Abstract
Previous studies have shown that the Wistar-Kyoto (WKY) rat strain is more sensitive to stressors and consumes significant quantities of alcohol under basal as well as stressful conditions when compared to other strains. Given that the glutamate neurotransmitter system has been implicated in depression and addiction, the goals of the present study were to investigate the effects of stress and stress-alcohol interactions on N-methyl-D-aspartate (NMDA) receptors in the rat brain. Thus this study measured the binding of [3H] MK-801 to NMDA receptors in the prefrontal cortex (PFC), caudate putamen (CPu), nucleus accumbens (NAc), hippocampus (HIP) and basolateral amygdala (BLA) in WKY rats in comparison to the Wistar (WIS) rat strain. Our results suggested that while voluntary alcohol consumption did not alter NMDA receptors in the PFC, CPu or NAc in either rat strain, it increased NMDA receptors in the HIP and BLA in both strains. In contrast, chronic stress increased NMDA receptors in the PFC, CPu, NAc in WKY rats but not in WIS rats. Chronic stress also decreased NMDA receptors in the HIP and increased NMDA receptors in the BLA in both strains. Alcohol co-treatment with stress increased NMDA receptors in the PFC, CPu and NAc in WKY rats but not in WIS rats. Interestingly, while alcohol co-treatment did not reverse stress induced decreases in NMDA receptors in the HIP, it reduced the binding of NMDA receptors in the BLA to control levels in both strains. Thus it appears that NMDA receptors in the PFC, CPu and NAc may be more sensitive to the effects of stress and could be implicated in the stress-induced alcohol consumption behavior seen in WKY rats. In contrast, NMDA receptors in the HIP and BLA may reflect an adaptive response and may not be responsible for the stress susceptible phenotype of the WKY rat strain.
Keywords: depression, animal model, [3H] MK-801 binding, glutamate
Numerous studies have suggested that negative emotional state (e.g. depression) and exogenous stressful stimulation contribute to the development of alcoholism in human subjects (Brown et al., 1995) and experimental animals (Rockman 1987; Coffey et al., 2002). According to the National Comorbidity Surveys (NSDUH2005) and other related investigations, alcohol dependency most commonly co-occurs with depressive disorder (Kessler et al., 1997, Jane-Llopis and Matytsina, 2006). Treatment with antidepressant drugs has been shown to reduce depressive symptoms as well as decrease alcohol consumption in alcoholic patients (Brown et al., 1997; Cornelius et al., 1997). Although the exact mechanism responsible for the possible linkage between stress and psychological disorders is not well understood, it has been suggested that the interaction between genetic-predisposition and environmental stressors influences the presentation of conditions such as major depression as well as addiction (Zubin and Spring, 1977; Kendler et al., 1995; Parnas, 1999).
It is well known that glutamate homeostasis and neurotransmission are disrupted in major depressive disorder (Paul and Skolnick, 2003; Feyissa et al., 2009). Stress-induced modulation of glutamate has been proposed to contribute to the etiology and progression of depressive illness (Moghaddam, 2002). Stress exposure increases excitatory amino acid neurotransmission in the PFC, CPu, HIP and amygdala (Lowy et al., 1993; Moghaddam, 1993; Bagley et al., 1997); brain regions that are implicated in stress, depression and reward. Alcohol consumption affects brain functions by interacting with several neurotransmitter systems including the glutamatergic system of which the NMDA receptor is a major molecular target (Alele and Devaud, 2005; Heinz et al., 2005; Larsson et al., 2005; Raeder et al., 2008; Ridge et al., 2008). A glutamatergic hypothesis of human alcoholism suggests that neuropsychological and pathological effects of alcohol may be mediated through the glutamatergic system (Samson and Harris, 1992; Tsai et al., 1995), especially the NMDA type of glutamate receptors (Nagy et al., 2005).
The WKY rat strain has been proposed as an animal model of depressive behavior (Lopez-Rubalcava and Lucki, 2000; Paré et al., 1989a and b; Redei et al., 1994; Tejani-Butt et al., 2003). Several studies have noted that WKY rats differ from other strains in their behavioral, physiological, and neuroendocrine responsiveness to environmental as well as pharmacological challenges (Lopez-Rubalcava and Lucki, 2000; Redei et al., 1994; Tejani-Butt et al., 1994; Tejani-Butt et al., 2003). Exposing WKY rats to stress stimulation results in behaviors which resemble human depressive behavior, such as anhedonia (Paré 1994a), psychomotor retardation (Paré 1994b), ambivalence (Paré 1993), and negative memory bias (Paré 1996). We have previously suggested that the WKY rat strain may represent a suitable model for studying the neurochemical mechanisms underlying depressive behavior and increased alcohol consumption (Paré et al., 1999; Jiao et al., 2006). Given the involvement of NMDA receptors in the effects of alcohol as well as stress, the present study investigated the effects of alcohol consumption, stress and stress-alcohol interactions on the binding of [3H] MK-801 to NMDA receptor sites in the brains of WKY rats and compared them to a control WIS rat strain.
Experimental Procedure
Animals
WIS rats were purchased from Harlan (Indianapolis, IN). WKY rats were obtained from Charles River Laboratories (Kingston, NY). Age matched male WKY and WIS rats (6-8 months) were used in this study. Animals were handled according with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, and with the Perry Point VAH Institutional Review Committee approval. All efforts were made to minimize the number of animals used and their suffering. Animals were individually housed at 22°C and placed on a 12-hr light/dark cycle. Both strains were assigned into four groups (n=8): (1) control group (Control), (2) no stress with 24-day alcohol treatment (AL), (3) 24-day chronic-stress (CS) and (4) 24-day chronic-stress with 24-day alcohol treatment (CS-AL).
Alcohol treatment
The alcohol procedure established by Sandbak and Murison (1996) was used with minor modification. Animals from alcohol groups were given access alcohol or water by offering two sipper-type drinking tubes with tap water in one and alcohol solution in the other. The position of the tubes was switched daily (left/right) to eliminate the possibility of location preference. On the other hand, control group was offered two sipper tubes containing water. A 3% solution was presented during the first (1-7 days), a 5% solution on the second 7-day (8-14 days) period, and a 7% solution was presented during the rest of time (15-24 days). Body weight and alcohol consumption was recorded daily at the same time.
Chronic mild stress procedure
A chronic mild stress procedure with a repeated-novel-stressor regimen, based on the procedure established by Katz et al. (1981), with minor modifications (Tejani-Butt et al., 1994), was used in this study. Experimental animals were exposed to a schedule of different daily stressors. The elements of the schedule consists of a scrambled foot shock (1.0 s., 1.0 mA scrambled foot shock presented with a variable interval of 10 s. between shocks in a 20×20×25 cm stainless steel cage for 30 min), food deprivation (remove food from home cage for 40 hr), cold swim (forced swim in 4°C water for 5 min), water deprivation (remove water bottle 24 h), restraint (placing the rat in ¼-in hardware cloth tube with a diameter of 38 cm and 18 cm long for 2 h), shaker stress (animal was in home cage and placed on shaker platform for 15 min), restraint (rat was placed in a 5mm hardware cloth tubes with a diameter of 3.8 cm and 18 cm long for 2 hr), heat stress (exposed to 40°C ambient temperature in a drying oven for 5 min), reverse light/dark cycle (artificial light on between 18.00 and 06.00h); switch cage mate; increased housing density (rats housed 5 per 18 × 18 × 32 cm cage for 24 hours). The order of stress administration is described in Table 1. Body weight was recorded daily at the same time.
Table1.
Chronic stress schedule
| Days | Stress treatment | Days | Stress treatment | Days | Stress treatment |
|---|---|---|---|---|---|
| 1 | Foot shock | 9 | Food deprivation | 17 | Reverse L/D cycle |
| 2 | Food deprivation | 10 | Heat stress | 18 | Increase housing density |
| 3 | Cold swim | 11 | Restraint | 19 | Foot shock |
| 4 | Water deprivation | 12 | Reverse L/D cycle | 20 | Water deprivation |
| 5 | Restraint | 13 | Cold swim | 21 | Heat stress |
| 6 | No stress | 14 | Foot shock | 22 | Food deprivation |
| 7 | Shaker stress | 15 | Switch cage mate | 23 | Shaker stress |
| 8 | Heat stress | 16 | Food deprivation | 24 | Switch cage mate |
Stress-Alcohol treatment
The stress-alcohol group received the same stress procedure as described above, but received free access to water or alcohol (as described above). Body weight was recorded daily at the same time (Fig. 1). Alcohol consumption was recorded daily at the same time and has been reported previously (Yaroslavsky and Tejani-Butt, 2010).
Fig.1. Mean body weights of WIS and WKY rats over 24 days of experiment.
Data are expressed as the mean body weights of 6-8 rats from each group over 24 days of experiment. No significant change in weights was noted following treatment with AL, CS and CS-AL, compared to their respective control group, Student's t test, P>0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Brain section preparation
On Day 25, all animals were sacrificed by rapid decapitation and the brains were removed immediately, dipped into −20°C isopentane and stored at -80°C until use. All of the brain tissue sections (16 μm) were cut at -18°C in a cryostat microtome according to the Brain Atlas of Paxinos and Watson (1998) and mounted onto gelatin-coated microscope slides. Sections from plate 12 and 30 including PFC, CPu and NAc, HIP and BLA were examined in the current study.
[3H] MK-801 binding assay
NMDA receptor autoradiography of [3H] MK-801 (Sigma Aldrich; 27.5 Ci/mmol) was performed as previously described (Lei et al., 2009). Briefly, brain sections were thawed to room temperature and pre-washed in 50 mM Tris–HCl buffer (pH 7.4) for 30 min at 4°C. Then, the sections were incubated in 50 mM Tris–HCl buffer containing 15 nM [3H] MK-801 and 30 μ M glutamate/15 μ M glycine for 120 min at room temperature, rinsed for 30 min with cold 50 mM Tris–HCl buffer, dipped once in ice-cold distilled water, and immediately dried in a stream of cool air. Non-specific binding was measured in the presence of 50 μM non-radioactive MK-801 and was less than 10% of total binding. Dried tissue sections were exposed to autoradiography film. Following a 4-week exposure period at 4°C, the film was developed in Kodak D19, at room temperature.
Quantitation and Statistics
The autoradiography films were analyzed with ImageJ version 1.41. Nonspecific binding was subtracted from the total binding to provide the specific binding in the regions of interest. The values of binding density were expressed as mean ± S.E.M specific binding (fmol/mg brain protein). Statistical analysis was performed with Sigma Stat for Windows. The data were analyzed using two-way analysis of variance (ANOVA), designed with strain (two levels) and treatment (three levels) as independent variables. Post hoc Tukey-HSD tests was conducted to locate significant differences (P<0.05).
Results
Alcohol drinking behavior and body weight changes during the experimental period
The average 24-day alcohol consumption was previously reported to increase in both stressed WKY and WIS rats compared to non-stressed animals. However, the average alcohol consumption was found to be significantly greater in non-stressed and stressed WKY compared to WIS rats (Yaroslavsky and Tejani-Butt, 2010). In contrast, there was no significant change in body weights among WKY and WIS rats following treatment with AL, CS and CS-AL, compared to their respective controls that received water throughout the 24-day experimental period (Fig.1).
Treatment related changes in [3H] MK-801 binding to NMDA receptors in the PFC
Significant strain effects [F (1, 47) = 28.26, P<0.001], treatment effects [F (3, 47) = 22.2 P<0.001] and strain × treatment interactions [F (3, 47) = 37.45, P<0.001] were identified in the PFC of WKY and WIS rats, following Control, AL, CS or CS-AL treatments. As shown in Fig. 2, WKY rats from Control and AL groups exhibited lower binding of [3H] MK-801 to NMDA receptors in the PFC compared to WIS rats [Tukey HSD test, P<0.05]. Significant differences within WKY rat strain, but not WIS strain, were found in the PFC following treatment with CS or CS-AL. Both CS and CS-AL increased the binding of [3H] MK-801 to NMDA receptors in the PFC of WKY rats compared to Control and AL groups [Tukey HSD test, P<0.05].
Fig.2. Effects of alcohol, chronic stress and co-treatment with alcohol and chronic stress on the binding of [3H] MK-801 to NMDA receptor in the prefrontal cortex in WKY and WIS rats.
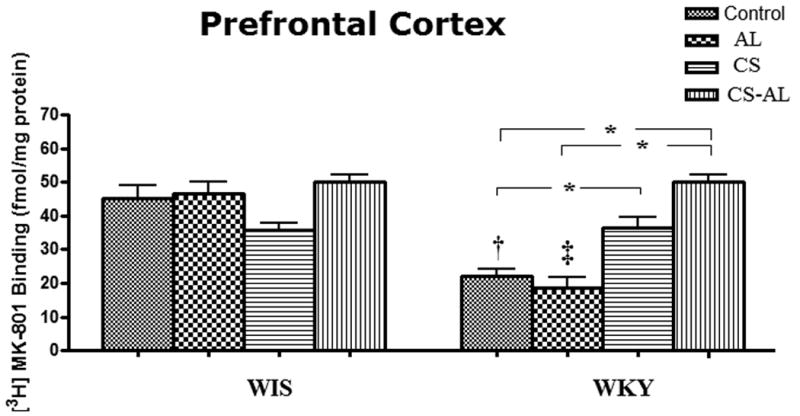
Data are expressed as the mean ± S.E.M. of measurements from 6-8 rats from each strain, with determinations made in duplicate sections from each brain. * Represents significant differences between treatment groups within strain, Tukey HSD test, P<0.05; † and ‡ represent significant differences between WKY and WIS within Control groups and AL groups respectively, Tukey HSD test, P<0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Treatment related changes in [3H] MK-801 binding to NMDA receptors in the CPu
Significant strain effects [F (1, 47) = 27.95, P<0.001], treatment effects [F (3, 47) = 6.31 P=0.001], and strain × treatment interactions [F (3, 47) = 26.94, P<0.001] were observed in the CPu in WKY and WIS rats. The binding of [3H] MK-801 to NMDA receptors in WKY rats was significantly lower in Control and AL groups compared to WIS rats [Tukey HSD test, P<0.05]. As shown in Fig. 3, CS increased the binding of [3H] MK-801 in WKY rats, but not in WIS rats [Tukey HSD test, P<0.05]. While CS-AL treatment increased NMDA receptors in the CPu in WKY rats, it decreased NMDA receptor binding in WIS rats compared to respective control and alcohol groups [Tukey HSD test, P<0.05].
Fig.3. Effects of alcohol, chronic stress and co-treatment with alcohol and chronic stress on the binding of [3H] MK-801 to NMDA receptor in the caudate putamen in WKY and WIS rats.
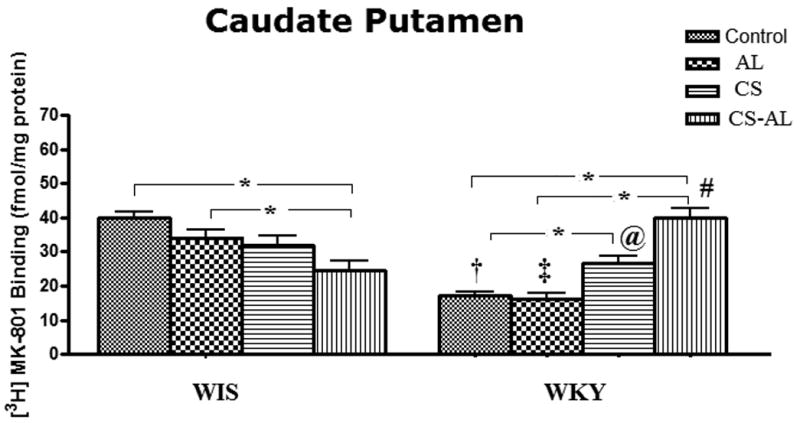
Data are expressed as the mean ± S.E.M. of measurements from 6-8 rats from each strain, with determinations made in duplicate sections from each brain. * Represents significant differences between treatment groups within strain, Tukey HSD test, P<0.05. †, ‡, @ and ♯ represent significant differences between WKY and WIS within their respective treatment groups, Tukey HSD test, P<0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Treatment related changes in [3H] MK-801 binding to NMDA receptors in the NAc
Significant treatment effects [F (3, 47) = 11.24, P<0.001] and strain × treatment interactions [F (3, 47) = 15.1, P<0.001] but not strain effects [F (1, 47) = 3.11, P>0.05] were observed in the NAc of WKY and WIS rats. As shown in Fig. 4, the binding of [3H] MK-801 to NMDA receptors was significantly lower in WKY Control and AL groups compared to WIS rats [Tukey HSD test, P<0.05]. However, the binding was increased in CS WKY rats compared to Control group [Tukey HSD test, P<0.05) and the increase in binding was maintained in CS-AL treated WKY rats [Tukey HSD test, P<0.05].
Fig.4. Effects of alcohol, chronic stress and co-treatment with alcohol and chronic stress on the binding of [3H] MK-801 to NMDA receptor in the nucleus accumbens in WKY and WIS rats.
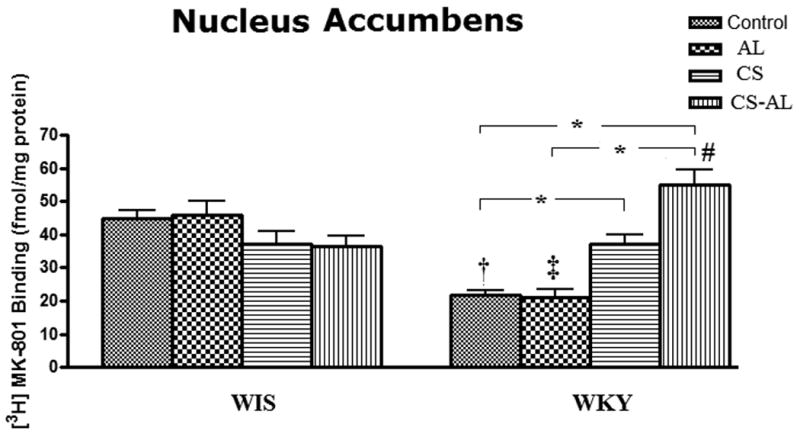
Data are expressed as the mean ± S.E.M. of measurements from 6-8 rats from each strain, with determinations made in duplicate sections from each brain. * Represents significant differences between treatment groups within strain, Tukey HSD test, P<0.05. †, ‡ and ♯ represent significant differences between WKY and WIS within their respective treatment groups, Tukey HSD test, P<0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Treatment related changes in [3H] MK-801 binding to NMDA receptors in the HIP
In the HIP (CA1 and CA2), significant strain effects [F (1, 47) = 50.36, P<0.001], treatment effects [F (3, 47) = 108.4, P<0.001] and strain × treatment interactions [F (3, 47) = 32.71, P<0.001] were observed between WKY and WIS rats. As shown in Fig. 5, the binding of [3H] MK-801 to NMDA receptor sites in the HIP of WKY rats from all the groups was significantly lower compared to the respective groups of WIS rats [Tukey HSD test, P<0.05]. Alcohol exposure increased the binding in both WKY and WIS rats when compared to their respective Control groups [Tukey HSD test, P<0.05]. In contrast, CS and CS-AL treatments decreased NMDA receptors in the HIP in both WKY and WIS rats [Tukey HSD test, P<0.05].
Fig.5. Effects of alcohol, chronic stress and co-treatment with alcohol and chronic stress on the binding of [3H] MK-801 to NMDA receptor in the hippocampus in WKY and WIS rats.
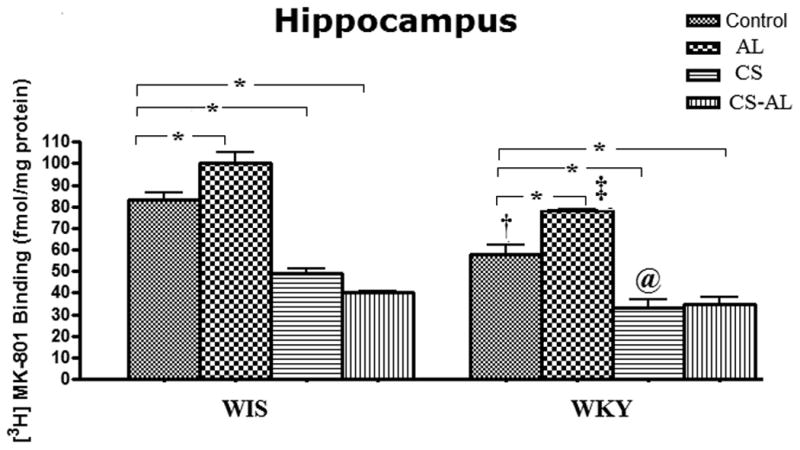
Data are expressed as the mean ± S.E.M. of measurements from 6-8 rats from each strain, with determinations made in duplicate sections from each brain. * Represents significant differences between treatment groups within strain, Tukey HSD test, P<0.05. †, ‡ and @ represent significant differences between WKY and WIS within their respective treatment groups, Tukey HSD test, P<0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Treatment related changes in [3H] MK-801 binding to NMDA receptors in the BLA
In the BLA, no significant strain effects [F (1, 47) = 2.9, P=0.096] nor strain × treatment interactions [F (3, 47) = 0.946, P = 0.065] were identified between WKY and WIS rats. However, a significant treatment effect [F (3, 47) = 85.7, P<0.001] was seen in both strains. As shown in Fig. 6, alcohol treatment led to a higher binding of [3H]-MK 801 to NMDA sites [Tukey HSD test, P<0.05]. CS also increased the binding of [3H]-MK 801 to NMDA receptors in both strains [Tukey HSD test, P<0.01]. In contrast, CS-AL treatment did not appear to have any significant effect on NMDA receptor binding in the BLA in either rat strain.
Fig.6. Effects of alcohol, chronic stress and co-treatment with alcohol and chronic stress on the binding of [3H] MK-801 to NMDA receptor in the basolateral amygdala in WKY and WIS rats.
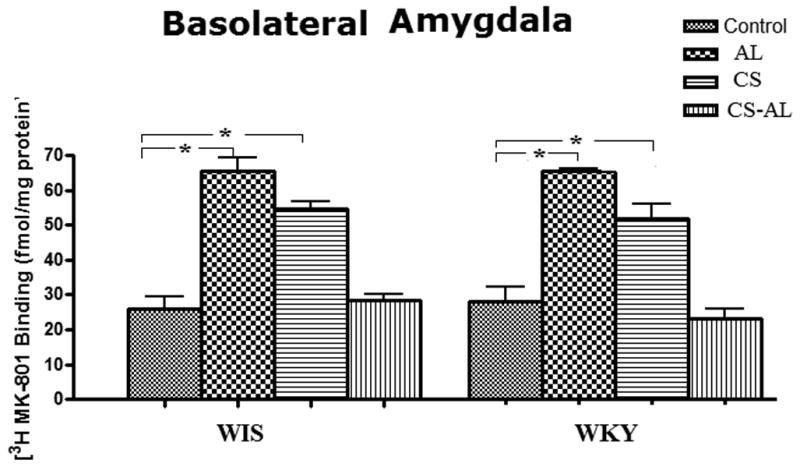
Data are expressed as the mean ± S.E.M. of measurements from 6-8 rats from each strain, with determinations made in duplicate sections from each brain. * Represents significant differences between treatment groups within strain, Tukey HSD test, P<0.05. Control: control group; AL: alcohol group; CS: chronic stress group; CS-AL: co-treatment with alcohol and chronic stress group.
Discussion
Effects of alcohol on [3H] MK-801 binding to NMDA receptors
We have previously reported that WKY rats consumed greater amounts of alcohol compared to WIS rats under basal condition (Jiao et al., 2006). Furthermore, when WKY and WIS rats were exposed to a 24 day schedule of novel stressors, both stressed WKY and WIS rats voluntarily consumed more alcohol compared to their non-stressed groups. However, the average alcohol consumption was significantly greater in non-stressed and stressed WKY rats compared to WIS rats (Yaroslavsky and Tejani-Butt, 2010). Given that the glutamate neurotransmitter system has been implicated in depression and addiction, the current study investigated the effects of alcohol, stress and stress-alcohol interactions on NMDA receptors in the PFC, CPu, NAc, HIP and BLA; brain areas implicated in stress and reward.
Previously, the binding of [3H] MK-801 to NMDA receptors was compared in naïve WKY and WIS rats (Lei et al., 2009). Since lower binding was observed in the CPu, NAc and HIP of WKY rats, it may reflect a reduced number of functional receptors, a deficiency in the NMDA receptor complex or a higher level of glutamate neurotransmission in these brain regions in naïve WKY rats compared to WIS rats (Lei et al., 2009). In the present study, alcohol consumption for 24 days did not alter NMDA receptors in the PFC, CPu and NAc in either strain, suggesting that alcohol consumption may not alter glutamate neurotransmission in these brain areas. Our results are in agreement with those of Tremwel and coworkers (1994) and Rudolph and coworkers (1997) who have reported that chronic alcohol exposure does not alter NMDA receptors in the PFC, CPu or NAc. In contrast, we observed an increase in NMDA receptors in the HIP and BLA in both rat strains following alcohol consumption for 24 days. Others have suggested that alcohol induced NMDA receptor up-regulation in the HIP (Trevisan et al., 1994; Rudolph et al., 1997; Winkler et al 1999; Maler et al., 2005) and the BLA (Floyd et al., 2003) may be due to an up-regulation of the NMDA-R1 subunit. Since different subunit compositions of NMDA-receptors exist in different brain areas (Laurie et al., 1995; Rafiki et al., 2000), it is possible that these subunits show differential sensitivity to alcohol treatment (Rammes et al., 2001). Increased binding in the HIP may be related to cognitive deficits associated with some aspects of alcohol dependency (Trevisan et al., 1994; Grant et al., 1990), whereas increased binding in the BLA may influence the expression of withdrawal anxiety, contributing to alcohol seeking behaviors (Floyd et al., 2003). Thus, one could speculate that the differential NMDA receptor binding observed in the HIP and BLA may reflect alcohol-induced changes in the subunit composition of NMDA-receptors. Furthermore, since these changes were observed in both rat strains, it is possible that NMDA receptors in the HIP and BLA may not be implicated in the increased voluntary alcohol consumption behavior that was previously noted in WKY rats. This is in agreement with previous reports which suggest that altered glutamate signaling in the BLA following drugs of abuse or alcohol may be a common characteristic shared by this class of addictive drugs (Silberman et al., 2009).
Effects of Stress on [3H] MK-801 binding to NMDA receptors
We have recently reported that chronic stress altered nigrostriatal and mesolimbic DA pathways, as measured by an increase in D2 receptor binding in the WKY rat strain (Yaroslavsky and Tejani-Butt, 2010). In the present study, chronic stress increased [3H] MK-801 binding to NMDA receptors in the PFC, CPu and NAc of WKY rats but not in WIS rats. Since glutamate projections to the limbic system regulate DA neurotransmission, it is possible that NMDA receptor-mediated regulation of DA may be compromised in this stress-sensitive rat as a consequence of increased glutamatergic neurotransmission (Moghaddam, 2002).
It was interesting to note that in this study, NMDA receptor binding was decreased in the HIP and increased in the BLA in both WKY and WIS rats following chronic stress. Stress is known to modulate synaptic plasticity and memory processing in the HIP (Kim and Yoon, 1998; Garcia, 2001; Kim and Diamond, 2002; Diamond et al., 2004; Huang et al., 2005) via sensory inputs from several afferent structures. The amygdala is an important input source and plays a major role in mediating the stress response by modulating plasticity in the HIP (Pikkarainen et al., 1999). Thus, increased anxiety related responses to stress exposure would be expected to increase BLA neuronal excitability leading to changes in the expression of plasticity genes, such as the glutamate receptors (Mozhui et al., 2010). Since it has been hypothesized that such changes reflect neuroadaptation (Mozhui et al., 2010), it is possible that glutamate regulation in the HIP and BLA are reflective of an adaptation response to stress in both strains and not to the stress susceptible phenotype reported in the WKY rat strain.
Effects of stress and alcohol on [3H] MK-801 binding to NMDA receptors
While alcohol consumption alone did not alter NMDA receptor binding in the PFC, CPu and NAc, alcohol consumption in the presence of chronic stress increased NMDA receptor binding in the same brain regions in WKY rats. Since alcohol consumption had a different effect on NMDA receptor binding in non-stressed vs. stressed WKY rats, it suggests that NMDA receptors in the PFC, CPu and NAc may be influenced by stress to a greater extent than alcohol exposure. Given that glutamate, acting through NMDA receptors, exerts an inhibitory action on DA release in the PFC, NAc and CPu in stressed rats (Hoffman and Tabakoff, 1996; Kretschmer, 1999), the noted up-regulation of NMDA receptors during stress and alcohol co-treatment could increase the tonic inhibitory control over DA release, resulting in decreased DA activity and reduction of DA release. This might be, in part, a neurochemical basis for the motivation to consume alcohol in WKY rats (Yaroslavsky and Tejani-Butt, 2010).
In the present study, co-treatment with stress and alcohol decreased NMDA receptors in the HIP in both WKY and WIS rat strains. Lower NMDA receptors in the HIP may reflect a reduced number of functional receptors or a deficiency in the NMDA receptor complex. Since the HIP is densely packed with GABA neurons (Banks et al., 2000), lower NMDA receptor binding may also be due to a shift in the balance between excitatory NMDA and inhibitory GABA receptors in the HIP (Lei et al., 2009). Given that co-treatment with alcohol and stress had no effect on NMDA receptors in the BLA, it is possible that GABAergic inhibition may counter glutamatergic excitation, leading to a “normalization” of NMDA receptors in the BLA. Alternately, a “normalization” of NMDA receptors in the BLA could be due to the stress reducing properties of alcohol in both rat strains.
In conclusion, the present study suggests that NMDA receptors in the PFC, CPu and NAc may be more sensitive to the effects of stress and could be implicated in the stress-induced alcohol consumption behavior that is reported in WKY rats. In contrast, NMDA receptor regulation in the HIP and BLA may reflect an adaptation response in both strains and may not be responsible for the stress susceptible phenotype reported in the WKY rat strain.
Acknowledgments
Support for this research was provided by USPHS grant AA 015921 (ST-B).
Abbreviations
- BLA
basolateral amygdala
- CPu
caudate–putamen
- DA
dopamine
- HIP
hippocampus
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartic acid
- PFC
prefrontal cortex
- WIS
Wistar
- WKY
Wistar Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29:1027–1034. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Banks MI, White JA, Pearce RA. Interactions between distinct GABA (A) circuits in hippocampus. Neuron. 2000;25:449–57. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans M, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Woodson J. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar Kyoto rat, a genetic animal model for depression. NeuroReport. 2000;11:627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;133:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, hippocampal plasticity, and spatial learning. Synapse. 2001;40:180–183. doi: 10.1002/syn.1040. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B. Alcohol dependence: a commentary on mechanisms. Alcohol Alcohol. 1996;31:333–340. doi: 10.1093/oxfordjournals.alcalc.a008159. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang CH, Hsu KS. Do stress and long-term potentiation share the same molecular mechanisms? Mol Neurobiol. 2005;32:223–235. doi: 10.1385/MN:32:3:223. [DOI] [PubMed] [Google Scholar]
- Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: A review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- Jiao X, Paré WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006;1073-1074:175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yoon KS. Stress: Metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD. Modulation of the mesolimbic dopamine system by glutamate: role of NMDA receptors. J Neurochem. 1999;73:839–848. doi: 10.1046/j.1471-4159.1999.0730839.x. [DOI] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic Ethanol and Withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Putzke J, Zieglgänsberger W, Seeburg PH, Tölle TR. The distribution of splice variants of the NR1 subunit mRNA in adult rat brain. Brain Res Mol Brain Res. 1995;32:94–108. doi: 10.1016/0169-328x(95)00067-3. [DOI] [PubMed] [Google Scholar]
- Lei Y, Yaroslavsky I, Tejani-Butt SM. Strain differences in the distribution of N-methyl-d-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain. Life Sci. 2009;85:794–799. doi: 10.1016/j.lfs.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swim test. Neuropsychopharmacol. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, Bleich S, Rüther E, Kornhuber J. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052:156–162. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increase extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurosci. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J, Kolok S, Boros A, Dezso P. Role of altered structure and function of NMDA receptors in development of alcohol dependence. Curr Neuropharmacol. 2005;3:281–297. doi: 10.2174/157015905774322499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré WP. Stress ulcer susceptibility and depression in Wistar-Kyoto rats. Physiol Behav. 1989a;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Paré WP. “Behavioral despair” test predicts ulceration in WKY rats. Physiol Behav. 1989b;46:483–487. doi: 10.1016/0031-9384(89)90025-5. [DOI] [PubMed] [Google Scholar]
- Paré WP. Passive avoidance behavior in Wistar Kyoto, Wistar and Fischer 344 rats. Physiol Behav. 1993;54:845–852. doi: 10.1016/0031-9384(93)90291-m. [DOI] [PubMed] [Google Scholar]
- Paré WP. Hyponeophagia in Wistar Kyoto rats. Physiol Behav. 1994a;55:975–978. doi: 10.1016/0031-9384(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994b;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Paré WP. Enhanced retrieval of unpleasant memories influenced by shock controllability, shock sequence, and rat strain. Biol Psychiatry. 1996;39:808–813. doi: 10.1016/0006-3223(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Paré AMT, Parè WP, Kluczynski J. Negative affect and voluntary alcohol consumption in Wistar-Kyoto (WKY) and Sprague-Dawley Rats. Physiol Behav. 1999;67(2):219–225. doi: 10.1016/s0031-9384(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Parnas J. From predisposition to psychosis: Progression of symptoms in schizophrenia. Acta Psychiatr Scand Suppl. 1999;395:20–29. doi: 10.1111/j.1600-0447.1999.tb05979.x. [DOI] [PubMed] [Google Scholar]
- Paul LA, Skolnick P. Glutamate and depression, Clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. Academic Press; New York: 1998. [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Raeder H, Holter SM, Hartmann AM, Spanagel R, Moller HJ, Rujescu D. Expression of N-methyl-d-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend. 2008;96:16–21. doi: 10.1016/j.drugalcdep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Rafiki A, Bernard A, Medina I, Gozlan H, Khrestchatisky M. Characterization in cultured cerebellar granule cells and in the developing rat brain of mRNA variants for the NMDA receptor 2C subunit. J Neurochem. 2000;74:1798–1808. doi: 10.1046/j.1471-4159.2000.0741798.x. [DOI] [PubMed] [Google Scholar]
- Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J. The anticraving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacol. 2001;40:749–760. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Redei E, Paré WP, Aird F, Kluczynski J. Strain differences in hypothalamic pituitary adrenal activity and stress ulcer. Am J Physiol. 1994;266:353–360. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Ho AM, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann N Y Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Hall A, Hong J, Glavin GB. Unpredictable cold-immobilization stress effects on voluntary ethanol consumption in rats. Life Sci. 1987;40:1245–1251. doi: 10.1016/0024-3205(87)90580-7. [DOI] [PubMed] [Google Scholar]
- Rudolph JG, Walker DW, Iimuro Y, Thurman RG, Crews FT. NMDA receptor binding in adult rat brain after several chronic ethanol treatment protocols. Alcohol Clin Exp Res. 1997;21:1508–1519. [PubMed] [Google Scholar]
- Samson HH, Harris RA. Neurobiology of alcohol abuse. Trends Pharmacol Sci. 1992;13:206–11. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- Sandbak T, Murison R. Voluntary alcohol consumption in rats: Relationships to defensive burying and stress gastric erosions. Physiol Behav. 1996;59:983–989. doi: 10.1016/0031-9384(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings (Office of Applied Studies NSDUH Series H-30, DHHS Publication No. SMA 06-4194) Rockville, MD: 2006. [Google Scholar]
- Tejani-Butt SM, Paré WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar-Kyoto rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Kluczynski J, Paré WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Tremwel M, Anderson KJ, Hunter BE. Stability of [3H] MK-801 binding sites following chronic ethanol consumption. Alcohol Clin Exp Res. 1994;18:1004–1008. doi: 10.1111/j.1530-0277.1994.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDARl receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- Winkler A, Mahal B, Kiianmaa K, Zieglgänsberger W, Spanagel R. Effects of chronic alcohol consumption on the expression of different NR1 splice variants in the brain of AA and ANA lines of rats. Brain Res Mol Brain Res. 1999;72:166–175. doi: 10.1016/s0169-328x(99)00218-1. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010;94:471–476. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubin I, Spring B. Vulnerability--a new view of schizophrenia. J Abnorm Psychol. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]


