Abstract
Neurologically normal observers misperceive the midpoint of horizontal lines as systematically leftward of veridical center, a phenomenon known as pseudoneglect. Pseudoneglect is attributed to a tonic asymmetry of visuospatial attention favoring left hemispace. Whereas visuospatial attention is biased toward left hemispace, some evidence suggests that audiospatial attention may possess a right hemispatial bias. If spatial attention is supramodal, then the leftward bias observed in visual line bisection should also be expressed in auditory bisection tasks. If spatial attention is modality specific then bisection errors in visual and auditory spatial judgments are potentially dissociable. Subjects performed a bisection task for spatial intervals defined by auditory stimuli, as well as a tachistoscopic visual line bisection task. Subjects showed a significant leftward bias in the visual line bisection task and a significant rightward bias in the auditory interval bisection task. Performance across both tasks was, however, significantly positively correlated. These results imply the existence of both modality specific and supramodal attentional mechanisms where visuospatial attention has a prepotent leftward vector and audiospatial attention has a prepotent rightward vector of attention. In addition, the biases of both visuospatial and audiospatial attention are correlated.
Keywords: Line Bisection, Pseudoneglect, Visuospatial Attention, Audiospatial Attention
INTRODUCTION
Hemineglect
Hemineglect refers to a deficit of attention towards stimuli located within contralesional (typically left) hemispace, defined in retinocentric, egocentric or allocentric coordinates (Bisiach, Capitani, Columbo & Spinnler, 1976; Heilman & Valenstein, 1979; Bisiach, Bulgarelli, Sterzi & Vallar, 1983; Karnath, Schenkel & Fischer, 1991; Driver, Baylis, Goodrich & Rafal, 1994; Bisiach, 1996). Left hemispatial neglect occurs most commonly after lesions to right inferior parietal or temporoparietal cortex, but may also result from lesions to frontal or cingulate cortex, or to subcortical structures (Heilman & Valenstein, 1972a; Watson, Valenstein & Heilman, 1981; Mesulam, 1981; Vallar & Perani, 1986). Line bisection tasks are commonly employed to assay asymmetries of spatial attention. Neglect patients bisect horizontal lines of moderate length significantly rightward of veridical center, as though ignoring the left-hand side of the stimulus or, alternatively, being hyperattentive to the right-hand side. Hemispatial neglect has also been reported to occur for auditory stimuli (Heilman & Valenstein, 1972b; Hugdahl, Wester & Asbjornsen, 1991).
Pseudoneglect
It is well established that visuospatial attention in neurologically normal subjects is asymmetrically distributed as well, resulting in a modest but systematic and significant leftward deviation of perceived line midpoint in line bisection tasks (Bradshaw, Nathan, Nettleton, Wilson & Pierson, 1987; McCourt & Olafson, 1997; McCourt & Jewell, 1999; Jewell & McCourt, 2000; McCourt, Garlinghouse & Slater, 2000; McCourt & Garlinghouse, 2000a;b; McCourt, 2001; McCourt, Freeman, Tahmahkera-Stevens & Chaussee, 2001; McCourt, Garlinghouse & Butler, 2001; Foxe, McCourt & Javitt, 2003; McCourt, Garlinghouse & Reuter-Lorenz, 2005; McCourt, Shpaner, Javitt & Foxe, 2008; Leone & McCourt, 2010), a left hemifield bias in perceived luminance in the greyscales task (Nicholls, Bradshaw & Mattingley, 1999; Nicholls & Roberts, 2002), a left hemispatial bias in perceived stimulus size (Nicholls, Bradshaw & Mattingley, 1999; Charles, Sahraie & McGeorge, 2007) and numerosity (Luh, Rueckert & Levy, 1991; Nicholls, Bradshaw & Mattingley, 1999), and a left hemifield advantage in the processing of faces (Levy & Heller, 1981). This constellation of left-biased asymmetries of spatial attention is called pseudoneglect (Bowers & Heilman, 1980; Jewell & McCourt, 2000). The phenomena of neglect and pseudoneglect, as their names suggest, are theorized to be twin manifestations of a common and fundamental hemispheric asymmetry in the neural substrates of visuospatial attention (McCourt & Jewell, 1999). Supporting this idea are experiments illustrating that a variety of stimulus and task-related variables modulate the magnitude and direction of both neglect and pseudoneglect in a complimentary manner (Anderson, 1996; McCourt & Jewell, 1999).
Visual and Auditory Spatial Attention
Most research on spatial attention has focused on visual processing, but environmental space is monitored by multiple sensory modalities (Stein & Meredith, 1993), and there is a burgeoning interest in developing a comprehensive understanding of multisensory attention and perception (Calvert, Spence & Stein, 2004).
Pseudoneglect arises due to a prepotent vector of visuospatial attention deployed into left hemispace by the dominant right cerebral hemisphere. There is some evidence for a rightward asymmetry in the deployment of spatial attention within the auditory modality (Cusak, Carlyon & Robertson, 2001; Dufour, Touzalin & Candas, 2007; Corral & Escera, 2008; see however: Bisiach, Cornacchia, Sterzi & Vallar, 1984; Vallar, Guariglia, Nico & Bisiach, 1995; Kerkhoff, Artinger & Ziegler, 1999). If the leftward bias observed in visuospatial attention arises from asymmetry in a supramodal attentional system, then both visual and auditory spatial attention should be similarly biased. If, however, a bias in auditory spatial attention is found which differs from that for visuospatial attention, then this implies that auditory and visual spatial attention are governed by modality-specific processes. Using a within-subjects design we investigate the relationship between biases in visual and auditory spatial attention using visual line bisection and auditory interval bisection tasks.
METHODS
Subjects
Subjects were 33 dextral students (18 male, mean age = 22.9 years; 15 female, mean age = 23.7 years). Handedness laterality quotients were assessed using a standard instrument (Oldfield, 1971) on which a composite score of −100 denotes exclusive left-handedness, and +100 denotes exclusive right-handedness. Mean handedness laterality quotients for males and females were +77.2 and +78.0, respectively. There was no significant difference in mean age or handedness laterality score between male and female subjects [F1, 31 = 0.15, p = 0.70, and F1, 31 = 0.02, p = 0.90, respectively]. Subsequent inferential statistical tests were therefore conducted on data collapsed across subject sex.
The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. Prior to their participation in the study all subjects provided written informed consent, and all procedures were approved by the Institutional Review Board of North Dakota State University.
Stimuli
Auditory Interval Bisection (AB)
Figure 1 illustrates a schematic of the horizontal array of 27 speakers used to deliver the auditory stimuli. At a distance of 110 cm the inter-speaker separation was 1.02° of spatial angle. The spatial interval to be bisected was defined by two speakers with a spatial separation of 26.6°. This spatial interval was defined on each trial by the delivery of two complex tones (200 Hz and 400 Hz squarewaves; 65 dB SPL) of 300 msec duration. The target consisted of a complex tone (300 Hz squarewave; 65 dB SPL) which was also 300 msec in duration. On a given trial the target tone could appear at one of 13 spatial locations ranging from ±10.2° with respect to veridical interval midpoint. Ambient noise level was 45 dB SPL. Auditory calibration was performed using a sound level meter (Extech, model 407764).
Figure 1.
A schematic diagram of the horizontal array of 27 speakers used to deliver the auditory stimuli. At a distance of 110 cm the inter-speaker separation was 1.02° of spatial angle. The spatial interval to be bisected was defined by two speakers with a spatial separation of 26.6°. On a given trial the target tone could appear at one of 13 spatial locations ranging from ±10.2° with respect to veridical interval midpoint.
Visual Line Bisection (VB)
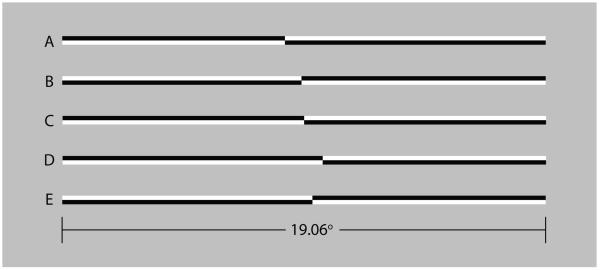
Figure 2 illustrates the stimuli used in the visual line bisection task. Horizontal lines of 100% Michelson contrast were tachistoscopically presented for 150 ms. At a viewing distance of 70 cm the lines subtended 19.06° × 0.33° of visual angle. Lines were pre-transected at 29 locations ranging from ±1.66° with respect to veridical line midpoint. Mean display luminance was 67 cd/m2. Display resolution was 640 × 480 pixels (26.52° × 19.89°), and the screen refresh rate was 60 Hz. Luminance and contrast calibration were performed using a spot photometer (Konica Minolta LS110).
Figure 2.
Examples of line stimuli used in the experiments. The members of the upper pair (A, B) are transected to the left of veridical line midpoint (by −0.75° and −0.08°, respectively). The members of the lower pair (D, E) are transected to the right of veridical center (by +0.70° and +0.33°, respectively). Line C is veridically transected. The members of line pairs (A, B) and (D, E) differ in contrast polarity. Lines of opposite polarity appeared with equal frequency and were counterbalanced within and across blocks of trials.
Procedure
Both AB and VB experiments were conducted in a single session. The order of presentation of the two tasks was counterbalanced across subjects. Subjects were seated in straight-backed chairs with their midsagittal plane aligned with the midpoint of the speaker array and the visual display. All subjects had normal or corrected-to-normal vision. Audiometric tests confirmed that all subjects had normal auditory thresholds. Subjects performed the AB task with eyes closed. In both the visual and auditory bisection tasks a programmed microcomputer sensed and collected subject responses (Presentation: Neurobehavioral Systems, Inc.).
Auditory Interval Bisection (AB)
Figure 1 illustrates the stimulus arrangement and experimental procedure used in the auditory interval bisection task. Subjects faced the array. Three tones were presented sequentially. The first two tones (t1 and t2) served to define the 26.6° spatial interval to be bisected. Following the presentation of the third (target) tone (t3), subjects judged whether the spatial location of the target tone was to the left or right of the midpoint of the spatial interval defined by the two fiducial tones. The sequence of fiducial tones was presented with an interstimulus interval (ISI) of 100 msec. The target tone was presented 300 msec after the second fiducial tone. Subsequent trials began 400 msec after subject response.
Subjects performed two blocks of trials. In one block the left fiducial tone preceded the right fiducial tone in a left-to-right sequence (ABL→R); the second block employed a right-to-left tone sequence (ABR→L). The order of presentation of the two sequences was counterbalanced across subjects. Within a block of trials the target tone (t3) could appear randomly at one of 13 different speaker locations. Subjects made fifteen bisection judgments in conjunction with each of the 13 target tone locations, such that the determination of perceived auditory interval midpoint was based on a total of 195 (13 target tone locations × 15 judgments per location) forced-choice trials. Subjects indicated decisions using the right hand to depress a left or right mouse button, as appropriate.
Visual Line Bisection (VB)
Lines were tachistoscopically presented for 150 ms; intertrial interval varied randomly, with a boxcar distribution, between 500-1000 ms following subject response. Lines of each contrast polarity appeared with equal frequency and the order of appearance of lines with different transector locations was randomized within blocks of trials. Subjects made ten bisection judgments at each of the 29 transector locations, such that the determination of perceived line midpoint was based on a total of 290 (29 transector locations × 10 judgments per location) forced-choice trials. Subjects indicated decisions using the right hand to depress a left or right mouse button, as appropriate.
Data Analysis
For both VB and AB tasks the dependent measure was the proportion of trials on which subjects judged that the visual line transector or auditory target was located to the left of the midpoint of the line (VB task) or spatial interval (AB task). Psychometric functions were derived using the method of constant stimuli. Multidimensional unconstrained nonlinear optimization (Nelder & Mead, 1965) was used to fit logistic functions to the psychometric data using maximum likelihood optimization. The logistic function is described by the equation
where x refers to the spatial location of the visual line transector or the auditory target, μ is the point of subjective equality (PSE), corresponding to the inflection point of the sigmoidal function, and σ is the standard deviation whose value is inversely proportional to discrimination precision. Line transector and auditory target locations corresponding to a 50% probability of “left” responses (μ), and corresponding standard deviations (σ) were estimated for each subject in each condition. Subsequent inferential statistical tests, including one-sample and paired sample t-tests, were conducted on these optimized values of PSE and standard deviation. The t-statistics were used to calculate estimates of effect size using the formula, d = 2t /√df (Cohen, 1988). By convention, an effect size of ±0.2 is considered to be small, a value of ±0.5 is moderate and a value of ±0.8 or greater is considered a large effect (Cohen, 1992).
RESULTS
Auditory Interval Bisection (AB) Accuracy (Bias)
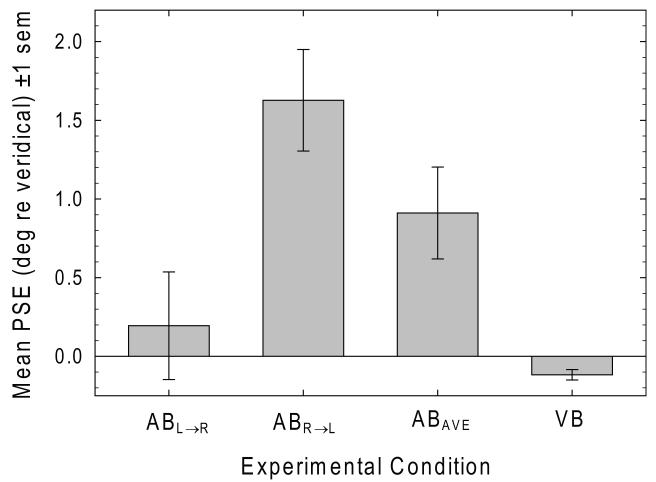
The leftmost bars of Figure 3 plot mean PSE (±1 sem) in the ABL→R and ABR→L conditions. Mean PSE was 0.195° (0.73% interval length) in the ABL→R condition and 1.627° (6.12% interval length) in the ABR→L condition. A paired-samples t-test reveals that mean PSE in the ABR→L condition is significantly rightward of the mean PSE in the ABL→R condition [t32 = 4.50, p < .001, d = 1.59], indicating a highly significant effect of directional attentional scanning. Single-sample t-tests reveal that mean bisection error was significantly rightward of veridical in the ABR→L condition [t32 = 5.04, p < .001, d = 1.78] but not in the ABL→R condition [t32 = 0.57, p = .573, d = 0.20]. The third bar in Fig. 3 plots the average bisection error in the AB condition collapsed across the two fiducial tone direction conditions (ABAVE). A single-sample t-test confirms that the average auditory bisection error (0.911°) deviates significantly rightward of veridical interval midpoint (3.42% interval length) [t32 = 3.12, p = .004, d = 1.10].
Figure 3.
Leftmost bars plot mean PSE in the ABL→R and ABR→L conditions. Mean PSE in the ABR→L condition is significantly rightward of the mean PSE in the ABL→R condition, indicating a significant effect of directional attentional scanning. Mean bisection error was significantly rightward of veridical in the ABR→L condition, but not in the ABL→R condition. The third bar plots the average bisection error in the AB condition. Average auditory bisection error deviates significantly rightward of veridical interval midpoint. The rightmost bar plots mean bisection error in the VB task, which deviates significantly leftward of veridical line midpoint.
Visual Line Bisection (VB) Accuracy (Bias)
The rightmost bar of Fig. 3 plots mean bisection error in the VB task which was −0.117° (0.61% line length). A single-sample t-test shows that visual bisection error is significantly leftward of veridical line midpoint [t32 = −3.51, p = .001, d = −1.24].
Auditory Interval versus Visual Line Bisection
Figure 4 plots VB versus ABAVE PSE for the entire sample of 33 subjects. Despite the significant difference in the direction of visual and auditory hemispatial bias, there is a significant positive correlation between bisection errors across the two sensory modalities [r32 = 0.38, p = .029].
Figure 4.
VB PSE plotted against ABAVE PSE for the entire sample of 33 subjects. Bisection error in the two tasks is significantly correlated.
Auditory Interval Bisection (AB) and Visual Line Bisection (VB) Precision
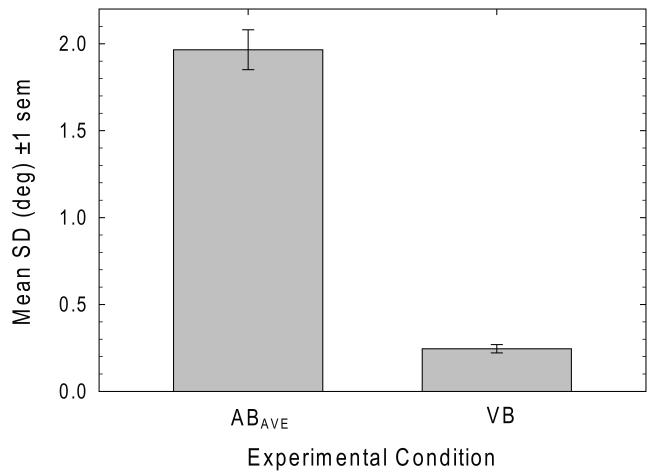
Figure 5 plots mean standard deviation (precision) values for the AB and VB tasks. There was no significant difference in mean precision between the ABL→R (1.87°) and ABR→L conditions (2.06°) [t32 = −1.60, p = .120, d = 0.57]. Average precision in the auditory interval bisection task (1.97°) is significantly poorer than in the visual line bisection task (0.25°) [t32 = 15.11, p<.001, d = 5.34].
Figure 5.
Mean standard deviations of the logistic function fits to the psychometric data AB and VB tasks. Average bisection precision in the AB task is significantly poorer than in the VB task.
DISCUSSION
Visual versus Auditory Spatial Processing
Whereas the neural basis for visual spatial localization is well understood, the neural mechanisms for sound source localization are still a subject of considerable debate (Zatorre, Bouffard, Ahad & Belin, 2002). The visual system is organized for spatial localization; it possesses numerous spatiotopically mapped low-level cortical areas (e.g., V1, V2, V3) in which the spatial location of stimuli is mapped explicitly. By contrast, auditory cortex is tonotopically mapped; sound localization depends primarily on interaural time and intensity differences, with some contribution from monaural spectral cues (Blauert, 1996). It is not known with certainty how these cues are processed by the auditory system to achieve sound localization (Richter, Schroger & Rubsamen, 2009). In addition, central auditory projections have a large ipsilateral component that is absent in the visual system, and whereas the neural networks subserving visuospatial attention are largely housed in the right hemisphere (Nobre, Sebestyen, Gitelman, Mesulam, Frackowiak & Frith, 1997; Kastner & Ungerleider, 2000), there is substantial evidence for both right and left hemisphere involvement in sound localization and audiospatial attention (Bellmann, Meuli & Clarke, 2001; Clarke et al., 2000; 2002; Zatorre, Bouffard, Ahad & Belin, 2002; Richter, Schroger & Rubsamen, 2009).
Visuospatial Attention
Our results for the visual line bisection task contribute to the growing consensus that visuospatial attention in neurologically normal subjects exhibits a small, but significant and consistent leftward bias, i.e., pseudoneglect (Bowers & Heilman, 1980; McCourt & Olafson, 1997; Nicholls, Bradshaw & Mattingley, 1999; McCourt & Jewell, 1999; Jewell & McCourt, 2000; McCourt, 2001; McCourt et al., 2005; 2008; Leone & McCourt, 2010; Dickinson & Intraub, 2009). The leftward bias of normal subjects and the profound rightward bias of neglect patients are twin manifestations of the specialization of neural networks in the right hemisphere for the deployment of visuospatial attention (Heilman & Valenstein, 1979; Mesulam, 2000; Kinsbourne, 1970; 1977; 1993; Nobre, Sebestyen, Gitelman, Mesulam, Frackowiak & Frith, 1997; Kastner & Ungerleider, 2000). The emerging consensus is that the (normally) dominant right hemisphere projects a prepotent vector of visuospatial attention into contralateral (left) hemispace, differentially increasing the salience of left hemispace in general (in egocentric coordinates), and the left-hand portions of visual stimuli such as lines (in allocentric coordinates), thereby biasing perceived midpoint leftwards (Anderson, 1996; McCourt & Jewell, 1999).
Audiospatial Attention
Our results for the auditory interval bisection task indicate that audiospatial attention in neurologically normal subjects exhibits a significant rightward bias. This finding is consistent with several previous reports. Cusak, Carlyon & Robertson (2001) manipulated the interaural time delay (ITD) of headphone-delivered noise bursts and found that six of seven control subjects perceived sounds with positive ITDs (consistent with a physical sound source located in right hemispace) as located on the midsagittal plane. Dufour, Touzalin & Candas (2007) delivered noise bursts from two speakers situated at ±30° with respect to the midsagittal plane. Subjects judged the location of the binaurally fused stimuli to be aligned with the auditory midline when the left speaker possessed a greater physical intensity, and stimuli with an interaural intensity difference of zero were perceived to be located rightward of the midsagittal plane. Corral & Escera (2008) embedded novel sounds (distracters) in a repetitive stream of auditory stimuli and presented these at various azimuthal locations relative to gaze direction while subjects were engaged in a demanding visual discrimination task. They found a significant effect of distracting sounds on visual task performance only for distracters delivered in right hemispace. Sosa, Clarke & McCourt (2009) used a tachistoscopic visual line bisection paradigm to assess whether exogenous lateral auditory cues can bias PSE and to characterize the manner in which auditory (A) and visual (V) cues combine to jointly influence PSE. They found a significant hemifield asymmetry in the weights assigned to A and V cues, where V cues were more heavily weighted in left hemispace and A cues were more heavily weighted in right hemispace.
Attentional Scanning
Our results from the auditory interval bisection task show significantly greater rightward error in the ABR→L versus ABL→R condition. This result is consistent with previous findings. In their review and meta-analytic treatment of the visual pseudoneglect literature Jewell & McCourt (2000) noted that directional scanning, as executed either overtly (e.g., occulomotor or limb scanning) or covertly (absent eye or limb movements), exerted a significant modulatory influence on perceived line midpoint such that that left-to-right scanning was associated with significantly larger leftward bisection errors than right-to-left scanning (Chokron, Bartolomeo, Perenin, Helft & Imbert, 1998; Chokron & Imbert, 1993a;b; Halligan, Manning & Marshall, 1991; Brodie & Pettigrew, 1996), which can sometime lead to rightward bisection errors. The asymmetrical effect of directional scanning on perceptual asymmetries has been studied behaviorally (McCourt & Jewell, 1999; McCourt, Garlinghouse & Slater, 2000; Nicholls & Roberts, 2002) but the neural basis for this effect is still poorly understood, particularly since covert shifts of spatial attention leftward or rightward from fixation are generally associated with increased activation of contralateral extrastriate and parietal cortical areas (Yamaguchi, Tsuchiya & Kobayashi, 1995; Kelley, Serences, Giesbrecht & Yantis, 2008). Thus, according to activation-orientation theory (Kinsbourne, 1970; 1977; 1993) the increased left hemisphere activation which accompanies a left-to-right attentional shift would predict smaller leftward bisection errors, whereas the opposite finding is observed. It is nonetheless interesting and potentially significant that directional scanning has a similar profound influence on interval midpoint estimation in both visual and auditory modalities.
Supramodal Spatial Attention
Based on findings that the severity and direction of inattention for visual and auditory stimuli is positively correlated in neglect patients (Pavani, Husain, Ladavas & Driver, 2004), and on neuroimaging studies of normal subjects which show largely coextensive regions of increased BOLD signal for shifts of spatial attention to visual and auditory cues, it has been suggested that spatial attention is supramodal (Krumbholz, Nobis, Weatheritt & Fink, 2009). To the extent that we likewise find a significant positive correlation between normal attentional biases in visual and auditory interval bisection tasks our results are consistent with this hypothesis. However, the quite different distributions of visual and auditory spatial attention are more difficult to reconcile with a supramodal mechanism.
Left Hemisphere Control of Audiospatial Attention: A Hypothesis
Five experimental/clinical findings that a model of visual and auditory spatial attention must explain in an integrated fashion are: 1) the leftward bias of normal visuospatial attention, i.e., pseudoneglect; 2) the rightward bias of normal audiospatial attention; 3) the positive correlation of these biases in normal observers; 4) the rightward bias of both visuospatial and audiospatial attention following right hemisphere damage, i.e., hemineglect; and 5) the positive correlation between errors of visuospatial and audiospatial attention in patients with hemineglect.
There is strong evidence for a lateralized neural network for visuospatial attention where the right hemisphere deploys attention into both contralateral (left) and ipsilateral (right) hemispace and the left hemisphere attends primarily to contralateral (right) hemispace (Mesulam, 1981; Anderson, 1996; Gitelman, Nobre, Parrish, LaBar, Kim, Meyer & Mesulam, 1999). Based on our present findings, as well as on cognate results from other laboratories (Cusak et al., 2001; Dufour et al., 2007; Corral & Escera, 2008), we postulate the existence of a left hemisphere based network governing audiospatial attention. Just as the right hemisphere is attentive to visual events in both contralateral and ipsilateral hemispace, there is converging evidence that sounds confined to left hemispace activate only the contralateral (right) hemisphere, whereas sounds located in right hemispace cause bihemispheric activation (Deouell, Bentin & Giard, 1998; Kaiser, Lutzenberger, Preissl, Ackermann & Birbaumer, 2000; Krumbholz, Schonwiesner, von Cramon, Rubsamen, Shah, Ziles & Fink, 2005; Krumbholz, Hewson-Stoate & Schonwiesner, 2007; Petit, Simon, Joliot, Andersson, Bertin, Zago, Mellet & Tzourio-Mazoyer, 2007; Schonwiesner, Krumbholz, Rubsamen, Fink & von Cramon, 2007; Hine & Debener, 2007). Further, just as the right hemisphere’s specialization for visuospatial attention increases the salience of visual stimuli in left hemispace and leads to tonic leftward error in tasks like visual line bisection, so the left hemisphere’s specialization for audiospatial attention causes a tonic rightward bias in auditory midline and interval judgments. If, as suggested by the activation-orientation theory (Kinsbourne, 1970; 1977; 1993) the left and right cerebral hemispheres compete for control of various functions (such as speech production or the deployment of spatial attention) via a process of mutual inhibition, then any phasic stimulation of the right hemisphere will up-regulate activity in visuospatial networks and so exacerbate the normal tonic leftward error in line bisection. Due to mutual interhemispheric inhibition the phasic stimulation of the right hemisphere will also result in the down-regulation of activity in the audiospatial attention networks of the left hemisphere. Since the strongest vector of audiospatial attention from the left hemisphere is directed towards right hemispace, the weakening of this vector will cause a leftward shift of PSE in auditory interval bisection tasks. The complimentary pattern of altered attentional biases will result from phasic stimulation of the left hemisphere (i.e., a rightward shift of both visual line bisection and auditory interval bisection). Thus, despite the different directions of the prepotent vectors of visuospatial and audiospatial attention, interhemispheric inhibition explains the correlated nature of these biases. While phasic changes of relative hemispheric activity serve to illustrate this point, it should be noted that any static imbalances in right versus left hemispheric activation (due to individual differences) will also result in correlated bisection errors across the visual and auditory modality.
In hemineglect following right hemisphere lesions the normal leftward vector of visuospatial attention from the right hemisphere is greatly weakened; the distribution of visuospatial attention which remains is dominated by the left hemisphere and is strongly right-biased (Anderson, 1996). In addition, according to activation-orientation theory, right hemisphere lesions will allow the left hemisphere to be released from inhibition. Such disinhibition increases the normally right-biased distribution of audiospatial attention. Thus, here too a correlated pattern of increased rightward errors is produced in both visual and auditory tasks. Finally, this theory makes a testable prediction: if audiospatial attention depends on left hemisphere neural networks, then lesions to the left hemisphere should cause leftward shifts in audiospatial attention, and a pattern of greater left bias in auditory bisection tasks.
CONCLUSIONS
In contrast to the significant leftward bias of visuospatial attention, a significant rightward attentional bias characterizes midpoint judgments of spatial intervals defined via the auditory modality. This dissociation implies that the neural architectures which underpin spatial attention are modality specific, and that distinct networks govern the deployment of visuospatial and audiospatial attention. This dissociation is moderated by a significant positive correlation between bisection errors across the visual and auditory modalities which is consistent with the activation-orientation theory of mutual interhemispheric inhibition.
ACKNOWLEDGMENTS
This work was supported by grants to MEM: NIH P20 RR020151 and NIH R15 EY12267. The National Center for Research Resources (NCRR) and the National Eye Institute (NEI) are components of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH, NCRR, or NEI. The authors thank Aaron Clarke for assistance with data analysis and Dan Gu for assistance with programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson B. A mathematical model of line bisection behavior in neglect. Brain. 1996;119:841–850. doi: 10.1093/brain/119.3.841. [DOI] [PubMed] [Google Scholar]
- Bellman A, Meuli R, Clarke S. Two types of auditory neglect. Brain. 2001;124:676–687. doi: 10.1093/brain/124.4.676. [DOI] [PubMed] [Google Scholar]
- Bisiach E. Unilateral neglect and the structure of space representation. Current Directions in Psychological Science. 1996;5:62–65. [Google Scholar]
- Bisiach E, Cornacchia L, Sterzi R, Vallar G. Disorders of perceived auditory lateralization after lesions of the right hemisphere. Brain. 1984;107:37–52. doi: 10.1093/brain/107.1.37. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Bulgarelli C, Sterzi R, Vallar G. Line bisection and cognitive plasticity of unilateral neglect of space. Brain and Cognition. 1983;2:32–38. doi: 10.1016/0278-2626(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Capitani E, Columbo A, Spinnler H. Halving a horizontal segment: A study on hemisphere-damaged patients with focal cerebral lesions. Archs. Suisses de Neurologie, Neurochirguire et de Psychaitre. 1976;118:199–206. [PubMed] [Google Scholar]
- Blauert J. The psychophysics of human sound localization. Revised Edition MIT Press; Cambridge, MA: 1996. [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nathan G, Nettleton NC, Wilson L, Pierson J. Why is there a left side underestimation in rod bisection? Neuropsychologia. 1987;25:735–738. doi: 10.1016/0028-3932(87)90067-4. [DOI] [PubMed] [Google Scholar]
- Brodie EE, Pettigrew LEL. Is left always right? Directional deviations in visual line bisection as a function of hand and initial scanning direction. Neuropsychologia. 1996;34:467–470. doi: 10.1016/0028-3932(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Spence C, Stein BE. The handbook of multisensory processes. MIT Press; Cambridge, MA: 2004. [Google Scholar]
- Charles J, Sahraie A, McGeorge P. Hemispatial asymmetries in judgment of stimulus size. Perception & Psychophysics. 2007;69:687–698. doi: 10.3758/bf03193771. [DOI] [PubMed] [Google Scholar]
- Chokron S, Imbert M. Egocentric reference and asymmetric perception of space. Neuropsychologia. 1993a;31:267–275. doi: 10.1016/0028-3932(93)90091-d. [DOI] [PubMed] [Google Scholar]
- Chokron S, Imbert M. Influence of reading habits on line bisection. Cognitive Brain Research. 1993b;1:219–222. doi: 10.1016/0926-6410(93)90005-p. [DOI] [PubMed] [Google Scholar]
- Chokron S, Bartolomeo P, Perenin M, Helft G, Imbert M. Scanning direction and line bisection: A study of normal subjects and unilateral neglect patients with opposite reading habits. Cognitive Brain Research. 1998;7:173–178. doi: 10.1016/s0926-6410(98)00022-6. [DOI] [PubMed] [Google Scholar]
- Clarke S, Bellmann A, Meuli RA, Assal G, Steck AJ. Auditory agnosia and auditory spatial deficits following left hemispheric lesions: Evidence for distinct processing pathways. Neuropsychologia. 2000;38:797–807. doi: 10.1016/s0028-3932(99)00141-4. [DOI] [PubMed] [Google Scholar]
- Clarke S, Thiran AB, Maeder P, Adriani M, Vernet O, Regli L, Cuisenaire O, Thiran J-P. What and where in human audition: Selective deficits following focal hemispheric lesions. Experimental Brain Research. 2002;147:8–15. doi: 10.1007/s00221-002-1203-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2nd ed Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Corral M-J, Escera C. Effects of sound location on visual task performance and electrophysiological measures of distraction. NeuroReport. 2008;19:1535–1539. doi: 10.1097/WNR.0b013e3283110416. [DOI] [PubMed] [Google Scholar]
- Cusak R, Carlyon RP, Robertson IH. Auditory midline and spatial discrimination in patients with unilateral neglect. Cortex. 2001;37:706–709. doi: 10.1016/s0010-9452(08)70620-8. [DOI] [PubMed] [Google Scholar]
- Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: Evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- Dickinson CA, Intraub H. Spatial asymmetries in viewing and remembering scenes: Consequences of an attentional bias? Attention, Perception, & Psychophysics. 2009;71:1251–1262. doi: 10.3758/APP.71.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Baylis GC, Goodrich SJ, Rafal RD. Axis-based neglect of visual shapes. Neuropsychologia. 1994;11:1353–1365. doi: 10.1016/0028-3932(94)00068-9. [DOI] [PubMed] [Google Scholar]
- Dufour A, Touzalin P, Candas V. Rightward shift of the auditory subjective straight ahead in right- and left-handed subjects. Neuropsychologia. 2007;45:47–453. doi: 10.1016/j.neuropsychologia.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Right hemisphere control of visuospatial attention: line-bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam MM. A large-scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioral and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Manning L, Marshall JC. Hemispheric activation versus spatio-motor cueing in visual neglect: A case study. Neuropsychologia. 1991;29:165–176. doi: 10.1016/0028-3932(91)90018-4. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Frontal lobe neglect in man. Neurology. 1972a;22:660–664. doi: 10.1212/wnl.22.6.660. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Auditory neglect in man. Archives of Neurology. 1972b;26:32–35. doi: 10.1001/archneur.1972.00490070050007. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Mechanisms underlying hemispatial neglect. Annals of Neurology. 1979;5:166–170. doi: 10.1002/ana.410050210. [DOI] [PubMed] [Google Scholar]
- Hine J, Debener S. Late auditory evoked potentials asymmetry revisited. Clin. Neurophysiol. 2007;118:1274–1285. doi: 10.1016/j.clinph.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Wester K, Asbjornsen A. Auditory neglect after right frontal lobe and right pulvinar thalamic lesions. Brain & Language. 1991;41:465–473. doi: 10.1016/0093-934x(91)90167-y. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Preissl H, Ackermann H, Birbaumer N. Right-hemisphere dominance for the processing of sound-source lateralization. J. Neurosci. 2000;20:6631–6639. doi: 10.1523/JNEUROSCI.20-17-06631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Schenkel P, Fischer B. Trunk orientation as the determining factor of the “contralateral” deficit in the neglect syndrome and as the physical anchor of the internal representation of body orientation in space. Brain. 1991;114:1997–2014. doi: 10.1093/brain/114.4.1997. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff G, Artinger F, Ziegler W. Contrasting spatial hearing deficits in hemianopia and spatial neglect. NeuroReport. 1999;10:3555–3560. doi: 10.1097/00001756-199911260-00017. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychologica. 1970;33:193–201. doi: 10.1016/0001-6918(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv. Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Kinsbourne M. Orientational bias model of unilateral neglect: Evidence from attentional gradients within hemispace. In: Robertson IH, Marshall JC, editors. Unilateral neglect: Clinical and experimental studies. Lawrence Erlbaum Associates; Hove, U.K.: 1993. pp. 63–86. [Google Scholar]
- Krumbholz K, Hewson-Stoate N, Schonwiesner M. Cortical response to auditory motion suggests an asymmetry in the reliance on inter-hemispheric connections between the left and right auditory corticies. J. Neurophysiol. 2007;97:1649–1655. doi: 10.1152/jn.00560.2006. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Nobis EA, Weatheritt RJ, Fink GR. Executive control of spatial attention shifts in the auditory compared to the visual modality. Human Brain Mapping. 2009;30:1457–1469. doi: 10.1002/hbm.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz K, Schonwiesner M, von Cramon DY, Rubsamen R, Shah NJ, Ziles K, Fink GR. Representation of interaural temporal information from left and right auditory space in the human planum temporale and inferior parietal lobe. Cerebral Cortex. 2005;15:317–324. doi: 10.1093/cercor/bhh133. [DOI] [PubMed] [Google Scholar]
- Leone L, McCourt ME. The effect of acute alcohol challenge on global visuospatial attention: Exaggeration of leftward bias in line bisection. Laterality: Asymmetries of Body, Brain and Cognition. 2010;15:327–342. doi: 10.1080/13576500902781745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Heller W. Perception and expression of emotion in right-handers and left-handers. Neuropsychologia. 1981;19:263–272. doi: 10.1016/0028-3932(81)90110-x. [DOI] [PubMed] [Google Scholar]
- Luh KE, Rueckert LM, Levy J. Perceptual asymmetries for free viewing of several types of chimeric stimuli. Brain and Cognition. 1991;16:83–103. doi: 10.1016/0278-2626(91)90087-o. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Freeman P, Tahmahkera-Stevens C, Chaussee M. The influence of unimanual response on pseudoneglect magnitude. Brain and Cognition. 2001;45:52–63. doi: 10.1006/brcg.2000.1255. [DOI] [PubMed] [Google Scholar]
- McCourt ME. Performance consistency of normal observers in forced-choice tachistoscopic visual line bisection. Neuropsychologia. 2001;39:1065–1076. doi: 10.1016/s0028-3932(01)00044-6. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Stimulus modulation of pseudoneglect: Effect of line geometry. Neuropsychologia. 2000a;38:520–524. doi: 10.1016/s0028-3932(99)00085-8. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Asymmetries of visuospatial attention are modulated by viewing distance and visual field elevation: Pseudoneglect in peripersonal and extrapersonal space. Cortex. 2000b;36:715–732. doi: 10.1016/s0010-9452(08)70548-3. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: Stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Olafson C. Cognitive and perceptual influences on visual line bisection: Psychophysical and chronometric analyses of pseudoneglect. Neuropsychologia. 1997;35:369–380. doi: 10.1016/s0028-3932(96)00143-1. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Butler J. The influence of viewing eye on pseudoneglect magnitude. Journal of the International Neuropsychological Society. 2001;7:391–395. doi: 10.1017/s1355617701003137. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Reuter-Lorenz PA. A common origin for the effects of unilateral cueing and line geometry in the modulation of pseudoneglect. Cortex. 2005;41:499–511. doi: 10.1016/s0010-9452(08)70190-4. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Slater J. Centripetal versus centrifugal bias in visual line bisection: Focusing attention on two hypotheses. Frontiers in Bioscience. 2000;5:d58–71. doi: 10.2741/a496. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Shpaner M, Javitt DC, Foxe JJ. Hemispheric asymmetry and callosal integration of visuospatial attention in schizophrenia: A tachistoscopic line bisection study. Schizophrenia Research. 2008;102(1-3):189–196. doi: 10.1016/j.schres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Attentional networks, confusional states, and neglect syndromes. In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. 2nd Edition Oxford University Press; Oxford: 2000. pp. 174–256. [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Nelder JA, Mead R. A simplex method for function minimization. Computer Journal. 1965;7:308–313. [Google Scholar]
- Nicholls MER, Roberts GR. Can free-viewing perceptual asymmetries be explained by scanning, pre-motor or attentional biases? Cortex. 2002;38:113–136. doi: 10.1016/s0010-9452(08)70645-2. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Bradshaw JL, Mattingley JB. Free-viewing perceptual asymmetries for the judgement of shade, numerosity and size. Neuropsychologia. 1999;37:307–314. doi: 10.1016/s0028-3932(98)00074-8. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pavani F, Husain M, Ladavas E, Driver J. Auditory deficits in visuospatial neglect patients. Cortex. 2004;40:347–365. doi: 10.1016/s0010-9452(08)70130-8. [DOI] [PubMed] [Google Scholar]
- Petit L, Simon G, Joliot M, Andersson F, Bertin T, Zago L, Mellet E, Tzourio-Mazoyer N. Right hemisphere dominance for auditory attention and its modulation by eye position: An event related fMRI study. Restor. Neurol. Neurosci. 2007;25:211–225. [PubMed] [Google Scholar]
- Richter N, Schroger E, Rubsamen R. Hemispheric specialization during discrimination of sound sources reflected by the MMN. Neuropsychologia. 2009;47:2652–2659. doi: 10.1016/j.neuropsychologia.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Schonwiesner M, Krumbholz K, Rubsamen R, Fink GR, von Cramon DY. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cerebral Cortex. 2007;17:492–499. doi: 10.1093/cercor/bhj165. [DOI] [PubMed] [Google Scholar]
- Sosa Y, Clarke A, McCourt ME. Multisensory cue integration in audiovisual spatial localization. Journal of Vision. 2009;9(8):722a. http://journalofvision.org/9/8/722/, doi:10.1167/9.8.722. [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Vallar G, Perani D. The anatomy of neglect after right hemisphere stroke lesions: A clinical CT scan correlation study in man. Neuropsychology. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Vallar G, Guariglia C, Nico D, Bisiach E. Spatial hemineglect in back space. Brain. 1995;118:467–472. doi: 10.1093/brain/118.2.467. [DOI] [PubMed] [Google Scholar]
- Watson RT, Valenstein E, Heilman KM. Thalamic neglect. Archives of Neurology. 1981;38:501–506. doi: 10.1001/archneur.1981.00510080063009. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S. Electrophysiologic correlates of visuo-spatial attention shift. Electroencephalography and Clinical Neurophysiology. 1995;94:450–461. doi: 10.1016/0013-4694(94)00315-c. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Bouffard M, Ahad P, Belin P. Where is ‘where’ in the human auditory cortex? Nature Neuroscience. 2002;5:905–909. doi: 10.1038/nn904. [DOI] [PubMed] [Google Scholar]