Abstract
Antidepressants require adaptive brain changes before efficacy is achieved, and they may impact the affectively disordered brain differently than the normal brain. We previously demonstrated metabolic disturbances in limbic and cortical regions of the congenitally helpless rat, a model of susceptibility to affective disorder, and we wished to test whether administration of fluoxetine would normalize these metabolic differences. Fluoxetine was chosen because it has become a first-line drug for the treatment of affective disorders. We hypothesized that fluoxetine antidepressant effects may be mediated by decreasing metabolism in the habenula and increasing metabolism in the ventral tegmental area. We measured the effects of fluoxetine on forced swim behavior and regional brain cytochrome oxidase activity in congenitally helpless rats treated for two weeks with fluoxetine (5 mg/kg, i.p., daily). Fluoxetine reduced immobility in the forced swim test as anticipated, but congenitally helpless rats responded in an atypical manner, i.e., increasing climbing without affecting swimming. As hypothesized, fluoxetine reduced metabolism in the habenula and increased metabolism in the ventral tegmental area. In addition, fluoxetine reduced the metabolism of the hippocampal dentate gyrus and dorsomedial prefrontal cortex. This study provided the first detailed mapping of the regional brain effects of an antidepressant drug in congenitally helpless rats. All of the effects were consistent with previous studies that have metabolically mapped the effects of serotonergic antidepressants in the normal rat brain, and were in the predicted direction of metabolic normalization of the congenitally helpless rat for all affected brain regions except the prefrontal cortex.
Keywords: cytochrome oxidase, brain mapping, animal model, post-traumatic stress disorder, depression, fluoxetine
1. NTRODUCTION
Antidepressant medications are the most commonly prescribed medications in the USA (Olfson & Marcus, 2009) and they require several weeks of administration before clinical efficacy is observed (Hickie et al., 1999; Nurnberg et al., 1999). This response delay follows myriad neurophysiological adaptations to enhanced aminergic transmission, including autoreceptor desensitization, multiple increases and decreases of postsynaptic receptor sites, altered second-messenger pathways, and enhanced neurotrophism (Blier & de Montigny, 1999; Duman et al., 1999; Hyman & Nestler, 1996). However, the individual brain regions mediating these events at the systems level are not well characterized.
Although the human imaging literature on antidepressant treatment effects is highly variable, fluorodeoxyglucose positron emission tomography (FDG-PET) studies of depressed patients using selective serotonin reuptake inhibitors (SSRIs) have most often found treatment-related increases in prefrontal and anterior cingulate cortical metabolism and decreases in hippocampal and ventromedial frontal, subgenual cingulate, and anterior insular cortical metabolism (Brody et al., 1999; Buchsbaum et al., 1997; Mayberg et al., 1999). For example, Mayberg et al. (2000) examined glucose metabolism in depressed males both one week and six weeks after initiating fluoxetine treatment. The authors found some changes that were common at both time points, some that were opposite, and some that were only present after depression remission. Changes present at one week which persisted at six weeks included increased metabolism in the rostral brainstem, premotor cortex, and inferior parietal cortex, and decreased metabolism in the caudate, medial thalamus, insular cortex, and cerebellum. Initial increases were observed in the hippocampus, medial temporal cortex, putamen, and pallidum, but net decreases were observed in these same regions at six weeks. The opposite was seen in the posterior cingulate, where an initial decrease was followed by a final increase. Changes in prefrontal and cingulate cortex were only observed after depression remission. These changes constituted a normalization of prefrontal cortex and dorsal anterior cingulate metabolism, but also a decrease of subgenual cingulate metabolism to below normal.
The present study examined changes in regional brain cytochrome oxidase activity associated with two weeks of fluoxetine treatment in congenitally helpless rats by using a metabolic mapping technique that allows higher spatial resolution than is possible with human neuroimaging. For example, the brainstem effects reported in human neuroimaging studies may result from alterations in any of a large number of regions, while effects occurring in small subcortical regions such as the habenula may go undetected. Quantitative histochemical mapping of cytochrome oxidase activity was chosen because cytochrome oxidase is a mitochondrial respiratory enzyme that quantifies neuronal metabolic capacity, providing a steady-state intracellular measure of metabolism (Sakata et al., 2005), and has been successfully applied to map the regional effects of the tricyclic antidepressant amitriptyline in normal rats (Gonzalez-Pardo et al., 2008). Fluoxetine was chosen for this study because it has become a first-line drug for the treatment of affective disorders, and Freo et al. (2000) previously examined resting glucose metabolism in over fifty regions following two weeks of daily fluoxetine administration. Although fluoxetine continued to cause acute metabolic changes in a number of regions, baseline metabolism was altered in only two regions: the lateral habenula and CA3 field of the hippocampus, which both showed significant decreases. However, fluoxetine may impact the affectively disordered brain differently than the normal brain. Congenitally helpless rats, a selectively bred line, were chosen as subjects because their behavior models aspects of depression and post-traumatic stress disorder (Enkel et al., 2010; King et al., 2001; Shumake et al., 2005; Vollmayr et al., 2004), and, using quantitative cytochrome oxidase histochemistry, we have identified significant brain changes in the line, including hypermetabolism in regions where fluoxetine reduced metabolism in normal rats, namely, the habenula and hippocampus (Shumake et al., 2002; Shumake et al., 2003). Thus, we were interested to examine to what extent two weeks of fluoxetine treatment could reverse these and other brain metabolic abnormalities found in congenitally helpless rats, including reduced metabolism in regions of frontal-cingulate cortex (Shumake et al., 2000) and the mesolimbic dopamine system (Shumake et al., 2003). Specifically, given that congenitally helpless rats show opposite metabolic changes in the habenula and ventral tegmental area, we hypothesized that fluoxetine may differentially modify the metabolism of these regions toward the direction of normalization. We also used the forced-swim test as a behavioral measure of drug efficacy (Detke et al., 1995).
2. Results
2.1. Forced-Swim Test
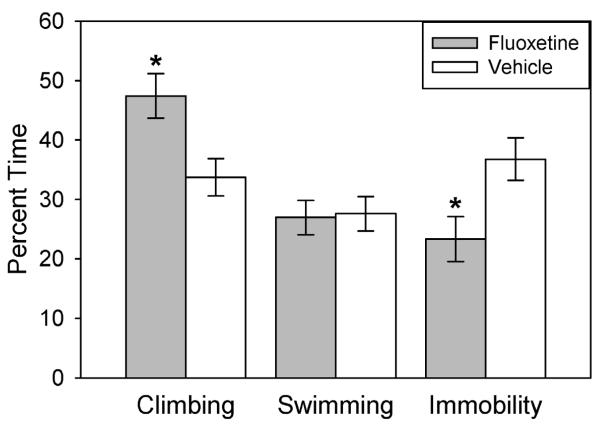
The forced-swim test showed that congenitally helpless rats treated for two weeks with fluoxetine (5 mg/kg, i.p., daily) had lower immobility scores, an index of antidepressant drug action. However, 9 subjects (out of 20) were unable to complete the initial swim session because they appeared to be drowning (remained completely submerged for 5 consecutive seconds, which was our criterion for removing the animal). For these subjects, the test was terminated between 5.5 and 14.5 minutes (mean = 11.5 min). Three of these subjects were from the fluoxetine-treated group (mean = 11.8 min), and six were from the vehicle-control group (mean = 10.9 min). This introduces a possible bias into the interpretation of the results, in that some rats experienced a shorter duration of swim stress than others. Thus, rats experiencing less stress might show reduced immobility in the final test, regardless of group assignment. However, this appears not to have been the case since a linear regression indicated that, if anything, earlier sink times on day 1 tended to predict more, not less, immobility on day 2, r(9) = −.65, p = .06. Therefore, all subjects were included in the univariate ANOVAs of the forced-swim scores. These tests indicated that fluoxetine-treated rats showed significantly less immobility, F(1,19) = 6.6, p = .02, and more climbing, F(1,19) = 7.8, p = .01, than vehicle-control rats, while swimming was not significantly different between groups, F(1, 19) = .024, p = .88 (Figure 1).
Figure 1.
Swim behavior during 5 minutes of the forced-swim test following 2 weeks of treatment with either fluoxetine (daily i.p., 5 mg/kg) or vehicle. *p < .05. N=10 per group.
2.2. Regional Brain Effects
There were both decreases and increases in regional cytochrome oxidase activity in the brains of fluoxetine-treated rats as compared to vehicle-control rats. As hypothesized, fluoxetine reduced metabolism in the habenula and increased metabolism in the ventral tegmental area. Means and standard errors for all regions of interest are reported in Table 1, along with p values determined from independent samples t tests, with equal variances not assumed for regions rejected by Levene’s test for equality of variances. Significant decreases in cytochrome oxidase activity were observed in three regions of the fluoxetine-treated group, including the habenula, the hippocampal dentate gyrus and the deeper layers of the dorsomedial prefrontal cortex (PFC). A significant increase in cytochrome oxidase activity was observed only in the midbrain ventral tegmental area (VTA), which showed the largest fluoxetine effect, a 20% increase in cytochrome oxidase activity.
Table 1.
Means and standard errors (S.E.) of cytochrome oxidase activity (micromole/min/gram wet tissue) of regions of interest in fluoxetine-treated (n=7) and vehicle (n=10) groups.
| Brain Region | Fluoxetine Group Mean ± S.E. |
Vehicle Group Mean ± S.E. |
p value |
|---|---|---|---|
| Habenula and Midbrain Regions | |||
| Habenula | 283.0 ± 6.4 | 305.0 ± 6.1 | .028* |
| Dorsal raphe nuc. | 221.8 ± 6.4 | 207.7 ± 6.8 | .163 |
| Periaqueductal gray | 256.4 ± 10.5 | 235.9 ± 7.1 | .116 |
| Midbrain reticular formation | 190.8 ± 5.2 | 178.7 ± 9.1 | .269 |
| Ventral tegmental area | 177.4 ± 4.5 | 148.2 ± 5.7 | .001* |
| Interpeduncular nuc. | 390.0 ± 8.2 | 381.5 ± 8.7 | .507 |
| Red nuc. | 242.7 ± 14.2 | 250.7 ± 9.8 | .638 |
| Substantia nigra, pars ret. | 236.2 ± 11.8 | 238.7 ± 7.0 | .851 |
| Substantia nigra, pars comp. | 183.9 ± 13.7 | 189.4 ± 7.4 | .711 |
| Superior colliculus, sup. | 251.9 ± 11.7 | 252.2 ± 5.5 | .984 |
| Superior colliculus, deep | 232.3 ± 13.1 | 232.4 ± 5.5 | .998 |
| Inferior colliculus | 349.5 ± 10.1 | 345.1 ± 6.9 | .712 |
| Oculomotor nuc. | 364.5 ± 8.2 | 351.0 ± 5.0 | .156 |
| Hippocampal Regions | |||
| Ant. hipp. CA1, oriens | 188.2 ± 9.6 | 183.3 ± 5.6 | .642 |
| Ant. hipp. CA1, pyr. | 184.1 ± 9.2 | 182.5 ± 5.3 | .871 |
| Ant. hipp., lac.-mol. | 274.9 ± 8.6 | 270.9 ± 3.7 | .646 |
| Ant. hipp., CA3, oriens | 206.5 ± 4.9 | 211.7 ± 5.8 | .531 |
| Ant. hipp., CA3, pyr. | 171.0 ± 4.3 | 174.8 ± 4.2 | .546 |
| Dentate gyrus | 292.5 ± 2.7 | 301.5 ± 3.0 | .046* |
| Post. hipp., CA1 | 208.4 ± 9.9 | 222.0 ± 3.3 | .234 |
| Post. hipp., CA2 | 222.3 ± 9.0 | 232.3 ± 3.0 | .321 |
| Post. hipp., CA3 | 236.6 ± 10.5 | 251.2 ± 4.9 | .242 |
| Subiculum | 252.5 ± 10.6 | 249.8 ± 7.9 | .835 |
| Frontal Cortical Regions | |||
| Infralimbic cortex, sup. | 228.8 ± 5.2 | 239.0 ± 7.2 | .305 |
| Infralimbic cortex, deep | 235.4 ± 5.6 | 245.9 ± 7.1 | .297 |
| Prelimbic cortex, sup. | 261.5 ± 8.6 | 265.2 ± 10.2 | .799 |
| Prelimbic cortex, deep | 257.3 ± 8.4 | 269.5 ± 7.9 | .316 |
| Dorsomedial frontal cortex, sup. | 289.2 ± 7.3 | 287.7 ± 5.8 | .874 |
| Dorsomedial frontal cortex, deep | 209.3 ± 3.8 | 232.3 ± 8.7 | .032* |
| Dorsal frontal cortex, sup. | 293.1 ± 5.9 | 296.7 ± 4.1 | .612 |
| Dorsal frontal cortex, deep | 158.7 ± 8.0 | 178.6 ± 15.5 | .330 |
| Lateral frontal cortex, sup. | 277.9 ± 3.6 | 287.8 ± 4.2 | .115 |
| Lateral frontal cortex, deep | 234.4 ± 7.9 | 241.7 ± 8.1 | .539 |
| Anterior cingulate cortex, sup. | 286.8 ± 17.6 | 293.8 ± 8.7 | .698 |
| Anterior cingulate cortex, deep | 264.2 ± 14.1 | 279.4 ± 7.2 | .310 |
| Thalamic Regions | |||
| Nuc. reuniens | 223.7 ± 10.0 | 228.0 ± 2.7 | .690 |
| Parafascicular nuc. | 228.1 ± 6.3 | 231.3 ± 5.5 | .709 |
| Ventral basal nuc. | 249.0 ± 6.2 | 252.3 ± 3.3 | .617 |
| Subthalamic nuc. | 334.9 ±4.0 | 330.9 ± 6.2 | .637 |
| Ant. dorsal thal. nuc. | 332.0 ± 9.7 | 342.2 ± 4.4 | .303 |
| Ant. ventral thal. nuc. | 259.4 ± 6.9 | 265.8 ± 3.7 | .391 |
| Ant. medial thal. nuc. | 238.7 ± 6.8 | 243.3 ± 4.7 | .574 |
| Lateral geniculate nuc. | 233.8 ± 6.6 | 244.0 ± 4.1 | .182 |
| Medial geniculate nuc. | 266.4 ± 6.7 | 265.7 ± 5.6 | .929 |
| Septal Regions | |||
| Lateral septal nuc. | 295.8 ± 15.4 | 309.6 ± 8.6 | .411 |
| Medial septal diagonal band | 218.6 ± 15.3 | 221.6 ± 8.4 | .856 |
| Basal Ganglia Regions | |||
| Caudate putamen | 320.0 ± 12.7 | 334.8 ± 6.8 | .287 |
| Nucleus accumbens | 259.5 ± 12.1 | 273.0 ± 8.2 | .352 |
| Ventral pallidum | 200.3 ± 10.5 | 201.2 ± 3.4 | .936 |
| Globus pallidus | 161.6 ± 9.7 | 155.8 ± 3.8 | .593 |
| Amygdaloid Regions | |||
| Bed nuc. stria terminalis | 232.0 ± 12.9 | 230.3 ± 3.0 | .908 |
| Basolateral amygdala | 246.8 ± 8.5 | 249.8 ± 6.4 | .778 |
| Central amygdala | 231.7 ± 12.8 | 230.6 ± 8.4 | .942 |
| Medial amygdala | 220.9 ± 13.7 | 219.3 ± 7.6 | .914 |
| Hypothalamic Regions | |||
| Paraventricular hypothal. | 160.2 ± 10.2 | 172.8 ± 4.9 | .229 |
| Lateral hypothal. | 201.3 ± 6.7 | 189.6 ± 4.3 | .142 |
| Suprachiasmatic nuc. | 241.2 ± 14.7 | 219.7 ± 5.5 | .140 |
| Lateral mammillary nuc. | 300.5 ± 7.1 | 311.3 ± 7.9 | .348 |
| Medial mammillary nuc. | 315.8 ± 4.8 | 324.4 ± 7.7 | .360 |
p > 0.05.
3. Discussion
There are two principal findings of this study. The first is that two weeks of fluoxetine (5 mg/kg/day) treatment reduced immobility of congenitally helpless rats in the forced-swim test. The second is that, out of various regions which showed cytochrome oxidase differences in congenitally helpless rats, only four responded significantly to this extent of fluoxetine treatment, with a metabolic decrease in the habenula, dentate gyrus, and deeper layers of the dorsomedial prefrontal cortex, and an increase in the VTA. All regional brain changes, except for the prefrontal cortex, were in the predicted direction of metabolic normalization.
3.1. Forced-Swim Test
Two weeks of fluoxetine administration reduced immobility in the forced-swim test, a finding consistent with studies in normal rats (Vazquez-Palacios et al., 2004). However, some of the results were unexpected. First, fluoxetine and other SSRIs typically reduce immobility by increasing swimming without affecting climbing (Detke et al., 1995; Vazquez-Palacios et al., 2004), whereas antidepressants targeting the norepinephrine system do just the opposite (Detke et al., 1995). The congenitally helpless rats in our experiment showed increased climbing with no change in swimming. Moreover, climbing is typically the least prevalent behavior in the forced-swim test (Detke et al., 1995; Vazquez-Palacios et al., 2004), whereas in our study it was the most prevalent. The reason for this difference is unclear, but it does not appear to be caused by the congenital helplessness phenotype since the only other study assessing forced swim behavior in this strain found the typical pattern in response to an experimental 5-HT2A receptor antagonist: increased swimming was solely responsible for decreased immobility, while climbing remained the least frequent behavior (Patel et al., 2004). One possible explanation for the discrepancy is that we may have identified as climbing many behaviors which other investigators would have considered swimming. However, climbing is a dramatic behavior, involving thrashing and splashing directed at the sides of the cylinder, which should be hard to mistake for swimming. Another possible explanation is that our rats were larger than those typically used (450-550 g vs. 250-300 g). Given the narrowness of the cylinder, the larger rats were forced into a mostly vertical posture which restricted their horizontal-rotational range of motion. This may have led to an increase in climbing and a decrease in swimming. In addition, fluoxetine is the least specific of the SSRIs (Hyttel, 1982), causing significant increases in brain catecholamine levels (Bymaster et al., 2002), which could help explain increased climbing behavior. In summary, the congenitally helpless rats showed a decrease of immobility after fluoxetine administration as expected by the literature but their pattern of climbing and swimming was different.
3.2. Regional Brain Effects
Quantitative cytochrome oxidase histochemistry identified the habenula, hippocampal dentate gyrus, dorsomedial prefrontal cortex, and VTA as the only regions responding to two weeks of fluoxetine (5 mg/kg/day) treatment in congenitally helpless rats. These results are fairly consistent with the 2-DG mapping study of normal rats by Freo et al. (2000), who found baseline changes in only the habenula and the hippocampal CA3 pyramidal layer of animals that had been administered fluoxetine daily for two weeks but were given only saline concurrent with 2-DG uptake testing. However, when fluoxetine was given concurrent with 2-DG, acute decreases were also observed in the frontal cortex, amygdala, and hypothalamus. However the VTA, where we found the largest fluoxetine effect in congenitally helpless rats, was not assessed in the 2-DG study of Freo et al. (2000). In normal rats, decreased 2-DG uptake in the medial prefrontal cortex was the only observed change after 2 weeks of treatment with the SSRI citalopram; however, in olfactory bulbectomized rats, the anterior olfactory nucleus, substantia nigra, and frontal, orbitofrontal, anterior cingulate, and visual cortex all showed decreased glucose metabolism (Skelin et al., 2009). Similar to our finding with fluoxetine, citalopram exacerbated rather than normalized the prefrontal hypometabolism associated with olfactory bulbectomy. A trend for decreased activity was also observed in the lateral habenula, but neither it nor the VTA showed significant citalopram-evoked changes (Skelin et al., 2009).
Decreased cytochrome oxidase activity in the medial prefrontal cortex and dentate gyrus were likewise observed as a main effect of amitriptyline administration to normal rats, along with decreases in several other limbic regions, in an inhibitory avoidance learning experiment (Gonzalez-Pardo et al., 2008), but the VTA and habenula were not assessed. However, in the chronic stress model of depression, a reduction and normalization of habenula metabolism was the only significant effect after three weeks of treatment with the monoamine oxidase inhibitor tranylcypromine (Caldecott-Hazard et al., 1988). Two human neuroimaging studies have also implicated the habenula in the therapeutic response to antidepressant treatment. Using a group of medicated depressed patients in recovery, Morris et al. (1999) demonstrated that habenula metabolism increased in response to tryptophan depletion, but only in subjects who experienced a relapse of depressive symptoms. This suggests that antidepressant effects dependent on serotonin are related to an inhibition of habenula activity. Tryptophan depletion also resulted in increased blood flow to the habenula in remitted depressed patients in response to emotional words (Roiser et al., 2009). Thus, the current finding of a fluoxetine-induced reduction of habenula metabolism adds to an extremely small but consistent literature.
The finding of increased VTA metabolism concurrent with decreased habenula metabolism adds to a large number of metabolic mapping studies which have consistently found opposite changes in these two regions (reviewed by Shumake & Gonzalez-Lima, 2003), and is consistent with the opposite behavioral effects of stimulating these two regions (Shumake et al., 2010). Out of all brain regions, the habenula has consistently shown the greatest response to various dopamine manipulations (Gomita & Gallistel, 1982; McCulloch et al., 1980; Pizzolato et al., 1984; Wechsler et al., 1979; Wooten & Collins, 1981). This suggests a potential dopaminergic effect of fluoxetine treatment, at least in congenitally helpless rats which show baseline hyperactivity in the habenula and hypoactivity in the VTA (Shumake et al., 2003), perhaps suggesting an abnormality in dopaminergic function and concomitant sensitivity to the dopaminergic actions of drugs. Here it is interesting to note that fluoxetine, but not other SSRIs, increases norepinephrine and dopamine levels in prefrontal cortex (Bymaster et al., 2002). Although one study showed that fluoxetine suppressed the firing rates of dopaminergic VTA neurons acutely but not after 3 weeks of chronic administration, this effect was achieved by administering fluoxetine intravenously at exponentially increasing doses (Prisco & Esposito, 1995). When fluoxetine was given intraperitoneally at lower doses—arguably more appropriate to the pharmacokinetics and dosages used in clinical practice—it caused a significant increase in the number of spontaneously active dopamine neurons in the VTA, both acutely and after 3 weeks of chronic administration (Sekine et al., 2007). The increased metabolic capacity of excitatory synapses innervating the VTA would be consistent with these findings and our finding of increased cytochrome oxidase activity in the VTA.
While our results are consistent with other findings regarding the antidepressant effects on glucose and oxidative metabolism in rat brains (Caldecott-Hazard et al., 1988; Freo et al., 2000; Gonzalez-Pardo et al., 2008; Skelin et al., 2009), we did not see more widespread cortical changes in line with those observed in human imaging studies of depression treatment, such as normalization of metabolic deficits in prefrontal cortex instead of the decrease also observed in normal rats. There are several possible explanations for this, the most simple being that the rat brain may not respond to antidepressants in the same way the human brain does, at least not in terms of regional cortical metabolism. Alternatively, we may have observed more widespread changes had we administered fluoxetine at a higher dose or for a longer time. For example, Mayberg et al. (2000) did not observe normalization of cortical metabolism until after 6 weeks of treatment. Although still more widespread than our observations, changes that Mayberg et al. (2000) observed after 1 week did include decreased metabolism in the medial thalamus, and increased metabolism in the brainstem. Respectively, the habenula and VTA might be involved in these effects, in which case the directions of change are consistent with our findings. Yet another possibility is that, despite a significant response to fluoxetine in the forced-swim test, congenitally helpless rats might be resistant to fluoxetine treatment or might respond better to another class of drug. It is also possible that regions not showing significant differences in our study might show changes with a larger sample size or with a different methodology for mapping neuronal activity. Regardless, the results suggest that the habenula, VTA, dentate gyrus, and dorsomedial prefrontal cortex are highly sensitive to chronic fluoxetine treatment and are among the first regions to adapt to it. These regions are then well-positioned to affect the functioning of other neural systems implicated in helpless and depressive behavior (Shumake & Gonzalez-Lima, 2003).
4. Experimental Procedures
4.1. Subjects
Experiments were done in accordance with NIH guidelines for the use of experimental animals and were approved by the University of Texas Institutional Animal Care and Use Committee. The rats used in this experiment were bred at the University of Texas Animal Resources Center derived from a selectively bred line originated by Henn et al. (1985) and reestablished by Vollmayr and colleagues (2004). The breeding protocol was to test Sprague-Dawley rats in an escape task 24 hours after an inescapable shock session and breed animals with more than 10 failures out of 15 trials across generations. The first four generations of breeding more than doubled the percentage of helplessness-susceptible offspring, and genetic vulnerability continued to increase in the helplessness-susceptible line until a plateau was reached in the 25th generation, when 95% of rats began to show spontaneous helpless behavior in the absence of a training session (Lachman et al., 1992). The originators of the strain have referred to it as “congenital learned-helpless.” Here, we have shortened this to “congenitally helpless” to emphasize that the rats used in this study did not undergo learned helplessness training and testing—they were merely derived from a line of rats that is highly predisposed to learned helplessness. The choice of the word “congenital” as opposed to “genetic” is important because, while the helplessness-susceptibility trait appears inborn, one cannot rule out a possible contribution of the prenatal environment to the phenotype. Congenitally helpless rats appear to be a good model of depressive behavior, demonstrating gene-environment interactions underlying the expression of anhedonia (Enkel et al., 2010).
Twenty congenitally helpless males were selected from the cohort of subjects used in the behavioral characterization study reported in Shumake et al. (2005), during which the rats underwent behavioral testing for open-field and sucrose-consumption behavior which took place between postnatal days 29 and 48. Approximately 3 months later, when subjects weighed an average of 450-550 g, rats were randomly assigned for either fluoxetine treatment (n = 10) or vehicle control (n = 10). Rats were individually housed at this time in 45 × 24 × 21 cm cages in a temperature-controlled room (22 ± 1°C). They were maintained on a 12/12 hour light cycle and provided continuous food and water.
4.2. Drug Administration
Fluoxetine hydrochloride (Sigma) was dissolved in a 25% dimethylsulfoxide (DMSO)-75% saline vehicle (2.5 mg/ml). For two weeks, rats received daily i.p. injections of either fluoxetine/vehicle or vehicle (5 mg/kg). Injections were administered during the last hour of the light cycle.
4.3. Forced-Swim Test
On the last day of drug administration, rats were placed in a glass cylinder (46 cm high × 20 cm in diameter) containing a 30-cm water column (24 ± 1 °C). Two swimming sessions were conducted: an initial 15-min pretest, followed by a 5-min test 24 h later. Test sessions were video-taped for scoring. A time-sampling technique was employed to score several behaviors during a single viewing. This previously described method has proven reliable and valid for detecting the effects of different antidepressant drugs (Detke et al., 1995). At the end of each 5-s period during the 5 min test, the scorer rated the rat’s behavior as belonging to one of the following behavioral categories: (1) immobility—floating passively in the water and only making slight movements to keep the head above the water line (Porsolt et al., 1977); (2) swimming—making active swimming motions, more than necessary to merely keep the head above water; or (3) climbing—making active movements with forepaws in and out of the water, usually directed against the walls. Two raters blind to the treatment conditions scored all of the behaviors. Scores for each behavior were expressed as percentages of time.
4.4. Tissue Processing
Immediately after the conclusion of the forced-swim test, rats were taken to a separate room and decapitated. Brain extraction and processing proceeded according to the protocol for quantitative cytochrome oxidase histochemistry outlined in Shumake et al. (2000; 2001; 2002; 2003; 2004). Following decapitation, brains were removed intact and frozen rapidly in isopentane. Using a cryostat (Reichert-Jung) at −20°C, brains were sectioned at 40 μm and kept frozen at −40°C until they were processed using qua ntitative cytochrome oxidase histochemistry. In brief, the cytochrome oxidase staining procedure involves a series of chemical exposures, the first of which (0.1 M phosphate buffer with 10% wt/vol sucrose and 0.5% vol/vol glutaraldehyde, pH 7.6, for 5 min) facilitates tissue adherence to the slides. The next series of exposures (four changes of 0.1 M phosphate buffer with 10% wt/vol sucrose, for 5 min each) removes red blood cells. Then the slides undergo metal intensification (0.05 M Tris buffer, pH 7.6, with 275 mg/l cobalt chloride, 10% wt/vol sucrose, and 0.5% vol/vol dimethylsulfoxide, for 10 min) to enhance staining contrast and reduce time spent in the following incubation procedure (350 mg diaminobenzidine tetrahydrochloride, 52.5 mg cytochrome c, 35 g sucrose, 14 mg catalase, and 1.75 ml dimethylsulfoxide in 700 ml of oxygen-saturated 0.1 M phosphate buffer, at 37 °C f or 1 hour). The reaction is stopped by fixing the tissue in buffered formalin (for 30 min at room temperature with 10% wt/vol sucrose and 4% vol/vol formalin). Finally, the slides are dehydrated in a series of ethanol baths (increasing from 30% to 100% vol/vol ethanol), cleared with xylene, and coverslipped with Permount.
To quantify enzymatic activity and to control for staining variability across different batches of cytochrome oxidase staining, sets of tissue-homogenate standards were included with each batch of slides. This tissue homogenate was obtained from 12 normal adult Sprague-Dawley male rats, whose brains were removed after decapitation, stored at 4°C (in sodium phosphate buffer, pH 7.6), and then homogenized at 4°C. The enzymatic activity of cytochrome oxidase in this homogenate was assayed using spectrophotometry, as described by Gonzalez-Lima and Cada (1994), and activity units were defined at pH 7 and 37°C, where 1 unit oxidizes 1 μmol of reduced cytochrome c per min (μmol/min/g tissue wet weight). Remaining tissue homogenate was frozen and stored at −40°C.
Immediately prior to each cytochrome oxidase staining procedure, the standard homogenate was sectioned at varying thickness (10, 20, 40, 60, and 80 μm), and these sections were thaw-mounted onto a slide and stained for cytochrome oxidase along with the experimental tissue. Activity values determined from the spectrophotometric procedure were assigned to each standard, and these values were correlated with corresponding optical density measurements of the cytochrome oxidase chromatic indicator, taken with an image-processing system as described by Gonzalez-Lima and Cada (1994). The resulting linear regression equations (r2 > .90) were used to convert optical density readings from brain regions of interest into cytochrome oxidase activity values, which were used in all statistical analyses in this study.
Using an image-processing system (JAVA, Jandel Scientific, Corte Madera, CA), optical density was sampled from the regions of interest. The size of the square-shaped sampling window was adjusted for each region so that it was as large as possible while still allowing four, non-overlapping readings to be taken bilaterally. For each region, optical density was sampled across three adjacent sections and averaged. These optical density values were then converted to cytochrome oxidase activity units, which were determined by spectrophotometry of cytochrome oxidase standards as described above. In addition to the regions of interest, whole-brain metabolism was assessed by taking the mean of the average activity for each section of the brain. Unfortunately, artifacts in tissue processing resulted in the exclusion of three subjects from the fluoxetine group, such that the final sample size for quantitative cytochrome histochemistry was fluoxetine (n = 7) and vehicle (n = 10).
4.5. Delineating Regions of Interest
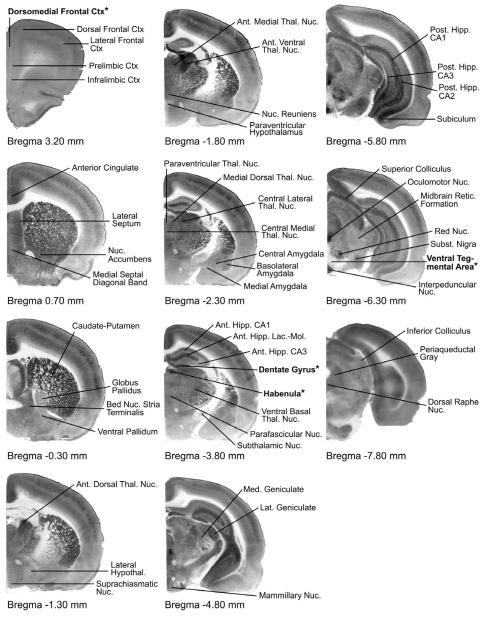
Regions of interest were those showing significant effects in the previous brain metabolic studies of congenitally helpless rats (Shumake & Gonzalez-Lima, 2003) as well as a number of neighboring regions. Regions were imaged according to the specifications given in Shumake et al. (2000; 2001; 2002; 2003; 2004). Because of previous work demonstrating highly collinear cytochrome oxidase activity between the lateral and medial nuclei of the habenula, the entire habenula was sampled as a unitary region in this analysis. See Figure 2 for details.
Figure 2.
Cytochrome-oxidase stained sections indicating regions of interest by Bregma level. Regions appearing in bold with asterisks (habenula, ventral tegmental area, dentate gyrus, and dorsomedial frontal cortex) showed significant differences (p < .05) in cytochrome oxidase (μmol/min/g tissue wet weight) between fluoxetine (N = 7) and vehicle (N = 10) groups.
Acknowledgements
The authors thank Drs. B. Vollmayr and F. Henn for breeding pairs from the congenital learned helpless line. This work was supported by NIH grants R01 MH076847 to FGL and T32 MH65728 fellowship to JS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR., Jr. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol. Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J. Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Enkel T, Spanagel R, Vollmayr B, Schneider M. Stress triggers anhedonia in rats bred for learned helplessness. Behav. Brain Res. 2010;209:183–186. doi: 10.1016/j.bbr.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Freo U, Ori C, Dam M, Merico A, Pizzolato G. Effects of acute and chronic treatment with fluoxetine on regional glucose cerebral metabolism in rats: implications for clinical therapies. Brain Res. 2000;854:35–41. doi: 10.1016/s0006-8993(99)02261-1. [DOI] [PubMed] [Google Scholar]
- Gomita Y, Gallistel CR. Effects of reinforcement-blocking doses of pimozide on neural systems driven by rewarding stimulation of the MFB: a 14C-2-deoxyglucose analysis. Pharmacol. Biochem. Behav. 1982;17:841–845. doi: 10.1016/0091-3057(82)90369-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Cada A. Cytochrome oxidase activity in the auditory system of the mouse: a qualitative and quantitative histochemical study. Neuroscience. 1994;63:559–578. doi: 10.1016/0306-4522(94)90550-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pardo H, Conejo NM, Arias JL, Monleon S, Vinader-Caerols C, Parra A. Changes in brain oxidative metabolism induced by inhibitory avoidance learning and acute administration of amitriptyline. Pharmacol. Biochem. Behav. 2008;89:456–462. doi: 10.1016/j.pbb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Henn FA, Johnson J, Edwards E, Anderson D. Melancholia in rodents: neurobiology and pharmacology. Psychopharmacol. Bull. 1985;21:443–446. [PubMed] [Google Scholar]
- Hickie IB, Scott EM, Davenport TA. Are antidepressants all the same? Surveying the opinions of Australian psychiatrists. Aust. N. Z. J Psychiatry. 1999;33:642–649. doi: 10.1080/j.1440-1614.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Citalopram--pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1982;6:277–295. doi: 10.1016/s0278-5846(82)80179-6. [DOI] [PubMed] [Google Scholar]
- King JA, Abend S, Edwards E. Genetic predisposition and the development of posttraumatic stress disorder in an animal model. Biol. Psychiatry. 2001;50:231–237. doi: 10.1016/s0006-3223(01)01071-x. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Weiner ED, Ramazankhana R, Hartnick C, Edwards E, Henn FA. Hippocampal neuropeptide Y mRNA is reduced in a strain of learned helpless resistant rats. Brain Res. Mol. Brain Res. 1992;14:94–100. doi: 10.1016/0169-328x(92)90015-4. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol. Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Savaki HE, Sokoloff L. Influence of dopaminergic systems on the lateral habenular nucleus of the rat. Brain Res. 1980;194:117–124. doi: 10.1016/0006-8993(80)91322-0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Nurnberg HG, Thompson PM, Hensley PL. Antidepressant medication change in a clinical treatment setting: a comparison of the effectiveness of selective serotonin reuptake inhibitors. J Clin. Psychiatry. 1999;60:574–579. doi: 10.4088/jcp.v60n0902. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Patel JG, Bartoszyk GD, Edwards E, Ashby CR., Jr. The highly selective 5-hydroxytryptamine (5-HT)2A receptor antagonist, EMD 281014, significantly increases swimming and decreases immobility in male congenital learned helpless rats in the forced swim test. Synapse. 2004;52:73–75. doi: 10.1002/syn.10308. [DOI] [PubMed] [Google Scholar]
- Pizzolato G, Soncrant TT, Rapoport SI. Haloperidol and cerebral metabolism in the conscious rat: relation to pharmacokinetics. J. Neurochem. 1984;43:724–732. doi: 10.1111/j.1471-4159.1984.tb12792.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prisco S, Esposito E. Differential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br. J. Pharmacol. 1995;116:1923–1931. doi: 10.1111/j.1476-5381.1995.tb16684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, Henn FA, Sahakian BJ, Drevets WC. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol. Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res. Brain Res. Rev. 2005;48:1–15. doi: 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR., Jr. Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse. 2007;61:72–77. doi: 10.1002/syn.20349. [DOI] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav. Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Shumake J, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F. Brain differences in newborn rats predisposed to helpless and depressive behavior. Brain Res. 2004;1030:267–276. doi: 10.1016/j.brainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Hypermetabolism of paraventricular hypothalamus in the congenitally helpless rat. Neurosci. Lett. 2001;311:45–48. doi: 10.1016/s0304-3940(01)02142-5. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Dissociation of septo-hippocampal metabolism in the congenitally helpless rat. Neuroscience. 2002;114:373–377. doi: 10.1016/s0306-4522(02)00297-x. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav. Cogn. Neurosci. Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J. Neurosci. 2010;30:5876–5883. doi: 10.1523/JNEUROSCI.3604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Poremba A, Edwards E, Gonzalez-Lima F. Congenital helpless rats as a genetic model for cortex metabolism in depression. Neuroreport. 2000;11:3793–3798. doi: 10.1097/00001756-200011270-00040. [DOI] [PubMed] [Google Scholar]
- Skelin I, Sato H, Kovacevic T, Diksic M. Chronic therapy with citalopram decreases regional cerebral glucose utilization in OBX, and not sham-operated, rats: an autoradiographic study. Psychopharmacology (Berl) 2009;207:315–323. doi: 10.1007/s00213-009-1659-4. [DOI] [PubMed] [Google Scholar]
- Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine. Pharmacol. Biochem. Behav. 2004;78:165–169. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav. Brain Res. 2004;150:217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Wechsler LR, Savaki HE, Sokoloff L. Effects of d- and l-amphetamine on local cerebral glucose utilization in the conscious rat. J. Neurochem. 1979;32:15–22. doi: 10.1111/j.1471-4159.1979.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Collins RC. Metabolic effects of unilateral lesion of the substantia nigra. J. Neurosci. 1981;1:285–291. doi: 10.1523/JNEUROSCI.01-03-00285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]