Abstract
BACKGROUND
Cancer stem cells (CSCs) are thought to be a critical subpopulation in tumor development, progression, metastasis and recurrence, and the identification of these cells is an initial step in understanding their role in oncogenesis and in seeking valuable markers for diagnosis or development of targeting therapeutics.
AIMS
To identify CSCs in hepatocellular carcinoma (HCC) specimens and define their tissue specificity.
METHODS
Immunohistochemical staining of CSC markers: CD44, CD90, CD133 and aldehyde dehydrogenase (ALDH) was performed in 25 HCC specimens, 4 hepatoblastomas, 8 peri-malignant tissues, and 19 cases of viral hepatitis.
RESULTS
The positivity of CD44 staining in HCC specimens was significantly lower than in viral hepatitis specimens. The positive rate of CD133 in HCC was similar to viral hepatitis specimens. CD133+ cells were largely localized to ALDH-positive cells in HCC as revealed by confocal microscopy. In contrast, the co-expression of both markers was visualized within vessels or in the portal areas in viral hepatitis. Moreover, among 7 liver specimens adjacent to HCC tissue, 3–6 samples were positive for CD44, CD90, CD133 and ALDH, especially in dysplastic cells. One of 4 hepatoblastoma cases was positive for all these markers; whereas, the other three specimens were negative for all these CSC markers.
CONCLUSIONS
In HCC and dysplastic tissues, clusters of CD133+/ALDHhigh cells were identified. The use of cancer stem cell markers to screen tissues with chronic liver diseases provides limited guidance in the identification of malignant cells.
Keywords: Aldehyde dehydrogenase, Cancer stem cells, CD44, CD90, CD133, Hepatocellular carcinoma and Hepatoblastoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most deadly and the fifth most common malignancy worldwide, with an estimation of 600,000 to 1 million new cases per year. The incidence will keep increasing in the next 2–3 decades due to the high incidence of HCV and HBV infection (El-Serag and Mason, 1999; Tanaka et al., 2002; El-Serag and Rudolph, 2007). Surgical removal and liver transplantation remain the most effective therapy for HCC. Although various novel approaches to treat non-resectable HCC are available, the efficacy and three year survival rate (30–40%) are still not promising (Lau and Lai, 2009; Tanaka and Arii, 2009). The pathogenesis of HCC is not fully delineated. The great majority of HCC occurs in the setting of cirrhosis due to chronic viral infections with hepatitis B or C, alcohol or genetic/metabolic deficiencies. The profound genetic changes identified in HCC include activation of proto-oncogenes, gene deletion or mutation, reactivation of telomerase activity, and epigenetic abnormalities (Aravalli et al., 2008). However, these changes and existing theories of oncogenesis do not explain the full picture of tumor development, progression, metastasis and recurrence.
Cancer stem cells (CSCs) are a newly identified subpopulation that possesses stem cell properties and may differentiate into heterogeneous progenies of malignant cells. They are probably the progenitor cells that undergo unknown genetic mutations, and lose potential for tissue repair, but retain stem cell characteristics, such as self renewal and plasticity to differentiate into different cell types in tumor tissues (Clarke et al., 2006; Dalerba et al., 2007). CSCs are thought to be the cells that are least sensitive to chemotherapy or radiotherapy, and develop resistance to pharmacological, biological or radiotherapeutic treatments (Park et al., 2009). These cells are probably the source for tumor metastasis and relapse. Thus, the concept of CSCs is a new paradigm of cancer biology explaining many existing basic and clinical challenges. Developing a better understanding of their pathobiology may well aid in a break-through in developing novel therapeutic strategies. CSCs have been identified in both solid malignancies (such as breast, prostate and colon cancers) and leukemia (Klonisch et al., 2008). The exploration of phenotypic, genetic, epigenomic, epigenetic or molecular abnormalities (Mishra et al., 2009), as well as the identification of molecular markers of CSCs (Klonisch et al., 2008) and the search for novel treatments of CSCs, are all areas of active investigation.
CD44 is a cell surface adhesion molecule mediating multiple signaling pathways (Fujita et al., 2002), which has been used as a CSC marker in leukemia, head or neck cancer, pancreatic, breast, and prostate cancer (Klonisch et al., 2008; Lee et al., 2008). CD133 is a cell surface glycoprotein (prominin-1) with a 97 kDa molecular size and 5 transmembrane domains. It has been used as a marker for immature hematopoietic stem cells or progenitor cells. A single CD133+ colon cancer cell formed a tumor in the kidney capsule but CD133− cells were not capable of forming tumors in immunodeficient mice (O'Brien et al., 2007). CD133+ HCC cells were chemoresistant and possessed a greater ability to form tumors in vivo (Ma et al., 2007). Aldehyde dehydrogenase (ALDH) is highly expressed in embryonic tissue as well as in adult stem cells isolated from bone marrow, cord blood, and other tissues, and has been employed to identify cancer stem cells in various tumor types (Moreb, 2008). Adult progenitor cells with high ALDH activity displayed a better tissue repopulation rate after transplantation (Zhou et al., 2009). CD90 (Thy-1) is a 25–37 kDa glysylphosphatidylinosital (GPI)-anchored glycoprotein which is expressed in bone marrow-derived mesenchymal stem cells, and hepatic stem/progenitor cells, and it is primarily involved in cell-cell and cell-matrix interactions. CD90+/CD44− HCC cells formed xenografts in NOD-SCID mice, whereas CD90+/CD44+-derived tumors were more metastatic (Yang et al., 2008a). Furthermore, CD45−/CD90+ cells were found in all HCC, but not in normal, cirrhotic and non-tumor tissues; and CD45−/CD90+ was identified in 90% of HCC blood samples, but not in blood from normal or cirrhotic patients [19]. Thus, one or more of these markers may be useful for identifying cancer stem cells in HCC or even as markers for the detection of HCC in liver or blood (Yang et al., 2008b). However, to our knowledge, no previous study has investigated the specificity of these CSC markers by comparing their expression profile in various disease categories, and the question remains whether these markers have any clinical value in the detection of HCC in liver tissue or serum samples. In the present study we collected liver specimens from healthy individuals, as well as from patients with hepatitis with or without cirrhosis, HCC or hepatoblastoma (HB), and from patients with liver tissue adjacent to HCC. We then stained these markers by immunohistochemistry in order to define their tissue specificity.
MATERIALS AND METHODS
1. Tissue collection and patient demographic information
Twenty-five HCC specimens in paraffin-embedded sections were provided through the UC Davis Cancer Center BioRepository (CCBR). The human subject protocols were approved by the Institutional Review Board (IRB) according to the NIH guidelines. All specimens provided by the CCBR were recoded without patient identification. The male/female ratio was 20/5; mean age: 56.4±14.4 (20–73) years. The tumor size ranged from 1.8 to 13 cm in diameter (mean: 5.76±3.1cm, median: 5.0 cm). In these specimens, HCC differentiation degree as determined by pathologists was as follows: I–II (4), II (7), III (9), III–IV (1), undetermined (4). Basic disease: 11 with viral hepatitis (5 cases of hepatitis Band 6 cases of hepatitis C), 9 with cirrhosis, 7 with active inflammation, 2 with steatosis, 2 with fibrosis, 5 without clear predisposing conditions (Table 1). Four hepatoblastoma (HB) cases were previously healthy pediatric subjects with age varying from 1 to 4 years old (male/female: 2/2). In 19 cases of the viral hepatitis group, 18 cases were hepatitis C and one with hepatitis B, with a male/female ratio of 16/3 and a mean age of 50±8.7 years. Eight patients were cirrhotic, 7 cases were accompanied by various degrees of fibrosis, and there was no obvious fibrosis in the remaining four cases (Table 2). Eight liver specimens adjacent to HCC in paraffin-embedded sections were provided by the First Affiliated Hospital, Nanjing Medical University, with patient consent, and they were all HBV-positive. Their male/female ratio was 5/3 with a mean age of 47.5±15.5 years. Tumor size ranged from 1.5 to 8 cm in a mean diameter of 4.4±2.7 cm (median: 4.5 cm) (Table 3). Four liver specimens from “healthy” subjects were from patients without known liver disease who had liver resection for other reasons.
Table 1.
Demographic information and base disease of HCC cases
| Case | Different. degree |
Age | TNM stage |
Tumor size (cm) |
Base disease | CD133/1 staining |
CD44 staining |
|---|---|---|---|---|---|---|---|
| 1 | III–IV | 67 | T3NoMo | 13 | Chronic active hepatitis | N | P |
| 2 | III | 73 | T3NoMo | 7.2 | No clear base disease | P | P |
| 3 | N/a | 20 | T3N1Mo | 9 | No clear base disease | P | P |
| 4 | I–II | 48 | T3NoMo | 6.3 | Multiple, chronic Hepatitis B | P | P |
| 5 | I–II | 49 | T3NoMo | 5 | 2 HCC nodules, transplanted, cirrhosis | P | P |
| 6 | I–II | 76 | T4N1Mo | 12 | Vascular infiltration, chronic portal infiltration |
N | N |
| 7 | II | 62 | T1NoMo | 2 | Chronic active hepatitis, grade I, stage 3 fibrosis |
P | P |
| 8 | III | 76 | T3NoMo | 10.5 | Portal inflammation, mild fibrosis. | n | P |
| 9 | N/a | 52 | T1NoMo | 1.8 | Cirrhosis, transplanted | N | N |
| 10 | II | 51 | T3NoMo | 7 | No clear base disease | N/A | N |
| 11 | III | 64 | T3NoMo | 5.5 | No clear base disease | N | P |
| 12 | II | 55 | T3NoMo | 5 | Cirrhosis | P | P |
| 13 | N/a | 40 | T3NoMo | 10.5 | Small incidental hemangioma (0.6 cm) | N | P |
| 14 | II | 49 | T3NoMo | 2.8 | No clear base disease | P | P |
| 15 | III | 71 | T3NoMo | 7 | No clear base disease | P | N |
| 16 | III | 53 | T2NoMo | 3.9 | 3 HCC nodules/cirrhosis | N | P |
| 17 | II | 75 | T2NoMo | 4.5 | Chronic portal inflammation, minimal steatosis |
N | N |
| 18 | II | 47 | T2NoMo | 5 | Chronic hepatitis (grade 2, stage 2), minimal steatosis |
N | N |
| 19 | II | 48 | T3NoMo | 3 | 3 HCC nodules/cirrhosis | N | N/a |
| 20 | N/a | 50 | T2NoMo | 4 | Chronic active hepatitis/cirrhosis | N | N |
| 21 | III | 59 | T3NoMo | 2 | Chronic hepatitis B, grade 3 inflammation, stage 4 cirrhosis |
N | N |
| 22 | III | 30 | T3NoMo | 4.5 | Cirrhosis | P | P |
| 23 | I–II | 72 | T3NoMo | 3.5 | 3 nodules, cirrhosis | N/a | N/a |
| 24 | III | 54 | T3N1Mo | 5 | Multiple nodules, portal vein invasion, lymphonode positive, cirrhosis |
N | P |
| 25 | III | 70 | T3N1Mo | 4 | Multiple nodules | P | P |
| 56.44± 14.4 |
5.76±3.11 | Pstv: 10 Ngtv:15 |
15 8 |
||||
Pstv = positive; Ngtv = negative. N/a = non applicable.
Table 2.
CSC marker staining in liver specimens of patients with viral hepatitis
| Case | Age | Gender | Hepatitis | Fibrosis/cirrhosis | CD44 | CD133 | ALDH | CD90 |
|---|---|---|---|---|---|---|---|---|
| 1 | 46 | F | HCV | Cirrhosis | + in fibrotic septa | + | + in portal track | ++ in fibrotic septa |
| 2 | 55 | F | HCV | Fibrosis +++/ cirrhosis |
++ in hepatocytes |
+++ in hepatocytes |
Overlapping with CD133 |
+++ in hepatocytes |
| 3 | 44 | M | HCV | Fibrosis++ | Negative | Few cells in portal track |
+/− | Negative |
| 4 | 40 | M | HBV | Not obvious | Negative | Negative | +/− | Negative |
| 5 | 58 | M | HCV | Not obvious | ++ in hepatocytes |
+ | + overlapping with CD133 |
+ faint staining |
| 6 | 55 | M | HCV | Fibrosis +/++ | ++ in hepatocytes |
++ in fibrotic septa & hepatocytes |
++ overlapping with CD133 |
++ |
| 7 | 51 | M | HCV | Fibrosis +/++ | Few positive cells | Few positive cells in portal track |
+/− | + |
| 8 | 52 | M | HCV | Fibrosis +++/cirrhosis |
+ in fibrotic septa | Negative | + | N/A |
| 9 | 33 | M | HCV | Cirrhosis | + in fibrotic septa | + | + | N/A |
| 10 | 55 | M | HCV | Cirrhosis/fatty liver | + | + | + in fibrotic septa | N/A |
| 11 | 50 | M | HCV | Cirrhosis | + | + | + | N/A |
| 12 | 47 | M | HCV | Cirrhosis | + in fibrotic septa | + | + | Negative |
| 13 | 68 | M | HCV | Fibrosis | + | + | + | Negative |
| 14 | 36 | M | HCV | Not obvious | +/− | Negative | + | Negative |
| 15 | 48 | M | HCV | Cirrhosis | + in fibrotic septa | Negative | +/− | N/A |
| 16 | 50 | M | HCV | Fibrosis | + in fibrotic septa | Negative | +/− | N/A |
| 17 | 65 | F | HCV | Fibrosis +++ | + in fibrotic septa | Few positive cells in portal track |
Few positive cells in portal track |
N/A |
| 18 | 49 | M | HCV | Fibrosis | + in fibrotic septa | Negative | Negative | N/A |
| 19 | 47 | M | HCV | No fibrosis | + in fibrotic septa | Negative | + | N/A |
| Mean | 50 ±8.7 |
M: 16 F: 3 |
HCV 18 HBV 1 |
With cirrhosis: 8 With fibrosis: 7 |
Positive: 17 Negative: 2 |
Positive: 12 Negative: 7 |
Positive: 18 Negative: 1 |
Positive: 5 Negative: 5 |
Table 3.
Demographic information and base disease of 8 peri-HCC cases as well as CD133 and CD44 staining in cancer tissue part.
| Case | Different. degree |
Age | TNM stage |
Tumor size (cm) |
Base disease | CD133/1 staining |
CD44 staining |
|---|---|---|---|---|---|---|---|
| 1 | II | 43 | T3NoMo | 3 | Fatty liver, dysplasia, HBV+ |
+/− | N/a |
| 2 | III | 39 | 2 | HBV+ | + | + | |
| 3 | I–II | 22 | T3NoMo | 6 | HBV+ | − | − |
| 4 | I–II | 70 | T3NoMo | 6 | HBV+ | + | − |
| 5 | II | 42 | T2NoMo | 1.5 | HBV+, clear cell type |
++ | +/− |
| 6 | III | 45 | T3NoMo | 8 | HBV+ | − | +/− |
| 7 | I | 52 | T3NoMo | 7 | HBV+ | +/− | +/− |
| 8 | II | 67 | T2NoMo | 1.5 | HBV+ | +/− | +/− |
| 47.5± 15.5 |
4.4±2.7 | Pstv:6 Ngtv:2 |
Pstv:5 Ngtv:2 |
||||
N/a = non applicable, Pstv = positive; Ngtv = negative.
2. Immunohistochemical staining
Paraffin-embedded sections were deparaffinized with xylene and a series of concentrations of ethanol, blocked with 20% rabbit serum, and stained with primary goat-anti-human ALDH1A1 from Santa Cruz Biotechnol ogies (Santa Cruz, CA), mouse anti-human CD44 and CD133/1 from (MiltenyiBiotec Inc. Aubum, CA) or CD90 from BD Pharmingen (San Diego, CA). Secondary antibodies [F(ab’)2-PE-Cy5 fluorescein-conjugated rabbit anti-mouse IgG and FITC-conjugated rabbit anti-goat IgG] were used for fluorescent imaging for double staining of CD133/1 and ALDH (Table 4). Some sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI) in mounting medium (from Vector Laboratories, Inc. Burlingame, CA) demonstrating nuclei. Negative controls were stained without primary antibodies. Stained sections were examined under either a Nikon fluorescent microscope or Zeiss LCM confocal microscope as indicated in the figure legends. Positive rates for CD44 and CD133 were counted in all HCC and hepatitis sections.
Table 4.
The first and second antibodies for immunohistochemical staining of CSC markers
| Tumor Marker |
Primary Antibody | Secondary Antibody |
|---|---|---|
| CD44 | Monoclonal mouse anti-human CD44 |
F(ab’)2-PE-Cy 5 fluorescein- conjugated goat anti-mouse IgG |
| CD133 | Monoclonal mouse anti-human CD133 |
F(ab’)2-PE-Cy 5 fluorescein- conjugated rabbit anti- mouse IgG |
| ALDH | Polyclonal goat anti- human ALDH |
FITC-conjugated rabbit anti- goat IgG |
| CD90 | Monoclonal mouse anti-human CD90 |
F(ab’)2-PE-Cy 5 fluorescein- conjugated rabbit anti- mouse IgG |
3. Statistical analysis
Quantitative data were expressed as means ± SEM, and the difference in positive rates of CD133 and CD44 in HCC specimens or hepatitis specimens was analyzed by the Chi-square test. A p-value of less than 0.05 was considered as statistically significant.
RESULTS
1. Identification of CD133+/ALDH+ cells in HCC specimens
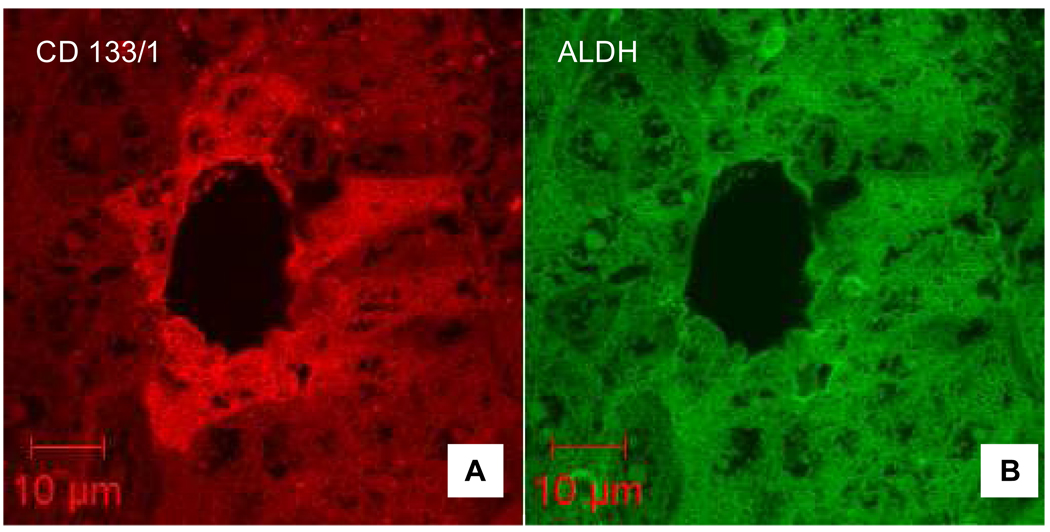
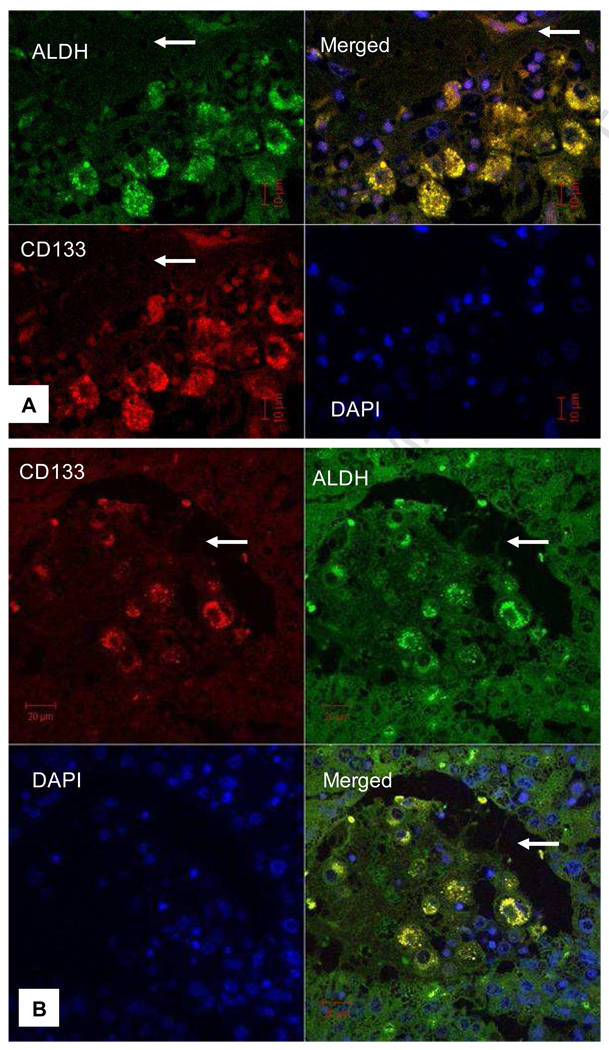
CD133+/ALDHhigh HCC cells had a high tumor-forming ability, and are considered to be a subpopulation of cancer initiating cells (Ma et al., 2008). We co-stained HCC specimens with anti-CD133 and anti-ALDH monoclonal antibodies, and found that many HCC cells were positive for ALDH staining. CD133-positivity was identified mostly in ALDH-positive cells and in nearly two third of HCC specimens (Table 1). However, we estimated that only a small portion (5%) of HCC cells was positive for both markers (double positive), as shown in Fig. 1. Moreover, doubly positive HCC cell clusters were co-localized by confocal microscopy in the areas adjacent to connective tissue (Fig. 2A) and within invaded vessels (Fig. 2B), suggesting that these cells are highly metastatic.
Fig. 1. Confocal imaging of immunohistochemical staining of HCC sections.
A. CD133-positive HCC cells (red) were identified around a vessel. B. ALDH highly-positive HCC cells (green) were located in the same areas as CD133+ cells. Please note that nuclei were not stained with these two markers. Scale bars indicate the magnification.
Fig. 2. CD133+/ALDH+ HCC cells were co-localized in the areas adjacent to connective tissue or within an invaded vessel.
A. CD133+/ALDH+-HCC cells were identified under a confocal microscope adjacent to connective tissue (arrow in A) and within an invaded vessel (arrow in B). Merged images show some HCC cells are positive (yellow) for both markers. DAPI staining was not overlapping with either CD133 (red) or ALDH (green) staining in the merged images.
2. CSC markers in peri-HCC liver tissue and hepatitis specimens
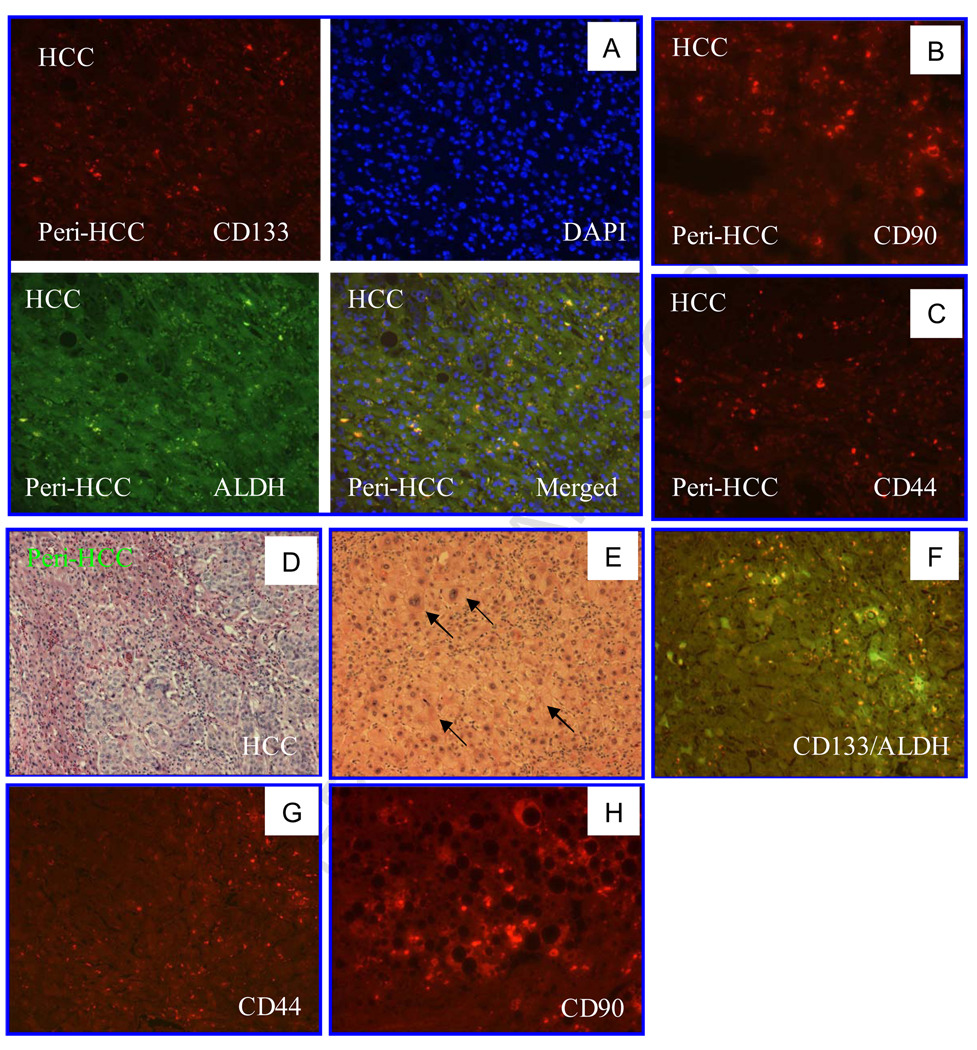
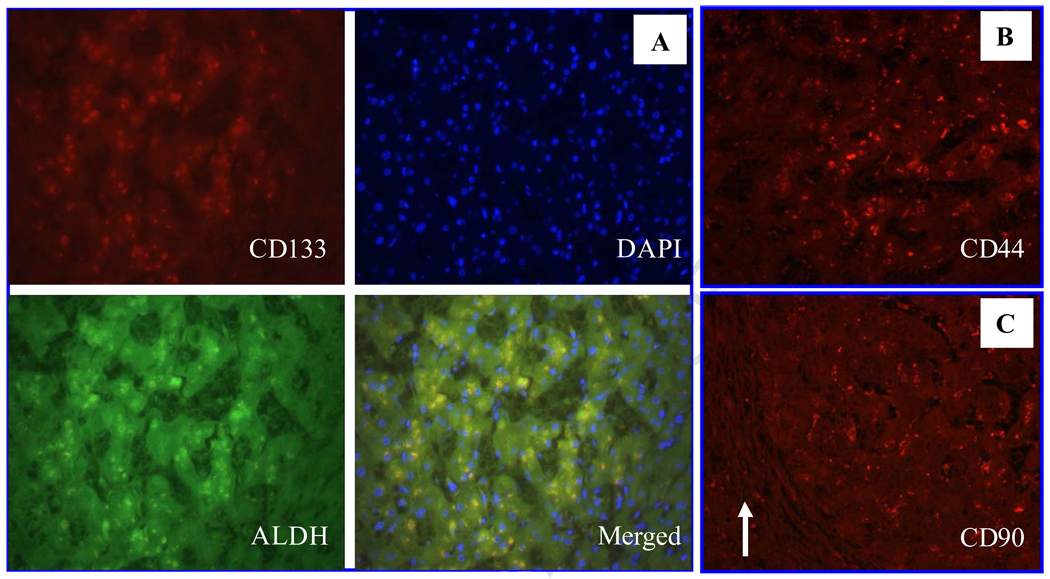
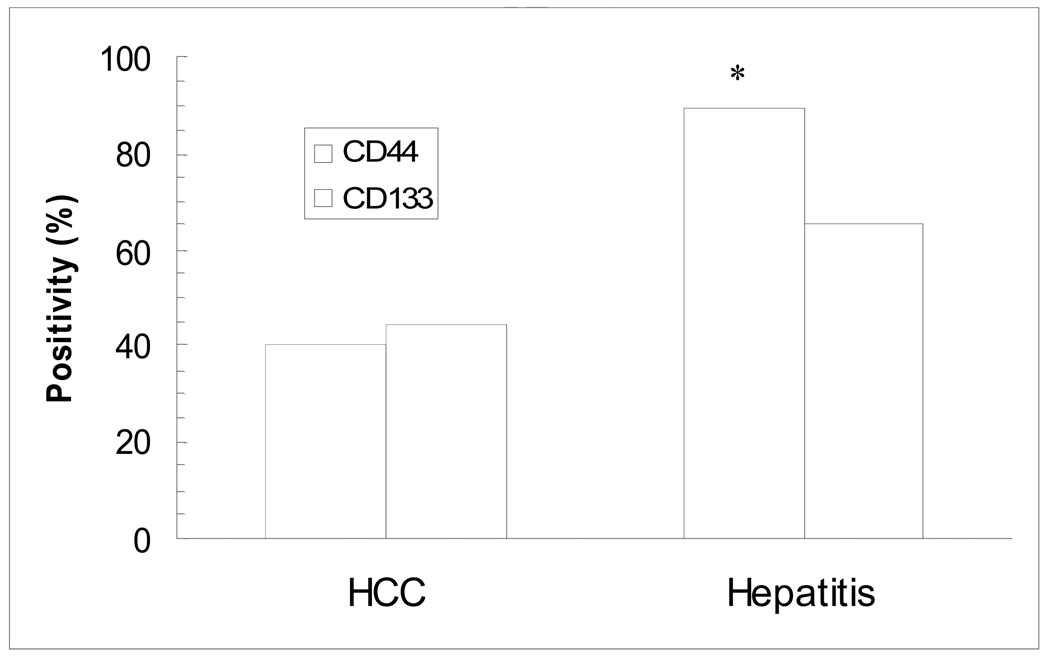
CSC markers are useful for identifying CSCs or cancer initiating cell populations in some cancer tissues. Whether these markers are valuable in identifying malignant cells in liver tissue is an intriguing question. We collected 8 peri-HCC liver tissue blocks and stained sections from these blocks with anti-CD133, ALDH, CD44 and CD90 antibodies. Among 7 specimens (1 unstained), 6 were found to be ALDH-positive and one in questionable; 5 CD133-positive; 3 CD44-positive; 4 slightly CD44-positive; and 4 CD90-positive (Fig. 3 A–D, Table 5), especial1y in tissue with dysplasia adjacent to HCC (Fig. 3E–H). The positivity rate for these markers in the peri-HCC tissue was at least similar to that in the corresponding HCC tissue (Table 3). Moreover, in hepatitis tissues, CD133, CD44 or CD90 positive cells were predominantly visualized in the portal zones or fibrous septa (Fig. 4). The CD44 positive rate in viral hepatitis was significantly higher than in HCC, but there was no statistical difference in CD133 positivity between HCC and hepatitis tissues (Fig. 5). In both peri-HCC tissues and hepatitis tissues (with or without cirrhosis) (Fig. 3 and 4) it is difficult to determine whether CD133, CD44 and CD90 positive cells are resident non-parenchymal cells or circulating blood cells because additional hematopoietic stem cell markers, such as CD34 and CD90, as well as endothelial epithelial cell markers, such as CD31, are needed. In addition, CD133, CD44 and a few CD90-positive cells, as well as ALDH-positive cells were also identified in 3 of 4 liver tissues whose hepatic biochemical tests were normal and who underwent an abdominal surgery for a non-hepatic disorder. Liver histology confirmed that two were normal, one with slight steatosis, and another one with moderate steatosis (Fig. 6). Compared to CD90, CD44 staining appeared to be positive in more cells in normal tissue (Fig. 6B and C), and more positive cells were seen around the central vein, which may be related to any on-going sub-clinical inflammation.
Fig. 3. CSC markers in both HCC tissue and peri-HCC liver tissues.
A. CD133/ADLH positive cells were visualized in both malignant and peri-HCC tissues. B. CD90-positive cells (red) were visualized in both HCC and peri-HCC tissue. C. CD44-positive cells (red) were identified in both HCC and peri-HCC tissues. D. H-E staining of HCC and adjacent liver tissue. E. Peri-HCC tissue with dysplasia (arrows). F. Merged image of CD133/ALDH staining of non-HCC area with dysplasia. G. CD44 staining in non-HCC area with dysplasia. H. CD90 staining in non-HCC area with steatosis. Images were visualized under a standard fluorescent microscope (200X).
Table 5.
Immunohistochemical staining of CSC markers in peri-HCC tissue.
| Case | CD44 | CD133 | ALDH | CD90 |
|---|---|---|---|---|
| 1 | + | + | ++ | N/A |
| 2 | +/− | + | + | + |
| 3 | +/− | + | + | + |
| 4 | N/A | N/A | N/A | N/A |
| 5 | +/− | − | +/− | N/A |
| 6 | +/− | − | + | + |
| 7 | + | + | + | + |
| 8 | + | + | + | N/A |
N/a = non-applicable.
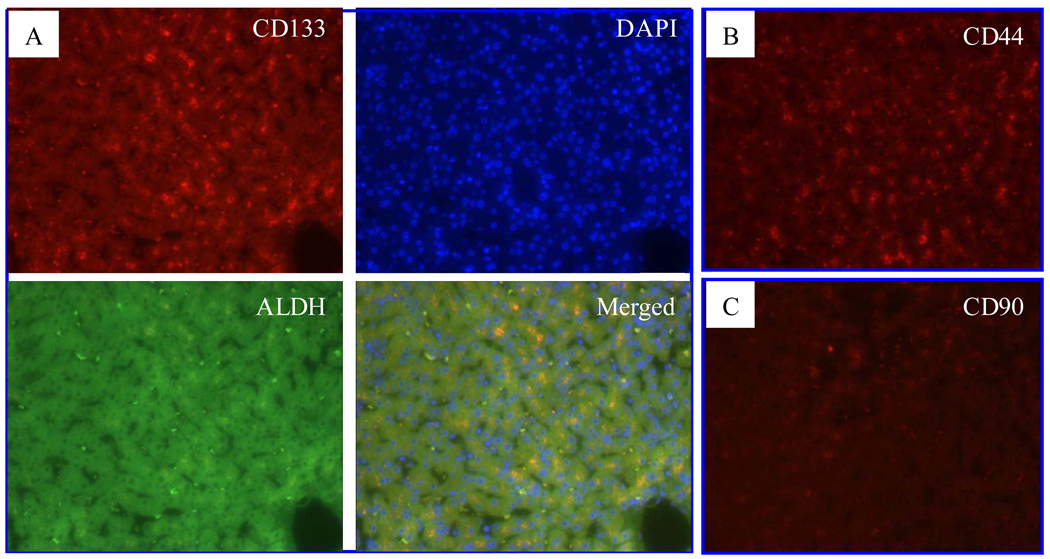
Fig. 4. CSC marker expression in viral hepatitis specimens.
A. CD133 (red) and ALDH positive (green) cells were shown in hepatitis C specimens with cirrhosis. CD44 (B) and CD90 (C)-positive cells (red) were visible in hepatitis specimens with cirrhosis. Fluorescent microscope images (200X). The arrow indicates thick fibrous septum.
Fig. 5. Positivity of CD133 and CD44 in HCC and hepatitis specimens.
Cells positive for CD133 and CD44 staining in HCC and hepatitis specimens were counted and analyzed by the Chi-square test. * p< 0.05 compared to HCC specimens.
Fig. 6. CSC marker expression in nearly normal liver.
Liver specimens collected from subjects with normal liver biochemical tests but with other GI disorders for surgery. Liver H&E staining confirmed normal histology. Some hepatocytes near the central vein were positive for CD133/ALDH (A), CD44 (B) and CD90 (C). More CD44+ cells were seen than CD90+ cells in this case.
3. CSC markers in hepatoblastoma tissues
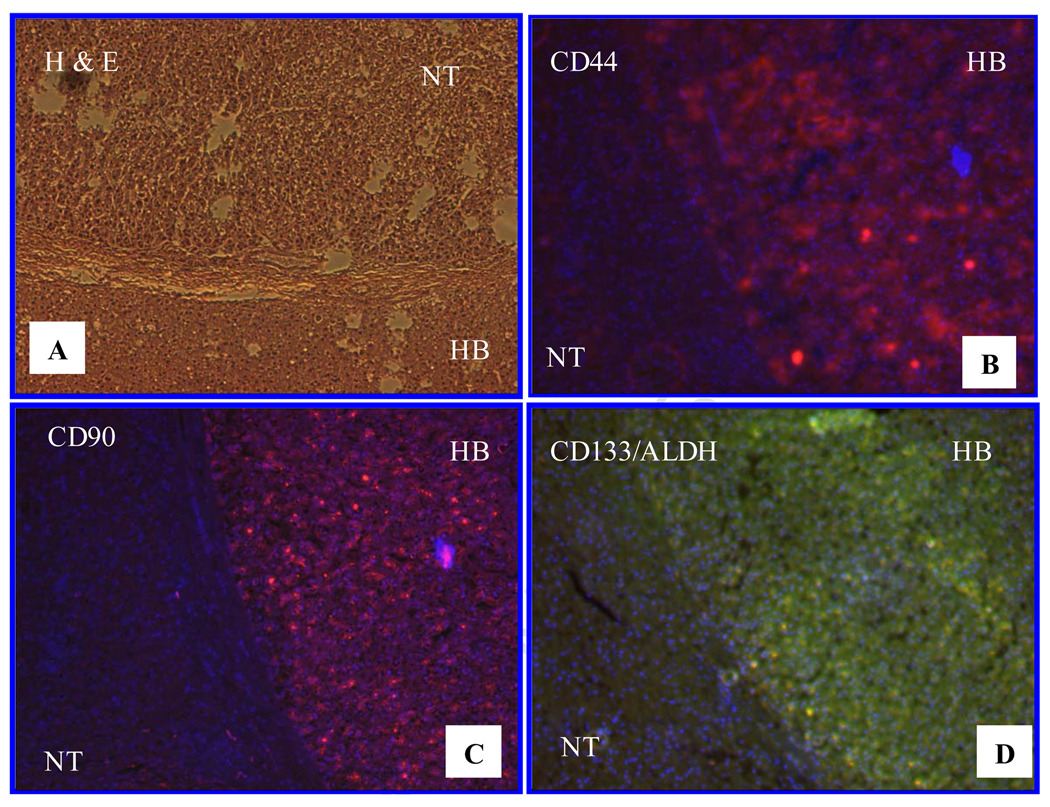
Hepatoblastoma (HB) is a malignant liver cancer occurring in normal liver of early childhood, and is thought to derive from liver stem cells/progenitor cells (Cairo et al., 2008). Therefore, it would be interesting to investigate the CSC marker profile in HB specimens. We found that one out of four HB specimens was positive for all markers stained (Fig. 7), whereas the other three were negative for all these markers. In the case with positive staining, HB was encapsulated by connective tissue, and the liver tissue surrounding the neoplasm was completely normal, and negative for all markers stained.
Fig. 7. CSC marker expression in hepatoblastoma (HB) specimens.
A. H & E stain of HB and non-tumor liver tissue (NT); separated from each other by connective tissue. B. CD44 positive cells were visualized in the HB area, but not in the NT area. C. Almost all HB cells are CD90 positive; in contrast, non-tumor liver tissue was negative for CD90. CD133+/ALDH+ cells are identified in the HB area but not in the NT region. B, C and Dare merged images for DAPI staining with CD44 (B), CD90(C) or CD133 (red) plus ALDH (green) staining (yellow) (D). Fluorescent images (100X).
DISCUSSION
In this study we stained HCC specimens with a number of cell surface molecules, such as CD133, CD44 and CD90, and ALDH which is a cytoplasmic enzyme. They have been selected as markers for identifying CSCs in HCC tissues or hepatoma cell lines in a number of recent studies (Ma et al., 2007; Yang et al., 2008a; Yang et al., 2008b). With the combination of CD133 and ALDH we identified a small subpopulation of doubly positive cells in HCC tissues, especially in the areas adjacent to connective tissue or within vascular lumens with HCC invasion. The separation of these cells as a subpopulation could enhance our understanding of the initiation, progression, metastasis or relapse of HCC at the molecular, genomic or epigenetic levels, as well as lead to the identification of novel molecules for the development of therapeutic agents targeting HCC(Aravalli et al., 2008; Kudo, 2008; Visvader and Lindeman, 2008; Mishra et al., 2009).
It has been claimed that the CD45−/CD90+ combination could be used as a marker for human liver cancer or as a target for its diagnosis and therapy (Yang et al., 2008b). In this study, we examined the expression profile of several CSC markers in HCC, peri-HCC tissue, viral hepatitis and normal liver tissues, and found that expression of individual or a combination of CSC markers in HCC is not a unique phenomenon. Cell surface markers, CD133, CD44 and CD90, as well as ALDH expressed in HCC tissue, suggesting stem/progenitor cell properties, were also expressed in inflamed or nearly normal liver tissue. The HCC specimens were from patients with either HBV or HCV infection with or without advanced fibrosis or cirrhosis, and it is surprising that nearly all peri-HCC tissue specimens were positive for these markers, and that many hepatitis specimens were positive too. However, in non-HCC tissue, positive cells were found mainly in the portal areas and fibrous septa. One speculation for this finding is that circulating, bone marrow-derived stem cells may be recruited into inflamed livers and facilitate tissue repair, because a significant number of CD44 or CD90 (a hematopoietic stem cell marker)-positive Cells were found in normal adult liver (epithelial cells with hepatobiliary phenotype) and in a variety of liver disorders (Khuu et al., 2007; Mao et al., 2008). Moreover, regenerative hepatocytes, hepatic oval cells or other resident progenitor cells in the liver may also yield positive staining in the liver with inflammation or from “healthy” individuals (Herrera et al., 2006). Therefore, it appears that the use of CSC markers for identifying malignant cells in normal livers or livers with inflammation has a limited value, which is consistent with a recent report that CSC markers were not useful in predicting prognosis of this malignancy (Salnikov et al., 2009). A panel of liver stem cell markers, such as O volvulus 6 (OV-6), glutathione S-transferase placental (GST-P), CK-19 and H19, was found to be positive for both HCC and cirrhotic tissues (Oliva et al.). Interestingly, HCC tissues had a higher positivity than cirrhotic tissues in liver stem cell marker staining, and these markers were positive in non-tumor liver tissues, such as alcoholic steatohepatitis, non-alcoholic steatohepatitis (NASH), HBV or HCV infection (Oliva et al.). Thus, it is consistent for our findings with this work that in both HCC and non-malignant liver tissues, there exist stem cell-like populations which are positive for either cancer stem cell markers or/and stem/progenitor cell markers, although the positivity of each marker may vary in different tissue types and a specific location, and the origin of stem/progenitor cells needs to be further defined.
HB is a malignant embryonal tumor of the liver usually diagnosed in children younger than 3 years of age (Cairo et al., 2008). In the absence of underlying liver disease or viral infection, genetic/epigenetic abnormalities of this tumor are linked to a stem cell origin of tumorigenesis. The distinctive stem cell expression profiles and molecular signatures divide this malignancy into two classes: well-differentiated (C1) and poorly-differentiated (C2). Poorly-differentiated HBs are more often the perivenous type, express fewer CSC genes and less liver-specific genes with more chromosomal abnormalities, and have an unfavorable outcome (Cairo et al., 2008). On the other hand, the well-differentiated HBs express more liver-specific genes, and most CSC markers are positive with a less aggressive tumor behavior. A recent study demonstrated that hedgehog (Hh) signaling activation is involved in this subclass, suggesting a possible role of Hh signaling activation in the malignant transformation of embryonal liver cells in early childhood HBs (Eichenmuller et al., 2009). In our limited number of HB cases, one out of 4 HB cases was positive for all CSC markers stained, whereas the other three were completely negative for these markers. Although more studies are needed to define their liver-specific gene expression profile and genetic abnormalities, it is possible that the case with all CSC markers positive may belong to the C1 subclass, whereas, the other three may belong to the C2 subclass.
In conclusion, in HCC and dysplastic tissue, clusters of cancer stem cells were identified by CD133+/ALDH or in combination with CD44 and CD90 staining. However, liver specimens from patients without malignancies and patients with normal liver also express these markers. Thus, it appears that the use of cancer stem cell markers to screen tissues with chronic liver disease provides limited guidance for the identification of malignant cells because proliferative liver cells or infiltrating bone marrow-derived stem cells in the damaged liver may display a similar expression pattern.
ACKNOWLEDGEMENTS
The authors are grateful to the UC Davis Cancer Center BioReposity (CCBR) and Dr. Vijay Khatri in the Department of Surgical Oncology, UC Davis Center Centre for providing tissue specimens used in this study.
The study is supported by the Technology Transfer Grant by the UC Davis Medical Center to JW, and an NIH grant to MAZ (DK075415). Dr. Yi-Yao Cui was supported by the China Scholarship Council.
Abbreviations used in this manuscript
- ALDH
aldehyde dehydrogenase
- CSCs
Cancer stem cells
- DAPI
4',6-diamidino-2-phenylindole
- HB
hepatoblastoma
- HCC
hepatocellular carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of the study was presented in the 60th Annual meeting of the American Association for the Study of Liver Diseases (AASLD), Oct. 30-Nov. 3, 2009, Boston, MA.
REFERENCES
- Aravalli RN, et al. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- Cairo S, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Clarke MF, et al. Cancer stem cells--per spectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Dalerba P, et al. Cancer stem cells: models and concepts. Annual Review of Medicine. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Eichenmuller M, et al. Blocking the hedgehog pathway inhibits hepatoblastoma growth. Hepatology. 2009;49:482–490. doi: 10.1002/hep.22649. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, et al. Rising incidence of hepatocellular carcinoma in the United States. N. Eng. J. Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, et al. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Fujita A, et al. In vivo activation of the constitutive androstane receptor beta (CARbeta) by treatment with dehydroepiandroster one (DHEA) or DHEA sulfate (DHEA-S) FEBS Letters. 2002;532:373–378. doi: 10.1016/s0014-5793(02)03712-2. [DOI] [PubMed] [Google Scholar]
- Herrera MB, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- Khuu DN, et al. Epithelial cells with hepatobiliary phenotype: is it another stem cell candidate for healthy adult human liver? World J. Gastroenterol. 2007;13:1554–1560. doi: 10.3748/wjg.v13.i10.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, et al. Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol. Med. 2008;14:450–460. doi: 10.1016/j.molmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Kudo M, et al. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl. 1:1–12. doi: 10.1159/000181865. [DOI] [PubMed] [Google Scholar]
- Lau WY, et al. The current role of radio frequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann. Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- Lee CJ, et al. Pancreatic cancer stem cells. Journal of Clinical Oncology. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- Ma S, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Ma S, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol. Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- Mao L, et al. Liver progenitor cells activated after 30% small-for-size liver transplantation in rats: a preliminary study. Transplant. Proc. 2008;40:1635–1640. doi: 10.1016/j.transproceed.2008.03.133. [DOI] [PubMed] [Google Scholar]
- Mishra L, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr. Stem Cell Res. Ther. 2008;3:237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Oliva J, et al. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp. Mol. Pathol. 2010 doi: 10.1016/j.yexmp.2010.01.003. In press (Doi,10.1016/j.yexmp.2010.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, et al. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol. Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikov AV, et al. Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Letters. 2009;275:185–193. doi: 10.1016/j.canlet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Tanaka S, et al. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1–8. doi: 10.1111/j.1349-7006.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, et al. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15584–15589. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, et al. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Yang ZF, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008a;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Yang ZF, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008b;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- Zhou P, et al. Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology. 2009;49:1992–2000. doi: 10.1002/hep.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]