Abstract
Catechol estrogens, especially 4-hydroxylated metabolites of 17β-estradiol (E2), are responsible for estrogen-induced carcinogenesis. 4-Hydroxyestradiol (4-OHE2), a major metabolite of E2 formed preferentially by cytochrome P-450 1B1, is oxidized to E2-3,4-quinone, which can react with DNA to yield the depurinating adducts 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua. The apurinic sites generated by the loss of these depurinating adducts induce mutations that could lead to cancer initiation. In the present study, we have evaluated the effects of N-acetycysteine (NAcCys) on the metabolism of two cell lines, MCF-10F (a normal human breast epithelial cell line) and E6 (a normal mouse mammary epithelial cell line), treated with 4-OHE2 or its reactive metabolite, E2-3,4-quinone. Extensive HPLC with electrochemical detection and UPLC-MS/MS analyses of the cell media demonstrated that the presence of NAcCys very efficiently shifted the estrogen metabolism towards protective methoxylation and conjugation pathways in multiple ways, while formation of depurinating DNA adducts was inhibited. Protection by NAcCys appears to be similar in both cell lines irrespective of their origin (human or mouse) or the presence of estrogen receptor-alpha. This finding suggests that NAcCys, a common dietary supplement, could be used as a potential chemopreventive agent to block the initial step in the genotoxicity caused by catechol estrogen quinones.
Keywords: catechol estrogen quinones; depurinating estrogen-DNA adducts; cancer prevention, N-acetylcysteine; homeostasis of estrogen metabolism; breast epithelial cells
Introduction
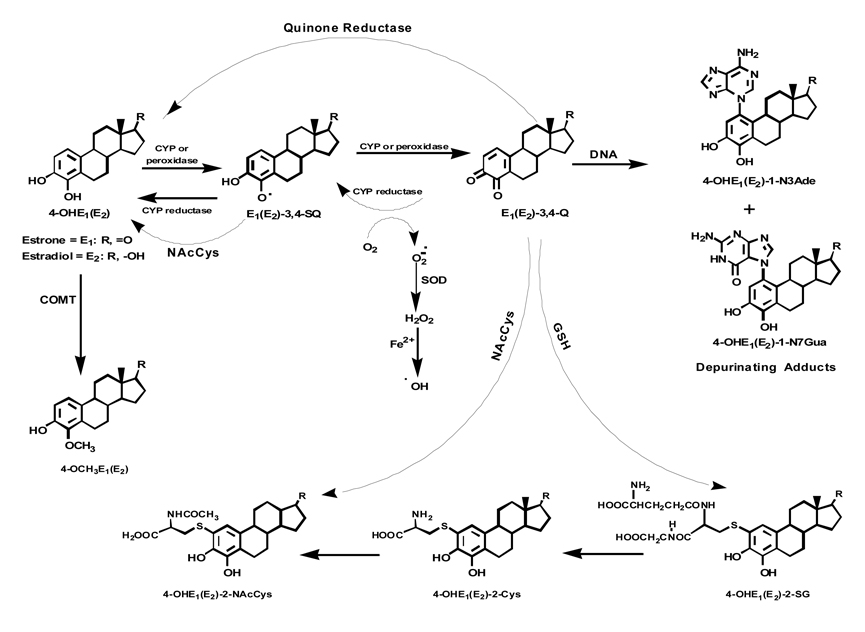
Breast cancer is the most commonly occurring neoplasm and the second most frequent cause of death in women in the United States [1]. Present evidence shows the involvement of estrogens in processes leading to cancers, including breast, endometrial and ovarian [2–5]. A substantial amount of evidence from experiments on estrogen metabolism, formation of DNA adducts, mutagenicity, cell transformation and carcinogenicity [6–24] provides the basis for the paradigm that certain estrogen metabolites, catechol estrogen-3,4-quinones, react with DNA to form predominantly depurinating adducts 4-hydroxyestrone(estradiol)-1-N3Ade [4-OHE1(E2)-1-N3Ade] and 4-OHE1(E2)-1-N7Gua (Fig. 1) [10–13]. Apurinic sites resulting from formation of these adducts can undergo error-prone repair to generate critical mutations that initiate cancer [14–16]. The paradigm of cancer initiation by natural or synthetic estrogens is based on estrogen metabolism that generates a disrupted homeostatic balance between activating (leading to DNA adducts) and deactivating (leading to methoxylation and conjugation) pathways. In support of this, we have recently shown that in healthy women, the level of estrogen-DNA adducts in urine is low and the levels of methoxylated estrogen metabolites and conjugates are high (Fig. 1) [9,25]. In contrast, higher levels of DNA adducts and lower levels of methoxylated estrogen metabolites and conjugates are present in the urine of breast cancer patients and women at high risk for the disease [9,25].
Figure 1.
Metabolic pathway for 4-OHE1(E2) in the absence or presence of NAcCys.
The oxidation of catechol estrogens to quinones can be reduced by the actions of Phase II enzymes. Specifically, catechol-O-methyltransferase (COMT) catalyzes the methylation of catechol estrogens, preventing their conversion to estrogen semiquinones and quinones (Fig. 1) [26]. We have documented that low COMT activity is a contributory factor in the increased formation of estrogen-DNA adducts [27,28]. In turn, the estrogen quinones are expected to undergo conjugation with glutathione (GSH), either nonenzymatically or more efficiently, via the catalytic action of glutathione-S-transferases, a superfamily of multifunctional phase II enzymes that play a key role in cellular detoxification [29–31]. Other phase II enzymes, NADPH quinone oxidoreductase 1 and 2 (NQO1 and NQO2) are also involved in detoxification of the quinones by their reduction back to catechol estrogens [32–34].
The activating and deactivating pathways leading to DNA adducts or methoxylation and conjugation can be modulated by using antioxidant compounds. These agents, in mechanism-based action, either prevent the formation of estrogen quinones and their reaction with DNA or covalently bind with the quinones to form nontoxic conjugates. Towards this goal, in a preliminary in vitro study, we have tested several antioxidants, NAcCys, GSH, cysteine (Cys), melatonin, resveratrol and reduced lipoic acid [35]. NAcCys was found to be one of the best inhibitors of the formation of depurinating adducts in reaction mixtures containing E2-3,4-Q or enzyme-activated 4-OHE2 and DNA.
NAcCys is an aminothiol, and its hydrolytic product, Cys, is a precursor of intracellular GSH [36]. Changes in GSH homeostasis have been implicated in the etiology and progression of a variety of human diseases, including cancer [37]. NAcCys was initially used as an antidote for paracetamol poisoning [38] and as a mucolytic drug [39]. Later it was shown to possess antioxidant, anticoagulant and platelet-inhibiting properties with low systemic toxicity [40–44]. In light of our recent findings on the mechanism of initiation of breast cancer and in vitro studies, it was of interest to investigate further the effect of NAcCys on estrogen metabolism in breast epithelial cells.
In the present study using the human breast epithelial cell line MCF-10F (estrogen receptor negative and aryl hydrocarbon receptor positive), a well-developed cell culture model for studying the carcinogenesis of non-estrogen receptor pathways, and E6 (a mouse mammary epithelial cell line), we have demonstrated that NAcCys modulates estrogen metabolism in multiple ways. The data presented in this article suggest that NAcCys could be used as a chemopreventive agent against the initiation of breast cancer.
Materials and Methods
Chemicals and Reagents
4-OHE2 and all standards were synthesized in our laboratory, as previously described [10,11,45,46]. For quinone treatments, E2-3,4-Q was freshly synthesized as previously described [12]. NAcCys and all other chemicals were purchased from Sigma (St. Louis, MO).
Cell culture and treatment
MCF-10F cells were obtained from the ATCC (Rockville, MD), and cultured in DMEM and Ham’s F12 media (Mediatech, Inc.) with 20 ng/ml epidermal growth factor, 0.01 mg/ml insulin, 500 ng/ml hydrocortisone, 5% horse serum and 100 µg/ml penicillin/streptomycin mixture. Estrogen-free medium was prepared in phenol red-free DME/F12 medium with charcoal-stripped FBS (HyClone, Logan, UT).
Mouse mammary epithelial cells (E6) were a generous gift from Dr. K.H. Cowan (University of Nebraska Medical Center, Omaha, NE) [47]. These cells were originally isolated from the mammary gland of a Brca1fl/fl mouse (loxP sites flanking exon 11 of the Brca1 gene). They were immortalized by infection with HPV-16E6 (Neo+) retrovirus to inhibit p53. The E6 cells express a full-length Brca1 protein and are considered “normal” cells. E6 cells were cultured in 1:1 Dulbecco’s minimal essential medium: Ham’s F-12 (DMEM:F-12, Mediatech, Herndon, VA) supplemented with 10% bovine growth serum (BGS, Hyclone, Logan, UT) in a 5% CO2 incubator at 37 °C. Cell viability of both cell lines was determined by the MTT [3,(4,5-dimethylthiazole-2-yl)-2,5-diphenyltrazolium bromide] assay [48].
The cells (0.75 × 105 cells) were seeded for 48 h in estrogen-containing medium. The medium was changed to estrogen-free medium and the cells were grown for another 72 h. After changing the medium, cells were treated with E2-3,4-Q or 4-OHE2 (1, 10 or 30 µM) for 24 h. To see the effect of NAcCys on the metabolic profile of each cell line, the cells were first pre-incubated for 2 h with a 1:1 or 1:0.5 ratio of NAcCys to estrogen and then treated with E2-3,4-Q or 4-OHE2 (1, 10 or 30 µM) for 24 h. Only MCF-10F were used to determine whether NAcCys can prevent the formation of estrogen-DNA adducts in a dose dependent manner. The cells were pre-incubated with 1, 3, 10 or 30 µM NAcCys for 2 h and then treated with a fixed moderate dose of 4-OHE2 (10 µM) for 24 h. To keep the concentration of organic solvents (0.001%) the same in all experiments, different stock solutions of E2-3,4-Q and 4-OHE2 (1–30 mM) were prepared. Similar media from five flasks, either treated with an equal amount of organic solvent or untreated, were used as controls.
After collecting the medium from five flasks, 1 ml of trypsin was added to each flask and incubated for 10 min. Once the cells had detached, the cells from all five flasks were combined and 10 ml of double serum medium was added to neutralize the trypsin. Cell numbers were estimated by using a Coulter counter (Beckman Coulter Inc., USA).
Analysis of estrogen-DNA adducts
Following the treatments, media were harvested, supplemented with 2 mM ascorbic acid (to prevent possible decomposition of the compounds) and processed immediately. Sample preparation and analysis by HPLC-ECD and UPLC-MS/MS were carried out as follows:
Sample Preparation: Culture media pooled from five flasks (50 mL) were processed by passage through C8 Certify II cartridges (Varian, Harbor City, CA), which were equilibrated by sequentially passing 1 mL of methanol, distilled water, and potassium phosphate buffer (100 mM, pH 8) through them. The harvested media (50 mL) were adjusted to pH 8.0 and passed through these cartridges. The retained analytes in the cartridges were washed with the above phosphate buffer, eluted with methanol:acetonitrile:water:trifluoroacetic acid (8:1:1:0.1), and processed as described previously [20,49].
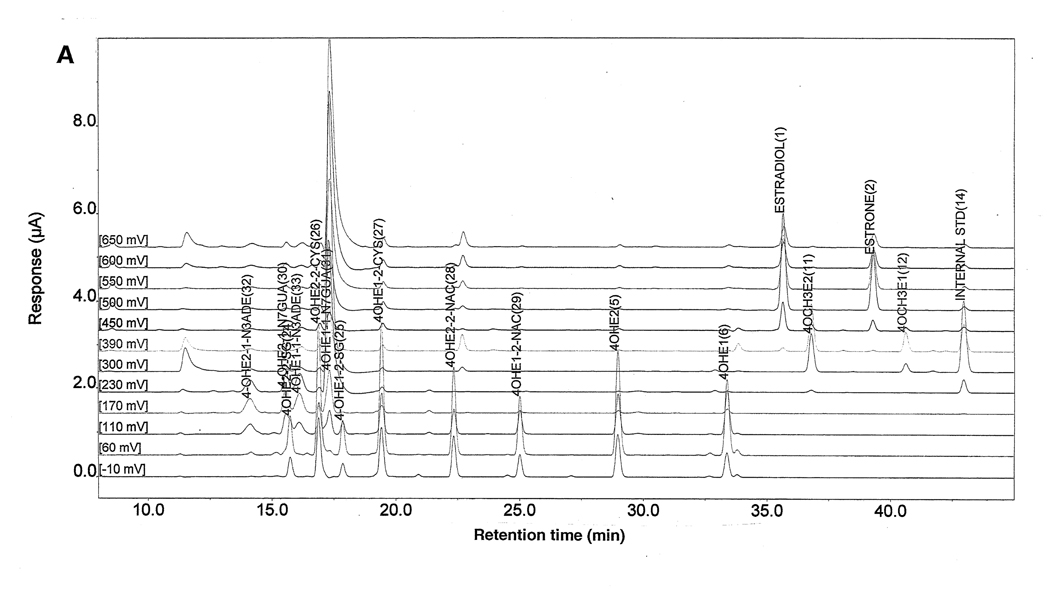
HPLC-ECD and UPLC-MS/MS: Analyses of all samples were conducted on an HPLC system equipped with dual ESA Model 580 solvent delivery modules, an ESA Model 540 auto-sampler and a 12-channel CoulArray electrochemical detector (ESA, Chelmsford, MA) [20,49] (Fig. 2). The analyte and adduct peaks were identified by their retention times and peak height ratios between the dominant peak and the peaks in the two adjacent channels. The data were quantified by comparison with known amounts of standards. The % recovery of each standard was used to normalize our data. To determine the % recovery for each experiment, a complete set of analytes was spiked into medium (same batch as used with the cells), processed as the other samples and analyzed. The % recovery for each analyte was calculated from this set of standards for the samples analyzed in this experiment. The metabolites and adducts showed better recovery (85–93%) than the quinone conjugates (56–78%).
Figure 2.
HPLC-ECD of (A) a 95 µl aliquot of the standard compounds at a concentration of 1.0 µg/µl each, and (B) a representative chromatogram of culture medium following 24 h of treatment of MCF-10F cells with 10 µM 4-OHE2.
The statistical significance of data was determined by using the t-test in which the value obtained from cells treated in the presence of NAcCys was compared individually with the control in which NAcCys was not added. The identity of the DNA adducts was further confirmed on an Acquity UPLC connected to a MicroMass QuattroMicro triple quadrupole mass spectrometer (Waters, Milford, MA), as described previously [9].
Immunoblotting analysis
After treatment for 24 h with NAcCys (0.1, 1, 10 or 30 µM), whole cell lysate was prepared by suspending the MCF-10F cells in RIPA buffer with protease inhibitor and lysing by three freeze-thaw cycles. Nuclei and unlysed cells were removed by centrifugation. The protein concentration was determined by using the BCA protein assay kit (Pierce Biotechnology, Inc, Rockford, IL). Protein samples (20 µg) were solubilized with SDS-polyacrylamide gel electrophoresis sample loading buffer and electrophoresed on 12% SDS-polyacrylamide gel at 200 V for 30 min. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane at 100 V for 60 min. The blots were blocked for 60 min at room temperature in fresh blocking buffer (0.1% Tween-20 in Tris-buffered saline, pH 7.4, 5% nonfat dried milk, PBST). Dilutions of the primary antibodies [COMT (1:2000, Chemicon International, Temecula, CA); NQO1 (1:2000, Abcam, Temecula, CA), CYP1B1 (1:1000, Genetest, Bedford, MA) and β-actin (1:10000, Genetest, Bedford, MA)] were made in blocking solution. The blots were incubated overnight with primary antibody at 4 °C. Following three washes with PBST for 10 min each, the blots were incubated with appropriate secondary antibody (horseradish peroxidase-conjugated) for 45 min at room temperature. The blots were washed again three times in PBST, incubated with Super Signal West Pico Chemiluminescent kit (Pierce Biotechnology, Inc., Rockford, IL) for 2 min, and visualized with radiographic film. The intensities of the bands were quantified by Alpha DigiDoc 1201 (Alpha Innotech, San Leandro, CA).
Results
The effects of NAcCys on estrogen metabolism were studied in MCF-10F (human) and E6 (mouse) cells, which were treated with E2-3,4-Q or 4-OHE2 alone or in the presence of NAcCys (Tables 1–4) for 24 h. The cell culture media from all the treatment groups were analyzed by HPLC-ECD. The presence of DNA adducts was confirmed by UPLC-MS/MS. MCF-10F and E6 cell treatments with either E2-3,4-Q or 4-OHE2 were divided into three groups. First, control group (A) was treated with 1, 10 or 30 µM E2-3,4-Q or 4-OHE2 alone. In the second group (B), cells were treated with NAcCys at half the molar concentration of E2-3,4-Q or 4-OHE2. In the third group (C), cells were treated with equal molar ratios of NAcCys and E2-3,4-Q or 4-OHE2.
Table 1.
Incubation of MCF-10F cells with E2-3,4-Q alone or in the presence of NAcCys1
| E2-3,4-Q alone | E2-3,4-Q: NAcCys (1:0.5) | E23,4-Q: NAcCys (1:1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected Compounds2 | 1 | 10 | 30 | 1 | 10 | 30 | 1 | 10 | 30 |
| pmol/10 million cells | |||||||||
| 4-OHE1(E2) | 4.05 ± 0.32 |
7.79 ± 0.34 |
14.2 ± 1.41 |
2.96 ± 0.24 |
5.79 ± 0.51 |
9.57 ± 1.15 |
1.17 ± 0.26 |
3.07 ± 0.32 |
5.11 ± 0.47 |
| 4-OCH3E1(E2) | 49.7 ± 4.27 |
437 ± 50.9 |
824 ± 120 |
41.9* ± 4.06 |
329* ± 62.3 |
593* ± 59.6 |
32.3* ± 5.48 |
196* ± 28.9 |
302* ± 51.3 |
| 4-OHE1(E2)-2-SG | 0.58 ± 0.05 |
3.13 ± 0.38 |
6.39 ± 0.77 |
0.65 ± 0.06 |
3.75 ± 0.59 |
8.10 ± 1.54 |
0.74* ± 0.06 |
4.36* ± 0.31 |
10.2* ± 1.71 |
| 4-OHE1(E2)-2-Cys | 3.95 ± 0.71 |
8.78 ± 0.56 |
28.1 ± 2.40 |
4.42 ± 0.46 |
10.6* ± 0.72 |
34.7* ± 2.49 |
5.02 ± 0.70 |
12.2* ± 1.28 |
42.1* ± 2.84 |
| 4-OHE1(E2)-2-NAcCys | 1.36 ± 0.28 |
6.37 ± 0.81 |
15.9 ± 2.82 |
1.63 ± 0.29 |
8.15* ± 1.04 |
21.6* ± 1.61 |
1.98* ± 0.26 |
10.3* ± 1.53 |
28.0* ± 3.41 |
| 4-OHE1(E2)-1-N3Ade | 0.39 ± 0.03 |
2.22 ± 0.24 |
5.42 ± 0.60 |
0.34* ± 0.01 |
1.70* ± 0.24 |
3.76* ± 0.26 |
0.28* ± 0.02 |
1.12* ± 0.14 |
1.67* ± 0.35 |
| 4-OHE1(E2)-1-N7Gua | 0.36 ± 0.02 |
2.08 ± 0.18 |
4.97 ± 0.44 |
0.30 ± 0.04 |
1.52 ± 0.39 |
3.50* ± 0.40 |
0.26* ± 0.03 |
0.99* ± 0.13 |
1.55* ± 0.19 |
MCF-10F cells were incubated at 37 °C for 24 h with E2-3,4-Q (1, 10 or 30 µM) alone or in the presence of different ratios of NAcCys.
The compounds were identified and quantified by HPLC-ECD and the adducts were confirmed by UPLC-MS/MS; values are an average of three replicates from three independent pools of medium each from a set of five flasks; ± standard deviation.
The value is significantly different from that in the cell s not treated with NAcCys (p<0.05) as determined by the t-test.
Table 4.
Incubation of E6 cells with 4 -OHE2 alone or in presence of NAcCys1
| 4-OHE2 alone | 4-OHE2: NAcCys (1:0.5) | 4-OHE2: NAcCys (1:1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected Compounds2 | 1 | 10 | 30 | 1 | 10 | 30 | 1 | 10 | 30 |
| pmol/10 million cells | |||||||||
| 4-OHE1(E2) | 1.29 ± 0.11 |
4.46 ± 1.01 |
20.1 ± 1.76 |
0.78 ± 0.09 |
2.86 ± 0.86 |
10.5 ± 2.40 |
0.21 ± 0.07 |
0.59 ± 0.10 |
3.56 ± 1.13 |
| 4-OCH3E1(E2) | 2077 ± 327 |
4669 ± 440 |
15112 ± 1652 |
2377 ± 338 |
5718* ± 342 |
19804* ± 1325 |
2785* ± 335 |
7599* ± 963 |
36372* ± 3355 |
| 4-OHE1(E2)-2-SG | 2.38 ± 0.47 |
13.9 ± 2.02 |
33.0 ± 4.19 |
2.67 ± 0.55 |
16.4 ± 0.57 |
40.8* ± 1.70 |
3.07 ± 0.70 |
18.6* ± 2.32 |
50.4* ± 3.23 |
| 4-OHE1(E2)-2-Cys | 6.19 ± 1.29 |
18.12 ± 2.53 |
38.5 ± 7.06 |
7.25 ± 1.16 |
22.3* ± 2.27 |
50.4 ± 7.53 |
8.80 ± 1.81 |
28.4* ± 2.92 |
64.2* ± 7.61 |
| 4-OHE1(E2)-2-NAcCys | 6.22 ± 0.66 |
17.9 ± 1.53 |
40.5 ± 6.46 |
7.23 ± 0.96 |
22.1* ± 1.92 |
54.3* ± 6.82 |
8.78* ± 1.07 |
26.8* ± 2.04 |
70.8* ± 11.9 |
| 4-OHE1(E2)-1-N3Ade | 1.33 ± 0.28 |
4.18 ± 0.87 |
41.9 ± 4.97 |
1.07 ± 0.05 |
2.81* ± 0.33 |
25.3* ± 1.88 |
0.53* ± 0.03 |
1.63* ± 0.26 |
4.00* ± 0.73 |
| 4-OHE1(E2)-1-N7Gua | 0.97 ± 0.11 |
3.97 ± 0.59 |
39.6 ± 4.65 |
0.79* ± 0.09 |
2.70* ± 0.51 |
24.3* ± 1.48 |
0.51* ± 0.04 |
1.58* ± 0.25 |
4.59* ± 0.83 |
E6 cells were incubated at 37 °C for 24 h with 4-OHE2 (1, 10 or 30 µM) alone or in the presence of different ratios of NAcCys.
The compounds were identified and quantified by HPLC-ECD and the adducts were confirmed by UPLC-MS/MS; values are an average of three replicates from three independent pools of medium each from a set of five flasks; ± standard deviation.
The value is significantly different from th at in the cell s not treated with NAcCys (p<0.05) as determined by the t-test.
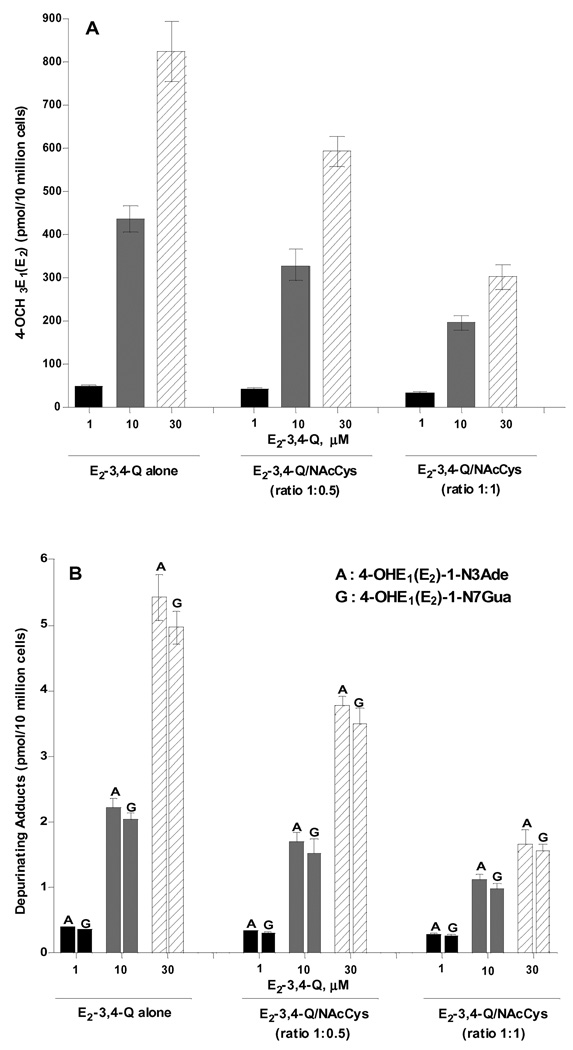
Effect of NAcCys on E2-3,4-Q metabolism and DNA adduct formation in MCF-10F and E6 cells treated with E2-3,4-Q
The control group, in which cells were treated with 1, 10 or 30 µM E2-3,4-Q, showed the formation of hydroxycatechols, methoxycatechols, conjugates and depurinating DNA adducts in a dose-dependent manner (Table 1). The methoxycatechol and depurinating DNA adduct levels are also shown in Figure 3. NAcCys treatment of MCF-10F cells resulted in a decrease in 4-hydroxycatechol and 4-methoxycatechol levels (Table 1, Fig. 3A), whereas there were significant increases in 4-OHE2-2-SG, 4-OHE2-2-Cys and 4-OHE2-2-NAcCys (Table 1). 4-OHE2-1-N3Ade adduct levels were decreased up to 31% when NAcCys was present at half the concentration of E2-3,4-Q and up to 69% when NAcCys and E2-3,4-Q were equimolar (Table 1, Fig. 3B). Similarly, 4-OHE2-1-N7Gua levels were reduced up 29% with NAcCys at half the molar level of E2-3,4-Q and up to 69% with equimolar NAcCys and E2-3,4-Q (Table 1, Fig. 3B).
Figure 3.
Effects of NAcCys on the formation of (A) 4-OCH3E1(E2) and (B) estrogen-DNA adducts in MCF-10F cells treated with E2-3,4-Q.
Similar effects of NAcCys were observed in E6 cells treated with E2-3,4-Q (Table 2). The levels of metabolites, conjugates and DNA adducts in the E6 cells were similar to or somewhat higher than those observed in the MCF-10F cells (Table 1). The levels of 4-hydroxycatechols, 4-methoxycatechols and depurinating DNA adducts were decreased in the E6 cells, whereas there were significant increases in quinone conjugates (Table 2).
Table 2.
Incubation of E6 cells with E2-3,4-Q alone or in the presence of NAcCys1
| E2-3,4-Q alone | E2-3,4-Q: NAcCys (1:0.5) | E2-3,4-Q: NAcCys (1:1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected Compounds2 | 1 | 10 | 30 | 1 | 10 | 30 | 1 | 10 | 30 |
| pmol/10 million cells | |||||||||
| 4-OHE1(E2) | 0.41 ± 0.07 |
1.34 ± 0.16 |
2.35 ± 0.30 |
0.27 ± 0.06 |
0.90 ± 0.05 |
1.50 ± 0.18 |
0.14 ± 0.03 |
0.65 ± 0.06 |
0.92 ± 0.09 |
| 4-OCH3E1(E2) | 60.2 ± 5.45 |
555 ± 29.4 |
1142 ± 74.8 |
48.9* ± 5.89 |
414* ± 29.9 |
761* ± 85.7 |
37.5* ± 2.47 |
264* ± 65.8 |
345* ± 81.3 |
| 4-OHE1(E2)-2-SG | 0.76 ± 0.05 |
6.38 ± 1.0 |
14.9 ± 2.36 |
0.86* ± 0.06 |
7.58 ± 2.26 |
18.5* ± 1.83 |
1.0* ± 0.09 |
8.98* ± 1.48 |
21.8* ± 2.41 |
| 4-OHE1(E2)-2-Cys | 4.52 ± 0.48 |
11.4 ± 0.95 |
32.7 ± 3.43 |
5.18 ± 0.48 |
14.1* ± 1.09 |
41.0* ± 3.23 |
6.27* ± 0.67 |
16.8* ± 1.35 |
48.2* ± 3.17 |
| 4-OHE1(E2)-2-NAcCys | 1.80 ± 0.37 |
8.53 ± 0.96 |
18.6 ± 3.26 |
2.30 ± 0.46 |
11.6 ± 2.74 |
28.5* ± 3.28 |
2.85* ± 0.40 |
15.2* ± 2.29 |
42.6* ± 2.08 |
| 4-OHE1(E2)-1-N3Ade | 0.49 ± 0.06 |
2.92 ± 0.27 |
6.22 ± 0.59 |
0.41 ± 0.04 |
2.36 ± 0.58 |
4.37* ± 0.32 |
0.32* ± 0.03 |
1.58* ± 0.16 |
1.86* ± 0.35 |
| 4-OHE1(E2)-1-N7Gua | 0.47 ± 0.08 |
2.76 ± 0.15 |
5.82 ± 0.54 |
0.39 ± 0.02 |
2.07* ± 0.36 |
3.94* ± 0.74 |
0.33* ± 0.02 |
1.42* ± 0.25 |
1.79* ± 0.21 |
E6 cells were incubated at 37 °C for 24 h with E2-3,4-Q (1, 10, or 30 µM) alone or in the presence of different ratios of NAcCys.
The compounds were identified and quantified by HPLC-ECD and the adducts were confirmed by UPLC-MS/MS; values are an average of three replicates from three independent pools of medium each from a set of five flasks; ± standard deviation.
The value is significantly different from th at in the cell s not treated with NAcCys (p<0.05) as determined by the t-test.
Effect of NAcCys on 4-OHE2 metabolism and formation of DNA adducts in MCF-10F and E6 cells treated with 4-OHE2
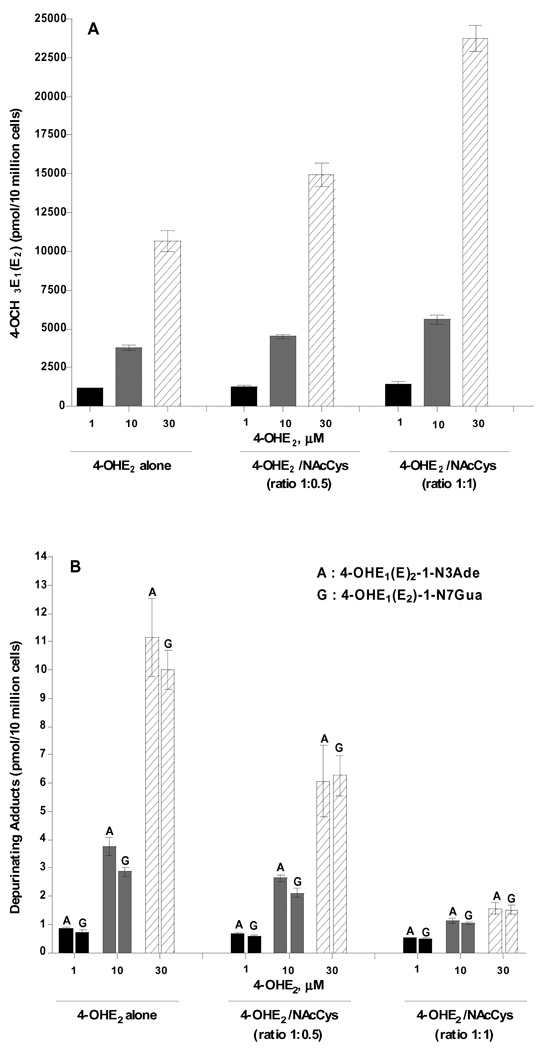
Analysis of the culture media from MCF-10F cells treated with 1, 10 or 30 µM 4-OHE2 alone showed an increase of 4-methoxycatechols, quinone conjugates and depurinating DNA adducts in a dose-dependent manner (Table 3, Fig. 4). In the presence of NAcCys, there was an increase in 4-methoxylation (Fig. 4A) and in quinone conjugates (4-OHE2-2-SG, 4-OHE2-2-Cys and 4-OHE2-2-NAcCys) (Table 3). The depurinating adduct, 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua, levels were reduced 46% and 37%, respectively, with NAcCys present at half the concentration of 4-OHE2. Their levels were reduced 86% and 85%, respectively, with equimolar NAcCys and 4-OHE2 (Table 3, Fig. 4B).
Table 3.
Incubation of MCF-10F cells with 4-OHE2 alone or in the presence of NAcCys1
| 4-OHE2 alone | 4-OHE2: NAcCys (1:0.5) | 4-OHE2: NAcCys (1:1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected Compounds2 | 1 | 10 | 30 | 1 | 10 | 30 | 1 | 10 | 30 |
| pmol/10 million cells | |||||||||
| 4-OHE1(E2) | 2.20 ± 0.45 |
9.49 ± 2.04 |
29.5 ± 3.45 |
1.11 ± 0.33 |
5.47 ± 0.71 |
14.2 ± 2.17 |
0.27 ± 0.07 |
2.42 ± 0.45 |
7.37 ± 1.4 |
| 4-OCH3E1(E2) | 1139 ± 106 |
3788 ± 353 |
10648 ± 1211 |
1282 ± 124 |
4500* ± 259 |
14944* ± 1316 |
1461* ± 200 |
5598* ± 500 |
23736* ± 1467 |
| 4-OHE1(E2)-2-SG | 1.10 ± 0.21 |
7.48 ± 0.77 |
12.2 ± 3.34 |
1.19 ± 0.16 |
8.57 ± 0.57 |
14.4 ± 2.09 |
1.32* ± 0.15 |
9.87 ± 2.19 |
17.3 ± 2.94 |
| 4-OHE1(E2)-2-Cys | 5.16 ± 0.55 |
9.98 ± 0.24 |
37.3 ± 1.26 |
5.74 ± 0.53 |
11.7* ± 0.72 |
46.2* ± 4.29 |
6.76* ± 1.08 |
14.2* ± 2.29 |
58.6* ± 3.30 |
| 4-OHE1(E2)-2-NAcCys | 5.0 ± 0.65 |
11.5 ± 2.0 |
31.0 ± 3.86 |
5.57 ± 1.10 |
13.2 ± 2.26 |
38.6* ± 3.95 |
6.84* ± 0.60 |
16.6* ± 3.31 |
51.2* ± 3.63 |
| 4-OHE1(E2)-1-N3Ade | 0.84 ± 0.09 |
3.76 ± 0.53 |
11.2 ± 2.38 |
0.68* ± 0.07 |
2.63* ± 0.20 |
6.07* ± 2.20 |
0.53* ± 0.03 |
1.14* ± 0.19 |
1.57* ± 0.34 |
| 4-OHE1(E2)-1-N7Gua | 0.70 ± 0.19 |
2.86 ± 0.28 |
9.99 ± 1.20 |
0.59 ± 0.05 |
2.11* ± 0.28 |
6.26* ± 1.24 |
0.51* ± 0.04 |
1.05* ± 0.08 |
1.52* ± 0.29 |
MCF-10F cells were incubated at 37 °C for 24 h with 4-OHE2 (1, 10 or 30 µM) alone or in the presence of different ratios of NAcCys.
The compounds were identified and quantified by HPLC-ECD and the adducts were confirmed by UPLC-MS/MS; values are an average of three replicates from three independent pools of medium each from a set of five flasks; ± standard deviation.
The value is significantly different from th at in the cells not treated with NAcCys (p<0.05) as determined by the t-test.
Figure 4.
Effects of NAcCys on the formation of (A) 4-OCH3E1(E2) and (B) estrogen-DNA adducts in MCF-10F cells treated with 4-OHE2.
The levels of 4-methoxycatechols, quinone conjugates and depurinating DNA adducts were similarly altered when E6 cells were exposed to 4-OHE2 in the presence of half or equimolar ratios of NAcCys (Table 4). The levels of metabolites, conjugates and DNA adducts were similar to those detected in the MCF-10F cells (Table 3), except that higher levels of DNA adducts were observed in the E6 cells. In fact, when the cells were treated with 30 µM 4-OHE2, about 3–4-fold higher levels of DNA adducts were seen in the E6 cells compared to MCF-10F cells, with or without NAcCys present.
Dose-response of NAcCys
To observe the effect of NAcCys on estrogen metabolism, we decided to use a moderate dose of 4-OHE2 in human breast epithelial cells (MCF-10F). The MCF-10F cells were treated with 10 µM 4-OHE2 and varying ratios (0, 0.1, 0.3, 1 and 3) of NAcCys (Table 5). The metabolic profile of cell media revealed a dose-dependent response in the elevation of 4-methoxycatechol and quinone conjugate levels (Table 5). Significant inhibition of 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua adducts was observed with increased NAcCys concentrations (Table 5). Complete inhibition of both depurinating adducts was observed when cells were treated with a 1:3 ratio of 4-OHE2 to NAcCys (Table 5).
Table 5.
Incubation of MCF-10F cells with 10 µM 4-OHE2 alone or in the presence of NAcCys1
| Treatment ratio: 4-OHE2: NAcCys | |||||
|---|---|---|---|---|---|
| Detected Compounds 2 | 1:0 | 1:0.1 | 1:0.3 | 1:1 | 1:3 |
| pmol/10 million cells | |||||
| 4-OHE1(E2) | 11.5 ± 2.73 |
9.38 ± 2.35 |
5.73 ± 0.92 |
3.03 ± 0.59 |
0.70 ± 0.09 |
| 4-OCH3E1(E2) | 3684 ± 346 |
3931 ± 427 |
4515 ± 275 |
5780* ± 386 |
8143* ± 942 |
| 4-OHE1(E2)-2-SG | 8.35 ± 0.75 |
8.52 ± 0.92 |
9.24 ± 1.12 |
10.4* ± 0.95 |
12.2* ± 1.78 |
| 4-OHE1(E2)-2-Cys | 11.9 ± 1.71 |
12.2 ± 1.06 |
14.3 ± 1.77 |
17.2* ± 1.96 |
20.8* ± 3.99 |
| 4-OHE1(E2)-2-NAcCys | 13.7 ± 2.07 |
14.2 ± 1.72 |
16.7 ± 1.72 |
20.2* ± 1.30 |
25.1* ± 2.30 |
| 4-OHE1(E2)-1-N3Ade | 4.43 ± 0.84 |
4.31 ± 0.45 |
3.23 ± 0.25 |
1.29* ± 0.45 |
nd3 |
| 4-OHE1(E2)-1-N7Gua | 3.56 ± 0.72 |
3.43 ± 0.51 |
2.62 ± 0.39 |
1.13* ± 0.27 |
nd |
MCF-10F cells were incubated at 37 °C for 24 h with 4-OHE2 (10 µM) alone or in the presence of different ratios of NAcCys.
The compounds were identified and quantified by HPLC-ECD and the adducts were confirmed by UPLC-MS/MS; values are an average of three replicates from three independent pools o f medium each from a set of five flasks; ± standard deviation.
not detected.
The value is significantly different from th at in the cells not treated with NAcCys (p<0.05) as determined by the t-test.
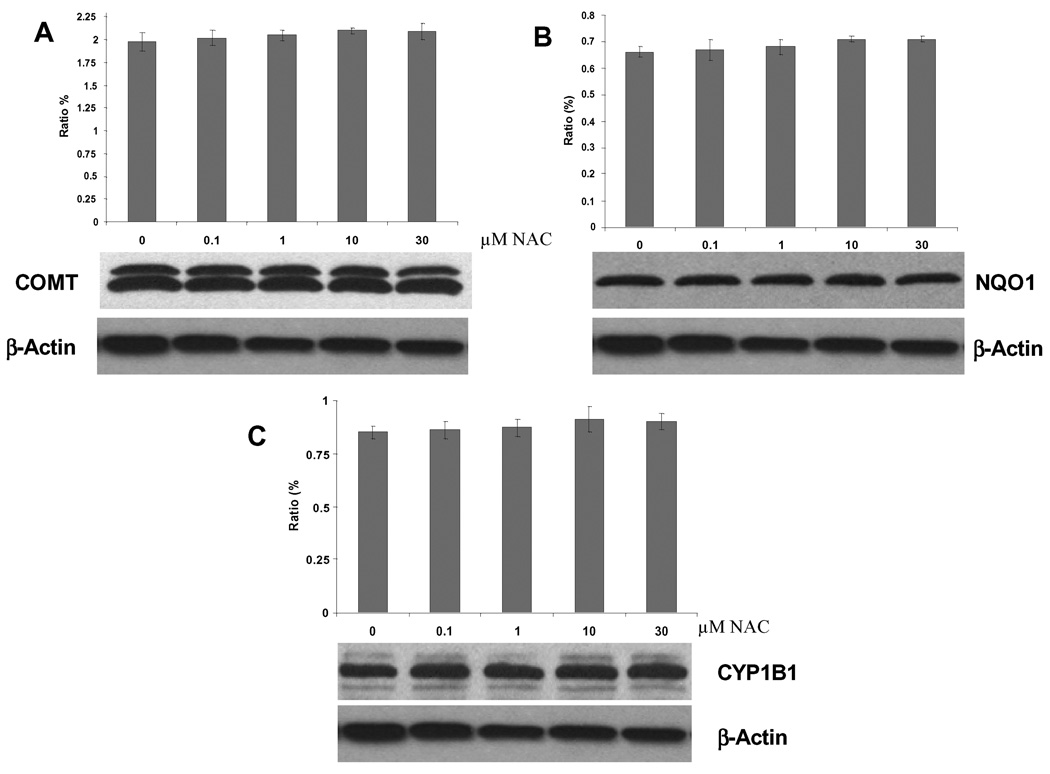
Effect of NAcCys on enzyme (COMT, NQO1 and CYP1B1) expression in MCF-10F cells
To see the effect of NAcCys on estrogen activating (CYP1B1) or protective (COMT and NQO1) enzymes (Fig. 1), MCF-10F cells were treated with various concentrations of NAcCys (1, 10 and 30 µM) for 24 h. These enzymes are of particular interest because breast tissue from women with breast cancer was found to contain higher levels of the activating enzyme and lower levels of the protective enzymes than breast tissue from control women [50]. Immunoblotting of the samples showed that the COMT, NQO1 and CYP1B1 protein expression remained unaffected (Fig. 5). Similar results were observed when E6 cells were treated with NAcCys (data not shown).
Figure 5.
Protein levels (COMT, NQO1, and CYP1B1) in MCF-10F cells after NAcCys treatment.
Discussion
To determine the antioxidant effect of NAcCys on estrogen metabolism, two different cell cultures were used: the MCF-10F human breast epithelial cell line (estrogen receptor-α negative and aryl hydrocarbon receptor positive) and the E6 mouse mammary epithelial cell line. In the treatment of MCF-10F cells with E2-3,4-Q alone, increased levels of the quinone led to increased levels of 4-OHE2, 4-OCH3E2, GSH conjugates and their derivatives, as well as increased levels of both depurinating DNA adducts (Table 1). When the cells were incubated with E2-3,4-Q plus NAcCys at two dose levels, decreased amounts of the catechol, the methoxycatechol and the depurinating DNA adducts were observed, due to the reaction of E2-3,4-Q with NAcCys [35,46]. This led to less formation of catechol from the reduction of E2-3,4-Q by NQO1 (Fig. 1). Thus, less methylation of 4-OHE2 by COMT to form 4-OCH3E2 could occur (Table 1, Fig. 3A). Reduced formation of the DNA adducts was also due to reaction of the quinone with NAcCys instead of DNA (Table 1, Fig. 3B).
The increased levels of GSH, Cys and NAcCys conjugates arose from the added NAcCys, its hydrolysis to Cys, and further formation of GSH. Similar results were obtained by incubation of the E6 mouse mammary cells with E2-3,4-Q alone or in the presence of NAcCys (Table 2).
When MCF-10F cells were treated with 4-OHE2 alone, a dose-response was observed for all of the analytes (Table 3). The catechol was detected almost entirely as the methoxycatechol. Most of the remaining catechol was converted to the quinone, which reacted with GSH, Cys and endogenous NAcCys or DNA. In the presence of NAcCys at half or equimolar concentrations, the levels of GSH conjugates and their derivatives increased, as expected (Table 3). The levels of 4-OHE2 decreased, but 4-OCH3E2 increased (Table 3, Fig. 4A). The 4-OCH3E2 arises partly from methylation of the added 4-OHE2 by COMT. The oxidation of 4-OHE2 to E2-3,4-Q proceeds through the E2-3,4-semiquinone. When NAcCys is present, some of the E2-3,4-semiquinone is efficiently reduced back to 4-OHE2 by NAcCys [51]. This results in greater formation of 4-OCH3E2 from the newly formed 4-OHE2 (Fig. 4A). The decreased levels of the 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua adducts (Fig. 4B) are due to two factors: first, the reduced amount of E2-3,4-Q formed and, second, its reaction with NAcCys [35,46].
The E6 cells treated with 4-OHE2 produced higher levels of the depurinating adducts (Table 4) than the MCF-10F cells (Table 3). The effects of NAcCys on the metabolism of 4-OHE2 in the E6 cells (Table 4) were, however, strikingly similar to those observed in the MCF-10F cells (Table 3). Use of the E6 cells enabled us to investigate the metabolism of 4-OHE2 and formation of estrogen-DNA adducts in cells that have the estrogen receptor, as well as MCF-10F cells, which lack the receptor. These results indicate that the presence of the estrogen receptor has no effect on how 4-OHE2 is metabolized and estrogen-DNA adducts are formed in mammary epithelial cells.
The effects of NAcCys on the metabolism of 4-OHE2 in MCF-10F cells was further studied over a dose range of 0 to 30 µM NAcCys with a constant 10 µM concentration of 4-OHE2 (Table 5). Over this expanded ratio of NAcCys to 4-OHE2 (0 to 3-fold), the level of 4-OHE2 decreased more than 90%, while the amount of 4-OCH3E2 more than doubled. The GSH and derivative conjugates also increased. The levels of the depurinating DNA adducts decreased with increasing amounts of NAcCys and were undetectable when the ratio of NAcCys to 4-OHE2 was 3:1 (Table 5).
To gain further insight into the various mechanisms by which NAcCys affects estrogen metabolism, the expression of three key enzymes, COMT, NQO1 and CYP1B1 (Fig. 1) [50], was determined in MCF-10F cells incubated with 0 to 30 µM NAcCys (Fig. 5). The presence of NAcCys had no effect on the expression of any of these three enzymes. These results are consistent with the known effects of NAcCys, i.e., reduction of catechol estrogen semiquinones to catechol estrogens [51] and reaction of NAcCys with catechol estrogen quinones [8,35,46].
Conclusions
NAcCys exerts its action through multiple protective mechanisms, including nucleophilicity, antioxidant activity and inhibition of DNA adduct formation. Its hydrolysis to Cys, the precursor to GSH, guarantees replenishment of this critical tripeptide in the cell. GSH has similar chemical properties to Cys and NAcCys. NAcCys reacts with E2-3,4-Q to prevent their reaction with DNA [35]. NAcCys, like Cys, also operates as an antioxidant in reducing catechol estrogen semiquinones to catechol estrogens [51]. All of these properties have been observed in human breast epithelial cells and mouse mammary epithelial cells, as reported here. In addition, NAcCys has been shown to prevent malignant transformation of E6 mouse mammary epithelial cells treated with 4-OHE2 or E2-3,4-Q [20]. These properties of NAcCys suggest that this compound can help keep estrogen metabolism balanced and help rebalance it if it becomes out of balance. Therefore, by blocking formation of estrogen-DNA adducts, NAcCys is a potential agent to prevent the initiation of cancer by estrogens.
Acknowledgment
The authors wish to thank Dr. Fang Lu and Dr. Nilesh W. Gaikwad for their contribution to these experiments. This research was supported by Prevention, LLC. Core support at the Eppley Institute was supported by Grant CA P30 36727 from the National Cancer Institute.
Abbreviations
- CE
catechol estrogen(s)
- CE-Q
catechol quinone(s)
- COMT
catechol-O-methytransferase
- Cys
cysteine
- E2-3,4-Q
estradiol-3,4-quinone
- GSH
glutathione
- GST
glutathione-S-transferase
- HPLC-ECD
high performance liquid chromatography with electrochemical detection
- NAcCys
N-acetylcysteine
- NQO1
NAD(P)H quinone oxidoreductase 1
- 4-OCH3E2
4-methoxyestradiol
- 4-OHE2
4-hydroxyestradiol
- -SG
glutathione moiety
- UPLC-MS/MS
ultraperformance liquid chromatography-tandem mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society, Cancer Facts and Figures. 2009. [Google Scholar]
- 2.Akhmed KA, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspective. Ann. N Y Acad. Sci. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 3.Persson I. Estrogen in the causation of breast, endometrial and ovarian cancer-evidence and hypotheses from epidemiological findings. J. Steroid Biochem. Mol. Biol. 2000;74:357–364. doi: 10.1016/s0960-0760(00)00113-8. [DOI] [PubMed] [Google Scholar]
- 4.Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol. Biomarkers Prev. 2000;9:575–579. [PubMed] [Google Scholar]
- 5.Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 6.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: Implications for estrogen-induced initiation of renal tumors. Chem. Res. Toxicol. 2001;14:1041–1050. doi: 10.1021/tx010042g. [DOI] [PubMed] [Google Scholar]
- 8.Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, Higginbotham SM, Cavalieri EL. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: Potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 9.Gaikwad NW, Yang Li, Muti P, Meza JL, Pruthi S, Ingle JN, Rogan EG, Cavalieri EL. The molecular etiology of breast cancer: Evidence from biomarkers of risk. Int. J. Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K-M, Todorovic R, Devanesan P, Higginbotham S, Kofeler H, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 12.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3,4-quinone versus estradiol-2,3-quinone with DNA in the formation of depurinating DNA adducts. Implications for tumor–initiating activity. Chem. Res. Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Gaikwad N, Meza J, Cavalieri E, Muti P, Trock B, Rogan E. Novel biomarkers for risk of prostate cancer. Results from a case-control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarti D, Mailander P, Li K-M, Higginbotham S, Zhang H, Gross ML, Cavalieri E, Rogan E. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–7953. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 15.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J. Steroid Biochem. Mol. Biol. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Kosinska W, Khmelnitsky M, Cavalieri EL, Rogan EG, Chakravarti D, Sacks PG, Guttenplan JB. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem. Res. Toxicol. 2006;19:475–479. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 17.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. BBA Reviews on Cancer. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int. J. Cancer. 2006;118:1862–1868. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 19.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. Faseb. J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 20.Venugopal V, Zahid M, Mailander PC, Meza JL, Rogan EG, Cavalieri EL, Chakravarti D. Reduction of estrogen-induced transformation of mouse mammary epithelial cell by N-acetylcysteine. J. Steroid Biochem. Mol. Biol. 2008;109:22–30. doi: 10.1016/j.jsbmb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catecholestrogens in Syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 23.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissue: Role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 24.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 25.Gaikwad NW, Yang L, Pruthi S, Ingle JN, Sandhu N, Rogan E, Cavalieri E. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer: Basic & Clinical Research. 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawling S, Roodi N, Mernaugh RL, Wang XY, Parl FF. Catechol-O-methyltransferase (COMT) –mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer. Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 27.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen-DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahid M, Saeed M, Lu F, Gaikwad N, Cavalieri EL, Rogan EG. Inhibition of catechol-O-methyltransferase increases estrogen-DNA adduct formation. Free Radic. Biol. Med. 2007;43:1534–1540. doi: 10.1016/j.freeradbiomed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyland E, Chasseaud LF. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- 30.Reed DJ. Glutathione: toxicology implications. Annu. Rev. Pharmacol. Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- 31.Strange RC, Spiteri MA, Ramachandran S, Fryer A. Glutathione S-transferase family of enzymes. Mutat. Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 32.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic. Biol. Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montano MM, Chaplin LJ, Deng H, Mesia-Vela S, Gaikwad N, Zahid M, Rogan E. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 34.Gaikwad NW, Yang L, Rogan EG, Cavalieri EL. Evidence for NQO2-mediated reduction of the carcinogenic estrogen ortho-quinones. Free Radic. Biol. Med. 2009;46:253–262. doi: 10.1016/j.freeradbiomed.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zahid M, Gaikwad N, Cavalieri EL, Rogan EG. Inhibition of depurinating estrogen-DNA adduct formation by natural compounds. Chem. Res. Toxicol. 2007;20:1947–1953. doi: 10.1021/tx700269s. [DOI] [PubMed] [Google Scholar]
- 36.Bonanomi L, Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. Suppl. 1980;111:45–51. [PubMed] [Google Scholar]
- 37.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human diseases. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flanagan RJ. The role of acetylcysteine in clinical toxicology. Med. Toxicol. 1987;2:93–104. doi: 10.1007/BF03260008. [DOI] [PubMed] [Google Scholar]
- 39.Webb WR. New mucolytic agents for sputum liquefaction. Postgrad. Med. 1964;36:449–453. doi: 10.1080/00325481.1964.11695324. [DOI] [PubMed] [Google Scholar]
- 40.Doelman CJ, Bast A. Oxygen radicals in lung pathology. Free Radic. Biol. Med. 1990;9:381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- 41.De Flora S, Cesarone CF, Balansky RM, Albini A, D'Agostini F, Bennicelli C, Bagnasco M, Camoirano A, Scatolini L, Rovida A, et al. Chemopreventive properties and mechanisms of N-acetylcysteine. The experimental background. J. Cell Biochem. Suppl. 1995;22:33–41. doi: 10.1002/jcb.240590806. [DOI] [PubMed] [Google Scholar]
- 42.Grandjean E;M, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 43.De Flora S, Izzotti A, D'Agostini F, Cesarone CF. Antioxidant activity and other mechanisms of thiols involved in chemoprevention of mutation and cancer. Am. J. Med. 1991;91:122S–130S. doi: 10.1016/0002-9343(91)90295-9. [DOI] [PubMed] [Google Scholar]
- 44.Niemi TT, Munsterhjelm E, Poyhia R, Hynninen MS, Salmenpera MT. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair abdominal aortic aneurysm. Blood Coagulation and Fibrinolysis. 2007;17:29–34. doi: 10.1097/01.mbc.0000195922.26950.89. [DOI] [PubMed] [Google Scholar]
- 45.Saeed M, Zahid M, Rogan E, Cavalieri E. Synthesis of the catechols of natural and synthetic estrogens by using 2-iodoxybenzoic acid (IBX) as the oxidizing agent. Steroids. 2005;70:173–178. doi: 10.1016/j.steroids.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine and glutathione. Chem. Res. Toxicol. 1998;11:909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 47.Sgagias MK, Wagner KU, Hamik B, Stoeger S, Spieker R, Huber LJ, Chodash LA, Cowan KH. Brca1-deficient murine mammary epithelial cells have increased sensitivity to CDDP and MMS. Cell Cycle. 2004;3:1451–1456. doi: 10.4161/cc.3.11.1211. [DOI] [PubMed] [Google Scholar]
- 48.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 49.Zahid M, Gaikwad N, Ali MF, Lu F, Saeed M, Yang L, Rogan EG, Cavalieri EL. Prevention of estrogen-DNA adducts in MCF-10F cells by resveratrol. Free Radic. Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S, Chakravarti D, Edney JA, Hollins RR, Johnson PJ, West WW, Higginbotham SM, Cavalieri EL, Rogan EG. Relative imbalances in the expression of estrogen-metabolizing enzymes in the breast tissue of women with breast carcinoma. Oncology Reports. 2005;14:1091–1096. [PubMed] [Google Scholar]
- 51.Samuni AM, Chuang EY, Krishna MC, Stein W, DeGraff W, Russo A, Mitchell JB. Semiquinone radical intermediate in catecholic estrogen-mediated cytotoxicity and mutagenesis: chemoprevention strategies with antioxidants. Proc. Natl. Acad. Sci. USA. 2003;100:5390–5395. doi: 10.1073/pnas.0930078100. [DOI] [PMC free article] [PubMed] [Google Scholar]