Abstract
Background:
Increased carotid artery intima-media thickness (IMT) is a noninvasive marker of systemic arterial disease, associated with atherosclerosis, abnormal arterial mechanics, myocardial infarction, and stroke. In the elderly, clinically elevated IMT is related to diminished attention-executive function. In this context, previous work involving paper-and-pencil measures of cognition has demonstrated that a threshold of pathology (i.e., IMT ≥ 0.9 mm) is needed before IMT consistently relates to poor neuropsychological test performance. Given the critical role of arterial health in the development of cognitive dysfunction, the goal of this study was to investigate early markers of brain vulnerability by examining subclinical levels of IMT in relation to a sensitive marker of neuronal integrity, cerebral N-acetyl-aspartate/creatine (NAA/Cr) ratio, in midlife.
Methods:
A total of 40 participants aged 50±6 years, underwent neuropsychological assessment, proton magnetic resonance spectroscopy (1H MRS) examination of occipitoparietal grey matter and B-mode ultrasound of the common carotid artery. IMT was defined as the distance between the luminal-endothelial interface and the junction between the media and the adventitia. The relation between IMT and cerebral metabolite ratios was modeled using a single multivariate multiple regression analysis adjusted for age and current systolic blood pressure.
Results:
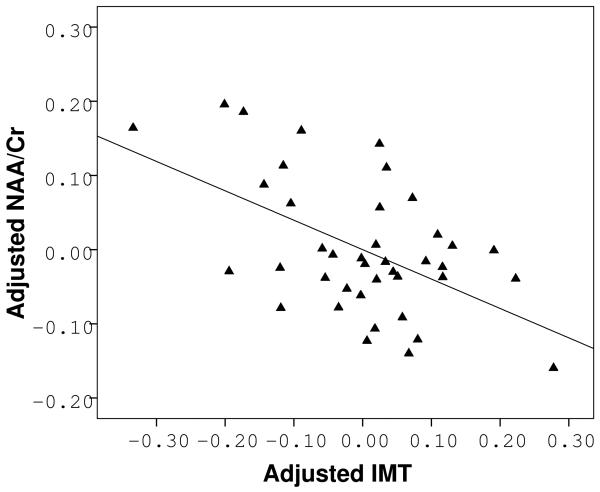
Increased IMT was associated with significantly lower NAA/Cr ratios (IMT beta=−0.62, p=0.001), independent of age and systolic blood pressure (F(3,36) = 4.928, p=0.006).
Conclusions:
Our study extends previous findings by demonstrating a significant relationship between IMT and NAA concentration, suggesting compromised neuronal viability even at IMT levels below thresholds for clinical end-organ damage.
Keywords: NAA, 1H MRS, Carotid Arteries, Atherosclerosis, Intima Media Thickness
1. Introduction
Midlife is a critical period of development when many chronic diseases first manifest and neurodegenerative processes are triggered (World Health Organization 2005). It presents an invaluable opportunity for implementing early interventions to prevent organ damage rather than striving to restore lost function later in life. Since cognition is the most important determinant of heath status, quality of life and functional ability in older age (Gaugler, et al. 2009), interventions that protect cognitive function are crucial for fostering the successful aging of our growing population of elderly. The timely evaluation of such interventions, however, depends on quantitative markers of brain health with higher sensitivity than the traditional paper-and-pencil tests of cognition. With this goal in mind, we set out to determine the relation between structural integrity of the common carotid arteries, the main blood supply to the brain, and cerebral metabolism in middle-aged adults with intact cognitive function.
Midlife vascular disease is the number one treatable risk factor for late-life cognitive impairment (Rigaud, et al. 2000). Carotid artery intima-media thickness (IMT) assessed by B-mode ultrasound, is a reliable, noninvasive measure of systemic arterial disease (Bots, et al. 2002). While small increases in IMT may be an adaptive response to changes in blood pressure and flow, IMT levels greater than 0.9 mm are indicative of atherosclerosis and end-organ damage (Van Bortel 2005). IMT values greater than 0.9 mm have been associated with significantly lower cognitive test performance in elderly patients with cardiovascular disease (Haley, et al. 2007). In addition, baseline IMT levels have proved to be a good independent predictor of new cognitive impairment in older adults (Sander 2009) and future cognitive decline in stroke patients (Talelli, et al. 2004). While it is acknowledged that atherosclerotic processes are triggered well before clinical symptoms of end-organ damage manifest (Singhal 2009), few studies of IMT and cognition have been performed in community-dwelling adults with normal IMT values. While one study found an association between IMT and cognitive impairment (Romero, et al. 2009), others have failed to detect a relationship between IMT and cognition in the absence of carotid plaques (Auperin, et al. 1996; Knopman, et al. 2001). This discrepancy may be due in part to the low sensitivity of paper-and-pencil cognitive screening measures. Thus, the timely identification of brain vulnerability and evaluation of interventions designed to protect cognitive function are contingent upon sensitive quantitative markers of neuronal integrity rather than the standard paper-and-pencil tests.
Proton magnetic resonance spectroscopy (1H MRS) potentially provides such markers. 1H MRS allows detection and quantification of a variety of cerebral metabolites of neurobiological significance: N-acetyl-aspartate (NAA), a marker of neuronal viability; choline-containing compounds, free choline, phosphocholine and glycerophosphocholine (Cho), markers of membrane breakdown and turn over; creatine (Cr), a marker of energy metabolism; myo-inositol (mI), an organic osmolyte and substrate for the synthesis of the secondary messenger, inositol triphosphate; and glutamate (Glu), a marker of excitatory neurotransmission and synaptic health (Danielsen 1999). In addition, previous reports of spectroscopic changes in areas of the brain where infarction subsequently occurred suggest that 1H MRS may have greater sensitivity than conventional MR imaging to detect tissue vulnerability (Barker, et al. 1994). Thus, 1H MRS appears uniquely suited for examining early changes in cerebral metabolism related to treatable medical conditions in midlife before visible brain volume losses have occurred and cognitive performance is clinically impaired.
With this information as background, we examined the relationship between carotid IMT and grey matter composition in a sample of cognitively normal middle-aged adults. We hypothesized that middle-aged adults at risk for future cognitive decline due to early atherosclerotic vascular changes will exhibit an altered cerebral metabolic profile. In addition, this profile changes will be significantly related to intima-media thickening despite currently intact global cognitive function.
2. Results
2.1 Selected subject characteristics
Selected demographic and physiological variables are reported in Tables 1 and 2. Raw cognitive test scores and standard deviations are reported in Table 3. Descriptive statistical analyses revealed a cognitively normal, ethnically diverse, middle-aged sample, well representative of the population of the state of Texas based on 2000 US census data for the state. The mean IMT value for the sample was 0.57±0.14 mm, well within the normal range according to the European Society of Hypertension – the European Society of Cardiology guidelines (Guidelines Committee 2003). Variable distributions were examined using the Shapiro-Wilk test of normality. All variables were deemed normally distributed and no transformations were performed (Shapiro-Wilk >0.949, p>0.072).
Table 1.
Selected Demographic Characteristics of Subjects
| Mean | SD | Number of Subjects |
Frequency | |
|---|---|---|---|---|
| Female | 23 | 57% | ||
| Caucasian | 17 | 43% | ||
| Hispanic | 17 | 43% | ||
| Asian | 1 | 2% | ||
| African American | 2 | 5% | ||
| Other/Did not report | 3 | 7% | ||
| Age | 50.1 | 6.5 | ||
| Education | 14.8 | 2.5 |
Table 2.
Selected Physiological Characteristic of Subjects
| Mean | SD | |
|---|---|---|
| IMT | 0.57 | 0.14 |
| Systolic Blood Pressure | 124 | 12 |
| Diastolic Blood Pressure | 76 | 8 |
| Body Mass Index | 28.8 | 5.4 |
| HDL-cholesterol (mg/dL) | 47.0 | 15.3 |
| Triglyceride (mg/dL) | 164.2 | 90.6 |
| LDL-cholesterol (mg/dL) | 112.5 | 38.9 |
| Fasting Glucose (mg/dL) | 105.1 | 33.8 |
| NAA/Creatine | 1.46 | 0.09 |
| Choline/Creatine | 0.18 | 0.02 |
| Myo-Inositol/Creatine | 0.71 | 0.08 |
| Glutamate/Creatine | 1.44 | 0.13 |
IMT=intima-media thickness, NAA=N-acetyl-aspartate
Table 3.
Cognitive Test Scores
| Test Measures by Domain | Sample Mean Score (SD) |
Total Possible Score |
|---|---|---|
| Global Cognitive Functioning | ||
| Mini Mental Status Exam (MMSE) | 28.4 (1.4) | 30 |
| Wechsler Abbreviated Scale of Intelligence (WASI) | 111.9 (11.6) | 95% is 149-160 |
| Language | ||
| WASI Vocabulary Subtest | 62.1 (10.3) | 80 |
| Category Fluency for Animals (Animals) | 24.2 (5.5) | 1 minute limit |
| Visual-Spatial | ||
| Complex Figure Test (CFT-Copy) | 30.8 (4.1) | 36 |
| WASI Matrix Reasoning Subtest | 25.9 (4.5) | 35 (12-44 yrs) or 32 (45-79 yrs) |
| Memory | ||
| California Verbal Learning Test (CVLT) | ||
| Immediate Recall | 10.4 (3.1) | 16 |
| Delayed Recall | 11.08 (3.2) | 16 |
| Recognition (Yes/No) | 3.18 (0.75) | −4 to 4 |
| Complex Figure Test (CFT) | ||
| Immediate Recall | 16.4 (5.2) | 36 |
| Delayed Recall | 15.9 (5.0) | 36 |
| Recognition Discrimination | 19.1 (4.0) | 24 |
| Attention-Executive-Psychomotor | ||
| Trail Making Test A, Time in seconds (Trails A) | 30.2 (9.4) | 5 minute limit |
| Trail Making Test B, Time in seconds (Trails B) | 74.7 (26.5) | 5 minute limit |
| Controlled Oral Word Association Test (COWAT) | 38.0 (10.7) | 3 minute limit |
| Grooved Pegboard, Dominant Hand, Time in seconds (Pegs-D) | 76.1 (14.3) | No limit |
| WAIS-III Digit Span Subtest (Digit Span) | 16.9 (4.0) | 30 |
2.2. Demographic and clinical variables in relation to IMT and cerebral metabolism
Older age was associated with significantly greater IMT values (r=0.43, p=0.006); however, none of the IMT values were above the clinical cut off of 0.9 mm for classification as atherosclerosis. As expected, higher resting systolic blood pressure was related to increased IMT, though the relationship did not reach statistical significance (r=0.24, p=0.14). As with previous studies of IMT and cognition in participants with normal IMT values, we found no relation between IMT and measures of global cognitive functioning (r=0.15, p=0.37), language (r=0.06, p=0.71), visual-spatial ability (r=0.003, p=0.98), or memory (r=0.001, p=0.996), independent of age, IQ and education.
Older age was not significantly associated with changes in cerebral metabolite ratios. Higher systolic blood pressure was associated with somewhat elevated levels of mI/Cr in the brain, but the relationship did not reach statistical significance (r=0.31, p=0.051). Consistent with mild cognitive impairment literature, higher levels of mI/Cr were associated with lower performance in the memory domain (r=−0.36, p=0.022) as well as the visual-spatial domain (r=−0.36, p=0.023). Higher levels of Glu/Cr, on the other hand, were associated with better performance in the memory domain (r=0.35, p=0.026). Once age, IQ and education were accounted for, however, only the relationships between higher mI/Cr and lower memory and visual-spatial ability remained (r=−0.38, p=0.021 and r=−0.34, p=0.041, respectively).
2.3. IMT and cerebral metabolism
Age and systolic blood pressure, being the two main predictors of IMT, were chosen a priori as covariates for our model (Spence 2004). The fully adjusted multivariate multiple regression model successfully predicted the level of NAA/Cr in the brain (F(3,36)=4.928, p=0.006), but not mI/Cr (F(3,36)=1.541, p=0.22), Cho/Cr (F(3,36)=0.132, p=0.94) or Glu/Cr (F(3,36)=1.213, p=0.32). Increased IMT was significantly associated with lower NAA/Cr (IMT beta=−0.62, p=0.001), independent of age and systolic blood pressure (Fig. 3). This relationship remained unchanged even after additional adjustment for sex (F(4,35)=3.612, p=0.014, IMT beta=−0.61, p=0.001). It also persisted after adjustments for use of antihypertensive (F(4,35)=3.623, p=0.014, IMT beta=−0.61, p=0.001) or lipid lowering medication (F(4,35)=3.809, p=0.011, IMT beta=−0.61, p=0.001).
Figure 3.
Partial correlation analysis showing an inverse relation between IMT and NAA/Cr after adjustments for age and systolic blood pressure.
3. Discussion
To our knowledge, this is the first study to specifically determine the relation between IMT and NAA. We found that increased carotid artery IMT was associated with lower NAA/Cr ratio in middle-aged adults with intact global cognitive performance. Importantly, the association was independent of age, sex, or systolic blood pressure. Previous findings of reduced NAA levels in patients with severe cognitive impairment due to ischemic vascular dementia (MacKay, et al. 1996) have proposed a direct link between vascular disease, cerebral metabolism and cognitive impairment. Similar association has also been observed in older, hypertensive adults (Ben Salem, et al. 2008). In the present study involving younger, cognitively normal middle-aged cohort, NAA/Cr was not significantly related to cognitive performance over and above demographic variables, yet lower NAA/Cr was associated with greater carotid artery IMT. These results suggest that reduced NAA/Cr in midlife might be an initial appearance of early neuronal vulnerability related to vascular risk/burden. Further studies are needed to assess the utility of the detected alterations in estimating risk for future cognitive decline.
NAA is exclusively found in neurons and oligodendrocyte-type-2 astrocyte progenitor cells (Birkenhager and Staessen 2006; Urenjak, et al. 1992) and is widely regarded as a marker of neuronal viability, synaptic health, and metabolism (Danielsen 1999). NAA is also considered a sensitive measure of cognitive status as higher NAA is related to better neuropsychological test performance in healthy young adults with equal levels of global intellectual ability (Jung, et al. 2000), and reductions in NAA are related to cognitive impairment in traumatic brain injury (Brooks, et al. 2001) and neurodegenerative diseases (Clarke and Lowry 2001; Valenzuela and Sachdev 2001). Moreover, NAA concentrations appear to be dynamic enough to reflect both neuronal death (Gillard, et al. 1996; Sappey-Marinier, et al. 1992) and transient mitochondrial impairment (Davie, et al. 1994; De Stefano, et al. 1995). Therefore, changes in NAA are at least partially reversible and a potential sensitive marker of treatment response for interventions designed to enhance neuronal viability and improve cognition. Our results indicate that NAA concentrations are associated with subtle alterations in vascular health as measured by early atherosclerotic changes in the large arteries supplying the brain. Thus, NAA may provide a quantitative marker of neuronal health for early interventions designed to minimize brain damage by improving peripheral vascular function. The use of NAA as a marker of early neuronal vulnerability is supported by previous reports of spectroscopic changes in areas of the brain where infarction subsequently occurred suggesting that MRS may have greater sensitivity than conventional MR imaging to detect tissue susceptibility to damage (Barker, et al. 1994). Mild, diffuse brain damage related to subclinical peripheral atherosclerosis may affect the brain's ability to process information quickly and efficiently even in the absence of discrete lesions. A reduction in cognitive processing speed is likely most detrimental to the highest order cognitive functions such as complex attention and planning ability. Thus, it is not surprising that in the elderly, increased IMT is related to poorer attention-executive-psychomotor performance (Haley, et al. 2007). In future investigations, additional assessments of white matter integrity may be useful in further elucidating the relationships detected in this study. While overt signs of microvascular damage in those 20-30 years of age are extremely rare, small white matter lesions have been reported in randomly selected individuals in their 40s and 50s (Sachdev, et al. 2008). Therefore, assessments of white matter integrity may lend further insights into cerebral metabolic health in middle-aged individuals and help estimate individual cognitive trajectories.
While considering future directions, it is also important to discuss the strengths and limitations of the present study. An important strength of the study is the thorough characterization of our sample in terms of cognitive and physiological functioning. The study employed a full cognitive battery as well as measures of metabolic and vascular health rather than relying on self-reported medical history. The confirmed cognitive and physiological health status allows us to interpret the detected neurochemical changes with greater confidence, without suspecting confounding effects of under-reported or undiagnosed medical conditions or cognitive disorders. On the other hand, the extensive assessments involved limited our study to a relatively small sample of self-selected community volunteers. Although ethnically diverse, the resulting sample was quite well educated (mean education level was 14.8 years), potentially limiting the generalizability of our results. Ideally, the external validity of this study should be tested in larger, randomly selected community samples. Finally, the cross-sectional nature of our study limits our ability to infer cognitive vulnerability from the detected neurochemical changes. Longitudinal studies that begin in mid-life can help validate the use of cerebral metabolic markers as indicators of long-term cognitive outcomes.
In summary, we demonstrated that increased IMT was associated with lower cerebral NAA/Cr in middle-aged adults with intact global cognitive function. These results suggest preclinical atherosclerosis in midlife is related to diminished neuronal viability. Studies including measurements of white matter integrity can help further elucidate the relationship between peripheral atherosclerosis and cognitive function. Understanding the early effects of vascular disease on the brain function is crucial for the development of early interventions that preserve cognitive function. Cognition is the most important determinant of health status, quality of life and functional ability in older age (Gaugler, et al. 2009) and preventing cognitive decline is central to ensuring successful aging for our growing population of elderly. An effective treatment that can delay the onset of cognitive impairment, would not only decrease the number of individuals with dementia but also improve patient quality of life, and reduce caregiver burden and medical care costs.
4. Experimental Procedures
4.1. Participants
Participants between the ages of 40 and 60 were recruited through flyers and newspaper advertisements. Two hundred and sixty two potential volunteers were screened. Volunteers were excluded from participation if they had a history of neurological disease (i.e., large vessel stroke, seizure disorder, Parkinson's disease, clinically significant traumatic brain injury, multiple sclerosis, or brain infection/meningitis), major psychiatric illness (e.g. schizophrenia, bipolar disorder), substance abuse (diagnosed abuse and/or previous hospitalization for substance abuse), or MRI contraindications. Forty-six participants passed the initial screening process. Three participants were excluded from further participation due to scoring below the normal range on the Wechsler Abbreviated Intelligence Scale (WASI < 85). IMT data for two participants and MRS data from one participant were unusable due to technical difficulties during data acquisition, resulting in a final sample size of 40.
The mean age (SD) of the sample was 50.1 (6.5) years. The mean education level (SD) was 14.8 (2.5) years. The mean full-scale IQ score (SD) was 111.9 (11.6), indicating high average global cognitive functioning according to published norms (Wechsler 1999). Enrollees identified themselves as follows: 43% - Caucasian, 43% - Hispanic, 2% Asian, 5% - African American, and 7% - Other. No participants registered IMT values equal to or greater than the 0.9 mm threshold necessary to be indicative of atherosclerotic vascular disease according to the European Society of Hypertension - the European Society of Cardiology guidelines (Guidelines Committee 2003). Eight participants were currently being treated with antihypertensive medications, 1 with an antiplatelet agent, 2 with lipid lowering agents, 3 with hypoglycemics, 2 with thyroid replacement therapy, 3 with bisphosphonates, and 1 with antidepressant medication. Further details about the demographic and physiological characteristics of the sample can be found in Tables 1 and 2.
4.2. Assessment Tools and Protocols
The study was approved by local Institutional Review Board and all volunteers provided written informed consent before enrollment. Participants were required to complete a medical history interview where medical conditions and treatments were coded as either present or absent according to participants' self-report. Participants underwent a full neuropsychological evaluation, brain imaging, and general health assessment including carotid ultrasound on separate days, completing the study within one month.
4.2.1. Neuropsychological evaluation
All participants completed a two-hour assessment battery including standard clinical neuropsychological instruments with established reliability and validity (Lezak 1995). The battery included measures of global cognitive functioning (Mini Mental Status Exam, MMSE (Folstein, et al. 1975); Wechsler Abbreviated Scale of Intelligence, WASI Full Scale IQ (Wechsler 1999), language (WASI Vocabulary Subtest; Category Fluency for Animals (Morris, et al. 1989), memory (California Verbal Learning Test-II, CVLT-II (Delis 2000); Rey Complex Figure Test, RCF (Lezak 1995), attention-executive functioning (Wechsler Intelligence Scale-III, WAIS-III, Digit Span Subtest (Wechsler 1979); Controlled Oral Word Association Test, COWA (Eslinger 1984); Trail Making Test A & B (Reitan 1958), psychomotor speed (Grooved Pegboard, Pegs (Klove and Forster 1963), visual-spatial ability (RCF copy; WASI Matrix Reasoning Subtest), and emotional functioning (Beck Depression Inventory-II, BDI (Beck, et al. 1996); Trait Anxiety Inventory, STAI-T (Niezel, et al. 1998). All tests were administered and scored by a trained research assistant using standard administration and scoring criteria.
4.2.2. Imaging protocols and MRS Data Processing
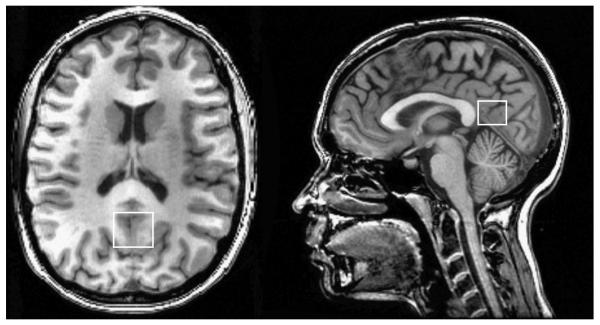
MRI data for each participant were acquired in a single session on a 3T GE Signa Excite MRI scanner equipped with a standard head coil. Structural imaging included a high-resolution Spoiled Gradient Echo (SPGR) sequence (256 x 256 matrix, FOV = 24 cm2, 1 mm slice thickness, 0 gap) anatomical scan of the entire brain in the saggital plane. Single voxel proton MRS was performed using the automated GE pulse sequence PROBE-P, which is a point resolved spectroscopy (PRESS) sequence with chemical shift selected (CHESS) water suppression. 1H-MRS parameters were as follows: echo time/ repetition time (TE/TR) = 35/3000 ms, 128 excitations, 5000 Hz spectral width, volume ~6 cm3 from the occipitoparietal gray matter (Fig. 1). The region was chosen due to its relevance for cognitive function. Spectroscopically detectable alterations in the neurochemical composition of this region occur in both mild cognitive impairment and Alzheimer's disease, in correspondence with severity of cognitive dysfunction (Kantarci, et al. 2000). In addition, early functional alterations in this region have been detected in healthy young adults at risk for developing vascular disease due to positive family history of hypertension (Haley, et al. 2008), suggesting early vulnerability of this region to vascular risk. Commercially available software, LCModel, was used to quantify and separate the metabolite resonances from the macromolecule background (Provencher 1993) (Fig. 2). In line with standard clinical protocols, the concentrations of NAA, choline containing compounds (choline, phosphocholine and glycerophosphocholine, Cho), myo-inositol (mI), and glutamate (Glu) were reported as ratios relative to creatine (Cr) (Kantarci, et al. 2000; Narayana 2005).
Figure 1.
Anatomic images with superimposed voxel borders indicating MRS data acquisition volume in occipitoparietal gray matter.
Figure 2.
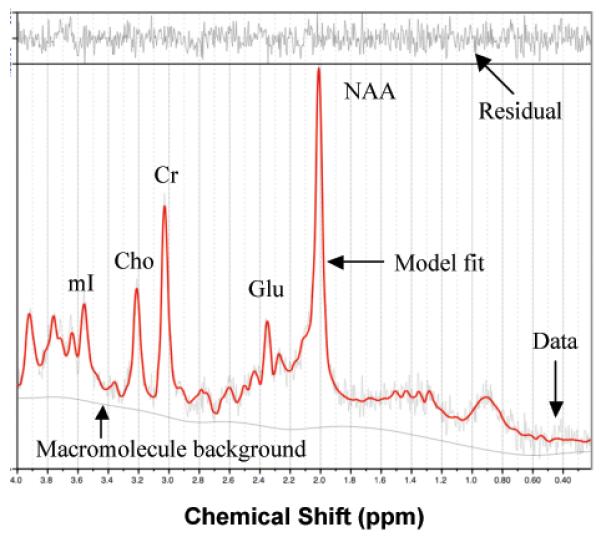
Representative 1H MRS spectrum. The narrow line width and small residual indicate excellent model fit. NAA = N-acetyl-aspartate; Glu = glutamate; Cr = creatine + phosphocreatine; Cho = choline + phosphocholine; mI = myo-inoitol
4.2.3. Ultrasound examination and IMT Data Processing
Carotid artery IMT was measured from the images derived from an ultrasound machine (iE 33 Ultrasound System, Philips, Bothell, Washington) equipped with a high-resolution linear-array transducer as previously described (Dinenno, et al. 2000; Tanaka, et al. 2002). A longitudinal image (B-mode) of the cephalic portion of the common carotid artery was acquired at 90° to the vessels so that the near and far wall interfaces were clearly discernible. These ultrasound-derived images were saved in DICOM format. Subsequently, ultrasound images were analyzed using a computerized image-analysis software (Vascular Research Tool Carotid Analyzer, Medical Imaging Applications, Coralville, IA). Carotid IMT was defined as the distance from the leading edge of the lumen-intima interface to the leading edge of the media-adventitia interface of the far wall. The mean values of at least 10 measurements of IMT were used for analysis. All image analyses were performed by the same investigator who was blinded to subject characteristics.
4.2.4. General health assessment
Fasting blood concentrations of glucose, triglyceride, HDLcholesterol, and LDL-cholesterol were determined using the standard enzymatic technique (Cholestech LDX system, Cholestech Corporation, Hayward, CA). Arterial blood pressure was measured using a standard oscillometric blood pressure monitor after at least 15 minutes of rest (VP-2000, Colin Medical Instruments, San Antonio, TX). Body mass index was calculated as weight in kilograms divided by the square of the height in meters. Prescription medications were coded as present or absent according to participant self-report and classified as antihypertensive, lipid lowering, hypoglycemic, antiplatelet, anti-inflammatory, antidepressant, antihistamine, hormone replacement, biphosphonates, or vitamins.
4.3. Data Analyses
Neuropsychological measures were grouped into one of five cognitive domains: 1) global cognitive functioning, 2) language functions, 3) visual-spatial abilities, 4) memoryfunctions, and 5) attention-executive-psychomotor functions. The following test scores were included in each domain, and raw total scores were utilized unless otherwise stated: 1) global: MMSE and WASI Full Scale IQ; 2) language: WASI Vocabulary Subtest and Category Fluency for Animals; 3) visual-spatial: RCF copy and WASI Matrix Reasoning Subtest; 4) memory: CVLT-II immediate recall, delayed recall, and recognition discrimination, RCF immediate recall, delayed recall, and recognition discrimination; 5) attention-executive-psychomotor functions: Trail making A and B time to completion, COWAT, WAIS-III Digit Span Subtest, and Grooved Pegboard-Dominant Hand time to completion. Participants' raw test scores were converted to z-scores using the study sample mean and standard deviation. Timed test scores were multiplied by −1 so that higher scores indicate better performance. Five composite cognitive domain z-scores were calculated for each participant by averaging the z-scores of all tests within that domain.
All variable distributions were examined using the Shapiro-Wilk test of normality recommended for small samples. Relationships between demographic and medical variables and IMT were explored using simple and partial correlations. The relationship between IMT and the 1H MRS markers (NAA/Cr, Glu/Cr, mI/Cr and Cho/Cr) was analyzed using a single multivariate multiple linear regression model with all MRS parameters entered in at once. Age and systolic blood pressure were chosen as covariates a priori, due to being the two main predictors of IMT (Spence 2004). All statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL). Since only a single multivariate multiple regression model was used, no multiple comparisons corrections were deemed necessary. A two-tailed alpha level of 0.05 was used as the criterion for statistical significance.
Acknowledgements
This work was funded in part by a pilot award supported by the National Institute of Nursing Research Center Grant P30 NR005051 (APH) and grants from the American Heart Association (09BGIA2060722, APH), the University of Texas Imaging Research Center, and Department of Psychology (APH). The authors thank the Imaging Center Staff for their help with the participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auperin A, Berr C, Bonithon-Kopp C, Touboul PJ, Ruelland I, Ducimetiere P, Alperovitch A. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59- to 71-year-olds. The EVA Study Group. Stroke. 1996;27(8):1290–1295. doi: 10.1161/01.str.27.8.1290. [DOI] [PubMed] [Google Scholar]
- Barker PB, Gillard JH, van Zijl PC, Soher BJ, Hanley DF, Agildere AM, Oppenheimer SM, Bryan RN. Acute stroke: evaluation with serial proton MR spectroscopic imaging. Radiology. 1994;192(3):723–732. doi: 10.1148/radiology.192.3.8058940. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II Manual. The Psychological Corporation; Fort Worth: 1996. [Google Scholar]
- Ben Salem D, Walker PM, Bejot Y, Aho SL, Tavernier B, Rouaud O, Ricolfi F, Brunotte F. N-acetylaspartate/creatine and choline/creatine ratios in the thalami, insular cortex and white matter as markers of hypertension and cognitive impairment in the elderly. Hypertens Res. 2008;31(10):1851–1857. doi: 10.1291/hypres.31.1851. [DOI] [PubMed] [Google Scholar]
- Birkenhager WH, Staessen JA. Progress in cardiovascular diseases: cognitive function in essential hypertension. Prog Cardiovasc Dis. 2006;49(1):1–10. doi: 10.1016/j.pcad.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Bots ML, Dijk JM, Oren A, Grobbee DE. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: current evidence. J Hypertens. 2002;20(12):2317–2325. doi: 10.1097/00004872-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 2001;16(2):149–164. doi: 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Lowry M. Systematic review of proton magnetic resonance spectroscopy of the striatum in parkinsonian syndromes. Eur J Neurol. 2001;8(6):573–577. doi: 10.1046/j.1468-1331.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Danielsen ER, Ross B. Magnetic resonance spectroscopy diagnosis of neurological diseases. Marcel Dekker, Inc.; New York: 1999. [Google Scholar]
- Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, McDonald WI. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117:49–58. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34(5):721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT-II) Manual. Harcourt Assessment Company; San Antonio: 2000. [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278(4):H1205–H1210. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- Eslinger P. The Iowa screening battery for mental decline. University of Iowa College of Medicine; Iowa City: 1984. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Yu F, Krichbaum K, Wyman JF. Predictors of nursing home admission for persons with dementia. Medical Care. 2009;47(2):191–198. doi: 10.1097/MLR.0b013e31818457ce. [DOI] [PubMed] [Google Scholar]
- Gillard JH, Barker PB, van Zijl PC, Bryan RN, Oppenheimer SM. Proton MR spectroscopy in acute middle cerebral artery stroke. Am J Neuroradiol. 1996;17(5):873–886. [PMC free article] [PubMed] [Google Scholar]
- Guidelines Committee European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- Haley AP, Forman DE, Poppas A, Hoth KF, Gunstad J, Jefferson AL, Paul RH, Ler AS, Sweet LH, Cohen RA. Carotid artery intima-media thickness and cognition in cardiovascular disease. Int J Cardiol. 2007;121(2):148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley AP, Gunstad J, Cohen RA, Jerskey BA, Mulligan RC, Sweet LH. Neural correlates of visuospatial working memory in healthy young adults at risk for hypertension. Brain Imaging Behav. 2008;2:192–199. [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Brooks WM. Myths of neuropsychology: Intelligence, neurometabolism, and cognitive ability. Clin Neuropsychol. 2000;14(4):535–545. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RJ. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H, Forster FM. Clinical Neuropsychology. The Medical Clinics of North America. Saunders; New York: 1963. [PubMed] [Google Scholar]
- Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press; New York: 1995. [Google Scholar]
- MacKay S, Meyerhoff DJ, Constans JM, Norman D, Fein G, Weiner MW. Regional gray and white matter metabolite differences in subjects with AD, with subcortical ischemic vascular dementia, and elderly controls with 1H magnetic resonance spectroscopic imaging. Arch Neurol. 1996;53(2):167–174. doi: 10.1001/archneur.1996.00550020079018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niezel M, Bernstein D, Russel R. In: Assessment of anxiety and fear. Bellack A, Hersen M, editors. Pergamon Press; Oxford: 1998. pp. 280–312. [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- Rigaud AS, Seux ML, Staessen JA, Birkenhager WH, Forette F. Cerebral complications of hypertension. J Hum Hypertens. 2000;14(10-11):605–616. doi: 10.1038/sj.jhh.1001118. [DOI] [PubMed] [Google Scholar]
- Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Chen X, Wen W. White matter hyperintensities in mid-adult life. Curr Opin Psychiatry. 2008;21(3):268–274. doi: 10.1097/YCO.0b013e3282f945d5. [DOI] [PubMed] [Google Scholar]
- Sander K, Bickel H, Forstl H, Etgen T, Briesenick C, Poppert H, Sander D. Carotid- intima media thickness is independently associated with cognitive decline. The INVADE study. Int J Geriatr Psychiatry, 2010;25(4):389–394. doi: 10.1002/gps.2351. [DOI] [PubMed] [Google Scholar]
- Sappey-Marinier D, Calabrese G, Hetherington HP, Fisher SN, Deicken R, Van Dyke C, Fein G, Weiner MW. Proton magnetic resonance spectroscopy of human brain: applications to normal white matter, chronic infarction, and MRI white matter signal hyperintensities. Magn Reson Med. 1992;26(2):313–327. doi: 10.1002/mrm.1910260211. [DOI] [PubMed] [Google Scholar]
- Singhal A. The early origins of atherosclerosis. Adv Exp Med Biol. 2009;646:51–58. doi: 10.1007/978-1-4020-9173-5_5. [DOI] [PubMed] [Google Scholar]
- Spence JD. Carotid intima-media thickness and cognitive decline: what does it mean for prevention of dementia? J Neurol Sci. 2004;223(2):103–105. doi: 10.1016/j.jns.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ellul J, Terzis G, Lekka NP, Gioldasis G, Chrysanthopoulou A, Papapetropoulos T. Common carotid artery intima media thickness and post-stroke cognitive impairment. J Neurol Sci. 2004;223(2):129–134. doi: 10.1016/j.jns.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol. 2002;92(4):1458–1464. doi: 10.1152/japplphysiol.00824.2001. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992;59(1):55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56(5):592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM. What does intima-media thickness tell us? J Hypertens. 2005;23(1):37–39. doi: 10.1097/00004872-200501000-00009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Third Edition. The Psychological Corporation; San Antonio: 1979. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. Harcourt Assessment Company; San Antonio: 1999. [Google Scholar]
- World Health Organization . Preventing chronic diseases: a vital investment: WHO global report. Geneva, Switzerland: 2005. [Google Scholar]