Abstract
The monkey's ability to learn a set of visual discriminations presented concurrently just once a day on successive days (24-hr ITI task) is based on habit formation, which is known to rely on a visuo-striatal circuit and to be independent of visuo-rhinal circuits that support one-trial memory. Consistent with this dissociation, we recently reported that performance on the 24-hr ITI task is impaired by a striatal-function blocking agent, the dopaminergic antagonist haloperidol, and not by a rhinal-function blocking agent, the muscarinic cholinergic antagonist scopolamine. In the present study, monkeys were trained on a short-ITI form of concurrent visual discrimination learning, one in which a set of stimulus pairs is repeated not only across daily sessions but also several times within each session (in this case, at about 4-min ITIs). Asymptotic discrimination learning rates in the non-drug condition were reduced by half, from ~11 trials/pair on the 24-hr ITI task to ~5 trials/pair on the 4-min ITI task, and this faster learning was impaired by systemic injections of either haloperidol or scopolamine. The results suggest that in the version of concurrent discrimination learning used here, the short ITIs within a session recruit both visuo-rhinal and visuo-striatal circuits, and that the final performance level is driven by both cognitive memory and habit formation working in concert.
Keywords: concurrent visual discrimination learning, haloperidol, scopolamine, visuo-striatal circuit, visuo-limbic circuit, monkey
INTRODUCTION
From the early to the mid 1900s, efforts of experimental psychologists to develop a unified approach to their discipline's subject matter generated instead the competing camps of behaviorists and cognitivists. Their major debate centered on the issue of learning - what was learned and what laws were followed (e.g. Hull, 1943; Tolman, 1932). Were the products stimulus-response connections or stimulus-stimulus associations? Was reinforcement necessary or was observation sufficient? Was the process one of trial-and-error or all-at-once insightful? The contrasts were sharp and plentiful (see Table 1), and yet, inexplicably to each side, the other side would not surrender. Then, in the 1950s, the story of H.M. began to unfold. First came the description of his tragic amnesia (Scoville & Milner, 1957); but soon after came the discovery of his remarkable ability to acquire new motor skills (Milner, 1962; Corkin, 1968). The relevance of H.M.'s selective memory disorder to the ongoing debate on learning theory was not widely appreciated at the time, but it soon would be, for his dramatic case motivated researchers to try to produce an animal model of his condition. By the 1980s these attempts had led to the identification of two parallel cerebral learning systems (Mishkin et al., 1984; Packard et al., 1989): One, cognitive and limbic-based, destroyed in H.M.; the other, behavioral and basal ganglia-based, which had apparently escaped intact. A valuable legacy of the prolonged debate, and of H.M., is the survival of both theoretical positions in psychology's conjunctive subtitle, behavioral and cognitive science.
Table 1.
Comparison of Behavioral and Cognitive Learning Systems
| Behavioral | Cognitive | |
|---|---|---|
| Internal state: | homeostatic drive | motive |
| Input: | stimulus element | stimulus configuration |
| Output: | response | act |
| Outcome: | homeostasis | information |
| Product: | habit | memory |
| Product formed by: | reinforcement | observation |
| Formation rate: | slow | fast |
| Product degraded by: | extinction | forgetting |
| Degradation rate: | slow | fast |
| Cerebral substrates: | cortico-basal ganglia | cortico-limbic |
| Major neuromodulator: | dopamine | acetylcholine |
Some of the properties that differentiate the two learning systems (adaped from Mishkin and Petri, 1984).
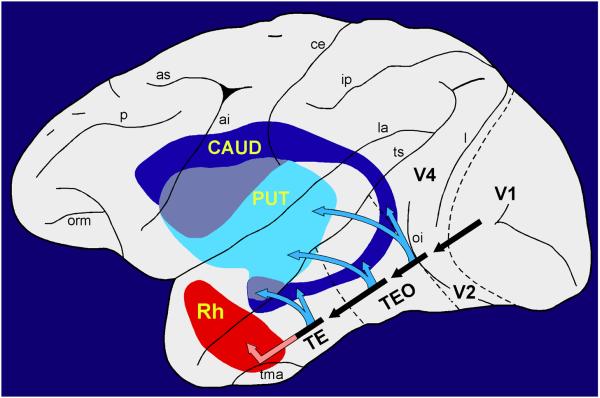
The initial segment of each of the two cerebral systems for visual learning is illustrated in Figure 1. The segment belonging to the cortico-basal ganglia system is the visuo-neostriatal pathway, which mediates visual habit formation, i.e. the incremental acquisition through reinforcement of stimulus-response connections; the segment belonging to the cortico-limbic system is the visuo-rhinal pathway, which supports visual recognition, enabling one-trial stimulus memory or stimulus-stimulus association often based on observation alone (Table 1). These two disparate learning systems are often competitive and mutually interfering (e.g. Poldrack & Packard, 2003; Foerde et al., 2006; Wingard & Packard, 2008), but this is unlikely to be their only form of interaction. In the present study we describe results suggesting that the two systems can also work together in a complementary way to achieve more rapid learning than either system can accomplish on its own.
Figure 1.
Nearly the entire length of the ventral visual stream (black arrows) projects to the ventrocaudal neostriatum, including both the tail of the caudate nucleus (CAUD) and the ventrocaudal part of the putamen (PUT), which thus receive relatively unprocessed input from early visual areas (e.g. V2) as well as highly processed input from the final station in the pathway (area TE). By contrast, the entorhinal/perirhinal or rhinal (Rh) cortex receives directly only highly processed visual input. The direct visuo-striatal connections (Saint-Cyr et al., 1990) form part of the cortico-basal ganglia learning system, whereas the visuo-rhinal connections (Van Hoesen and Pandya, 1975; Suzuki and Amaral, 1994; Saleem et al., 2007) form part of the cortico-limbic learning system. For simplicity, the cortico-striatal connections are shown projecting point-to-point, whereas in fact the terminal fields in the striatum often form longitudinal strips and, hence, can overlap densely. Abbreviations of sulci: ai, inferior arcuate; as, superior arcuate; ce, central; ip, intraparietal; l, lunate; la, lateral; oi, inferior occipital; orm, medial orbital; p, principal; tma, anterior middle temporal; ts, superior temporal.
A recent psychopharmacological study (Turchi et al., 2008) found that systemic blockade of cholinergic muscarinic receptors impaired one-trial visual recognition, a form of cognitive memory, but had little or no effect on concurrent visual discrimination learning, a type of habit formation. Conversely, systemic blockade of dopamine receptors impaired discrimination learning, but had no effect on recognition memory. The two tasks that yielded this pharmacologically induced, double dissociation of deficits differed in several ways, but one variable that might have been as important as the type of learning and memory tested was the length of the retention interval. In the recognition task (delayed nonmatching-to-sample with list lengths of 20 trial-unique stimuli), the interval between sample presentation and choice test involving that particular sample lasted only about 10 minutes. By contrast, in the discrimination task (concurrent discrimination learning with 20 stimulus pairs), the interval between successive trials on a given pair lasted 24 hours.
To investigate the potential role of this large retention-interval difference in yielding the double-dissociation of deficits on the two types of learning and memory, we exploited a more commonly used form of concurrent discrimination learning (e.g. Correll & Scoville, 1965; Mishkin, 1972; Teng et al., 2000), one in which a set of discrimination problems is presented several times within a session as well as across sessions, such that the retention interval between successive trials on the same pair within a session lasts only a few minutes. In the current study, the retention interval lasted about 4 min. We used this form of the test to determine whether the effects of systemic receptor blockade would be similar to those observed previously in the concurrent discrimination task with 24-hr retention intervals (viz. impairment induced by haloperidol but not by scopolamine), or whether they would be similar instead to those found in the recognition task with relatively short retention intervals (impairment induced by scopolamine but not by haloperidol). As described below, unlike the effects observed on either of the tasks used previously, blockade of both types of receptors produced significant impairment, suggesting that performance on the current task recruited both learning processes.
METHODS
Subjects
The subjects were three experimentally naïve monkeys (Macaca mulatta; 2 males, 1 female), ranging in weight from 3.7 to 4.8 kg. They were housed individually or in established pairs and fed a diet of primate chow (No. 5038, PMI Feeds, St. Louis, MO) supplemented with fruit; water was available ad lib. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (ILAR, NRC, 1996), the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985), and under an ACUC approved NIMH Animal Study Proposal.
Test apparatus
Testing was conducted in a sound attenuating operant chamber equipped with a 15-inch touch screen monitor (Microtouch, 3M Center, St. Paul, MN) programmed by LabView software (http://www.ni.com/labview/). The monkeys performed the task while seated in front of the monitor in a transport chair constructed to allow free arm movements. Correct responses were rewarded with pellets (50:50 mixture of banana and fruit punch flavors, Research Diets, 190 mg each) dispensed into a metal cup located just below the monitor.
Behavioral training
The animals were habituated to the transport chair and operant chamber, given several sessions of noncontingent access to food in the reward cup, and then shaped to touch colored squares on the monitor in order to obtain the food pellets. After this preliminary phase, which generally required one week, the animals began training on the concurrent visual discrimination task with repetition of the pairs within sessions (i.e. with relatively short delays between the trials on a given pair) as well as repetition across sessions.
In each daily test session, ten pairs of visual stimuli selected randomly from a library of 1,600 clip art pictures were presented in succession at 20-sec intervals, with one member of each pair arbitrarily designated as the positive stimulus, i.e. the one that led to delivery of food reward when touched. Each stimulus was displayed on the monitor at a size of 10 × 10 cm, and the members of each pair were presented simultaneously, spaced 10 cm apart. The same 10 pairs were presented five times in succession (a block of 5 trials/pair), always in the same order, and with the positive and negative stimuli in each pair remaining the same throughout; only the left-right position of the members within a pair was varied pseudorandomly across the presentations of the set. Touching either stimulus extinguished both, and there was no correction for errors. The minimum interval between presentations of the same pair within the 5-trial/pair-block was 3.3 min (20 s ITIs × 10), with the actual interval depending on the animal's response latencies (see below). In each daily session, two such blocks were presented successively, separated by a 5-min interval.
Testing continued on subsequent days with the same set of 10 stimulus pairs until the animal achieved the criterion of 90 percent correct responses in five consecutive repetitions of the set, either within or across the 5-trial blocks and/or test sessions. The three animals were then trained on a large series of additional sets of 10 concurrent visual discriminations, each composed of entirely new stimuli, to ensure that they had reached a stable learning rate. When criterion was attained on one set, testing was discontinued for that day and a new set introduced only on the next day.
Systemic injections
After reaching their stable learning rate (see Results), the animals' rate of learning additional sets was then examined after administration of one of the pharmacological agents on the first day only of testing a new stimulus set. Subsequent daily test sessions with that particular set, if needed, were given without the drug. Also, to ensure that the sets were learned reliably despite the one day of pharmacological interference, a more stringent criterion was introduced during drug (including vehicle-control) testing, with the animals now being required to maintain an average of 90 percent correct responses for two successive presentations of the set beyond the original criterion.
For three days prior to the start of drug testing, animals were habituated while off task to daily intramuscular injections of sterile saline (pH 7.4, 0.1 ml/kg). Thereafter, on the first day in which each new discrimination set was presented for acquisition, the animals received an injection either of scopolamine HBr (Sigma-Aldrich), a nonselective muscarinic cholinergic receptor antagonist, or of haloperidol (Ortho-McNeil), a nonselective dopaminergic antagonist. Each of these agents was administered in a dose of either 10.0 or 17.8 μg/kg (scopolamine doses were based on the salt form of the drug), the same doses as those used in the previous study. Each compound was dissolved in sterile saline and injected intramuscularly in a volume of 0.1 ml/kg, 20 minutes prior to task onset, and each dose of each drug was tested twice. In addition, the animals were tested on two sets of discriminations after receiving systemic injections of the saline vehicle, one before and one at or near the end of each injection series. The order of injections in the haloperidol (H) series was: saline, H10.0, H17.8, H10.0, saline, and H17.8. This was followed by the series of scopolamine (S) injections, in the order: saline, S10.0, S17.8, S17.8, S10.0, saline. Successive drug injections were separated by a minimum interval of three noninjection days.
RESULTS
By the end of pre-injection training on a total of 29 sets, the three animals had achieved an asymptotic learning rate that averaged about one block of 5 presentations per set, or 5 trials/pair, to achieve the criterion of 90 percent correct responses across the next block of 5 presentations. In short, the animals were now learning new sets of concurrent discriminations and performing the criterion runs at the rate of one 10-pair set per day. During this period of performance (sets 24-29), the animals' mean response latency was 3.7 sec, which, when added to the 20-sec interstimulus interval, resulted in a retention interval of about 4 min between successive trials on a given pair within a block. (As indicated above, there was an additional 5-min rest period between the two blocks of five presentations of the set in a daily session, leading to an interval of about 9 minutes between the 5th and 6th presentations of a given pair.)
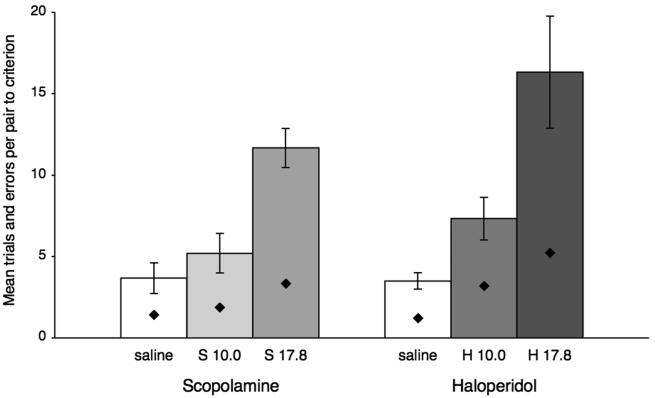
The effects of each pharmacological agent were evaluated with one-way analyses of variance (ANOVAs) that compared the number of trials/pair preceding criterion across the three conditions (saline and the two drug doses). This was followed by post-hoc comparisons between the effects of saline and of each drug dose separately with Tukey's least significant difference tests (LSDs). The results are illustrated in Figure 2. The systemic injections of both doses of haloperidol had a significant effect on learning rate: F(2,10) = 10.50, p = 0.003; H17.8 vs. saline, p = 0.019; H10.0 vs. saline, p = 0.039; H17.8 vs. H10.0, p = 0.029 [trials/pair to criterion, means (and SEs): saline, 4 (±1); H10.0, 7 (±1); H17.8, 16 (±3)]. And the systemic injections of the higher dose of scopolamine (but not the lower one) also had a significant effect: F(2,10) = 21.28, p = 0.000; S17.8 vs. saline, p = 0.027; S10.0 vs. saline, p = 0.40 ns; S17.8 vs. S10.0, p = 0.29 ns [trials/pair to criterion, means (and SEs): saline, 4 (±1); S10.0, 5 (±1); S17.8, 12 (±1)].
Figure 2.
Scores are mean trials per pair (±SE) and mean errors per pair (symbols within bars) preceding criterion on the two concurrent discrimination sets presented under saline and each of the two drug doses (in μg/kg), one ascending and one descending. H, haloperidol; S, scopolamine.
The same pattern of results was obtained with ANOVAs followed by LSDs when errors/pair to criterion instead of trials/pair to criterion were examined. Both doses of haloperidol and the higher dose of scopolamine increased error rates significantly (for haloperidol: F(2,10) = 7.893, p = 0.009; H17.8 vs. saline, p = 0.022; H10.0 vs. saline, p = 0.003; H17.8 vs. H10.0, p = 0.147; [errors/pair to criterion, means (and SEs): saline, 1.23 (±0.25); H10.0, 3.20 (±0.42); H17.8, 5.23 (±1.22)]). And for scopolamine: F(2,10) = 10.155, p = 0.004; S17.8 vs. saline, p = .007; S10.0 vs. saline, p = 0.348 ns; S17.8 vs. S10, p = 0.024; [errors/pair to criterion, means (and SEs): saline, 1.42 (±0.34); S10.0, 1.88 (±0.34); S17.8, 3.35 (±0.41)]).
Finally, the same results as those just described both for trials/pair to criterion and for errors/pair to criterion were obtained with the nonparametric Friedman test followed by two-tailed Wilcoxon signed rank tests. This was the case because none of the three animals' scores on either dose of haloperidol or on the higher dose of scopolamine overlapped with their saline scores (p < 0.05 in all cases).
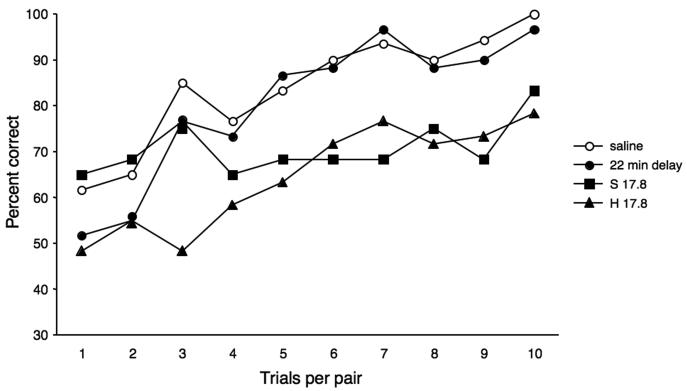
One possible explanation of the effects of haloperidol on our concurrent discrimination learning task is that the animals' response latencies were seriously slowed - from a mean of less than 4 s under saline conditions to means (and SEs) of 23 (±11) and 111 (±25) s under the low and high haloperidol doses, respectively. As a result, the intervals between successive trials on a given pair of discriminanda rose under the two haloperidol doses from a mean of < 4 min to means of 7 (± 2) and 22 (± 4) min, respectively. Administration of scopolamine, on the other hand, led to only modest increases in response latencies: means of 4 s (±1) and 7 s (±2) following S10.0 and 17.8 μg/kg, respectively. To determine whether the haloperidol-induced impairment could have been due to the increase in ITIs alone, we retested the animals without drugs on two new sets in which the interval between successive trials on a given stimulus pair was extended to match the 22-min intervals that were observed under the higher dose (17.8 μg/kg) of haloperidol. Despite these greatly increased ITIs, the animals learned the new sets about as rapidly as they had learned new sets under the control conditions, i.e. in an average of 5 trials/pair (Fig. 3), a value that did not differ significantly (p = 0.115) from their mean of 4 trials/pair when the ITIs were only about 4 minutes. (Interestingly, despite the slowed reaction time caused by its systemic administration, haloperidol also had no effect on accuracy of one-trial recognition memory in the previous study [Turchi et al., 2008]).
Figure 3.
Mean percent correct per trial on the single sessions under saline (total of 12 sets, i.e. 3 sets/animal), the nondrug condition with 22-min retention intervals (total of 6 sets), and the higher dose of haloperidol (H 17.8 μg/kg; total of 6 sets) and of scopolamine (S 17.8 μg/kg; total of 6 sets). H, haloperidol; S, scopolamine.
A possible ancillary explanation for the haloperidol-induced impairment is that the higher dose, in particular, may have prolonged the retardation in learning through its residual effect on motor function beyond the day of injection. This was reflected in an average response latency of 11 (±3) s on the post-drug day as compared with < 4 s on saline and post-saline days, resulting in retention intervals of 5+ minutes between trials on a particular pair. To address this possibility of residual drug effects, we administered a single, high dose of haloperidol (17.8 μg/kg) the day before presentation of a new 10-pair set. This second control procedure likewise failed to affect the rate of learning new sets (i.e. criterion was still attained within an average of 5 trials/pair), despite the persistence of mild motor difficulties beyond the day of injection. (Such residual effects on response latency followed only the higher dose of haloperidol; mean response latency on the day after the lower dose (10.0 μg/kg) remained about the same as those on post-saline injection days.) The two control assays in combination suggest that the learning deficits we observed were not due to the haloperidol-induced bradykinesia either on the day of or on the day after the injection.
Similarly, it is unlikely that the discrimination learning deficit produced by systemic administration of scopolamine was due to nontargeted drug effects, inasmuch as the same doses of systemic scopolamine as those used here failed to produce significant impairment on the 24-hr ITI task in the earlier study (Turchi et al., 2008).
DISCUSSION
Cross-study comparison of drug effects
Unlike concurrent visual discrimination learning at retention intervals of 24 hours, which was impaired only by systemic haloperidol (Turchi et al., 2008), and also unlike one-trial visual recognition at retention intervals of just a few minutes, which was impaired only by systemic scopolamine (Turchi et al., 2008), concurrent visual discrimination learning at within-session retention intervals of just a few minutes was impaired by both of these drugs. Restated, the task in the current study combined the type of learning previously impaired only by haloperidol with retention intervals previously impaired only by scopolamine, with the result that both pharmacological agents now interfered with learning.
Cross-study comparison of drug-dose effects suggests that the low dose of each drug (10.0 μg/kg) was less effective in the present study than in the previous one. On each task in the earlier report, the low as well as the high dose of the critical drug (haloperidol in the case of the 24-hr ITI task, and scopolamine in the case of the one-trial recognition task) produced marked deficits (see Fig. 1 in Turchi et al., 2008). In the present study, however, only the high dose (17.8 μg/kg) of each drug produced a marked deficit, with the low dose of haloperidol leading instead to a significant but relatively mild deficit that was significantly smaller than that produced by the high dose, and with the low dose of scopolamine causing no significant deficit at all (see Fig. 2). The results are consistent with the proposal that, whereas one-trial recognition memory is largely cholinergic-dependent, and concurrent discrimination learning with 24-hr ITIs is chiefly dependent on dopamine, within-session concurrent discrimination learning utilizes both neuromodulators and so is less sensitive than the other tasks to low doses of either of the two neuromodulator-receptor blockers.
Neuromodulatory circuitry
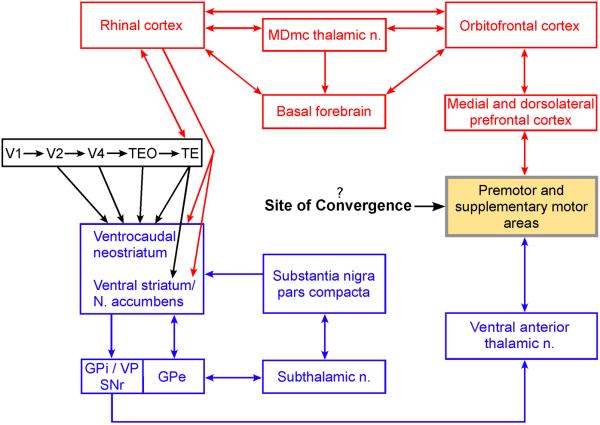
Before considering the implications of the present findings, we need to review again what is currently known about the neurobiological underpinnings of each of the tasks used in the two pharmacological studies. One-trial visual recognition at retention intervals of a few minutes, a form of cognitive memory, is mediated by a circuit that includes projections from rostral parts of the ventral visual stream to the perirhinal and entorhinal (or rhinal) cortices (Fig. 1; Mishkin et al., 1984; Mishkin and Phillips, 1990; Meunier et al., 1993). Further, performance on this task depends on cholinergic modulation of those same visuo-rhinal connections (Tang et al., 1997, Turchi et al., 2005), and also presumably of the other connections in the extended cortico-limbic circuit illustrated in Figure 4, suggesting that the differential effects of systemic scopolamine versus haloperidol in the earlier study that compared the two effects (Turchi et al., 2008) were probably likewise due to blockade of the cholinergic modulation of those cortico-limbic synapses. In contrast to one-trial recognition at delays of a few minutes, multi-trial concurrent discrimination learning at retention intervals of 24 hours, a form of habit formation, is mediated by a circuit that includes projections from the ventral visual stream to the ventrocaudal neostriatum (Fig. 1; Mishkin et al., 1984; Malamut et al., 1984; Phillips et al., 1988; Saint-Cyr et al., 1990; Fernandez-Ruiz et al., 2001) and from there to the other components of the cortico-basal ganglia circuit, also illustrated in Figure 4. Although there is as yet no direct intracerebral evidence regarding the role of neuromodulation in concurrent discrimination learning, the differential effects of systemic haloperidol versus scopolamine in the previous investigation (Turchi et al., 2008) suggest that these effects may well have been due to blockade of an essential dopaminergic modulation of the cortico-basal ganglia connections that mediate this ability.
Figure 4.
The neural underpinnings of the behavioral and cognitive learning systems in vision, illustrating information flow through some of the major components of each circuit. The ventral visual stream (in black) projects to both the ventrocaudal neostriatum and the rhinal cortices (see also Fig. 1), which constitute the initial components of the cortico-basal ganglia system (in blue; Alexander et al., 1986; Parent & Hazrati, 1995) and the cortico-limbic system (in red; Mesulam & Mufson, 1984; Mesulam et al., 1986; Kondo et al., 2005; Aggleton & Mishkin, 1983; Meunier et al., 1997), respectively. The principal neuromodulator in the cortico-basal ganglia system is dopamine, which is synthesized by neurons in the substantia nigra, pars compacta; and a principal neuromodulator in the cortico-limbic system is acetylcholine, synthesized by neurons in the basal forebrain, which projects not only to structures within this system but also to the entire cortical mantle including the ventral visual stream. The role of the direct projections from the rhinal cortex to the striatum is unknown, but one possibility is that they provide the visuo-striatal circuit with the products of stimulus configural learning (see Discussion). Major output targets of the basal ganglia are the premotor and supplementary motor areas of the frontal cortex. Because these motor-related areas are also targeted by the cortico-limbic system via the medial and dorsolateral prefrontal cortex, they could be the site of integration and collaboration between the two learning systems (see Discussion). Abbreviations: GPe, external segment of the globus pallidus; GPi, internal segment of the globus pallidus; MDmc, magnocellular portion of the medial dorsal nucleus; N., nucleus; SNr, reticulata portion of substantia nigra; VP, ventral pallidum.
As for the 4-min ITI task employed in the present study, two reports in the literature suggest that, unlike either of the tasks described above, this one recruits both the visuo-rhinal and visuo-striatal circuits. In one of these studies, Buckley and Gaffan (1997) found impairment after bilateral perirhinal lesions on a concurrent discrimination task in which a set of 40 pairs of visual stimuli (ASCII characters) was presented three times in succession each day, with retention intervals of about 7.5 min. In the other study, Teng and colleagues (2000) found an impairment after lesions of the tail of the caudate nucleus on a concurrent discrimination task in which eight pairs of objects were each presented five times per session, randomly intermingled with the other pairs, at a mean retention interval of about 2.5 min. (Interestingly, the observation in the Teng study was serendipitous, in that the aim of the experiment was to examine the effects on discrimination learning of selective hippocampal damage, but impairment was noted only when there was adventitious bilateral damage to the tail of the caudate nucleus.) The results of these two lesion studies, taken together with our pharmacological findings, suggests that within-session learning of concurrent visual discriminations normally depends on both cholinergic modulation of visuo-rhinal connections and dopaminergic modulation of visuo-striatal connections. If so, then such discriminations may be acquired not only as stimulus-response habits by the behavioral learning system but also as stimulus-reward associative memories (a form of stimulus-stimulus association) by the cognitive learning system (see Table 1). This contribution from the visuo-rhinal circuit, precluded by the 24-hr retention intervals in the earlier study (Turchi et al., 2008), was apparently enabled in the present study by the relatively short retention intervals.
Collaboration between the circuits
One benefit of the proposed cooperation between the two very different circuits could well be the fast discrimination learning that was observed. Normal animals performing the 24-hr ITI task, given pretraining on 17 successive, 20-pair sets of concurrent discriminations, reached an asymptotic learning rate of 11 trials/pair (Turchi et al., 2008). By comparison, after equally extensive pretraining on the present 10-pair version with retention intervals of a few minutes, the normal animals' asymptotic rate improved substantially to just 5 trials/pair. This more rapid acquisition can not have been due simply to some benefit afforded the visuo-striatal circuit by the reduced retention interval, or by the reduced set size, or even by both together, for when the contribution of the visuo-rhinal circuit in the present study was blocked by the high dose of scopolamine, the learning rate slowed to 12 trials/pair, about the same as that in the 20-pair, 24-hr ITI task, despite the reduced retention interval and set size. These results add support to the notion that within-session learning of concurrent visual discriminations adaptively recruits both the visuo-neostriatal and the visuo-rhinal circuits.
Although the two circuits have many connections, a few of which are illustrated in Figure 4, it is uncertain how these might operate to yield faster concurrent discrimination learning than either circuit can yield alone. For example, the rhinal cortex itself projects directly both to the ventrocaudal neostriatum in the medial temporal lobe (Saint-Cyr et al., 1990) and to the ventral striatum/nucleus accumbens in the orbitofrontal region (Kondo et al., 2005). These initial links between the two systems must mediate some form of systems interaction; yet, it is unlikely that they could speed acquisition by completely integrating the dopaminergic- and cholinergic-dependent learning circuits at such an early stage, because the encoding and storage mechanism supporting these two disparate circuits would seem to be incompatible and so require isolation from each other until their separate products are formed following information transmission through the entire circuit. A more likely possibility therefore – one that is consistent with the notion that integration of the two circuits be delayed until their separate products are formed – is that their outputs converge closer to the final common motor path, e.g. in one or more premotor areas, as proposed in Figure 4. Delaying their convergence until this late stage would seem to offer the best possibility that habit strength and memory strength could summate trial-by-trial and thereby speed mastery of the discriminations to a high criterion.
As for the earlier links between the two circuits, a plausible form of systems interaction enabled by these direct rhinal-striatal connections is an asymmetrical one in which, under special conditions, rhinal-based stimulus learning would be recruited to assist striatal-based habits. Such habit-formation assistance by the rhinal cortex may help explain why animals with rhinal lesions were impaired in concurrent discrimination learning with 24-hr ITIs when stimulus differentiation was made particularly difficult by the introduction of either a very large number of stimulus foils or stimulus pairs (Buckley & Gaffan, 1997), or of stimuli whose orientations were varied from trial to trial (Buckley & Gaffan, 1998). Under those special conditions, stimulus-response learning may have been aided by the rapid perceptual learning afforded by the rhinal cortex, e.g. through the binding of stimulus elements into a configural representation (see Table 1), which would then have formed an important part of the stimulus input to the visuo-striatal circuit. However, when there was no such special demand on stimulus differentiation, which was the case in most of the studies that utilized the 24-hr ITI task, rhinal damage had little or no effect (Malamut et al., 1984; Overman et al., 1990; Gaffan & Murray, 1992; Eacott et al., 1994; Buckley & Gaffan, 1997). It therefore seems unlikely that the impairment caused by pharmacological blockade of the visuo-rhinal connections under the same undemanding stimulus conditions in the present study was due to the loss of an unneeded stimulus-learning contribution to the visuo-striatal circuit. Rather, as proposed above, it may have been due instead to the loss of a totally independent contribution from the complete visuo-limbic circuit, one that normally results in a stimulus-reward (or stimulus-stimulus) associative memory.
The proposal that cognitive memory and habit formation can sometimes work in concert points to the need for research on the conditions under which visuo-limbic and visuo-striatal circuits not only operate singly, or even sometimes compete and interfere with each other, but also those circumstances under which they may collaborate to improve learning. Among the questions that need to be addressed are: What are the outer limits of the task parameters, including types of learning and durations of the retention interval, within which both circuits can contribute; and, within these limits, what degree of impairment in learning is produced by combined lesions or combined pharmacological blockade of both systems. Although the systemic pharmacological methods used in the present study proved to be a valuable first step, future investigations of these issues will clearly need to include intracerebral interventions with, among others, infusions of pharmacological agents directly into the various components of the two different learning circuits to test the proposals advanced here.
Acknowledgments
The authors thank George Dold for valuable assistance with computer programming and Kadharbatcha Saleem for expert neuroanatomical advice. This work was supported by the Intramural Research Program of NIMH/NIH/DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement
The authors declare they have no competing financial interests.
References
- Aggleton JP, Mishkin M. Memory impairments following restricted medial thalamic lesions in monkeys. Exp Brain Res. 1983;52:199–209. doi: 10.1007/BF00236628. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111:467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Learning and transfer of object-reward associations and the role of the perirhinal cortex. Behav Neurosci. 1998;112:15–23. doi: 10.1037//0735-7044.112.1.15. [DOI] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–265. [Google Scholar]
- Correll RE, Scoville WB. Effects of medial temporal lesions on visual discrimination performance. J Comp Physiol Psychol. 1965;60:175–181. doi: 10.1037/h0022290. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Euro J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wang J, Aigner TG, Mishkin M. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proc Natl Acad Sci USA. 2001;98:4196–4201. doi: 10.1073/pnas.061022098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci USA. 2006;103:11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys with rhinal cortex lesions succeed in object discrimination learning despite 24-hour intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of behavior: An introduction to behavior theory. Appleton-Century-Crofts; New York: 1943. [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Malamut B, Saunders RC, Mishkin M. Monkeys with combined amygdalo-hippocampal lesions succeed in object discrimination learning despite 24-hour intertrial intervals. Behav Neurosci. 1984;98:759–769. doi: 10.1037//0735-7044.98.5.759. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH. Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res. 1986;367:301–8. doi: 10.1016/0006-8993(86)91607-0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107:253–74. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Milner B. Les troubles de la mèmoire accompagnant des lésions hippocampiques bilatérales. In: Passouant P, editor. Physiologie de l'hippocampe. Centre National de la Recherche Scientifique; Paris: 1962. (English translation [1965] in P.M. Milner & S. Glickman [Eds.], Cognitive processes and the brain. New York: Van Nostrand.) [Google Scholar]
- Mishkin M. Cortical visual areas and their interaction. In: Karczmar AG, Eccles JC, editors. The Brain and Human Behavior. Springer-Verlag; 1972. pp. 187–208. [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J. Memories and habits: two neural systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of human learning and memory. The Guilford Press; New York: 1984. pp. 287–296. [Google Scholar]
- Mishkin M, Petri HL. Memories and habits: some implications for the analysis of learning and retention. In: Squire LR, Butters N, editors. Neuropsychology of Memory. Cambridge University Press; New York: 1984. pp. 196–210. [Google Scholar]
- Mishkin M, Phillips RR. A cortico-limbic memory path revealed through its disconnection. In: Trevarthen C, editor. Brain circuits and functions of the mind: Festschrift for Roger Wilcott Sperry. Cambridge University Press; New York: 1990. pp. 196–210. [Google Scholar]
- Overman WH, Ormsby G, Mishkin M. Picture recognition vs. picture discrimination learning in monkeys with medial temporal removals. Exp Brain Res. 1990;79:18–24. doi: 10.1007/BF00228870. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Phillips RR, Malamut BL, Bachevalier J, Mishkin M. Dissociation of the effects of inferior temporal and limbic lesions on object discrimination learning with 24-hour intertrial intervals. Behav Brain Res. 1988;27:99–107. doi: 10.1016/0166-4328(88)90035-6. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:241–244. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Price JL, Hashikawa TH. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 2007;500:973–1006. doi: 10.1002/cne.21141. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatr. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci USA. 1997;94:12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Stefanacci L, Squire LR, Zola SM. Contrasting effects on discrimination learning after hippocampal lesions and conjoint hippocampal-caudate lesions in monkeys. J Neurosci. 2000;20:3853–3863. doi: 10.1523/JNEUROSCI.20-10-03853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Purposive behavior in animals and men. Century; New York: 1932. [Google Scholar]
- Turchi J, Saunders RC, Mishkin M. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci USA. 2005;102:2158–2161. doi: 10.1073/pnas.0409708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Buffalari D, Mishkin M. Double dissociation of pharmacologically induced deficits in visual recognition and visual discrimination learning. Learn Mem. 2008;15:565–8. doi: 10.1101/lm.966208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey: I. Temporal lobe afferents. Brain Res. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 2008;193:126–131. doi: 10.1016/j.bbr.2008.05.002. [DOI] [PubMed] [Google Scholar]