Abstract
Objective
To determine the potential of periosteal cells to infiltrate poly-ε-caprolactone (PCL) nanofiber scaffolds in vivo and subsequently produce cartilage in vitro.
Design
PCL nanofiber scaffolds, with or without chitosan coating were implanted under periosteum in six-month old rabbits. Transforming growth factor-β1 (TGF-β1) or vehicle was injected into each implant site. After 1, 3, 5 or 7 days, scaffolds were removed, separated from the periosteum, and the scaffolds and periosteum were cultured separately for six weeks under chondrogenic conditions. Sulfated glycosaminoglycan (GAG), type II collagen and DNA content, cartilage yield, and calcium deposition were then analyzed.
Results
Cell infiltration was observed in all the scaffolds. Cartilage formation in the uncoated scaffolds increased with duration of implantation (maximum at 7 days). Cells in the uncoated scaffolds implanted for 7 days produced significantly higher levels of both GAG (560 (95% CI, 107–1013) vs. 228 (95% CI, 177–278) μgGAG/μgDNA) and cartilage yield (9% (95% CI, 3–14%) vs. 0.02% (95% CI, 0–0.22%)) compared to chitosan-coated scaffolds (p=0.006 or less). There was no significant difference in GAG content or cartilage yield between the TGF-β1-injected and vehicle-injected scaffolds. However, significantly more mineral deposition was detected in TGF-β1-injected scaffolds compared to vehicle-injected scaffolds (p<0.0001). Cartilage yield from the periosteum, moreover, was significantly increased by subperiosteal TGF-β1 injections (p<0.001). However, this response was reduced when chitosan-coated scaffolds were implanted.
Conclusions
This study demonstrates that it is possible to seed PCL nanofiber scaffolds with periosteal cells in vivo and subsequently produce engineered cartilage in vitro.
Key Indexing Terms: Cartilage, Chondrogenesis, Nanofibers, Periosteum, Transforming Growth Factor-beta, Poly-ε-Caprolactone
Introduction
Tissue engineering represents a viable option to repair cartilage and restore joint function1, 2. This approach requires a cell source, matrix or scaffold, and appropriate growth factors to promote chondrogenesis3. Current cell-based cartilage repair approaches such as autologous chondrocyte transplantation require in vitro culture to expand cells, and seed them onto a scaffold if applicable4–6. We are interested in developing autologous cell-based cartilage repair approaches that do not require harvest of healthy cartilage or in vitro culture.
Periosteal grafts meet these criteria and can be used clinically to resurface joints7. Periosteum contains chondrogenic and osteogenic cells in the form of mesenchymal stem cells (MSCs)7–18. In addition to using periosteum as a graft, a space can be created between the cambium layer and underlying bone to serve as an in vivo bioreactor, in which a graft, or scaffold can be implanted. For example, Cohen and LaCroix19 implanted free periosteal grafts under the adjacent periosteum of rabbits and demonstrated cartilage formation within the grafts. Recent studies have also demonstrated that periosteal cells can infiltrate hyaluronic acid gels or porous PCL scaffolds when implanted subperiosteally and form cartilage or bone in situ13, 14, 20.
We hypothesize that a scaffold in the form of a folded or rolled thin sheet could be implanted under the periosteum to capture chondrogenic cells. Subsequently, the cell-seeded scaffold could be separated from the periosteum, unfolded and used to resurface a large chondral defect or perhaps an entire joint surface. The first step in this process is to determine if a synthetic scaffold with suitable properties could capture periosteal cells in vivo and if these captured cells would form cartilage upon removal from the implantation site.
Electrospun polymeric nanofibers have emerged as potential scaffolds for cartilage tissue engineering21–30. Nanofibers mimic the natural extracellular matrix (ECM) and are suitable candidates for cartilage tissue engineering22, 31. PCL nanofiber sheets are also flexible and can be rolled or folded and contoured to cover the surface of a joint. Therefore, PCL nanofiber scaffolds are excellent candidates for this study.
Chitosan, a biopolymer found in the shells of crustaceans, is known for its biocompatibility, antibacterial properties, degradability, wound-healing capability, and support of chondrocyte differentiation32–34. Therefore, we hypothesized that coating PCL nanofiber scaffolds with chitosan would enhance the chondrogenic potential of periosteal cells.
This study was designed to determine the potential of periosteal cells to infiltrate uncoated and chitosan-coated PCL nanofiber scaffolds in vivo after subperiosteal implantation and form cartilage in vitro within the scaffolds after removal. We also, examined the effect of injecting TGF-β1 in the implantation site on the chondrogenic potential of the periosteal cells in the scaffolds and the implant-site periosteum. The in vitro culture step used in this experiment was employed as a method to determine proof-of-concept. From a clinical standpoint, we envision that a culture step would not be necessary for cartilage repair. Rather, after implanting the scaffold under the periosteum for a suitable duration, the resulting cell-seeded scaffold would be harvested and used directly to repair the defective cartilage during the same surgical procedure, in the same manner as periosteal transplantation.
MATERIALS AND METHODS
NANOFIBROUS SCAFFOLD SYNTHESIS
A 9.5 wt% homogeneous solution of PCL was prepared by dissolving 1.05 g of 80,000 Mn PCL (Sigma-Aldrich, St. Louis, MO, cat. #440744, batch #12331AB) in 0.8 g N, N-dimethylformamide (Fisher Scientific), 6.0 g chloroform (Fisher Scientific), and 3.2 g acetone (Fischer Scientific). The solution was stirred gently for 4 hours and transferred to a 30 ml glass syringe fitted with a 10 cm, 20-gauge needle. A 30 kV electric field was applied to the solution as it was dispensed at 3.3 ml/h. The nanofibers were collected on a glass plate at a distance of 15 cm from the tip of the needle. Approximately four batches were needed to make a 1 mm thick scaffold. The average diameter of fibers was approximately 400 nm based on light microscope observation.
NANOFIBER IMPLANT PREPARATION
256 cylindrical samples were cut from the 1 mm thick nanofiber sheet using a 3.5 mm dermal punch (Miltex Inc., York, PA) and divided into 2 groups. Chitosan was used to coat 128 samples, while the other 128 samples were left uncoated. Control samples were soaked in 50 wt% acetic acid solution, while the coated samples were soaked in 50 wt% acetic acid solution with 0.5 wt% of 85% deacetylated chitosan (Sigma, St. Louis, MO) for 12 hours. Both groups were freeze-dried and gas sterilized using ethylene oxide.
SCAFFOLD CELL SEEDING/IMPLANTATION
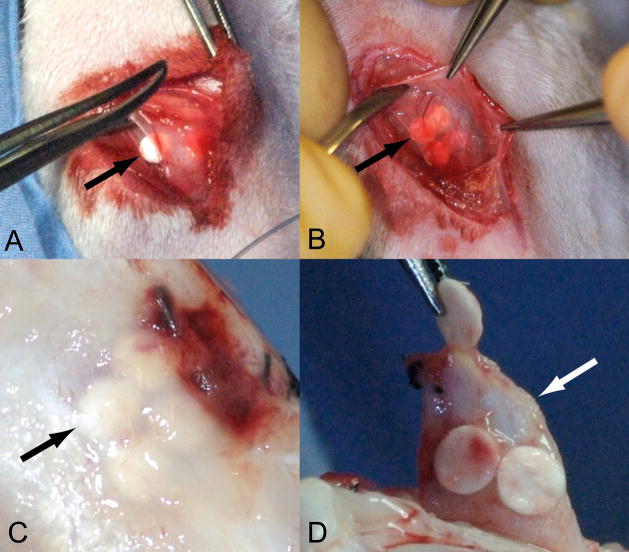
All procedures were conducted with the approval of our Institutional Animal Care and Use Committee. Thirty-two skeletally mature six-month old New Zealand white rabbits (4.2 to 5.2 kg) were used in this study. Under general anesthesia induced by an intramuscular injection of ketamine/xylazine/acepromazine (50mg/5mg/0.75mg/Kg of body weight respectively), both hind limbs were shaved and prepared with Techni-care® surgical scrub (Care-Tech Laboratories, St. Louis MO). A 1 cm skin incision was made over the medial proximal tibia. The underlying deep fascia was retracted and a 4 mm incision was made in the underlying periosteum. Inserting a sharp 2.5 mm periosteal elevator into the incision, the periosteum was elevated off the bone creating a 1 cm × 1 cm subperiosteal space. Individual rabbits received either uncoated scaffolds or chitosan-coated scaffolds not both. Four uncoated or chitosan-coated PCL nanofiber scaffolds were implanted into each subperiosteal space (Fig. 1A and 1B). After closure of the subperiosteal space with 4–0 silk suture, TGF-β1 (200 ng) was injected in one limb while vehicle was injected in the contralateral limb using a 30 gauge, ½ inch needle (Precision Glide Needle, Franklin Lakes, NJ) into the subperiosteal space under each of the four scaffolds. The fascia and skin were closed with 3–0 Vicryl subcuticular sutures. After 1, 3, 5 or 7 days, the rabbits were euthanized using sodium pentobarbital (100 mg/kg body weight) and the scaffolds were removed by separating them from the periosteum (Fig. 1C and Fig. 1D). The overlying periosteum was harvested and cut into four explants corresponding to the regions previously occupied by the scaffolds.
Fig. 1.
Implantation (A & B) and harvesting (C & D) of PCL nanofiber scaffolds. (A) Subperiosteal implantation. (B) Closure with scaffolds under periosteum. (C) Exposure of scaffolds after 7 days of implantation. (D) Harvesting of scaffold from periosteum for in vitro culture. The black arrows indicate the location of PCL nanofiber scaffolds. The white arrow shows the periosteum after removal of PCL nanofiber scaffold.
IN VITRO CULTURE OF SEEDED SCAFFOLDS AND PERIOSTEUM
The periosteal cell-seeded scaffolds and corresponding periosteal explants were obtained within 15 minutes of death and placed in Dulbecco’s Modified Eagle Media (DMEM), 10% FBS with penicillin/streptomycin and 1 mM proline at 4° C. Within 90 minutes, the scaffolds and explants were transferred to chondrogenic culture as previously described12. Briefly, 24-well culture plates were coated with 1% agarose (Bio-Rad, Hercules, CA) followed by 0.25 ml of 1% low-melt agarose (Bio-Rad, Hercules, CA) in DMEM. After gelling the agarose on ice, periosteal explants or scaffolds were placed on the agarose bed (one sample/well). Another 0.75 ml of 1% low-melt agarose in DMEM were then added and agarose was gelled on ice. One milliliter of liquid medium was then added to each well. The medium was supplemented with 10 ng/ml TGF-β1 for the first two days of culture and 50 μg/ml L-ascorbic acid throughout the culture period. The liquid medium was replaced three times each week. The cultures were maintained at 37° C, 5% CO2 and 95% air. After 6 weeks, the scaffolds were removed from culture and cut in half. One half was analyzed for cartilage content, mineral deposition and immunohistochemistry for type II collagen. The other scaffold halves were analyzed for GAG and dsDNA content (two samples from each limb), or collagen typing analysis35 (two samples from each limb).
CARTILAGE CONTENT AND MINERAL DEPOSITION
The half scaffolds and periosteal explants were weighed, fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 45 minutes, washed through a series of graded ethanol (50, 70, 95 and 100%), followed by washing in a 50:50 mixture of 100% ethanol and Histoclear, 100% Histoclear, a 50:50 mixture of Histoclear and Paraplast X-tra, and embedded in 100% Paraplast X-tra. For the scaffold samples, the edge where the scaffold had been cut in half was the section exposed for analysis. The periosteal explants were randomly shaped after the culture period and were not embedded in any particular orientation. The samples were cut into 3 μm thick sections, and stained with safranin O/fast green. Computerized histomorphometry was applied by a blinded technician to determine percentage cartilage yield (% area stained red versus total tissue area) in each sample using Vidas Image Analysis Program (Zeiss, software version 2.1)36. Positive staining occurred when hue and intensity threshold for red appropriate to cartilage was reached and the area over which this occurred was recorded. The paraffin embedded samples from the 7-day implantation group were also stained using the von Kossa technique and mineral deposition was evaluated using a simple histological method (based on a previously validated histological scoring technique37). The specimens were assigned a score from 0 to 3 by a blinded technician where 0 = no black von Kossa staining; 1 = von Kossa staining in less than half the specimen; 2 = von Kossa staining in more than half the specimen; and 3 = von Kossa staining throughout or nearly throughout the specimen.
GAG AND DNA CONTENT ANALYSIS
The other half of the scaffolds were rinsed in 1 x PBS, digested in 1 ml of 50 μg/ml proteinase K (Roche, IN, USA) dissolved in 100 mM K2HPO4 (pH 8.0) for 16 hours at 60° C in a water bath and digestion was inactivated in a 90° C water bath for 10 minutes. Using 100 μl of this working solution, GAG content was quantified following instructions provided by the manufacturer in the dimethyl-methylene blue assay kit (DMMB, Blyscan™ Sulfated Glycosaminoglycan Assay Kit; Biocolor Ltd., NI, UK). Bound dye values were quantified at 656 nm using a SpectraMax Plus spectro-photometer (Molecular Devices, CA, USA). Yield was normalized to dsDNA content. dsDNA content was determined using Quant-iT PicoGreen dsDNA Kit (invitrogen Eugene, OR) with a Fluorostar Plate Reader (BMG Labtechnologies, Offenburg, Germany) and 100 μl working solution from digest described above. The background values obtained from unseeded PCL and chitosan-coated PCL were subtracted from the dsDNA values to get the final dsDNA content of the engineered tissue.
COLLAGEN TYPING
Quantitative collagen typing was performed using a published technique for measuring the relative amount of type II collagen with respect to type I collagen in tissue samples38. This technique has been modified to permit the analysis of very small samples (1 – 10 mg) without initial purification of the collagen35. Samples were weighed, and collagen peptides were cleaved with 0.5 ml 5% cyanogen bromide (CNBr) in deaerated 88% formic acid. In preparation for electrophoresis, samples were dissolved in 0.063 M Tris–HCl, pH 8, 3.3% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.001% bromophenol blue, at a concentration of 8 μg (wet weight) of sample per micro liter of sample buffer. A 1 μl volume of sample was loaded onto 20% gels, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using a Phast System (Pharmacia LKB, Uppsala, Sweden). A standard derived from articular cartilage was run in each gel to ensure that the banding pattern for 100% type II collagen was accurately represented. The gels were stained with Coomassie blue and scanned on a laser densitometer. Typically, the percentage of type II collagen with respect to type I collagen would be determined by measuring the ratio of the α1(II)CB10 to the α1(I)CB7, 8 and α1(II)CB11 peaks in each lane. Unfortunately, the only detectable bands on the gels were those from the articular cartilage controls. Therefore, the collagen typing analysis was inconclusive due to interference by PCL in the samples and the data are not presented.
IMMUNOHISTOCHEMISTRY
Immunohistochemistry for type II collagen was also performed on select samples. The formalin fixed paraffin embedded samples were sectioned at 5 microns, deparaffinized and treated with 3% H202 to inactivate endogenous peroxidase, followed by incubation with protein block (Dako X0909, DAKO North America, Inc., Carpinteria, CA). Mouse monoclonal anti-collagen type II antibody, II-II6B3 (Developmental Studies Hybridoma Bank, The University of Iowa, Department of Biology, Iowa City), was applied using a 1/400 dilution from 432 μg/ml Ig stock (concentration provided by supplier) overnight at room temperature. Visualization was performed using Mach3 Mouse HRP Polymer Detection (REF#M3M530 Biocare Medical, Concord, CA) followed by incubation with diaminobenzidine. Sections were counterstained with hematoxylin. Rabbit osteochondral tissue was used for positive controls. Negative controls were prepared in the absence of primary antibody.
STATISTICAL ANALYSIS
Data are expressed as means with a 95 % confidence interval (lower limit, upper limit). Statistical differences between each treatment group and corresponding vehicle control were evaluated using one-way Analysis of Variance (ANOVA). Statistical differences between treatment groups were evaluated using the Least Squares Means Differences Student’s t-Test.
Results
PERIOSTEAL CELL INFILTRATION INTO PCL NANOFIBER SCAFFOLDS
During sample harvest, the uncoated scaffolds were more tightly integrated with the periosteum compared to chitosan-coated scaffolds. The uncoated scaffolds implanted for 5 and 7 days were particularly well attached to the surrounding periosteum leaving visible imprints in the periosteum upon removal (Fig. 1D). After the six-week culture period, light microscopy (Fig. 2) and scanning electron microscopy (not shown) revealed cell penetration into the scaffolds and attachment to PCL fibers with or without chitosan coating or TGF-β1 injection. The DNA content (Table 1) in the uncoated scaffolds increased with duration of implantation reaching maximum in scaffolds implanted for 7 days (Vehicle: 0.525 (0.473–0.577) μg, n=8; TGF-β1: 0.511 (0.455–0.566) μg, n=7). DNA content in chitosan-coated scaffolds reached maximum in after 3 days (Vehicle: 0.638 (0.443–0.832) μg, n=7; TGF-β1: 0.582 (0.387–0.776) μg, n=7). TGF-β1 injection did not significantly alter DNA content in any of the groups.
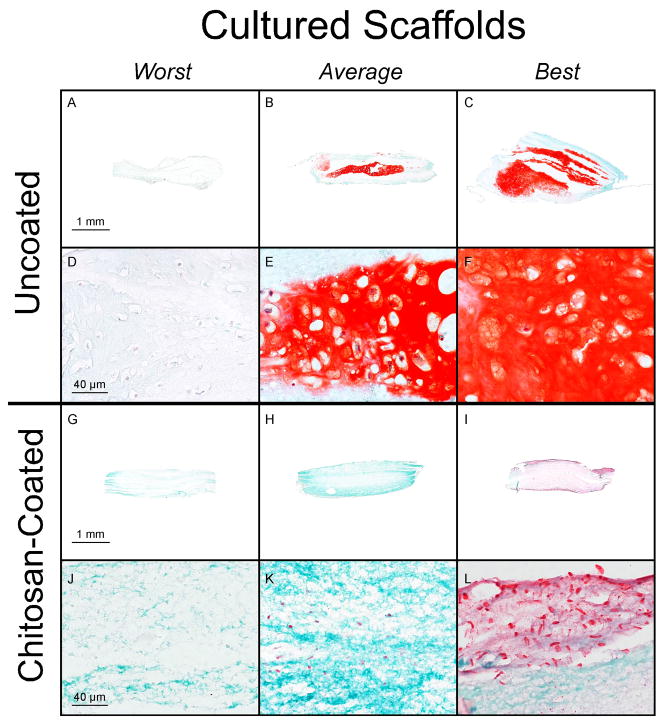
Fig. 2.
Safranin O/fast green stained histological specimens of uncoated (A–F) and chitosan-coated (G–L) PCL nanofiber scaffolds after 7 days of subperiosteal implantation and 6 weeks of culture. The specimens are the worst, a representative of the average, and the best based on percent cartilage in the tissue sections. The samples without chitosan are from the 7-day implantation group. For the samples with chitosan, the “Worst” is from the 7-day no TGF-β1 implantation group, which is lacking in extra cellular matrix and has no cartilage staining. The “Average” is from the 7-day TGF-β1 implantation group and has little to no cartilage staining. The “Best” comes from the day 3 no TGF-β1 implantation group, stains red for cartilage on the periphery, and pink throughout the center.
Table 1.
DNA Content in Periosteal Cell-Laden PCL Nanofiber Scaffolds
| Uncoated | Chitosan-Coated | ||||
|---|---|---|---|---|---|
| Duration of Implant (Days) | Vehicle | TGF-β1 | Vehicle | TGF-β1 | |
| DNA Content (g) | 1 | 0.439 (0.4388-0.439) | 0.439 (0.439–0.4394) | 0.425 (0.413–0.436) | 0.418 (0.406–0.429) |
| 3 | 0.483 (0.446–0.520) | 0.456 (0.421–0.490) | 0.638* (0.443–0.832) | 0.582** (0.387–0.776) | |
| 5 | 0.519 (0.484–0.554) | 0.464 (0.429–0.500) | 0.470 (0.363–0.576) | 0.512 (0.412–0.611) | |
| 7 | 0.525 (0.473–0.577) | 0.511 (0.455–0.566) | 0.399 (0.390–0.408) | 0.397 (0.389–0.404) | |
The data are presented as means with 95% confidence intervals. The group marked (*) has significantly higher DNA content than all other groups in the table except the group marked with (**). The highest p-value occurring between this group and the uncoated, day 7 vehicle injected group at p=0.029. All other p-values when compared to this marked (*) value are below this p-value. The group marked (**) had significantly higher values of DNA content than all groups in the table with the exception of vehicle and TGF-β1 injected, day 7 uncoated scaffolds and vehicle injected day 5 and day 3 uncoated scaffolds. The highest p-value amongst the groups significantly different from (**) occurred between this group and TGF-β1 injected, day 5 uncoated scaffolds with p=0.0228. All other p-values when compared to this marked (**) value are below this p-value. These p values are based on the results of post-hoc testing using Student’s t-test (n=7 or 8).
GLYCOSAMINOGLYCAN SYNTHESIS
Normalized GAG content (μg GAG/μg DNA) (Fig. 3A) in uncoated scaffolds increased with duration of implantation reaching maximum in scaffolds implanted for 7 days (Vehicle: 560 (107–1013), n=8; TGF-β1: 728 (244–1213), n=7), which was significantly higher than uncoated scaffolds implanted for 1 day (Vehicle: 11 (1–21), n=7, p<0.0001; TGF-β1: 2.2 (0–12), n=7, p=0.0001), 3 days (Vehicle: 57 (20–94), n=7, p<0.0001; TGF-β1: 19 (0–54), n=8, p<0.0001), or 5 days (Vehicle: 160 (56–265), n=8, p=0.0004; TGF-β1: 186 (80–291), n=8, p<0.0001). There was also significantly more GAG produced in the uncoated scaffolds implanted for 7 days compared to all chitosan-coated scaffold groups including those implanted for 7 days (Vehicle: 217 (166–268), n=8, p=0.0024; TGF-β1: 228 (177–278), n=7, p<0.0001). These findings are reflected in the histology (Fig. 2) and cartilage content results presented below (Fig. 3B). The mean normalized GAG content in chitosan-coated scaffolds implanted for 1 and 3 days were higher than uncoated scaffolds, but no significant difference was found. There were no significant differences between TGF-β1 injected scaffolds and vehicle controls across all durations of implantation.
Fig. 3.
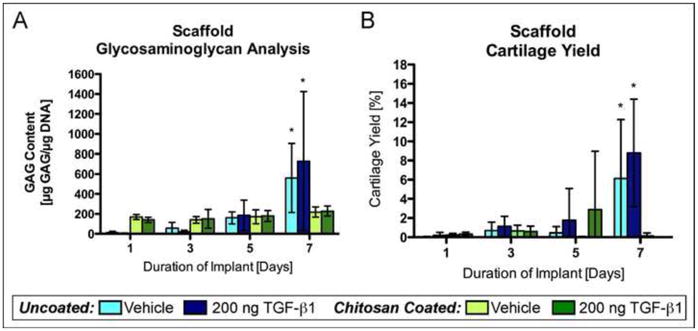
Glycosaminoglycan content and cartilage yield in periosteal cell-laden PCL nanofiber scaffolds. Uncoated and chitosan-coated PCL nanofiber scaffolds were implanted under the periosteum of rabbits for 1, 3, 5, or 7 days followed by six weeks of culture. The implant sites were injected with either 200 ng TGF-β1 or vehicle. (A) GAG content in periosteal cell-laden scaffolds after six weeks of culture (n=7 or 8). (B) Cartilage yield in periosteal cell-laden scaffolds after six weeks of culture (n=16). The data presented are means with 95% confidence interval. The asterisks indicate values that are significantly different than all other time points based on post-hoc testing (p<0.0001).
CARTILAGE CONTENT
Cartilage content was determined in the scaffolds and periosteal explants after the six-week culture period based on safranin O/fast green staining as described in the methods. Similar to the GAG content results, percent cartilage produced in the scaffolds was dependent on duration of implantation and whether the scaffold was coated with chitosan or not (Fig. 3B). In the scaffolds, cartilage content was negligible (<1.2%) except for uncoated scaffolds implanted for 7 days (vehicle: 6.1% (0.5–11.8%), n=16; TGF-β: 8.8% (3.2–14.4%), n=16), which produced significantly more cartilage than all other groups (p<0.0001). Also, this group produced significantly more cartilage than chitosan-coated scaffolds for all durations of implantation (p=0.006 or less). However, cartilage content in uncoated PCL scaffolds implanted for 7 days was highly variable with a minimum = 0 and maximum = 45 %. This was not a rabbit-specific effect because there was no correlation between the rabbit used and amount of cartilage produced in the scaffolds. The TGF-β1 injected scaffolds were not significantly different from vehicle injected scaffolds in cartilage content for all durations of implantation regardless of being uncoated or chitosan-coated. Overall, the cartilage yield results are consistent with the GAG content results described above (Fig. 3A). Also, similar to the GAG content results and safranin O/fast green staining, immunohistochemistry revealed darker staining for collagen type II in uncoated scaffolds compared to chitosan-coated scaffolds (Figure 5).
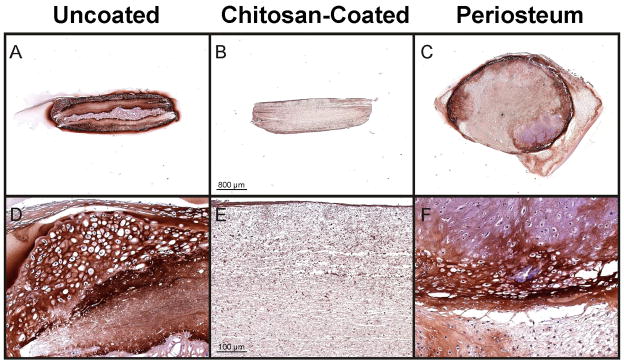
Fig. 5.
Typical type II collagen immunohistochemical staining of uncoated (A & D) and chitosan-coated (B & E) PCL nanofiber scaffolds implanted under the periosteum of rabbits for 7 days followed by six weeks of culture. (C & F) Representative implant-site periosteal explant after 7 days of scaffold implantation followed by six weeks of culture. For reference, the uncoated-scaffold (A & D), the chitosan-coated scaffold (B & E) and the periosteal explant (C & F), are from serial sections of the samples shown in Fig. 2(B & E), Fig. 2(H & K), and Fig. 8(A & B) respectively.
Periosteum from the implant sites was also harvested (Fig. 1) and cultured separately from the scaffolds for six weeks and cartilage yield was determined. Significantly more cartilage was produced in the periosteum when the implant site was injected with TGF-β1 compared to vehicle regardless of duration of implantation (uncoated scaffolds p < 0.0001; chitosan-coated p = 0.0014) (Fig. 4A & 8). Periosteum harvested from above uncoated, TGF-β1 injected implantation sites produced significantly more cartilage than periosteum from above chitosan-coated implantation sites (p=0.0028). Interestingly, the wet weights of the periosteum from above uncoated, TGF-β1 injected implantation sites were also significantly higher (p<0.0001) than periosteum from above chitosan-coated implantation sites (Fig. 4B). Unlike the scaffolds, no obvious differences in immunohistochemistry staining for type II collagen in the periosteal explants were observed between the treatment groups (Figure 5). The uncoated, TGF-β1 injected groups produced mean amounts of cartilage comparable to or greater than historical data of periosteum from rabbits 2 and 6-month old after culture (Fig. 4A)39. Notably, these groups produced a cartilage yield statistically the same as results from our previous study in which we injected TGF-β1 subperiosteally and waited 1, 3, 5, and 7 days before harvesting the tissue and culturing under the same conditions40. This study indicates that local TGF-β1 injections can rejuvenate periosteum to the same extent despite the presence of scaffolds and loss of cells when the scaffolds were removed.
Fig. 4.
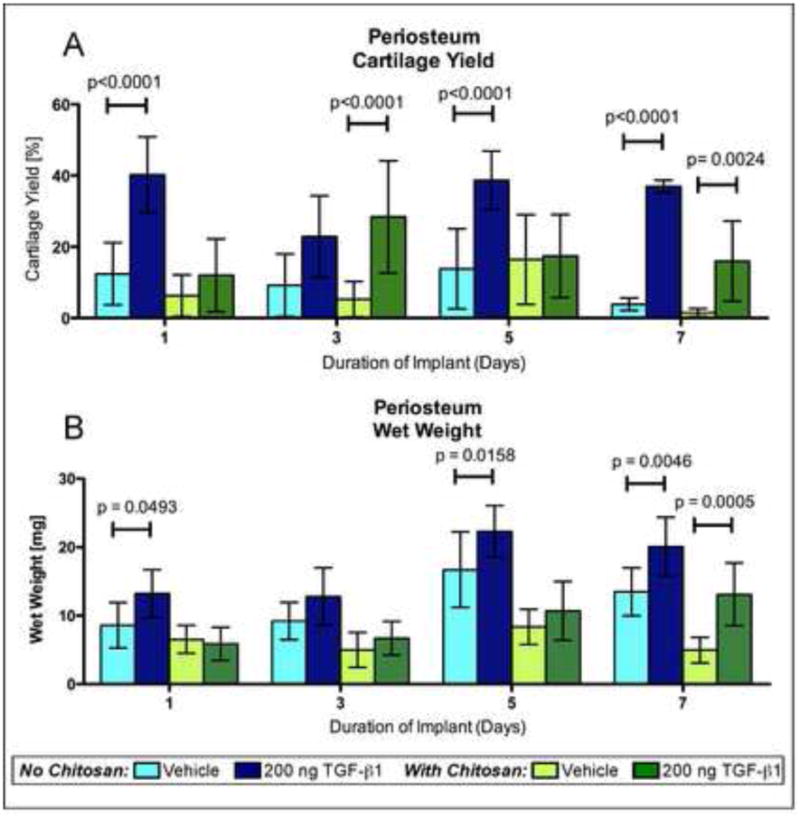
Cartilage yield and wet weights of implant-site periosteum. Uncoated and chitosan-coated PCL nanofiber scaffolds were implanted under the periosteum of rabbits for 1, 3, 5, or 7 days followed by six weeks of culture. The implant sites were injected with either 200 ng TGF-β1 or vehicle. (A) Cartilage yield and (B) wet weights of implant-site periosteum after six weeks of culture. The data presented are means with 95% confidence interval (n=16). The brackets indicate values in the TGF-β1 injected groups that are significantly different than the corresponding vehicle controls based on post-hoc testing.
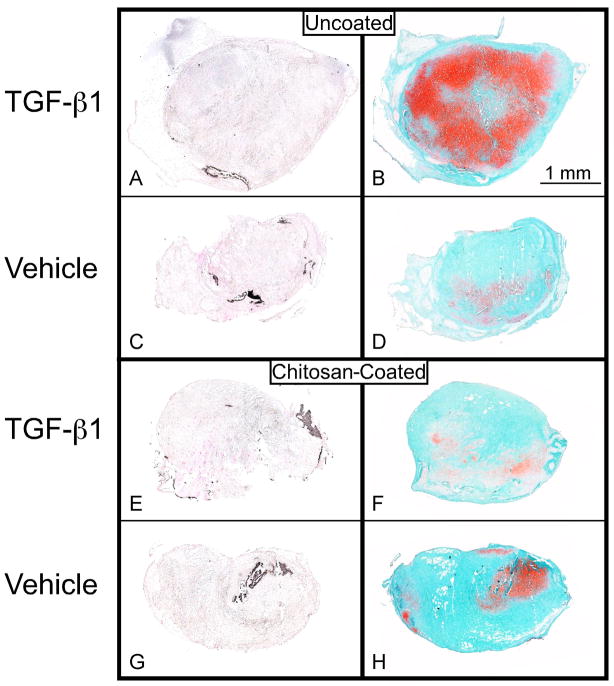
Fig. 8.
Von Kossa and Safranin O/fast green stained histological sections. These sections show mineralization of periosteal explants from the implantation sites of uncoated (A & C) and chitosan-coated (E & G) PCL nanofiber scaffolds and Safranin O/fast green stained serial sections (B, D, F & H) of periosteal explants from the 7-day subperiosteal implant group with TGF-β1 injection (A, B, E & F) and vehicle injection (C, D, G & H) at the implant site after six weeks of culture. The asterisks indicate values that are significantly different than the corresponding vehicle control based on post-hoc testing (p < 0.0001).
MINERAL DEPOSITION
Histological specimens from the 7-day implantation groups were stained using von Kossa technique to detect mineral deposition (Fig. 6–8). There was no significant difference in mineral deposition between uncoated and chitosan-coated scaffolds. However, significantly more mineral deposition was observed in scaffolds receiving TGF-β1 injection versus vehicle controls (p<0.0001) in both uncoated (vehicle: 0.75 (0.35–1.15), n=16; TGF-β1: 1.3 (0.91–1.71), n=16) and chitosan-coated scaffolds (vehicle: 0.44 (0.04–0.83), n=16; TGF-β1: 1.5 (1.11–1.89), n=16)(Fig. 6 & 7). Mineral deposition was minimal in the implant-site periosteum and no significant differences were observed between implant types or between injections of vehicle vs. TGF-β1 (Fig. 6 & 8).
Fig. 6.
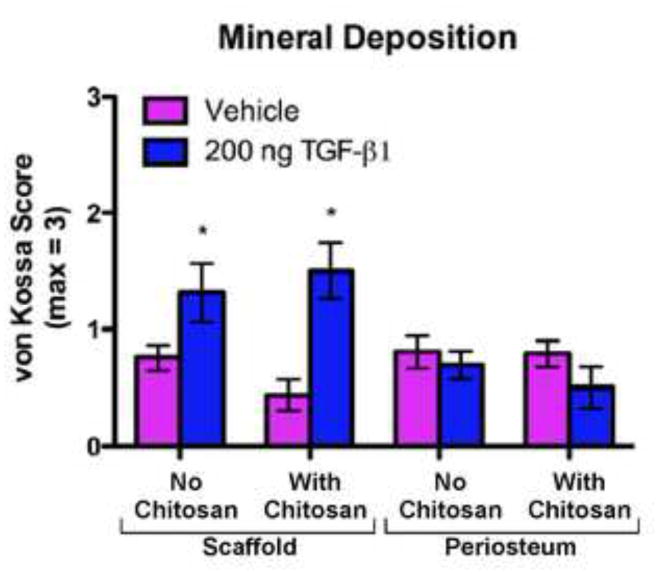
Mineral Deposition (based on von Kossa stained histological sections) in uncoated (No Chitosan) and chitosan-coated (With Chitosan) PCL nanofiber scaffolds and periosteum from the implant sites after 7 days of implantation and 6 weeks of culture. The specimens were scored from 0 to 3 (based on a previously validated histological scoring method37) by a blinded technician where 0 = no staining; 1 = partial staining < 50%; 2 = partial staining > 50 %; and 3 = nearly complete or complete staining of the section. The data presented are means with 95% confidence interval (n=16). The asterisks indicate values that are significantly different than the corresponding vehicle control based on post-hoc testing (p < 0.0001).
Fig. 7.
Von Kossa and Safranin O/fast green stained histological sections of periosteal cell-laden PCL nanofiber scaffolds. These sections show mineralization of uncoated (A & C) and chitosan-coated (E & G) PCL nanofiber scaffolds and Safranin O/fast green stained serial sections (B, D, F & H) of the periosteal cell-laden scaffolds from the 7-day subperiosteal implant group with TGF-β1 injection (A, B, E & F) and vehicle injection (C, D, G & H) at the implant site after six weeks of culture.
Discussion
This study demonstrates that chondrogenic cells can infiltrate PCL nanofiber scaffolds in vivo after subperiosteal implantation and produce cartilage in vitro after removal from the periosteum. This finding supports the notion that the subperiosteal space can be used as an “in vivo bioreactor” for musculoskeletal tissue engineering13, 14, 20. Another interesting finding is that periosteum from the implant sites retained the ability to form cartilage in vitro in response to in vivo injection of TGF-β1. These results mimic our previous finding that subperiosteal injection of TGF-β1 significantly increases in vitro periosteal cartilage yield in 6, 12 and 24-month rabbits40. However, as Simon et al. previously demonstrated, this result may be due in part to stimulation of cambium cells by elevation of the periosteum41. In any case, it may be possible to use both the periosteal cell-laden PCL nanofiber scaffold and the implant-site periosteum for chondral or osteochondral tissue regeneration. Specifically, since TGF-β1 injection increased mineralization in the cell-seeded scaffold but not in the surrounding periosteum, the cell-seeded scaffold in this case might be used as a bone layer of an osteochondral defect while using the periosteum as the cartilage layer. Of course this is speculation based on the results from in vitro culture and would need to be examined in vivo.
Cartilage formation in PCL nanofiber scaffolds was dependent on duration of the implantation period. However, because the scaffolds were only analyzed after the six-week culture period, the initial cell density of the scaffolds when placed into culture is unknown. This makes it unclear if optimal cell density in the scaffolds or initiation of cellular differentiation in vivo prior to removal of the scaffolds from the periosteum is responsible for the increase in cartilage formation over time. Nevertheless, after the six-week culture period, cell infiltration was observed in all scaffolds regardless of injection with TGF-β1 or vehicle, duration of implantation, and whether the scaffolds were chitosan-coated or not. Based on GAG and cartilage content analyses, significant amounts of cartilage formed in the uncoated, 7-day implantation group with no significant difference observed between TGF-β1 and vehicle-injected scaffolds. The amount of cartilage produced by this experimental group is comparable to historical data of in vitro cartilage yield produced from periosteum harvested from 6-month rabbits39. However, based on the present study, we hypothesize that longer implantation periods would result in greater and more consistent cartilage production in the periosteal cell-seeded PCL nanofiber scaffolds.
Interestingly, the chitosan-coated scaffolds did not yield significant amounts of cartilage even though cell infiltration was observed. In general, there was significant homology in cellular morphology and matrix within the chitosan-coated scaffolds. This was noticeably different from uncoated groups as seen in Figure 2. While it is possible that the chitosan-coating may have slowed cellular infiltration into the PCL nanofiber scaffolds resulting in decreased in vitro chondrogenesis, it is also possible that the chitosan-coating inhibited cell proliferation and/or chondrogenic differentiation. The latter possibility is supported by the observation that periosteum from chitosan-coated scaffold implant sites had significantly lower wet weights and formed significantly less cartilage than periosteum from uncoated scaffold implant sites. A potential biocompatibility issue with the chitosan coating cannot be ruled out from this experiment. The observed decrease in periosteal explant wet weights in the chitosan-coated groups support this notion, however, the DNA content of the scaffolds do not. The observed lack of chondrogenesis in the chitosan-coated scaffolds is in contrast to our previous results, which demonstrated chitosan-coating of macro porous PCL scaffolds resulted in higher GAG production from seeded human chondrocytes (unpublished results) as well as published reports demonstrating chitosan supports chondrocyte differentiation32–34. However, these apparently conflicting results may be accounted for by the obvious differences in approach between this study and other studies such as cell type, scaffold design (pore size, fiber size, architecture etc.), cell-seeding technique and culture conditions.
Because no significant cartilage was observed in chitosan-coated scaffolds, we speculated that the chitosan-coating might be inducing the periosteal cells to differentiate into osteoblasts rather than chondrocytes. Interestingly, while no difference in mineral deposition was observed between uncoated and chitosan-coated samples, a significant increase in mineral deposition was observed in the scaffolds when the implant site was injected with TGF-β1 compared to vehicle (Fig. 6 & 7). This observation raises an obvious concern for the use of TGF-β1 injections in combination with the periosteal cell-seeded PCL nanofiber scaffolds for cartilage tissue engineering. However, if the periosteal cell-seeded scaffolds were surgically implanted into a cartilage defect immediately after removal from the periosteum or if the scaffolds were exposed to continuous TGF-β1 in the culture medium (as opposed to only the first 2 days) such mineral deposition might be inhibited42–44. Additional studies are needed to test these approaches.
The approach described herein has the potential to generate surgically implantable prechondrocyte-laden scaffolds with the potential advantage of obviating the need for separate ex-vivo culturing of cell-seeded scaffolds or cells. However, it is not clear how results obtained in this study with six month-old rabbits will apply to an aged human population for cartilage repair. Future experiments in older rabbits or other species may be helpful in this regard, but no animal model can be directly translated to humans. Nevertheless, evidence in humans demonstrates that although the number of multi-potent mesenchymal cells in the cambium of periosteum is greatly reduced with age, they still exist and maintain their multi-potential and proliferative capacity even in elderly humans45, 46. In addition, we recently demonstrated in rabbits, that it is possible to increase the number of cambium cells in aged rabbits and rejuvenate the chondrogenic potential of the periosteum using local growth factor injection40.
The next step will be to determine if the periosteal cell-seeded scaffolds can be implanted directly into a cartilage defect upon removal from the periosteum for in vivo cartilage regeneration without the use of an in vitro culture step. In addition, it will be important to further characterize the cells that infiltrate the scaffold and if necessary to customize the scaffold to specifically select the mesenchymal stem cells.
Acknowledgments
We wish to thank Mr. Scott I. Gamb from the Electron Microscopy Core Facility, for producing the SEM images and Bridget Hoesley from the Tissue and Cell Molecular Analysis (TACMA) lab at Mayo Clinic for performing immunohistochemistry. The collagen II antibody (II-II6B3) developed by Thomas F. Linsenmayer was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This manuscript is dedicated to the memory of our dear friend and colleague Dr. James J. Stone.
Role of the funding source
Funding for this project was provided by NIH/NIAMS through grant AR52115 and supplements to this grant for MEC, and JJS. Mayo Clinic and the Department of Orthopedic Surgery provided additional funding support for this project. Dr. Olivos-Meza also received support from the Government of Veracruz State, and the University of Veracruz, Mexico. These funding sources were not involved in any other aspect of the study.
Footnotes
Competing interests
Mayo Clinic has filed a patent application related to this study on behalf of authors GGR, JSF and SWO. The remaining authors have no competing interests to declare.
Contributions
M.E. Casper contributed to acquisition, analysis and interpretation of data, drafting and critically reviewing the manuscript and final approval of the submitted version.
J.S. Fitzsimmons contributed to conception and design, obtaining funding, acquisition, analysis and interpretation of data, drafting and critically reviewing the manuscript and final approval of the submitted version.
J.J. Stone contributed to conception and design, obtaining funding, acquisition, analysis and interpretation of data, and drafting the manuscript.
Y. Huang contributed to acquisition, analysis and interpretation of data, drafting and critically reviewing the manuscript and final approval of the submitted version.
A. Olivos-Meza was the lead surgeon for the study and contributed to the acquisition, analysis and interpretation of the data, drafting and critically reviewing the manuscript and final approval of the submitted version.
T.J. Ruesink contributed to acquisition of data, revising the manuscript and final approval of the submitted version.
S.W. O’Driscoll contributed to conception and design, obtaining funding, critically reviewing the manuscript and final approval of the submitted version.
G.G. Reinholz contributed to conception and design, obtaining funding, acquisition, analysis and interpretation of the data, drafting and critically reviewing the manuscript and final approval of the submitted version, and accepts responsibility for the integrity of the study as a whole from inception to finished article (reinholz.gregory@mayo.edu).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caplan AI. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 2000;6:1–8. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- 2.Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5:235–238. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 5.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft. A preliminary report. J Bone Joint Surg Br. 2005;87:330–332. doi: 10.1302/0301-620x.87b3.15552. [DOI] [PubMed] [Google Scholar]
- 7.O’Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop. 2001:S190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 8.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg. 1986;68A:1017–1035. [PubMed] [Google Scholar]
- 9.O’Driscoll SW. Articular cartilage regeneration using periosteum. Clin Orthop. 1999;367 (Suppl):186–203. doi: 10.1097/00003086-199910001-00020. [DOI] [PubMed] [Google Scholar]
- 10.Nakahara H, Bruder SP, Haynesworth SE, Holecek JJ, Baber MA, Goldberg VM, et al. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone. 1990;11:181–188. doi: 10.1016/8756-3282(90)90212-h. [DOI] [PubMed] [Google Scholar]
- 11.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 12.O’Driscoll SW, Recklies AD, Poole AR. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994;76:1042–1051. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9 (Suppl 1):S45–64. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 14.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg. 1988;70A:595–606. [PubMed] [Google Scholar]
- 16.O’Driscoll SW, Salter RB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1986:131–140. [PubMed] [Google Scholar]
- 17.Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mardones RM, Reinholz GG, Fitzsimmons JS, Zobitz ME, An KN, Lewallen DG, et al. Development of a biologic prosthetic composite for cartilage repair. Tissue Eng. 2005;11:1368–1378. doi: 10.1089/ten.2005.11.1368. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J, LaCroix P. Bone and cartilage formation by periosteum. Assay of experimental autogenous grafts. J Bone Joint Surg. 1955;37-A:717–730. [PubMed] [Google Scholar]
- 20.Huang Q, Goh JC, Hutmacher DW, Lee EH. In Vivo Mesenchymal Cell Recruitment by a Scaffold Loaded with Transforming Growth Factor beta1 and the Potential for in Situ Chondrogenesis. Tissue Eng. 2002;8:469–482. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 21.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 23.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 24.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 25.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17:103–119. doi: 10.1163/156856206774879126. [DOI] [PubMed] [Google Scholar]
- 27.Thorvaldsson A, Stenhamre H, Gatenholm P, Walkenstrom P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules. 2008;9:1044–1049. doi: 10.1021/bm701225a. [DOI] [PubMed] [Google Scholar]
- 28.Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-Shaped, Nanofiber Scaffold-Based Cartilage Engineering Using Human Mesenchymal Stem Cells and Bioreactor. J Surg Res. 2008 doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A. 2008;14:639–648. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva MA, Crawford A, Mundy J, Martins A, Araujo JV, Hatton PV, et al. Evaluation of Extracellular Matrix Formation in Polycaprolactone and Starch-Compounded Polycaprolactone Nanofiber Meshes When Seeded with Bovine Articular Chondrocytes. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0327. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 32.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 35.O’Driscoll SW, Commisso C, Fitzsimmons JS. Type II collagen quantification in experimental chondrogenesis. Osteoarthritis Cartilage. 1995;3:197–203. doi: 10.1016/s1063-4584(05)80054-8. [DOI] [PubMed] [Google Scholar]
- 36.O’Driscoll SW, Marx RG, Fitzsimmons JS, Beaton DE. Method for automated cartilage histomorphometry. Tissue Eng. 1999;5:13–23. doi: 10.1089/ten.1999.5.13. [DOI] [PubMed] [Google Scholar]
- 37.O’Driscoll SW, Marx RG, Beaton DE, Miura Y, Gallay SH, Fitzsimmons JS. Validation of a simple histological-histochemical cartilage scoring system. Tissue Eng. 2001;7:313–320. doi: 10.1089/10763270152044170. [DOI] [PubMed] [Google Scholar]
- 38.O’Driscoll SW, Salter RB, Keeley FW. A method for quantitative analysis of ratios of types I and II collagen in small samples of articular cartilage. Anal Biochem. 1985;145:277–285. doi: 10.1016/0003-2697(85)90362-8. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 40.Reinholz GG, Fitzsimmons JS, Casper ME, Ruesink TJ, Chung HW, Schagemann JC, et al. Rejuvenation of periosteal chondrogenesis using local growth factor injection. Osteoarthritis Cartilage. 2009;17:723–734. doi: 10.1016/j.joca.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon TM, Van Sickle DC, Kunishima DH, Jackson DW. Cambium cell stimulation from surgical release of the periosteum. J Orthop Res. 2003;21:470–480. doi: 10.1016/S0736-0266(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 42.Mehlhorn AT, Schmal H, Kaiser S, Lepski G, Finkenzeller G, Stark GB, et al. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393–1403. doi: 10.1089/ten.2006.12.1393. [DOI] [PubMed] [Google Scholar]
- 43.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. Embo J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwasaki M, Nakahara H, Nakata K, Nakase T, Kimura T, Ono K. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-β and basic fibroblast growth factor. J Bone Joint Surg. 1995;77A:543–554. doi: 10.2106/00004623-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Jansen EJ, Emans PJ, Guldemond NA, van Rhijn LW, Welting TJ, Bulstra SK, et al. Human periosteum-derived cells from elderly patients as a source for cartilage tissue engineering? J Tissue Eng Regen Med. 2008;2:331–339. doi: 10.1002/term.100. [DOI] [PubMed] [Google Scholar]
- 46.De Bari C, Dell’Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]