Abstract
Cytochrome P4502E1 (CYP2E1) potentiated TNFα-toxicity by a mechanism involving increased oxidative stress and activation of JNK and p38 MAPKs. The current study evaluated what are the upstream mediators of this MAPK activation with a special focus on studying whether Apoptosis Signal Regulating Kinase-1 (ASK-1) is activated in the CYP2E1-TNFα hepatotoxic model. Wild type and CYP2E1−/− mice were treated with pyrazole (PY) for three days to induce CYP2E1 and challenged with TNFα on day three. Liver injury occurred between 8–12h after TNFα addition only to the wild type PY-treated mice. Oxidative stress was elevated in the PY mice at 4h, a time prior to the liver injury. ASK-1 was dissociated from the thioredoxin-ASK1 complex, and was activated at 4h after addition of TNFα to PY mice. This was followed by activation of MKK3/MKK6 and MKK4/MKK7 at 4–8 or 12h and then JNK/p38 MAPK at 8 to 12h. MAPK phosphatase-1 was decreased 12 to 24h after TNFα addition. This may promote a sustained activation of JNK. Bax was elevated while Bcl-2 and cFLIPS/L were lowered at 4h after addition of TNFα. These changes were followed by increases in caspase 8 and 3 activities and apoptosis. None of the above changes were found when TNFα was added to PY-treated CYP2E1−/− mice. These studies show that TNFα increases oxidative stress in mice with elevated CYP2E1 with subsequent activation of ASK-1 via a mechanism involving thioredoxin/ASK-1 dissociation, followed by activation of downstream MAPKK and MAPK. We speculate that similar interactions between CYP2E1 and TNFα may be important for alcohol-induced liver injury.
Key works: CYP2E1, TNFα, ROS, ASK-1, MAP kinases, Oxidative stress, Liver Injury
Tumor necrosis factor alpha (TNFα), plays an important role in alcoholic liver injury [1–3]. Chronic alcohol consumption promotes the permeability of bacteria through the intestinal epithelia into the bloodstream [4]. The elevation in lipopolysarcharide (LPS) induces activation of Kupffer cells and release of cytokines such as TNFα [5–7]. Alcohol-induced liver injury is prevented by destruction of Kupffer cells, by antibodies which neutralize TNFα, and in TNFα receptor 1 knockout mice [3, 8, 9]. Chronic alcohol consumption also induces cytochrome P4502E1 (CYP2E1) [10], an important pathway by which ethanol elevates oxidative stress [11–12]. CYP2E1 is a loosely coupled cytochrome P450, producing superoxide and hydrogen peroxide during its catalytic cycle [13]. In general, TNFα is not toxic to hepatocytes because it also activates NF-κB, with the subsequent production of survival factors [14]. Besides elevating TNFα, alcohol is believed to sensitize the liver to become susceptible to TNFα [6]. CYP2E1 may be one of these sensitizing factors. For example, in-vitro combined treatment with ethanol plus TNFα was toxic to hepatocytes and HepG2 E47 cells which express high levels of CYP2E1 [15]. RALA hepatocytes with increased CYP2E1 were sensitized to TNFα mediated cell death [16]. These results suggest that increased oxidant stress from CYP2E1 may sensitize isolated hepatocytes to TNFα-induced toxicity. Administration of TNFα to mice pre-treated with pyrazole to induce CYP2E1 caused significant liver injury, under conditions in which pyrazole alone, or TNFα administered to saline-treated mice did not cause liver injury [17]. This injury was mediated through a pathway involving elevated oxidative stress and activation of JNK and p38 MAPK [17, 18] and was blocked by inhibitors/scavengers of ROS, iNOS, JNK or p38 MAPK [18]. The potentiation by PY was blunted in CYP2E1−/− mice [17], suggesting an important role for CYP2E1 in the potentiated injury by TNFα plus PY. The upstream mediators of JNK and p38 MAPK activation were not identified in these previous studies. For mechanistic and therapeutic implications, it would be important to evaluate the MAP kinase kinase kinase and MAP kinase kinase which activate JNK and p38 MAPK in this PY plus TNFα model.
Apoptosis signal-regulating kinase 1 (ASK-1) is a member of the MAP3K family which is responsive to stress-induced cell damage. Activation of ASK-1 can determine cell fate by regulation of both the MKK4/MKK7-JNK and the MKK3/MKK6-p38 MAPK signaling cascades [19]. ASK-1 is activated by oxidative stress, ER stress, and inflammatory cytokines such as TNFα [20]. In resting cells, ASK-1 forms an inactive complex with reduced thioredoxin (Trx). Under conditions of stress by TNFα or ROS, ASK-1 dissociates from Trx and becomes activated [21]. Oxidation of Trx by ROS causes dissociation of ASK-1 from the oxidized Trx which switches the inactive form of ASK-1 to the active kinase. The Trx-ASK complex is thought to be a redox sensor, which functions as a molecular switch turning the cellular redox state into a MAP kinase signaling pathway [22]. Activated ASK-1 then promotes activation (phosphorylation) of the downstream MAPKK, MKK4/MKK7 which can activate JNK, and MKK3/MKK6 which can activate p38 MAPK [19–22]. Since previous studies showed that in mice treated with TNFα and PY, JNK and p38 MAPK were activated [17, 18], we evaluated whether CYP2E1 plus TNFα induced ROS promote release of ASK-1 from the Trx-ASK1 complex and activate ASK-1 followed by the phosphorylation of MKK4/MKK7 and/or MKK3/MKK6 which subsequently regulate the phosphorylation of JNK and p38 MAPK and contribute to the liver injury.
Previous studies were all carried out in samples collected 24h after administration of TNFα. As a secondary goal, we determined the time course for development of oxidative stress, activation of MAPK, induction of CYP2E1, in relation to the development of liver injury in order to understand the sequence of events which occur when TNFα is added to mice pretreated with pyrazole to induce CYP2E1.
Materials and Methods
Materials
Antibodies against ASK-1, Trx, pMKK4/MKK4, MKK7, pMKK3/MKK3, pMKK6/MKK6, pJNK/JNK, pp38 MAPK/p38 MAPK, MKP-1, Bcl-2, Bax, cFLIPL/S and β-actin were from Santa Cruz Biotechnology Inc (Santa Cruz, Ca). Antibody against pMKK7 was from Cell Signaling Technology, Inc. (Danvers, MA). Antibody against CYP2E1 was a generous gift from Dr. Jerry Lasker. Murine TNFα was from Fitzgerald (Concord, MA). Pyrazole, was from Sigma Chemical Co. (St Louis, MO). DNA Fragmentation Detection Kit, caspase 3 and caspase 8 substrates were from Calbiochem (La Jolla, Ca). ALT and AST Measuring Kit was from Thermo Fisher Scientific Inc. (Waltham, MA). Fluorescence conjugated second antibodies used in the Odyseey Infrared Imaging System were from Li—Cor Biosciences, Inc (Lincoln, NE).
Animal Model
Breeder pairs of CYP2E1 knockout mice were a generous gift from Dr. Frank Gonzalez, NCI. Wild type control mice and the CYP2E1 knockout mice as described in [17, 18] received humane care according to the outline in the NIH Guide and with approval of the Mount Sinai Animal Care and Use Committee. Male mice, 19–22 g body weight were injected intraperitoneally with PY, 150 mg/Kg body weight once per day for 3 days to induce CYP2E21. Control mice were injected with saline. On the third day, all mice received an injection of TNFα, 50 μg/kg body weight, intraperitoneally one hour after the PY or saline injection. At 0, 4, 8 and 12h after the TNFα injection, the mice were sacrificed, blood was collected from the retroorbital venous under anesthesia, and livers were removed.
Liver pathology
Mice liver samples were fixed in 10% formalin solution for pathological evaluation and DNA fragmentation study. Serum ALT and AST were determined with the THERMO detection kit. Liver lysates were prepared by homogenizing in phosphate-buffered saline buffer containing a cocktail of proteinase inhibitors (Sigma).
Nuclear DNA fragmentation, caspase 3 and 8 activity
Liver sections were used to evaluate DNA fragmentation as outlined in the Calbiochem DNA Fragmentation Detection Kit. The percentages of positive cells were counted. Activities of caspase 3 and 8 were determined with 50 μmol/L caspase 3 substrate II, AC-DEVD-AMC and caspase 8 substrate II, Granzyme B, Z-IETD-AFC, and 200 μg liver lysate protein. After overnight incubation at 37°C, fluorescence associated with the released aminomethylcoumarin (AMC) (excitation at 380 nm, emission at 460 nm) and aminofluoromethylcoumarin (AFC) (excitation at 400 nm, emission at 505 nm) were determined and caspase activity expressed as arbitrary fluorescence units per milligram liver protein.
Release of ASK-1 from the Trx-ASK1 complex
Release of ASK-1 from the Trx-ASK1 complex was determined using a Thermo Immunoprecipitation Kit (Product # 45332) followed by immunoblot study of the immunoprecipitate. 15 μg Trx1 antibody was coupled to 50 μl AminoLink Plus Coupling Gel. 100 μg liver homogenate protein extract (using M-PER Mammalian Protein Extract Reagent) was incubated with Trx1 antibody coupled to AminoLink Plus Coupling Gel overnight at 4°C to precipitate the Trx-ASK1 complex. The precipitated protein was washed several times with Immunoprecipitaion Sample Buffer. Trx-ASK1 complex protein was then eluted from the AminoLink Plus Coupling Gel with 50 μl Elution Buffer. Immunoblots were carried out with ASK-1 and Trx1 antibodies to determine the levels of ASK-1 and Trx.
Activation of ASK-1, MKK3/6, MKK4/7, JNK and p38 MAPK
Immunoblots were carried out to determine the levels of pASK-1, pMKK3/6, pMKK4/7, pJNK and pp38 MAPK. The blots were evaluated and quantified by the Odyssey Infrared Imaging System. The membranes were rinsed with Re-Blot Plus Strong Solution (Chemicon International) and re-incubated with ASK-1, MKK3/6, MKK4/7, JNK and p38 MAPK antibodies followed by Li-Cor secondary antibodies to determine total levels of the ASK-1, MAPKK and MAPK. The activation levels were determined by calculating the ratios of phosphorylated MAPKs to the non-phosphorylated MAPKs.
Intracellular glutathione and lipid peroxidation
Glutathione (GSH) levels and lipid peroxidation were determined as described previously [17, 18] and results expressed as arbitrary unit (GSH) or nmol (TBARS) per milligram of protein.
CYP2E1, cFLIP, MPK-1, Bcl-2 and Bax content
CYP2E1 protein content was determined by immunoblot of liver microsomal preparations. Levels of MPK-1, Bcl-2, Bax and cFLIP were determined by immunoblots of liver lysates. All results were expressed as the protein/β-actin ratio. CYP2E1 catalytic activity was evaluated from the oxidation of p-nitrophenol [17, 18].
Statistical Analysis
Statistical analysis was carried out using the SPSS (17.0) program with Nonparametric Test analysis of variance with subsequent two-independent samples comparison by the Mann- Whitney Test (1-tailed Sig). Comparisons were made between the effects of TNF plus saline versus TNF plus pyrazole for each specific time point. Values reflect mean ± standard error and are from either 3 or 4 mice treated with either TNF or TNF plus pyrazole at each time point. Immunoblots show results from one experiment but numbers ( ratios) under the blots are from 3 mice as indicated in the Figure legends.
Results
TNFα plus pyrazole induced liver injury
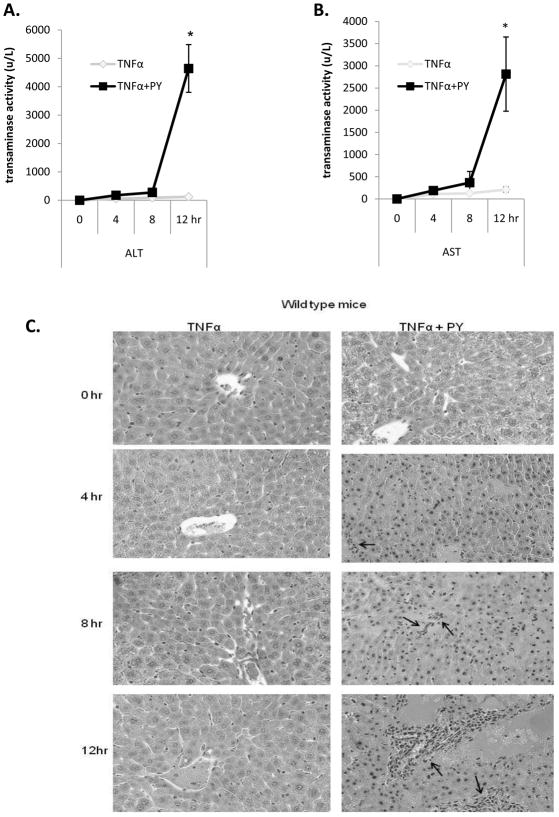
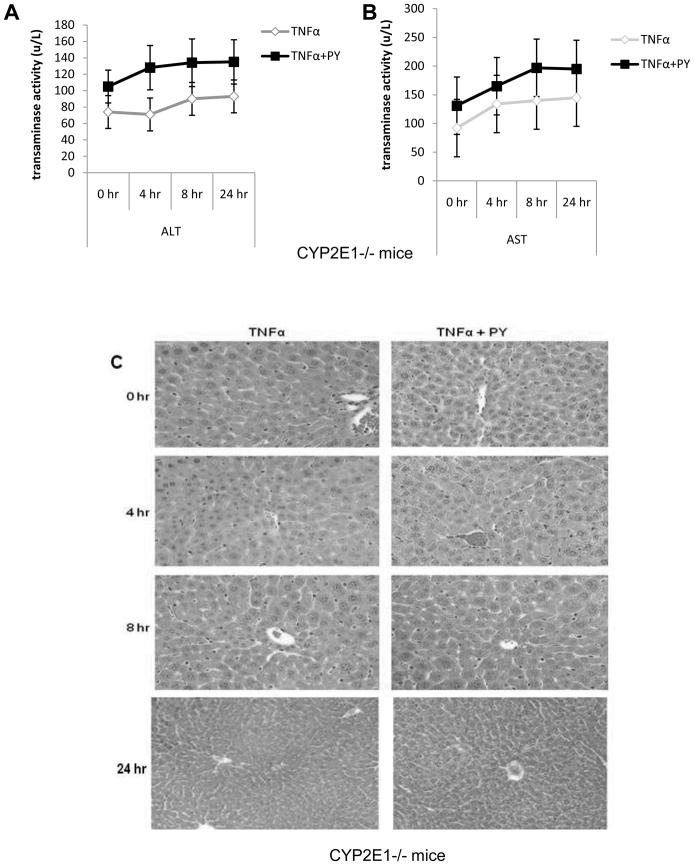
Wild type and CYP2E1−/− mice were treated with TNFα alone or TNFα plus pyrazole and sacrificed immediately, or at several time points after the TNFα injection. Wild type mice treated with PY plus TNFα developed liver injury between 8 and 12h after TNFα administration as reflected by the high levels of ALT and AST at 12h (Fig. 1A, B). PY plus TNFα treatment induced some liver cell nuclear shrinkage and infiltration of inflammatory cells in both periportal and pericentral areas of the liver at 8h, with more striking pathological changes at 12h (Fig. 1C). CYP2E1−/− mice, treated with PY plus TNFα did not show significant liver injury, even 24h after TNFα administration (Fig. 2). These results indicate that CYP2E1 is necessary for TNFα to induce liver injury in this model and that injury does not develop until at least 8 to 12h after the TNFα administration.
Fig. 1. CYP2E1 plus TNFα-dependent liver injury.
Wild type mice were treated with saline or pyrazole for 3 days as described in Methods and Materials. On day 3, TNFα was administered immediately after the saline or pyrazole treatment and the mice were sacrificed at 0, 4, 8 and 12 hours, after the TNFα. ALT (A), AST (B) and histopathology (H&E staining) (C). Arrows indicate foci of inflammatory infiltration focus. Results are the average from 4 mice in each group at each time point.. P<0.05 for the 12h TNFα+PY versus TNFα.
Fig. 2. Hepatotoxicity evaluation in CYP2E1−/− mice.
CYP2E1−/− mice were treated as mentioned in the legend to Fig. 1 and sacrificed at 0, 4, 8 and 24h, respectively, after the TNFα. ALT (A), AST (B), H&E staining (C). Results are the average of 4 mice. There were no significant differences between the TNFα versus the TNFα plus PY.
Oxidative stress
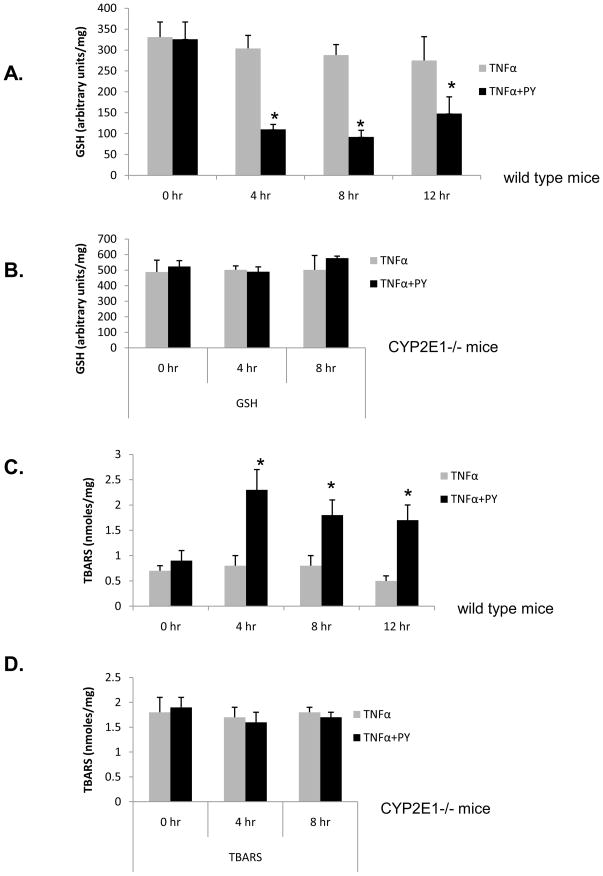
Oxidative stress is a likely key factor to trigger signaling and liver injury in CYP2E1-mediated hepatotoxicity [23]. A time course for oxidative stress after PY plus TNFα treatment was studied. GSH was decreased in wild type mice after 4h and remained at lower levels for at least 12h as compared to the TNFα alone group (Fig. 3A). Lipid peroxidation increased significantly at 4h in the PY plus TNFα treated mice, but not in the TNFα alone mice. TBARS remained elevated up to 12h (Fig. 3C). In the CYP2E1−/− mice, treatment with PY plus TNFα did not induce oxidative stress as GSH levels (Fig. 3B) or TBARS levels (Fig. 3D) remained the same as the 0 hour control and the TNFα alone levels. These results show that oxidative stress occurs at an earlier time after administration of TNFα than does liver injury in the TNFα plus PY treated mice, and CYP2E1 plays a major role in the increase of oxidative stress.
Fig. 3. CYP2E1 plus TNFα-dependent ROS stress.
WT mice (A, C) or CYP2E1−/− mice (B, D) were treated as described in the legend to Fig. 1, and at the indicated times after administration of TNFα, GSH levels (A, WT; B, 2E1−/−) and lipid peroxidation (C, WT; D, 2E1−/−) evaluated. Results are each from three mice. *, P< 0.05, compared with TNFα alone at different time points.
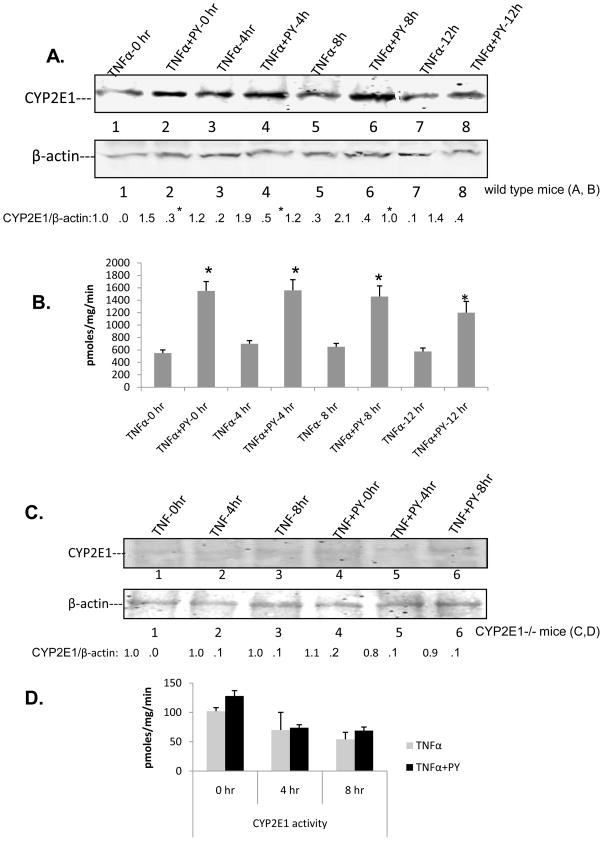
CYP2E1 levels and activity
Treatment with PY increased the levels of CYP2E1 prior to the administration of TNFα (Fig. 4A, TNFα-0h and TNFα+PY-0h blots) and CYP2E1 levels remained about 2-fold elevated at least until 8h after administration of TNFα in the PY-treated mice. TNFα alone had no effect on basal levels of CYP2E1 when administered into the saline treated mice. PNP oxidation activity was 2.5 fold higher in the PY plus TNFα treated mice at the 0h point (the mice were pretreated with PY for 2 days already) and remained at high levels up to 12 hours (Fig. 4B). CYP2E1 protein could not be detected and the PNP oxidation remained at very low levels in the CYP2E1−/− mice (Fig. 4C, D), likely catalyzed by other CYPs e.g. CYP3A.
Fig. 4. CYP2E1 levels.
Immunoblots for CYP2E1 and oxidation of PNP at 0, 4, 8, 12h after administration of TNFα to saline-or pyrazole-treated WT mice (A,B) or CYP2E1−/− mice (C,D). Numbers under the blots refer to the CYP2E1/β-actin ratio. For A and B, CYP2E1 levels and oxidation of PNP were significantly higher (*, P<0.05) for the TNFα+PY compared to the TNFα. Results are from 4 mice per group at each time point
Activation of ASK-1
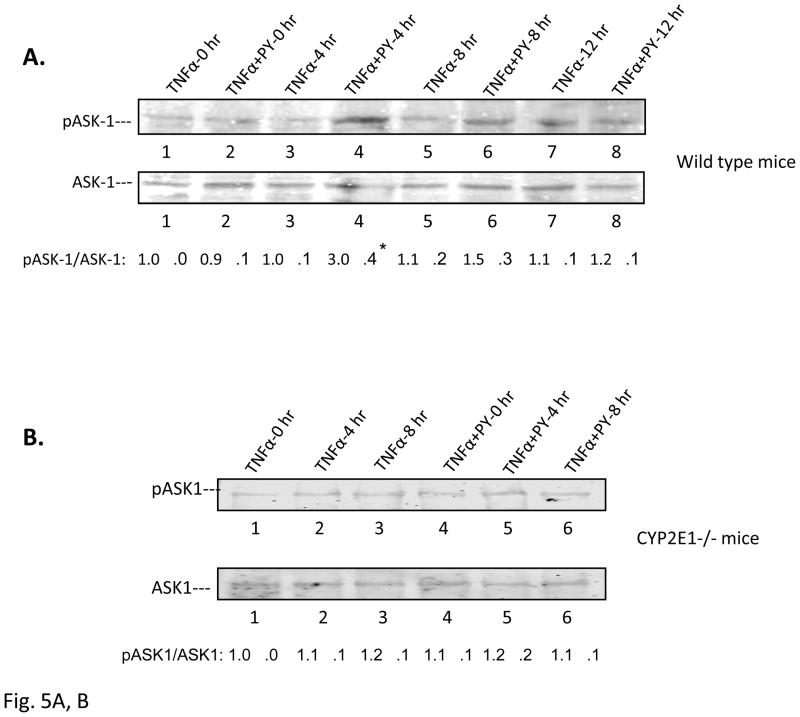
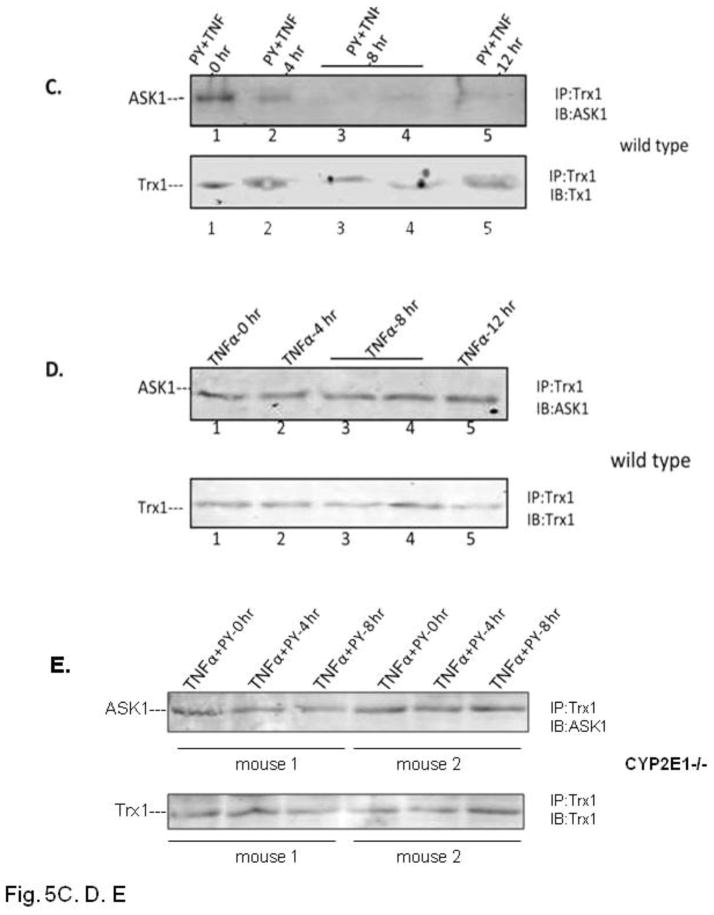
Since previous results showed a key role of JNK and p38 MAPK in the TNFα plus PY-induced liver injury, we evaluated whether upstream MAPKK and MAPKKK were activated, the time course for their activation in relation to the hepatic injury, and the role of CYP2E1. We focused on ASK-1 since this MAPKKK has been shown to be important as a target for TNFα signaling [19, 20, 22]. TNFα or pretreatment with PY alone did not activate ASK-1 (Fig. 5A, lanes 1, 3, 5 and 7 for TNFα alone and lane 2 for PY treatment alone). TNFα plus PY treatment activated ASK-1–3-fold compared with the 0 hour control at 4h after TNFα treatment (Fig. 5A, lane 4). Activation of ASK-1 decreased at 8 and 12h. Immunoprecipitation experiments showed that ASK-1 was bound to Trx-1 at 0h but was released from the Trx-ASK1 complex at 4h and remained free from binding to Trx1 at 8 and 12h (Fig. 5C). No ASK-1 release from the Trx-ASK1 complex was found in TNFα alone treated mice (Fig. 5D). ASK-1 was not activated in PY plus TNFα treated CYP2E1−/− mice (Fig. 5B) and no ASK-1 was released from the Trx-ASK1 complex in CYP2E1−/− mice (Fig. 5E). Thus, activation of ASK-1 by treatment with TNFα plus PY is associated with its release from the Trx-ASK1 complex, occurs prior to the liver injury, and requires CYP2E1.
Fig. 5. Activation of ASK-1.
The activation of ASK-1 was determined in liver lysate from WT (A) or CYP2E1−/− mice (B) by immunoblot analysis. The activation was expressed as the pASK/ASK ratio and ratios from 3 experiments are shown below the blots. * P<0.05 compared with TNFα alone at 4 H. Trx antibody was used to immunoprecipitate the Trx-ASK complex from TNFα plus PY (C) or TNFα alone (D) treated mouse liver lysates from WT (C, D) or CYP2E1−/− (E) mice. ASK-1 and Trx-1 levels in the immunoprecipitate were determined by immunoblot..
Activation of MKK4/7, MKK3/6, JNK and p38 MAPK
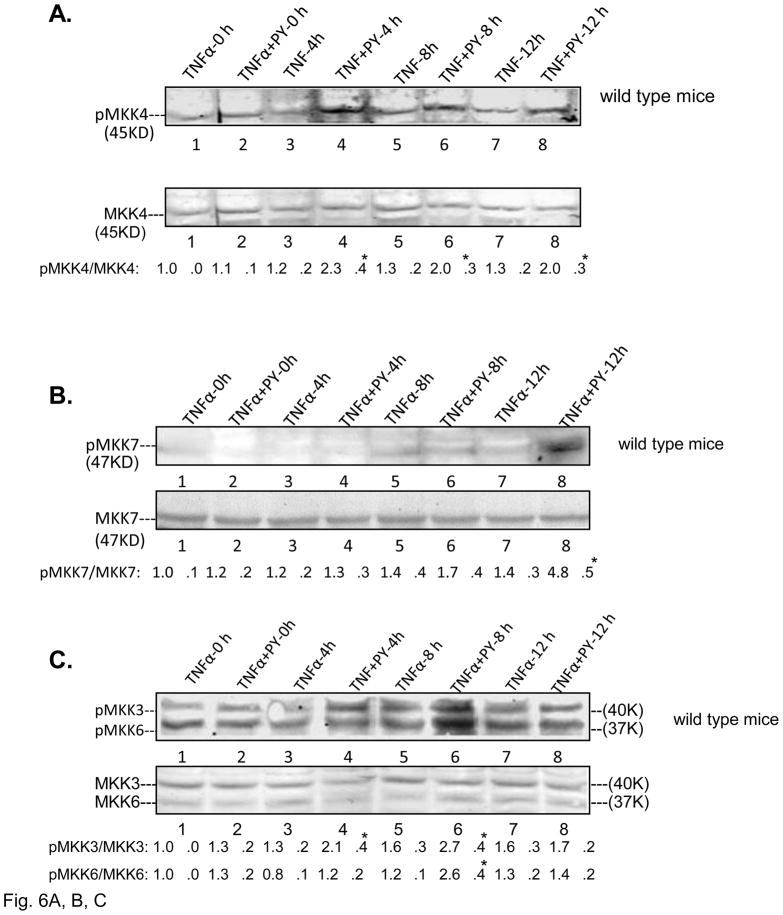
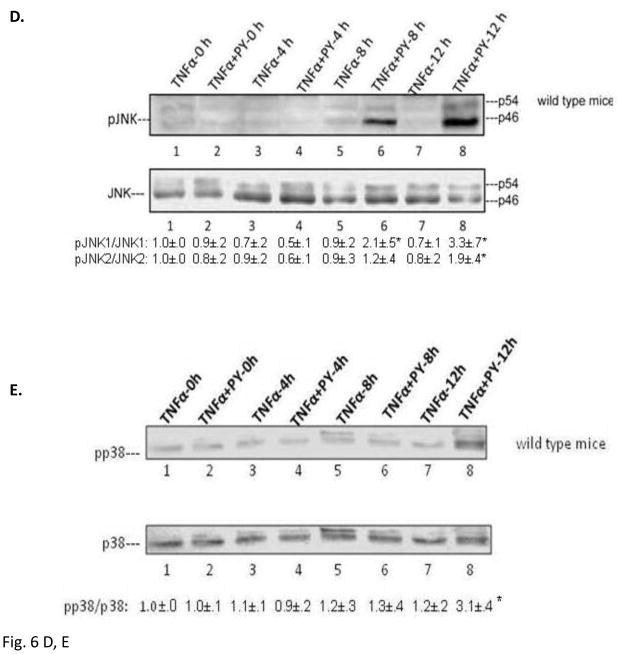
MKK4/7 and MKK3/6 are the MAPKK which activate downstream JNK or p38 MAPK, respectively [24]. They are also targets for activation by ASK-1 [25, 26]. Treatment of wild type mice with PY plus TNFα activated MKK4 at 4, 8 and 12h compared with the TNFα alone groups (Fig. 6A). No activation of MKK4 was found in TNFα or TNFα+PY treated CYP2E1−/− mice (data not shown). MKK7 was activated only at 12h (Fig. 6B). MKK3 was activated as early as 4h in the TNFα plus PY treated mice (Fig. 6C) while MKK6 was activated at 8h (Fig. 6C). JNK was activated in the TNFα+PY mice at 8 and 12h and p38 MAPK was activated at 12h when compared with TNFα alone (Fig. 6D, 6E). In CYP2E1−/− mice, neither MKK4/7, MKK3/6, JNK or p38 MAPK were activated ( data not shown). Thus, the time course experiments suggest MKK4 may be the MAPK responsible for activation of JNK, while either MKK3 or MKK6 may be the MAPKK responsible for the activation of p38 MAPK.
Fig. 6. Activation of MKK4/MKK7, MKK3/MKK6, JNK and p38 MAPK.
The activations of MKK4 (A,); MKK7 (B); MKK3, MKK6 (C); JNK (D ) and p38 MAPK (E ) in wild type mice were analyzed from liver lysates by immunoblot analysis. The activation was expressed as the phosphorylated over non-phosphorylated form and ratios from 3 different mice are shown under the blots. *, P<0.05 compared with their TNFα alone control respectively.
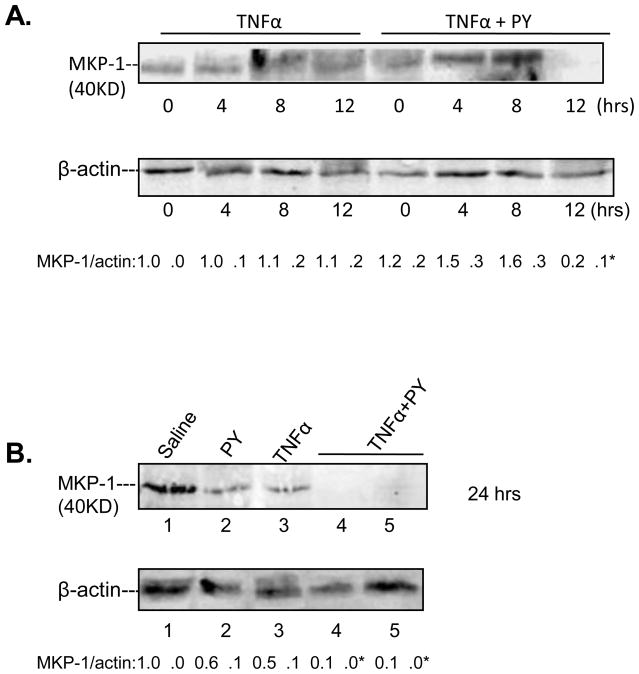
Effect of PY plus TNFα on MKP-1
MKP-1 is a MAPK phosphatase which dephosphorylates MAPKKK, MAPKK and MAPK [27] and which is sensitive to ROS [28]. Since CYP2E1 generates ROS, we evaluated whether decreases in MKP-1 in TNFα plus PY treated mice, may contribute to the active phosphorylated states of ASK-1, MKK4/7, MKK3/6, JNK and p38 MAPK. No effect was found on MKP-1 levels after treatment of wild type mice with TNFα alone (Fig. 7A). Treatment of mice with PY plus TNFα for 4 or 8 hours slightly increased MKP-1 levels, however, MKP-1 levels decreased at 12h (Fig. 7A), and remained low at 24h (Fig. 7B). The lowering of MKP-1 by PY plus TNFα treatment at later times (12–24h) after administration of TNFα may play a role in the sustained activation of JNK or p38 MAPK at 12h (Fig. 6D,6E,) and as previously found, 24h after the administration of TNFα [17].
Fig. 7. Levels of MKP-1.
Immunoblots were carried out to determine MKP-1 levels in liver lysates from TNFα alone or TNFα plus PY treated wild type mice. The levels of MKP-1 were determined from the MKP-1/β-actin ratios; ratios from 3 different mice are expressed below the blots. *, P<0.05 compared with TNFα alone groups for 12h or 24h respectively.
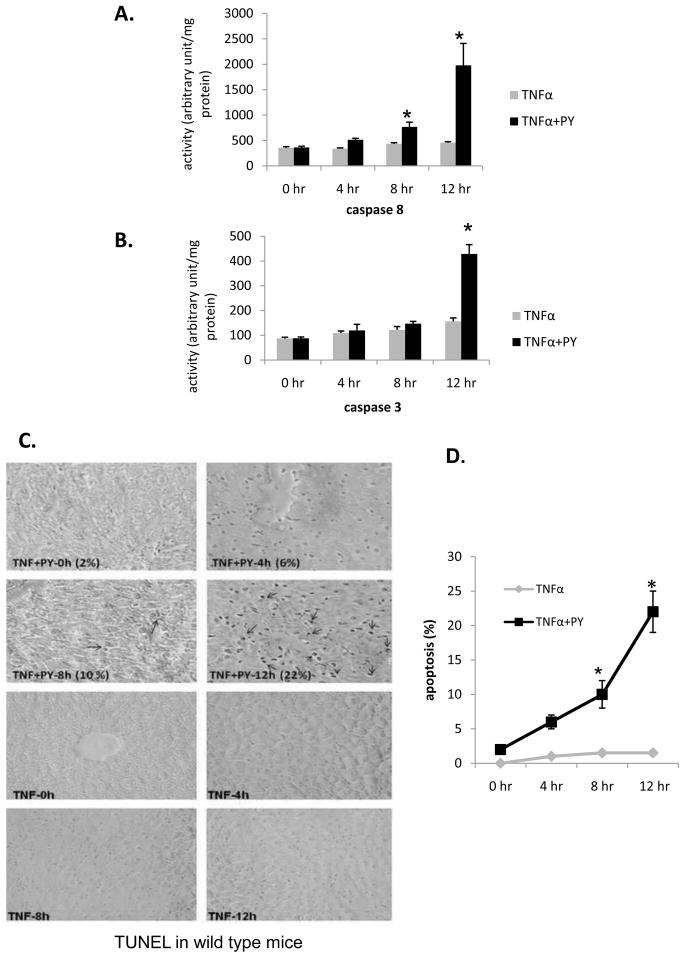
Liver apoptosis
Previous experiments showed that the liver injury found 24 h after administration of TNFα to PY mice was largely necrotic [17, 18]. Experiments were carried out to determine if apoptosis occurs in the TNFα plus PY-treated mice. Caspase 8 began to increase at 8h and reached high levels at 12h after TNFα addition to PY-treated mice (Fig. 8A). TNFα addition to saline-treated mice did not increase caspase 8 or 3 activity. Caspase 3 was activated at 12h in the TNFα plus PY treated mice (Fig. 8B). TUNEL evaluation indicated that TNFα plus PY treatment produced 2, 6, 10 and 22% positive cells after 0, 4, 8 and 12h treatment while TNFα alone did not cause DNA fragmentation (Fig. 8C, D). In CYP2E1−/− mice, treatment with PY plus TNFα did not activate caspase 8 or caspase 3 (data not shown). Thus, the liver injury has features of both necrosis and apoptosis which occur after the elevation of CYP2E1 and oxidative stress.
Fig. 8. Activation of caspase 8, 3 and DNA fragmentation.
Caspase 8 (A) and caspase 3 (B) activation in liver lysates from wild type mice treated with saline or with pyrazole was determined at the indicated time points after administration of TNFα. * P< 0.05 compared with TNFα alone. Experiments were repeated with three different mice. Liver slices from PY plus TNFα or TNFα alone treated mice were used for TUNEL analysis as described in Methods. Arrows indicate TUNEL-positive nuclei (stained dark brown). The average percent of TUNEL-positive cells are shown in (C) and graph (D), *, P<0.05 compared with TNFα alone treated groups.
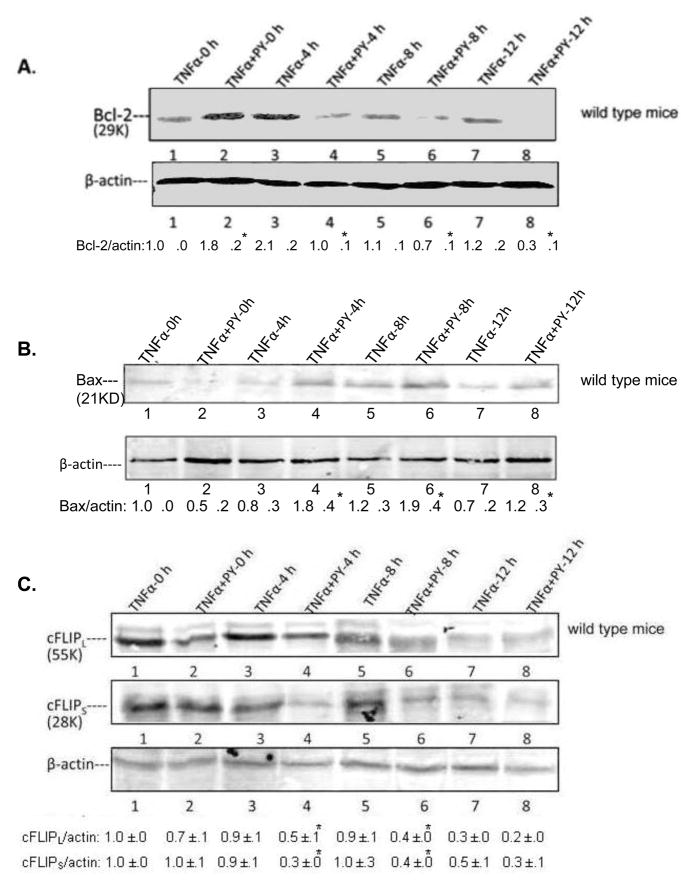
Levels of pro-and anti-apoptotic factors
Bcl-2 family members play a critical role in TNFα-induced liver apoptosis and necrosis [14, 25]. Levels of some pro-and anti-apoptotic Bcl-2 family members were evaluated. Bcl-2 rapidly decreased at 4h; levels of Bcl-2 continued to decrease at 8 and 12h (Fig. 9A), being 69, 63 and 36 % the levels of the TNFα alone groups at 4, 8 and 12h, respectively (Fig. 9A). Levels of Bcl-XL, an anti-apoptotic member of the Bcl-2 family, declined at 4h to 33% of the 4h TNFα alone group (data not shown). In contrast, Bax a major pro-apoptotic proteins increased at 4 or 8 and 12h after TNFα addition to the PY-treated mice as compared to the TNFα alone treatment (Fig. 9B). cFLIP blocks TNFα complex II formation and blocks the activation of caspase 8 [29]. cFLIPL/S levels were rapidly decreased to 55% and 50% of the TNFα alone levels after treatment with TNFα plus PY for 4h (Fig. 9C).
Fig. 9. Levels of pro-and anti-apoptotic factors.
Bcl-2 (A) and Bax (B) levels in liver lysates from wild type mice were determined by immunoblot. Blot intensities were compared with β-actin as shown below the blots. The long form and short form of cFLIP, cFLIPL and cFLIPS were also determined from liver lysates by immunoblots (C). All immunoblots were repeated with three different mice and the ratios are shown below the blots. *, P<0.05 compared with their TNFα alone control respectively.
Taken as a whole, pro-apoptotic factor such as Bax increased at 4, 8 and 12h after administration of TNFα to PY-treated mice, in association with a decrease of anti-apoptotic factors such as Bcl-2, and c-FLIP. Such changes are followed by activation of caspases 8 and 3, and elevated DNA fragmentation, largely between 8 and 12h after the administration of TNFα to the PY-treated mice. In contrast, treatment of CYP2E1−/− mice with TNFα plus PY did not change or only slightly affected the levels of Bcl-2, cFLIP, or Bax ( data not shown) suggesting the necessary role of CYP2E1 in the effects by TNFα plus PY.
Discussion
TNFα–induced ROS plays an important role in alcoholic liver injury [1, 3, 8], but TNFα alone usually fails to induce liver injury as TNFα activation of NF-κB blunts TNFα induced liver damage [14, 25]. The induction of CYP2E1 by ethanol is one important pathway by which ethanol induces oxidative stress [10–12]. Increasing CYP2E1 by pyrazole treatment sensitizes the liver to TNFα-induced liver injury. We used a concentration of TNFα which by itself was too low such that TNFα signaling and injury was minimal which allows the potentiation of TNFα actions by induction of CYP2E1 to be studied. In this model, liver injury is associated with activation of JNK and p38 MAPK and elevated production of ROS and RNS [17, 18]. JNK and p38 MAPK inhibitors such as SP600123 and SB203580 and ROS or RNS inhibitors such as NAC or 1400W blocked the CYP2E1 plus TNFα liver injury. How JNK or p38 MAPK were activated and the upstream signaling MAPKKKs and MAPKKs, are not known and were a major focus of the current study. A time course of in vivo liver injury induced by PY plus TNFα was carried out to determine the sequence of events and relationships between induction of CYP2E1, oxidative stress, the activation of ASK-1, MKK3/MKK6, MKK4/MKK7, p38 MAPK and JNK with the development of liver injury. Another goal was to evaluate whether apoptosis occurs in this injury model. Table I summaries a comparison of the effects of TNFα plus PY relative to TNFα alone on what we believe to be important contributors to mechanisms by which TNFα plus PY produce liver injury in WT mice, but not in CYP2E1 knockout mice. The table shows time courses for changes in liver parameters produced by TNFα plus PY at 4, 8 or 12h after administration of TNFα to PY-treated mice relative to any changes produced when TNFα was given to saline-treated mice. The liver injury occurs at 8 to 12h after the addition of TNFα. It would therefore be expected that increases (or decreases) in critical components for this injury would likely change prior to the liver injury and this is the basis for the discussion below.
Table 1.
Comparison of the effects of TNFα plus pyrazole as compared to TNFα alone on liver parameters
| Parameter | Time after TNFα administration (Hr) | |||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| CYP2E1 activity | ↑ | ↑ | ↗ | ↑ |
| GSH | - | ↓↓ | ↓↓ | ↓↓ |
| TBARS | - | ↑↑ | ↑ | ↑ |
| Dissociation of ASK1 | - | ↑ | ↑ | ↑ |
| pASK1 | - | ↑↑ | ↑ | - |
| pMKK4 | - | ↑↑ | ↑ | ↑ |
| pMKK7 | - | - | - | ↑↑ |
| pMKK3 | - | ↑ | ↑↑ | - |
| pMKK6 | - | ↗ | ↑↑ | - |
| Level of MKP-1 | - | ↗ | ↗ | ↓↓ |
| pJNK | - | - | ↑ | ↑↑ |
| pp38 | - | - | ↗ | ↑↑ |
| BCL-2 | - | ↓ | ↓ | ↓↓ |
| cFLIPL | - | ↓↓ | ↓↓ | - |
| cFLIPS | - | ↓↓ | ↓↓ | - |
| Bax | - | ↑ | ↑ | ↑ |
| caspase 8 | - | - | ↗ | ↑↑ |
| caspase 3 | - | - | - | ↑↑ |
| TUNEL | - | ↗ | ↑ | ↑↑ |
| ALT/AST | - | - | - | ↑↑ |
| Histopathology | - | - | ↗ | ↑↑ |
Results compare the changes in the indicated parameters produced by combined treatment with TNFα plus pyrazole relative to those produced by TNFα alone at 0, 4, 8 and 12 hr after the administration of TNFα. No difference, -; decrease, ↓; large decrease, ↓↓; small increase, ↗; Increase, ↑; large increase, ↑↑.
Since ROS is postulated to be a critical factor in the mechanism by which TNFα plus PY induce liver injury, development of ROS should precede the liver injury. Indeed, hepatic GSH levels were decreased and TBARS levels were elevated 4h after administration of TNFα to PY-treated mice. Thus oxidative stress precedes the liver injury. This is consistent with the ability of the antioxidant NAC to blunt the liver injury [18]. A likely contributor to the increase in oxidative stress is the induction of CYP2E1 by the pyrazole treatment as no injury or oxidative stress was observed in CYP2E1 knockout mice. CYP2E1 levels were already elevated at the time of TNFα administration (0h) since the mice were treated for two days prior to this injection of TNFα on day 3.
ASK-1, a member of the MAPKKK family, activates both MKK4/MKK7-JNK and MKK3/MKK6-p38 MAPK signaling cascades. ASK-1 is activated in cells subjected to stresses such as ROS or TNFα [25, 26]. In non-stressed cells, ASK-1 forms an inactive high-molecular-mass complex with Trx [30]. Trx is an oxidation/reduction (redox) regulatory protein, which inhibits the kinase activity of ASK-1. Upon ROS stress, the oxidized Trx is dissociated from ASK-1, resulting in activation of ASK-1 [21, 22]. ASK-1 was activated in PY-treated mice at 4h after the administration of TNFα. Immunoprecipitation analysis showed that ASK-1 was dissociated from the inactive Trx-ASK complex at 4h, consistent with the activation of ASK-1 at 4h. In CYP2E1−/− mice, pyrazole plus TNFα treatment failed to activate ASK-1 and ASK-1 was not dissociated from the Trx-ASK1 complex. TNFα alone failed to activate ASK-1 or release ASK-1 from the inhibitory Trx complex. If CYP2E1 generated ROS is important for the release and activation of ASK-1, elevation of CYP2E1 and in oxidative stress should occur as early events. Increases in CYP2E1 and ROS occur at 4h, at least consistent with the activation of ASK-1 at 4h, although future experiments with shorter time intervals will be necessary to evaluate these relationships in more detail. Recently, ASK-1 was shown to be important for acetaminophen-induced liver injury [31]. Our results implicate a role for ASK-1 in CYP2E1 potentiation of TNFα-induced liver injury. Future experiments with ASK-1 knockout mice [31] are planned to further validate the role of ASK-1 in the PY/TNFα model.
JNK or p38 MAPK activities are increased upon phosphorylation by MAPK kinase (MKK4/MKK7 or MKK3/MKK6) [24]. The activity of ASK-1 modulates and regulates the phosphorylation of MKK4/MKK7 and MKK3/MKK6. PY plus TNFα treatment increased MKK4 phosphorylation at 4, 8 and 12h while activation of MKK7 was delayed until 12h. MKK3 and MKK6 phosphorylation were also increased at 4 to 8h. In CYP2E1−/− mice, no MAPKK was activated at any observation time point. TNFα alone did not significantly activate the MAPKK in wild type or CYP2E1−/− mice. The activation of MKK4 and MKK3/6 (4–8h) occur prior to the onset of liver injury (8–12h).
Activated MAPK can be inactivated through dephosphorylation of threonine and/or tyrosine residues within the activation loop. The dephosphorylation can be catalyzed by serine/threonine phosphatase, tyrosine phosphatase and dual-specificity phosphatases or by MAP kinase phosphatase (MKP) [27]. In the MKP family, MKP-1 plays a critical role in the negative regulation of JNK and p38 MAPK in response to stress including oxidative stress [32]. MKP-1 levels and activity are very sensitive to ROS [28]. This raised the question as to whether CYP2E1-generated ROS activated MAPKKK, MAPKK, and MAPK not only via removal of Trx from the Trx-ASK1 complex, but also by decreasing MPK-1. PY plus TNFα treatment slightly increased levels of MKP-1 at 4 and 8h. MKP-1 has been shown to be induced by moderate oxidative stress [27, 33]. Perhaps the modest increase in MKP-1 is an adaptive response to CYP2E1 plus TNFα oxidative stress. However, MKP-1 is decreased by more pronounced oxidative stress. This may explain the large decrease at 12h after administration of TNFα to PY-treated mice. It is interesting to speculate that the ROS induced decrease of MKP-1 at 12h and 24h may play a role in the sustained activation of JNK and p38 MAPK found 24h after addition of TNFα to PY-treated mice [17, 18].
TNFα induced cell apoptosis involves activation of the caspase 8 pathway [34]. Pyrazole plus TNFα treatment activated caspase 8 and caspase 3 at 12h, occurring after the increase in oxidative stress (4h). The large increases in TUNEL staining at 12h occur in association with the activation of caspase 8 and 3 at this time point. The smaller increases in TUNEL at 4 and 8h are not associated with increases in caspase 8 and 3 activity and possibly may be caspase-independent or may reflect non-apoptotic DNA fragmentation. In CYP2E1−/− mice, pyrazole plus TNFα treatment do not increase TUNEL-positive cells or activate caspases 8 and 3. Thus, combined treatment with TNFα plus PY leads to cell death with features of either apoptosis or necrosis or both, a form of cell death termed necroptosis [35, 36]. An extensive signaling network regulating necroptosis, including Bcl-2 family members, is required for TNFα mediated death-receptor-induced necroptosis [37, 38]. Whether liver injury in the PY plus TNFα model reflects a model of necroptosis will require further study, e.g. does inhibition of apoptosis inhibit necrosis?
Bcl-2, a major anti apoptotic protein was decreased as early as 4h and remained at low levels at 8 and 12h after TNFα administration to the PY mice. Bax, a major pro-apoptotic protein was increased at 4, 8 and 12h. Levels of cFLIPL and cFLIPS which prevent TNFα induced complex II formation and block the activation of caspase 8 [29] were decreased at 4h. Thus, the TNFα plus PY treatment results in an early decline in antiapoptotic factors such as Bcl-2, and c-FLIP and an increase in pro-apoptotic factors such as Bax at 4 to 12h. These changes, which would promote activation of caspases, occur prior to the increases in caspase 8 and 3 activity (12h). Treatment of mice with PY plus TNFα was previously shown to induce mitochondrial damage, causing a decline in membrane potential and an increase in membrane permeability transition [17, 18]. Whether the decline in Bcl-2 and/or the increase in Bax contribute to the mitochondrial dysfunction remains to be determined.
The role of CYP2E1 in the activation of ASK-1, MKK4/MKK7 or MKK3/MKK6 is apparent, since TNFα treatment only induced such activations in wild type mice treated with PY to induce CYP2E1 but not in CYP2E1−/− mice. We hypothesize that TNFα alone-or CYP2E1 alone- generated ROS stress is not sufficient to trigger the dissociation of ASK-1 from the Trx-ASK complex. The CYP2E1 sensitization of TNFα induced liver injury may occur through a synergistic effect with TNFα to produce an enhanced ROS stress consistent with the so call —Two Hit hypothesis. ASK-1 can activate NF-κB in a redox-dependent manner and this may serve as a survival signal in hepatocytes early during injury. We previously found that while TNFα alone caused some activation of NF-κB and increased production of NF-κB-dependent survival factors such manganese SOD2, this upregulation was blocked in the livers from the TNFα plus pyrazole treated mice [18]. Thus, a possible TNFα-ASK-1-increase in the NF-κB survival pathway is blunted by the increase in CYP2E1 (and ROS) produced by the pyrazole treatment.
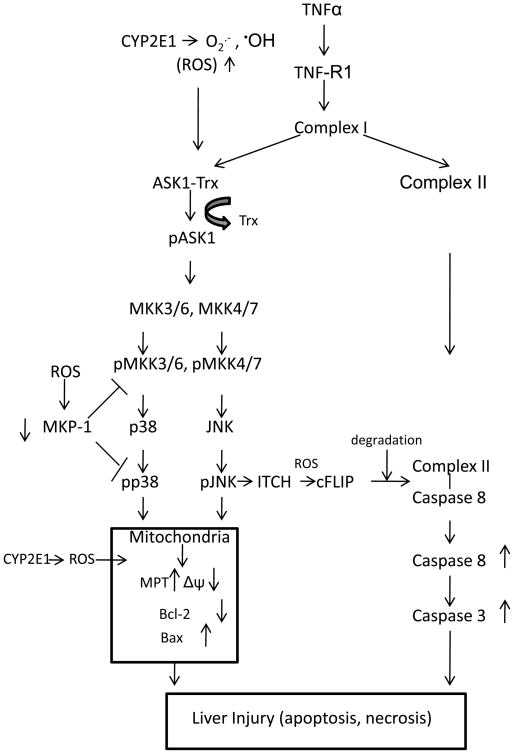
The scheme shown in Fig. 10 summarizes mechanisms that we believe play a role in the potentiation of TNFα liver injury by CYP2E1. We hypothesize that CYP2E1-derived ROS are critical to the TNFα–induced dissociation of ASK-1 from the Trx-ASK complex and lead to the activation of ASK-1. The fact that TNFα alone added to wild type mice, or TNFα plus PY added to CYP2E1−/− mice failed to activate ASK-1, and ability of the ROS/RNS inhibitors NAC and 1400W to block the activation of JNK or p38 MAPK [18] suggests that TNFα plus CYP2E1 derived ROS/RNS are —Two Hits to activate MAPKKKs like ASK-1, followed by activation of the downstream MAPKK and subsequently JNK and p38 MAPK. CYP2E1 plus TNFα also induced the degradation of MKP-1, a MAPKK or MAPK negative regulator. Levels of MKP-1 remain low after 12 or 24h treatment, which may contribute to sustained activation of JNK or p38 MAPK by the PY plus TNFα treatment. The decrease of Bcl-2 and the increase of Bax suggest that the ASK-1-MKK3/6, MKK4/7-p38 MAPK, JNK signaling pathway may target the mitochondria and produce mitochondrial dysfunction and subsequently liver injury. cFLIP blocks the ability of TNFα induced complex I to recruit caspase 8 to form complex II followed by activation of caspase 8. The decrease in cFLIP produced by the TNFα plus PY treatment may result in enhancement of caspase 8 activity. Activated caspase 8 may increase proapoptotic Bcl-2 family members such as Bax and Bid which causes mitochondria to release apoptosis factors to activate caspase 3 and induce apoptosis. Although further studies to explore aspects of this scheme are required, this study may offer possible mechanisms to explain how two independent major risk factors in alcohol-induced liver injury, TNFα and CYP2E1, interact to promote liver injury.
Fig. 10. Scheme. CYP2E1 sensitizes TNFα to activate ASK-1 and its downsteam signaling targets.
We hypothesize that CYP2E1-generated ROS cause dissociation of ASK-1 from the Trx-ASK1 complex in the presence of TNFα. This results in activation of the MAPKKK, ASK-1, followed by activation of MAPKK and eventually JNK and p38 MAPK. The combination of elevated ROS, activated JNK and p38 MAPK, increases in proapoptotic and decrease in anti-apoptotic Bcl-2 family members promote mitochondrial dysfunction and liver injury. Apoptosis occurs from the elevation of ROS, activation of MAPK, decline in cFLIP, which contributes to the liver injury. The decrease in MKP-1, likely from CYP2E1-generated ROS, results in prolonged and sustained activation of JNK and p38 MAPK.
Acknowledgments
These studies were supported by USPHS Grants AA017425 and 018790 from the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- TNFα
tumor necrosis factor alpha
- CYP2E1
cytochrome P4502E1
- PY
pyrazole
- ROS
reactive oxygen species
- ASK-1
apoptosis signal-regulating kinase-1
- Trx
thioredoxin
- MAPK
Mitogen activated protein kinase
- MAP3Ks
mitogen activated protein kinase kinase kinase
- MKK
MAP kinase kinase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GSH
glutathione
- TBARS
thiobarbituric acid reactive substances
- MKP-1
MAP kinase phosphatase 1
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling
- AMC
aminomethylcoumarin
- AFC
aminofluoromethylcoumain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thurman GR. Mechanisms of hepatic toxicity. II Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 2.McClain CJ, Barve S, Deaciu I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 3.Yin M, Wheeler MD, Kono H, Bradford BU, Galluci RM, Luster MI, Thurman RG. Essential role of TNFα in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 4.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 5.Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Kadiisda M, Mason RP. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13:S39–S50. [PubMed] [Google Scholar]
- 6.Tsukamoto H. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S–181S. doi: 10.1097/00000374-200105051-00029. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25(6 Suppl):51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 8.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 9.Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver tocicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944–950. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 10.Lieber CS. Cytochrome P4502E1; its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 14.Schwabe RF, Brenner DA. Mechanism of liver injury-TNFα-induced liver injury:role of IKK, JNK and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 15.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2002;282:G257–G266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Cederbaum AI. Cytochrome P4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology. 2008;47:1005–1017. doi: 10.1002/hep.22087. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Xu C, Cederbaum AI. Role of nitric oxide and nuclear factor-κB in the CYP2E1 potentiation of tumor necrosis factor α hepatotoxicity in mice. Free Rad Biol Med. 2008;46:480–491. doi: 10.1016/j.freeradbiomed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase:physiological roles of ASK-1 MAP kinase pathway in stress signaling. Biochim Biophy Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Kyraiakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathway activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 21.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Nishitoh H, Ichijo H, Kyriakis M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Sem Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773:1349–1357. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Wallaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-κB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK-1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 27.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 29.Chang L, Kamata H, Solinas G, Luo JB, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase Itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP L turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of preformed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai KS, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N—Terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Venugopal SK, Chen J, Zang Y, Clemens D, Follenzi A, Zern MA. Role of MAPK phosphatase-1 in sustained activation of JNK during ethanol-induced apoptosis in hepatocyte-like VL-17A cells. J Biol Chem. 2007;282:31900–31908. doi: 10.1074/jbc.M703729200. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q, Konta T, Nakayama K, Furusu A, Moreno-Manzano V, Lucio-Cazana J, Ishikawa Y, Fine LG, Yao J, Kitamura M. Cellular defense against H2O2-induced apoptosis via MAP Kinase-MKP1 pathway. Free Radical Biol Med. 2004;36:985–993. doi: 10.1016/j.freeradbiomed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Siegmund D, Mauri D, Peters N, Juo P, Thome M, Reichwein M, Blenis J, Scheurich P, Tschopp J, Wajant H. Fas-associated death domain protein (FADD) and caspase-8 mediate up-regulation of c-Fos by Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via a FLICE inhibitory protein (FLIP)-regulated pathway. J Biol Chem. 2001;276:32585–32590. doi: 10.1074/jbc.M100444200. [DOI] [PubMed] [Google Scholar]
- 35.Degterev AHuang, Boyce ZM, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury rat. Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 36.Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumor necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9:801–808. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 37.Hitomi J, Christofferson DE, Ng A, Yao J, Gegterev A, Cavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, Denecker G, Depuydt B, De Valck D, De Wilde G. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1995;47:67–75. [PubMed] [Google Scholar]