaelis M, Kabickova H, Kotchetkov R, Vogel J-U, et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003;63:1508–1514. [PubMed] [Google Scholar]
- 22.Fu X, Zhang X. Potent systemic antitumor activity from an oncolytic herpes simplex virus of syncytial phenotype. Cancer Res. 2002;62:2306–2312. [PubMed] [Google Scholar]
- 23.Wong R, Chan M-K, Yu Z, Kim T, Bhargava A, Stiles B, et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12. Clinical Cancer Research. 2004;10:251–259. doi: 10.1158/1078-0432.CCR-0197-3. [DOI] [PubMed] [Google Scholar]
- 24.Nakamori M, Fu X, Pettaway C, Zhang X. Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancer. The Prostate. 2004;60:53–60. doi: 10.1002/pros.20056. [DOI] [PubMed] [Google Scholar]
- 25.Delman KA, Bennett JJ, Zager JS, Burt BM, McAuliffe PF, Petrowsky H, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2000;11(18):2465–72. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody L, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17(12):1214–24. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 27.Fong Y, Kim T, Bhargava A, Schwartz L, Brown K, Brody L, et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol Ther. 2009;17(2):389–94. doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;13(23):7155–65. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 29.Duerner LJ, Schwantes A, Schneider IC, Cichutek K, Buchholz CJ. Cell entry targeting restricts biodistribution of replication-competent retroviruses to tumour tissue. Gene Ther. 2008;15(22):1500–10. doi: 10.1038/gt.2008.92. [DOI] [PubMed] [Google Scholar]
- 30.Varghese S, Rabkin SD, Nielsen GP, MacGarvey U, Liu R, Martuza RL. Systemic therapy of spontaneous prostate cancer in transgenic mice with oncolytic herpes simplex viruses. Cancer Res. 2007;67(19):9371–9. doi: 10.1158/0008-5472.CAN-07-0674. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74(10):4765–75. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakimoto H, Ikeda K, Abe T, Ichikawa T, Hochberg FH, Ezekowitz RA, et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol Ther. 2002;5(3):275–82. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 34.Kottke T, Diaz RM, Kaluza K, Pulido J, Galivo F, Wongthida P, et al. Use of Biological Therapy to Enhance Both Virotherapy and Adoptive T-Cell Therapy for Cancer. Mol Ther. 2008;16(12):1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15(4):660–5. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- 36.Kottke T, Galivo F, Wongthida P, Diaz RM, Thompson J, Jevremovic D, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol Ther. 2008;16(7):1217–26. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen T, Cullis P. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 38.Gimenez-Cassina A, Lim F, Diaz-Nido J. Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. J Neurosci Res. 2006;84(4):755–67. doi: 10.1002/jnr.20976. [DOI] [PubMed] [Google Scholar]
- 39.Yu Z, Li S, Huang YY, Fong Y, Wong RJ. Calcium depletion enhances nectin-1 expression and herpes oncolytic therapy of squamous cell carcinoma. Cancer Gene Ther. 2007;14(8):738–47. doi: 10.1038/sj.cgt.7701062. [DOI] [PubMed] [Google Scholar]
- 40.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88(12):1979–86. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benencia F, Courreges MC, Conejo-Garcia JR, Buckanovich RJ, Zhang L, Carroll RH, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16(6):765–78. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 42.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64(18):6616–25. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 43.Sharp R, Recio J, Jhappan C, Otsuka T, Liu S, Yu W, et al. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nature Medicine. 2002;8:1276–1280. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 44.Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68(4):1170–9. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong R, Boucher Y, Kozin S, Winkler F, Hicklin D, Jain R. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.