Abstract
Alterations in exploratory behavior are a fundamental feature of bipolar mania, typically characterized as motor hyperactivity and increased goal-directed behavior in response to environmental cues. In contrast, abnormal exploration associated with schizophrenia and depression can manifest as prominent withdrawal, limited motor activity, and inattention to the environment. While motor abnormalities are cited frequently as clinical manifestations of these disorders, relatively few empirical studies have quantified human exploratory behavior. This article reviews the literature characterizing motor and exploratory behavior associated with bipolar disorder and genetic and pharmacological animal models of the illness. Despite sophisticated assessment of exploratory behavior in rodents, objective quantification of human motor activity has been limited primarily to actigraphy studies with poor cross-species translational value. Furthermore, symptoms that reflect the cardinal features of bipolar disorder have proven difficult to establish in putative animal models of this illness. Recently, however, novel tools such as the Human Behavioral Pattern Monitor provide multivariate translational measures of motor and exploratory activity, enabling improved understanding of the neurobiology underlying psychiatric disorders.
Keywords: bipolar disorder, animal models, rodent, open field test, exploration, hyperactivity, behavior pattern monitor, dopamine
1. Introduction
Bipolar Disorder (BD), a severe and chronic psychiatric illness where patients alternate between episodes of depression and mania (Goodwin and Jamison, 1990), affects between 1% and 4% of the world’s population, depending upon the criteria used to define the disorder (Gould and Einat, 2007). Episodes of mania and depression have been documented since antiquity, first described by Hippocrates and linked together as manifestations of one illness by Aretaeus of Cappadocia (c. 150 AD) (Jackson, 1986). In the 19th century, several authors described a longitudinal pattern of illness that included periods of depression, mania and symptom-free intervals later labeled by Kraepelin as manic-depressive insanity (Kraepelin, 1899) and eventually classified as bipolar disorder in the third version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) (APA, 1980).
Phases of depression associated with BD are generally indistinguishable from unipolar depression, while manic episodes are marked by increased energy and hyperactivity, euphoric mood, irritability, racing thoughts and pressured speech, reduced sleep, impulsive behavior, hypersexuality, and excessive self-confidence (Einat, 2007a). The cumulative effect of repeated bouts of depression and mania often result in significant functional impairment, including family and marital breakdown, unemployment or career difficulties, and financial distress (Kleinman et al., 2003; Woods, 2000). The economic burden of BD is also substantial; recent studies indicate that the lifetime cost of treating individuals with BD could reach 24 billion US dollars in the United States alone, with an average cost of $11,720 for a single manic episode and a total of $624,785 per individual for chronic treatment (Begley et al., 2001).
Alterations in the quantity and pattern of motor activity have long been recognized as a fundamental feature of psychiatric disorders including BD (Lawrence et al., 1992). In fact, the assessment of motor activity has played an important role in formulating criteria for the diagnosis of mental illness. While only 7 types of psychiatric disorders in the Diagnostic and Statistical Manual for Mental Disorders (DSM) versions I and II included criteria that required clinicians to assess motor activity (Tryon, 1986), that number increased to 16 for DSM-III and subsequently to 30 for DSM-IV (Lawrence et al., 1992; Teicher, 1995). Just as the assessment of motor activity is increasingly relevant as a diagnostic tool, the ability to develop objective, precise and meaningful measures of human movement has become a crucial issue. In contrast to the well-developed and sophisticated methods of assessing locomotion in rodents (Crusio, 2001; Geyer and Paulus, 1996; Paulus and Geyer, 1996), the vast majority of studies on human motor activity have used self-report and observer rating scales that are deficient in several ways. Such measures are inherently subjective, have limited ability to detect subtle alterations in motor activity, and generally lack the ability to assess qualitative differences that could indicate alterations in neural circuitry unique to different psychiatric disorders (Young et al., 2007). In this review, we will discuss: 1) methods used to assess motor and exploratory activity in rodents; 2) related genetic and pharmacological animal models of BD; 3) methods used to quantify human motor and exploratory activity in BD, including the Human Behavior Pattern Monitor (hBPM).
2. Assessment of motor and exploratory behavior in rodents
Exploration refers to a broad category of behaviors that function to provide an organism with information about its external environment, representing a fundamental characteristic of all living species (Berlyne, 1960). In the first half of the 20th century, under the influence of Darwinian theory and Cannon’s concept of homeostasis, it was widely believed that such behavior was motivated only by the prerequisites of survival, dictated solely by the need to find food, reproduce and avoid injury (Cannon, 1953). However, subsequent empirical investigations suggested that exploration persists even in the presence of stimuli that are neither biologically beneficial nor harmful (Welker, 1961). Some behavioral theorists focused on the concept of “curiosity”, proposing that animals engage in exploration to achieve a level of stimulation that will optimize the function of the brain in the absence of any explicit threat or danger (Berlyne, 1966). Berlyne proposed that exploratory behavior can be divided into two distinct categories: 1) inspective exploration, when an organism will examine a particular location or source in response to a specific need for information; 2) diversive exploration, a general drive to seek out stimulation anywhere in the environment, resulting in contact with distant stimuli (Berlyne, 1966). More recent work has emphasized the theory that novel environments create conflict by inducing both approach and avoidance behaviors, pitting the desire to explore, or approach, against the tendency to fear, and thus avoid, novelty (Dulawa et al., 1999).
The assessment of locomotor and exploratory behavior in rodents is one of the most widely used behavioral methods to determine the effects of genetic, physiological, and pharmacological manipulations. An extensive variety of measures have been used to quantify motor activity in rodents, but the oldest and most common paradigm is the open field test (Robbins, 1979; Whimbey and Denenberg, 1967). The open field apparatus typically consists of a circular, square, or rectangular novel open space either enclosed by a surrounding wall or elevated above the floor to prevent escape. A small rodent, either a rat, mouse, gerbil or vole, is placed in the field for a fixed time interval and a number of activity variables are quantified, including the distance covered per unit time, the number of regions visited (central or peripheral), rearing, latency to initial movement, stereotypic behaviors such as sniffing or grooming, and physical responses such as defecation or urination (Walsh and Cummins, 1976). The reliability of the test is critically dependent on uniform experimental conditions, including how rodents are handled and housed prior to the test, the degree of illumination, the extent of ambient noise, the time of testing during the light-dark cycle, and the visibility of the experimenter in the testing room (Gould et al., 2009).
The simplest and cheapest way to conduct the open field test is to use a container where the floor is marked into discrete regions using parallel lines delineated by marker pen or tape. An observer (either present in the test room or remotely viewing a video) can manually score rodent transitions between regions and quantify peripheral vs. center entries (Gould et al., 2009). To avoid problems associated with direct observation of rodents and simplify data collection, a number of automated open field instruments have been developed, including photobeam and video recording systems (Geyer et al., 1986; Sanberg et al., 1985; Vorhees et al., 1992; Young et al., 1993). A photobeam apparatus consists of an open field container embedded with an array of infrared photobeams that cross the enclosure, but are invisible to the rodent being tested. When the animal crosses in front of a beam, the photobeam interruption is recorded by a computer, allowing measurement of the position and motor activity of the animal. In contrast, a video recording system records data from a camera placed above the open field. The video images of the animal are converted into digitized data that are transferred to a computer and analyzed with the appropriate computer software.
Rodent behavior in the open field test has been interpreted in myriad ways and has been described by different groups as representing diversive and inspective exploration, arousal, inhibition, and anxiety (Archer, 1973; Geyer et al., 1986; Robbins, 1979; Walsh and Cummins, 1976). The paradigm was originally developed several decades ago by Hall (Hall and Ballachey, 1932), who suggested that emotional responses must occur at a higher frequency in a novel environment and decrease with repeated exposure as the increasing familiarity of the situation evokes less fear or anxiety (Hall, 1934). Given the difficulty in differentiating the internal and external factors that may motivate locomotor activity, a number of researchers have chosen to use the holeboard apparatus to characterize exploratory behavior. Originally developed in the early 1960s (Boissier and Simon, 1962), the holeboard apparatus consists of a test chamber containing holes where a rat or mouse is able to dip its head, thus “exploring” a specific stimulus. Typically, the number of holes entered and the spatial and temporal pattern of head-dipping is assessed and the complexity and novelty of these stimuli can be manipulated by placing objects in the holes. Holepoke measures have been demonstrated to exhibit good test-retest reliability and have been validated as measures of inspective exploration, as determined by decreased habituation when a novel object is placed in a specific hole (File and Wardill, 1975a; File and Wardill, 1975b; Flicker and Geyer, 1982). Many holeboard chambers contain holes placed in the floor, a design that may increase the probability of erroneous counts if animals inadvertently step into the holes; however, holes placed into the wall increase the reliability of this measure (Flicker and Geyer, 1982).
Most reviews of activity measures conclude that it is advantageous or even necessary to conduct multiple simultaneous measures of locomotion and exploration in order to accurately characterize multifaceted types of behavior (Geyer, 1990; Reiter and MacPhail, 1979; Robbins, 1979). Such multivariate assessment improves the ability of the investigator to determine the validity of hypothetical constructs, to be more confident about comparing results to similar studies in the literature, to examine the generality or specificity of a certain effect, and to identify the contribution of competing behavioral responses.
There have been a number of multivariate approaches to characterizing motor and exploratory behavior using both automated and direct observational methods (Drai and Golani, 2001; Eilam and Golani, 1989; Szechtman et al., 1985). For example, one group has successfully used a video-tracking system in conjunction with sophisticated data analysis (Software for Exploring Exploration or SEE) to differentiate the effect of stimulants on patterns of locomotion and acceleration in rats and mice (Drai and Golani, 2001; Kafkafi and Elmer, 2005; Kafkafi et al., 2003). More than 25 years ago, our own lab developed a computer-monitored open field paradigm called the Behavior Pattern Monitor (BPM) (Adams and Geyer, 1982; Flicker and Geyer, 1982). The BPM was designed to combine the features of open field and holeboard chambers by simultaneously measuring locomotion and exploratory behaviors in rats such as holepoking and rearing. The rat BPM consists of a large (30.5 by 60 cm) black Plexiglas box containing 3 floor and 7 wall holes equipped with infrared beams and a wall touch plate for the detection of rearing. The chamber is criss-crossed by a pattern of infrared beams sampled by a microcomputer and is enclosed in an electrically shielded and ventilated cabinet. The computer records the sequences of holepokes, rearing, and current location of the animal as determined by the status of beam breaks with a temporal resolution of 55 msec and a spatial resolution of 3.8 cm (Geyer and Paulus, 1996). Measures of locomotion include the total beam breaks, transitions and number of entries into specific regions of the BPM, and time spent in the center (Geyer et al., 1986). An open black cylinder is located behind each hole in the chamber, where novel objects or lights can be placed, allowing for manipulation of the novelty or complexity of specific holes. Finally, a wide-angle lens permits observation of the chamber without disturbing the animal.
In addition to categorizing the type of behavior that is measured, there have also been efforts to describe and quantify the structure and pattern of motor activity. This work has been motivated by the observation that drugs such as amphetamine not only affect overall locomotion, but also induce very pronounced changes in the structure of the activity, such as increasing stereotyped, or repetitive patterns of motor activity (Lat, 1963). In order to characterize patterns of behavior in the BPM objectively, several measures have been developed to characterize the organization of behavior over both distance and time, independent of the resolution of measurement and the length of the sequences being measured. Two of these measures have been used most widely. The spatial scaling exponent, d, is a measure of the structure of the dimensionality of movement over distance, the degree to which an animal travels in a straight line versus a more convoluted, meandering path. This measure is based on the concept that the observed distance of a non-straight line depends on the size of the ruler used to calculate the distance. If one is trying to accurately measure the distance of a small convoluted and curved line, a small ruler with a high resolution will provide a more accurate and larger measure of distance on average compared to a large, crude ruler with low resolution. Spatial d is calculated by plotting the measured path length against the ruler length using a double-logarithmic coordinate system and the d value is generated from the slope of the line of fit between these two variables (Geyer et al., 1986; Paulus and Geyer, 1996). Spatial d typically ranges between 1 (a straight line) and 2 (a filled plane), with values close to 1 indicating linear, straight movements, and values close to 2 indicating more circumscribed, local movements. Near the extremes, high or low spatial d values indicates a predictable patterns of movement (repeated linear movements if d is close to 1, or a repeated pattern of circumscribed movement if d is close to 2), while a value in the middle of the range indicates a more random, unpredictable path.
While spatial d measures the structure of motor behavior over the distance traveled, dynamical entropy h measures the organization of behavior over time, determining whether the activity is ordered and predictable or more random and disorganized (Paulus and Geyer, 1996; Paulus et al., 1990). This measure quantifies the degree of uncertainty of predicting the next movement based on the sequence of preceding movements and is based on similar measures assessing the emergence of uncertainty in nonlinear dynamical systems (Eckmann and Ruelle, 1985). Low values of entropy indicate predictable and repetitive patterns of movement over time, while higher values indicate more disordered, random movement.
The spatial d and entropy h measures enable objective assessment of behavior organization in the BPM without relying on predefined or arbitrary categories of activity and are also independent of resolution scale and sequence length. A factor analysis of 137 rats run in the BPM revealed that the measures quantified in this open field paradigm load on to three primary factors: amount of activity total transitions and distance traveled); exploratory behavior (holepokes and rearing); and a third independent factor described as “sequential response organization” (including spatial d and entropy h) (Paulus and Geyer, 1993). The factor loadings support the argument that the structure of rat motor activity (both spatial d and entropy h) varies independently from the amount of activity.
Exploratory and motor behavior has been studied extensively in mice as well as rats using a variety of paradigms, including simple open field tests, light-dark boxes, elevated-plus mazes, and photobeam activity chambers (Crawley, 1999; Crusio, 2001; Lalonde and Strazielle, 2008; Mathis et al., 1995). Amphetamine administration increases mouse activity and spatial d values (at high doses) in a video-tracked open field apparatus (Ralph et al., 2001b), indicating that this drug can increase local, circumscribed movements in both rodent species. A factor analysis of 84 mice run with the video-tracking system demonstrated that, similar to rat, transitions and distance measures loaded on to amount of activity, while spatial d and entropy h values loaded on to a behavioral organization factor (Paulus et al., 1999).
More recently, a mouse version of the BPM (Risbrough et al., 2006) has been developed, equipped with a infrared photobeam system to measure locomotion and rearing behavior. Mouse exploration is quantified by evaluating murine investigation (holepoking) into the 11 holes placed in the enclosure floor and walls. Administration of 3,4-methylenedioxymethamphetamine (MDMA) in wildtype mice increased activity and decreased spatial d (Risbrough et al., 2006) in a similar response pattern as previously observed in rat (Gold et al., 1988), demonstrating the ability of the BPM to generalize results across different rodent species.
In summary, multivariate assessment of motor and exploratory behavior has significant utility in sensitively discriminating genetically and pharmacologically manipulated rodents, rendering these paradigms ideal for testing animal models of psychiatric disorders. The following section will discuss the current status of animal models of BD and highlight some of the challenges in this field.
3. Animal models of bipolar disorder
The paucity of fully validated and appropriate animal models of BD has significantly limited progress in our understanding of the neurobiology of the disorder. BD is problematic to model in animals for a variety of reasons and the cyclic nature of the disorder creates some unique challenges to establishing a multifaceted animal model. As with all animal models of psychiatric illness, it is difficult to infer affective states in rodents that mirror the disruption of human affect that characterize this illness. Other major limitations include a lack of established biomarkers for the disease, continuing uncertainty about how existing mood-stabilizing medications exert their therapeutic actions, and limited (although improving) knowledge regarding the genetic basis for the disorder (Gould and Einat, 2007). Ideally, a comprehensive model for BD will include common mechanisms shared by the model and the human disorder (etiological validity), common observable features (face validity), and similar treatment efficacy for both the model and human BD (pharmacological predictive validity) (Einat et al., 2003; Machado-Vieira et al., 2004). Recent attempts to model mania in rodents have focused on utilizing paradigms that mirror specific facets or individual symptoms associated with a manic episode, such as hyperactivity or aggressive behavior (Table 1). While these tests attempt to satisfy the requirement of face validity and have been used to determine predictive validity (e.g., overactive behavior is muted by treatment with mood stabilizers), a comprehensive multi-symptom model remains elusive.
Table 1.
Animal models of motor/exploratory behavior proposed to reflect facets of bipolar mania
| Manic Symptoms | Rodent paradigm | Citation |
|---|---|---|
| Hyperactivity / increased goal-directed behavior |
Activity in open field test Home cage locomotion Novel object exploration |
Jornada et al., 2009 Shaltiel et al., 2008 Roybal et al., 2007 |
| Risk-taking behavior / reduced anxiety |
Center entries in open field test Open arm activity in elevated plus maze Approaching predator odor |
Chen et al., 2010 Maeng et al., 2008 Kavaliers et al., 2001 |
| Decreased need for sleep | Circadian activity monitoring Home cage wheel running |
Berridge and Stalnaker, 2002 Engel et al., 2009 |
| Aggressive behavior | Resident-intruder paradigm | Beneditti et al., 2008 Einat, 2007 |
| Irritability | Vocal / Jumping reactions to touch | Rasmussen et al., 1990 Wei, 1973 |
Current models of BD can be classified into several broad categories, including the development of pharmacological, environmental, genetic, and endophenotypic models of the disorder. The administration of amphetamine, which induces hyperactivity in rodents, is the most commonly used and generally accepted standard animal model for BD (Mamelak, 1978; O'Donnell and Gould, 2007). While low doses of amphetamine primarily increase locomotor activity, higher doses induce more locally oriented activity such as sniffing, gnawing, and rearing (stereotypy) (Kuczenski and Segal, 1999). Both types of behavior can be attenuated by treatment with mood-stabilizers such as lithium in both rats and mice (Flemenbaum, 1977; Wielosz, 1976). Recent studies have demonstrated that such effects may be strain-dependent (Gould et al., 2007; Gould et al., 2001), while some groups have reported inconsistent effects of lithium on amphetamine-induced activity (Gould et al., 2007; Miyauchi et al., 1981). Often, the effects of lithium on the locomotor activity of control animals are inadequately assessed due to prior habituation of the rodent to the test chamber. In most published reports, rodents are first habituated to the chamber and tested in the light (inactive) phase of their diurnal cycle, leading to a floor effect that precludes the detection of locomotor-suppressant effects of lithium in the absence of amphetamine treatment. Thus, the effects of lithium specifically on amphetamine-stimulated activity remain unclear. Other studies have utilized a mixture of amphetamine and the benzodiazepine chlordiazepoxide (CDP) as a model of mania, reporting that mood stabilizers (including lithium, valproic acid, and carbamazepine) are effective at blocking rodent hyperlocomotion induced by the combination of these two drugs (Arban et al., 2005; Aylmer et al., 1987; Cao and Peng, 1993). More recent work has suggested that compounds such as valproic acid may actually potentiate the hypolocomotor effects of CDP, instead of blocking stimulation induced by the amphetamine-CDP mixture (Kelly et al., 2009). Finally, amphetamine administration is reported to induce mania-like symptoms in healthy human subjects (Strakowski and Sax, 1998) and euthymic BD individuals (Anand et al., 2000), providing support for the use of this drug as a model of BD.
It is relevant to note that the amphetamine model of BD has a number of major limitations. First, the hyperactivity induced by the drug has been interpreted as a model for a number of different disorders, including SCZ (Peleg-Raibstein et al., 2008), based on evidence that amphetamine administration can produce psychosis in humans (Ellison and Eison, 1983) and that dopamine blockers attenuate amphetamine-induced rodent hyperlocomotion and stereotypy (Depoortere et al., 2007). Second, bipolar mania is characterized by a broad set of symptoms and not all manic individuals exhibit motor hyperactivity (Einat, 2007b). Third, while acute lithium treatment has been demonstrated to attenuate amphetamine-induced hyperactivity in rodents (Berggren et al., 1981), the specificity of this effect is debatable and the therapeutic effect of lithium in bipolar patients may not appear for weeks (Reischies et al., 2002).
While amphetamine has traditionally been used as a model of bipolar mania, there have also been a few attempts to use pharmacological manipulations to mimic the cyclic nature of BD. One group reported that chronic lithium treatment blocked cocaine-induced oscillations in dopamine efflux released by in vitro preparations of rat nucleus accumbens (Antelman et al., 1998). Similarly, lithium exposure diminished dopamine oscillations during a stress-induced hypoalgesia response associated with subchronic cocaine injections in rat. However, the oscillations in the response to a painful stimulus (a heated hotplate) are not well related to cyclic BD symptoms and the in vitro assay has obviously limited translational value when compared to human behavior. A more recent study examined the effect of BD treatments on a pattern of biphasic locomotion induced by quinpirole, a dopamine D2/D3 receptor agonist (Shaldubina et al., 2002). The authors report that treatment with quinpirole (0.5 mg/kg) initially inhibited, and then later increased rat locomotor activity in a manner resembling the cyclic nature of BD. They observed that treatment with valproate and carbamazepine decreased quinpirole-induced hyperactivity, but did not affect quinpirole-induced hypoactivity (Shaldubina et al., 2002).
In contrast to pharmacological models of BD, some groups have attempted to use environmental stressors to induce behavior in animals that resembles human symptoms associated with the disorder. Previous studies demonstrated that sleep deprivation in rats can induce hyperactivity, irritability, aggressive behavior, hypersexuality, stereotypy, and insomnia (Fratta et al., 1987; Hicks et al., 1979; Morden et al., 1967), a set of behaviors that matches many of the symptoms that characterize bipolar mania. One study reported that 10 days of lithium treatment significantly decreased locomotion in rats after 72 hours of sleep deprivation (Gessa et al., 1995). A more recent report proposed sleep deprivation as a model of mania in mice, indicating that 24 hours of sleep deprivation in male CD1 mice induced hyperlocomotion and aggressive behavior compared to control animals that were allowed a regular sleep pattern (Benedetti et al., 2008).
There have been many attempts to utilize rodent genetic differences and genetic manipulations to model symptoms or facets of BD. For example, Flinder sensitive line (FSL) rats exhibit reduced activity and anhedonia after exposure to stressors (Overstreet, 1986) and have been used as a model of depression; in contrast, Black Swiss mice have been reported to show mania-like behavior (Einat, 2007a). However, no strain has yet been demonstrated to exhibit the cycling behavior integral to BD.
Animal models of BD have also been derived from rare cases of the disorder caused by documented genetic mutations. Disruptions of the gene DISC1 (disrupted in SCZ 1) in members of a Scottish family have been linked with cases of SCZ and in one instance, BD, suggesting the possibility that mutant DISC1 mice could be used as model of BD (Ishizuka et al., 2006; O'Tuathaigh et al., 2007). Another genetic abnormality linked with mental disorders in the human population is the deletion at 22q11 (Paylor and Lindsay, 2006), associated with a high risk of developing SCZ or BD. Df1 mice carrying a deletion in this same chromosomal region exhibit SCZ or bipolar-like behavior, including deficits in sensorimotor gating (Paylor and Lindsay, 2006). Although an often-cited problem with both of these mutant mice is the lack of specificity, given that they could serve as equally valid models for two different psychiatric disorders, it must be acknowledged that the clinical separation of psychiatric disorders is not necessarily definitive.
A third type of genetic model is based on targeted mutations of specific genes or neurotransmitter systems hypothesized to play a role in BD, including dopamine (Ralph-Williams et al., 2003), serotonin (Lesch and Mossner, 2006) and intracellular pathways that include glycogen synthase kinase 3 (GSK3) (O'Brien et al., 2004), BCL-2 (Einat et al., 2005) and inositol (Shaldubina et al., 2006). Transgenic mice that overexpress GSK3β, an enzyme inhibited by both lithium and valproate (Chen et al., 1999; Klein and Melton, 1996), exhibit hyperactivity and hypophagia (Prickaerts et al., 2006), but the effect of lithium in these mice has not yet been demonstrated. Another group has proposed that mutant POLG (polymerase gamma) transgenic mice represent a model of BD (Kato et al., 2007). These animals exhibit increased activity during their nocturnal period, interpreted as a representation of insomnia. The mice also exhibit a shift towards hyperactive behavior when treated with the antidepressant amitriptyline, described as a phenomenon similar to antidepressant-induced manic behavior in human BD patients (Kasahara et al., 2006).
There are many lines of evidence that implicate dysregulation of the dopamine system in BD. Administration of the dopamine precursor levodopa or drugs that increase synaptic dopamine (such as amphetamine) can induce manic symptoms in healthy individuals (Jacobs and Silverstone, 1986; Krauthammer and Klerman, 1978). It is interesting to note that long term use of levodopa is associated with an “on-off” effect where patients experience abrupt swings from involuntary movements to akinesia, often accompanied by mood swings that range from elation to depression; these effects may be mediated by sudden changes in synaptic dopamine concentrations (Berk et al., 2007). Amphetamine withdrawal is also frequently associated with depression (Jacobs and Silverstone, 1986), similar to a transition from mania to depression in BD. In addition, administration of the antihypertensive agent reserpine, a classic model of depression, decreases intracellular stores of dopamine (Long and Kathol, 1993). Finally, dopamine antagonists such as haloperidol and chlorpromazine are effective at reducing manic symptoms (Tohen et al., 2003; Vieta and Sanchez-Moreno, 2008).
To explore the potential relationship between dysfunction of the dopamine system and BD, our group has assessed the behavior of dopamine transporter (DAT) deficient or knockout (KO) mice Ralph-Williams et al., 2003; Ralph et al., 2001a). Use of these animals is supported by several findings, including: 1) recent studies that indicate a genetic linkage between the DAT and BD Greenwood et al., 2001; Hayden and Nurnberger, 2006); 2) the observation that amphetamine increases dopamine levels in part by inhibiting dopamine transport; and 3) evidence that both DAT deficient and KO mice exhibit significant increases in extracellular dopamine activity (Giros et al., 1996; Zhuang et al., 2001).
Initial studies with the DAT KO mice indicated that these animals exhibited significantly increased locomotor activity in a novel environment and were characterized by a 100-fold increase in the duration of released extracellular dopamine compared to wildtype animals (Giros et al., 1996). When tested in a video-tracker open field apparatus, both male and female DAT KO mice exhibited increased motor activity, lower spatial d values (indicative of more repetitive, straight line movements), and a more restrictive pattern of activity along the walls of the enclosure relative to control (Powell et al., 2004;Ralph et al., 2001a).
The complete removal of DAT results in a number of physiological changes, including anterior pituitary hypoplasia, dwarfism, lactation deficits, and a high mortality rate (Bosse et al., 1997). To avoid these effects, a line of DAT knockdown (KD) mice was generated that lacks 90% of the DAT, but does not differ in body weight, pituitary weight, litter size, or lactation activity compared to wildtype animals (Zhuang et al., 2001). DAT KD mice exhibit hyperactivity and lower spatial d relative to wildtype littermates, similar to the DAT KO mice (Ralph-Williams et al., 2003). Furthermore, acute administration of valproate reduced both locomotor hyperactivity and the repetitive pattern of motor behavior observed with these animals, supporting the contention that the mice represent a model of BD mania (Ralph-Williams et al., 2003).
More recently, DAT KD mice have been assessed in the mouse Behavior Pattern Monitor (mBPM). As expected, these mice exhibit locomotor hyperactivity in the mBPM, as well as increased exploration (holepokes) and reduced spatial d compared to wildtype littermates, establishing a multivariate profile of their exploratory and motor activity (Young et al., 2007).
In conclusion, current efforts to model BD in animals have met with limited success and thus far, no single model has successfully encapsulated the key aspects of this disorder, including the cyclic nature of this illness. Most animal models are defined primarily, if not exclusively, by increased motor activity, but more recent endeavors, such as the work with the DAT manipulated mice, have attempted to provide a more detailed and comprehensive picture of abnormalities in motor and exploratory behavior. The following section will describe similar efforts to quantify these behaviors objectively in humans.
4. Assessment of exploration and motor behavior in psychiatric illness
The ability to provide an accurate and consistent assessment of human movement and exploratory behavior is in many ways a far greater challenge than quantifying animal behavior in a carefully controlled laboratory setting. LaPorte and colleagues suggested that 30 different methods of measuring human physical activity could be grouped into seven broad categories, including calorimetry, job classification, survey procedures, physiological markers, behavioral observation, mechanical and electronic monitors, and dietary measures (LaPorte et al., 1985). Some of these methods are precise, but relatively impractical, such as direct calorimetry, which requires subjects to be placed in special chambers that allow measurement of heat production. Other methods, such as diary surveys, are superior measures of normal day-to-day activity, but are prone to errors in self-assessment (Sims et al., 1999).
In the past 50 years, however, there have been a number of attempts to quantify locomotion using mechanical or electronic monitors. Mechanical activity monitors worn on the wrist were first described by Schulman (Schulman and Reisman, 1959) and were developed originally from modifications to the self-winding watch. Within a few years, other electronic devices were created to measure motor activity. One mechanism consisted of a wrist-worn unit where the movement of a steel ball activated a frequency modulated (FM) transmitter, allowing detection of movements that deviated from a horizontal plane (McPartland et al., 1976). A second type of wrist monitor contained a piezoelectric transducer to detect acceleration, a device that stored up to 256 readings of movement intensity (Colburn et al., 1976). More recent advances in the development of ambulatory activity monitors, commonly called actigraphs, have allowed various groups to quantify and record thousands of measurements taken from portable devices usually placed on the wrist or leg (Redmond and Hegge, 1985; Rothney et al., 2008; Tryon, 1991). Actigraphy has been used in multiple ways to characterize alterations in motor activity associated with psychiatric illness: 1) to assess overall activity level during the day or night as a measure of hyperactivity, agitation, or psychomotor retardation; 2) to measure patterns of sleep behavior, such as latency to fall asleep and number of awakenings; 3) to study circadian rhythms using data collected over several days (Teicher, 1995).
Several groups have used wrist actigraphy to characterize motor activity in BD mania. One study observed that inpatient BD subjects in the manic phase exhibited greater motor activity during a 24-hour period compared to a group of healthy controls and were particularly active at night (Wehr et al., 1980). A second report indicated that an inpatient BD sample demonstrated greater activity during the manic phase compared to the depressed phase of their illness (Wolff et al., 1985); these authors also found that BD patients showed lower motor activity during the day compared to healthy comparison subjects who were housed in the same psychiatric unit for the duration of the study. This result, however, is complicated by the fact that comparison subjects in this sample were college students averaging half the age of the patient group and were allowed the freedom to engage in off-unit activities. A more recent study (Gardos et al., 1992) compared motor activity of manic, depressed, and SCZ inpatients using a piezoelectric monitor attached to the non-dominant ankle. This group reported that depressed individuals (including both BD and unipolar patients) exhibited less activity compared to manic and SCZ patients during the daytime hours.
Additional studies have utilized wrist actigraphy to quantify locomotion during depression. Individuals diagnosed with unipolar depression exhibit greater motor activity relative to subjects with BD depression (Kupfer et al., 1974), but demonstrate lower activity compared to BD subjects in a manic state (Weiss et al., 1974). Other groups have reported both increases and decreases in motor activity in depressed subjects (Foster et al., 1978; Teicher et al., 1988).
The objective quantification of medication effects on motor activity in psychiatric populations has not been extensively documented. One report observed that amitriptyline treatment decreased motor activity in a subset of agitated unipolar depressed patients, but had little effect in other subjects (Kupfer et al., 1974). Treatment with lithium also appears to produce inconsistent effects on motor activity in BD mania (Heninger and Kirstein, 1977; Weiss et al., 1974). Klein and colleagues (Klein et al., 1992) evaluated locomotor activity in BD patients before and after discontinuation from lithium therapy using a wrist actigraph. They observed that individuals who relapsed into mania within 3 months of lithium treatment cessation showed higher levels of motor activity while still taking the drug. The authors suggest that elevated locomotor activity may be a marker for subclinical mania and could be used to identify patients at high risk for relapse.
Actigraphy has been also used to characterize motor activity in attention deficit hyperactivity disorder (ADHD), with studies reporting that children with ADHD are 25% to 30% more active than comparison subjects (Porrino et al., 1983). ADHD hyperactivity quantified by actigraphy can also be attenuated significantly with treatment such as methylphenidate (Borcherding et al., 1989). In the past decade, one group has developed a more comprehensive motion analysis system to assess motor activity in ADHD (Teicher et al., 1996; Teicher et al., 2008). In this paradigm, children are asked to sit in a chair and complete a continuous performance task (CPT) while their motor activity is tracked by an infrared video camera recording the location of reflective markers placed on their head, shoulders, and elbow. One study observed that ADHD children ages 6 to 14 moved their head 2.3 times more often than normal children during this procedure, exhibited a 3.4 fold increase in total distance traveled, and showed a significant decrease in spatial d for their head, shoulder, and elbow movements (Teicher et al., 1996).
In contrast to the relatively extensive work describing the quantification of general motor activity in humans, few attempts have been made to assess human exploration of a novel environment. The examples in the existing literature usually involve evaluating activity in children with ADHD. For example, Roberts and colleagues developed a playroom observation procedure to assess movement and attention in boys with this disorder (Roberts et al., 1984). Subjects were placed in a room divided into 16 equal regions with toys located on 4 tables situated around the room. The children were given instructions to play with all or a subset of the toys available. The results showed that boys with ADHD demonstrated increased locomotor activity (region transitions) and more frequent non-purposeful repetitive movements during play with a specific toy when they were compared to healthy comparison subjects. A subsequent study using this paradigm observed that ADHD children touched toys more frequently relative to comparison subjects, but did not exhibit a difference in total motor activity (broadly defined as walking or shifting posture in a seated position) (Handen et al., 1998). More recently, exploratory behavior has been assessed in autistic children (Pierce and Courchesne, 2001). In this study, subjects were asked to wait for eight minutes in a large room filled with 20 unique objects characterized by a variety of shapes, colors, and textures. The authors observed that autistic children exhibited decreased exploration (reduced object interaction) and increased stereotyped or repetitive movements when compared with healthy comparison subjects.
Assessment of human motor and exploratory behavior remains in an early stage of development relative to the refined and detailed tools available to describe these behaviors in animal models. While a few studies have reported a more comprehensive approach to measuring motor and exploratory activity in ADHD (Roberts et al., 1984; Teicher et al., 2008), the human Behavior Pattern Monitor (hBPM) represents the first translational tool utilized to quantify exploratory behavior in humans based on the rodent open field paradigm (Perry et al., 2009) (Figure 1). Typically, translational paradigms that assess similar measures in both human and animal models, such as the prepulse inhibition of the eyeblink response, are first developed in the human population (Braff, 1978), then extended and adapted for use in animals such as rodents (Geyer et al., 2001; Geyer and Markou, 2002). In contrast, the hBPM represents a “reverse-translational” model of the rodent open field BPM, now extended and adapted to assess similar measures in humans.
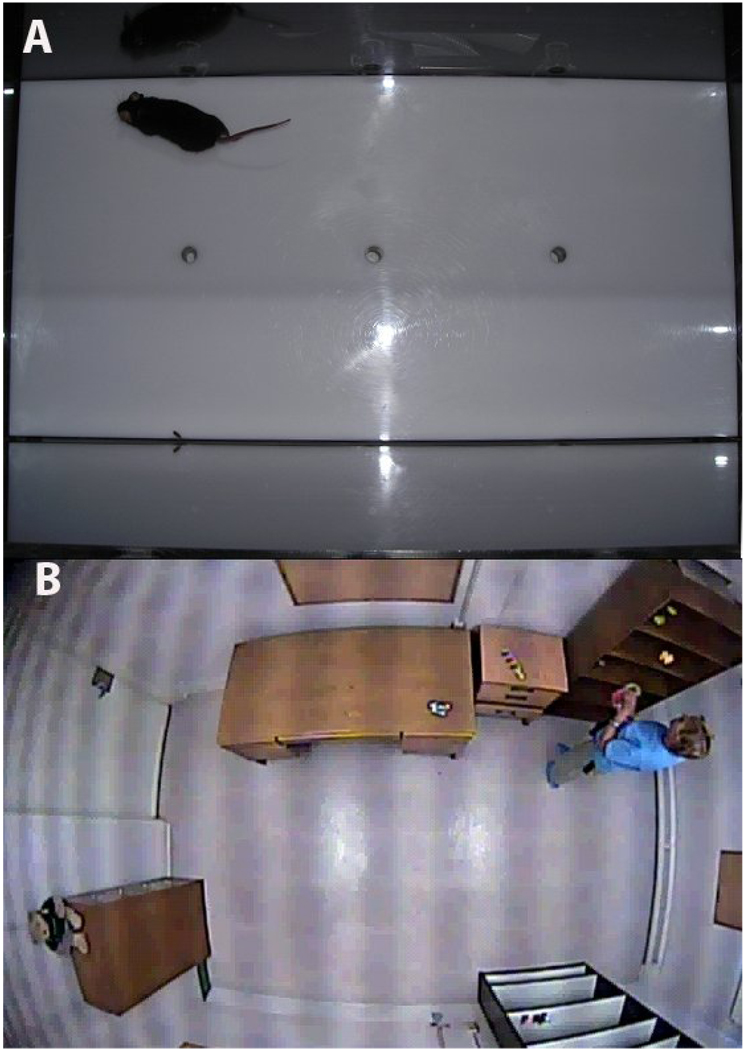
Figure 1.
The mouse Behavior Pattern Monitor (mBPM) (A) and Human Behavior Pattern Monitor (B). The mBPM consists of a 30.5 by 61 cm Plexiglas chamber equipped with 3 floor holes and 8 wall holes that serve as objects to stimulate exploratory behavior. Mouse locomotor activity is monitored by a grid of 12 by 24 infrared photobeams placed 1 cm above the floor. Additional photobeams are situated in each hole to record holepokes. The hBPM is a 2.7 by 4.3 meter room that serves as a novel environment for human subjects who receive no explicit instructions or directions during the test. The room contains several 11 small objects dispersed across several items of furniture, but does not include a chair. Motor activity and object interactions are recorded during the 15 minute testing session by a digital camera concealed in the ceiling.
The hBPM consists of a rectangular room novel to the human participants, outfitted with furniture that includes two bookcases and a desk, but no chairs. Subjects are asked to wait in the room for 15 minutes without explicit instructions or directions except to wait for the experimenter. Eleven colorful and tactile objects are placed around the room to stimulate exploration (Pierce and Courchesne, 2001); these objects act as an analogue of the exploratory holes in the walls and floor of the rodent BPM (Geyer et al., 1986; Risbrough et al., 2006). In addition, similar to the rodent BPM, multiple measures of motor activity, including spatial d, entropy h, transitions between various regions of the room, and total distance traveled can be assessed (Minassian et al., 2010; Perry et al., 2009; Young et al., 2007).
hBPM data are acquired from three principal sources: 1) collection of physiological information from an ambulatory monitoring vest worn by the subjects, 2) x-y coordinates of a subject’s spatial location in the hBPM, recorded by a digital video apparatus, and 3) experimenter ratings of exploratory activity. Collectively, these measures facilitate a multivariate assessment of behavior in this paradigm and describe primary factors of interest: 1) overall activity (accelerometry, region transitions); 2) exploratory behavior (object interactions); 3) the organization and structure of the motor activity (spatial d, entropy h).
Subjects in the hBPM wear an ambulatory monitoring device called the Lifeshirt System (LS) (Vivometrics, 2002). This device is a sleeveless undergarment that collects a variety of physiological data via multiple sensors, including respiratory inductive plethysmography bands that monitor pulmonary activity, a 3-lead electrocardiogram (ECG) that assesses electrical activity of the myocardium, and a two-axis accelerometer that measures motor activity. The accelerometer is placed over the sternum and can be used to detect periods of activity and rest. An on-board PDA continuously encrypts and records the patient’s activity and stores the information on a compact memory card. Accelerometer data are sampled at 10 Hz and stored in digital units. These data can be quantified over any chosen length of time; typically averaged over five-minute epochs of a 15-minute session within the hBPM. In addition to the accelerometer data, the LS records information about autonomic functions, including respiratory rate, heart rate, and heart rate variability, measures that are disrupted in psychiatric illness and affected by psychotropic medications (Henry et al., 2010; Rechlin et al., 1994; Valkonen-Korhonen et al., 2003).
The hBPM is equipped with a hidden ceiling camera furnished with a fish-eye lens capable of viewing the entire room. Video images are stored in digital format on a computer in an adjacent room, recorded at a frequency of 30 frames per second. Digital videos of the 15-minute hBPM session are subjected to frame-by-frame analysis with proprietary software (Clever Systems Inc. 1999) that generates x-y coordinates of the subject’s position by tracking the individual’s torso. The x-y coordinate information is used to generate measures that assess the quantity and pattern of motor activity in replication of the rodent BPM, including activity counts (defined as the number of discrete instances of movement or the smallest measured change in x-y coordinates), region transitions, entropy h, and spatial d.
Exploration is evaluated by measuring an individual’s interaction with the 11 objects that are placed around the room (analogous to holepokes in the rodent BPM). A trained observer analyzes the hBPM video images and records the number of objects a subject interacts with (i.e. touches), the duration of these interactions, and the quantity of multiple or repetitive object contacts. The combination of both automated and observer-derived data obtained from the Lifeshirt, x-y data analysis, and digital videos enable a uniquely descriptive and detailed assessment of motor and exploratory behavior with this translational paradigm.
Manic BD patients exhibit a hyperactive profile in the hBPM and display an increase in the average acceleration and total activity counts compared to healthy comparison subjects (Perry et al., 2009). They also demonstrate alterations in the pattern of their motor activity, as represented by a decrease in spatial d and an increase in entropy h, indicating more variable and disorganized activity in the room. Finally, manic BD patients display increased exploration as reflected by a greater number of object interactions. In addition, they show a tendency to engage in more disinhibited exploration (e.g., wearing a mask or opening the desk drawers).
BD individuals also exhibit a distinctive signature of motor and exploratory activity in the hBPM in comparison to other psychiatric disorders (Minassian et al., 2010; Paulus et al., 2007; Perry et al., 2009). Paulus and colleagues assessed the behavior of manic BD inpatients compared to an outpatient sample of adults with ADHD (Paulus et al., 2007). BD subjects demonstrated greater motor activity (distance traveled) in the first 5 minutes of the session, lower spatial d overall, and spent more time in the center of the room compared to both the ADHD and comparison groups. In contrast, while both healthy comparison and BD participants exhibited less activity over time, ADHD participants did not show habituation of locomotion during the 15 minute session. Although children diagnosed with ADHD demonstrated lower spatial d relative to comparison in a stationary paradigm (Teicher et al., 1996), ADHD adults and comparison subjects did not differ in this measure when allowed free movement in the hBPM.
It is often difficult to discriminate between BD mania and schizophrenia because the behavioral presentation in both patient groups is dominated by psychotic and mood symptoms (Pini et al., 2004). A recent study (Perry et al., 2009) assessed the behavior of both manic BD and SCZ inpatients in the hBPM. The results indicated that age- and education-matched manic BD subjects exhibited increased motor activity as measured by LS acceleration and activity counts relative to both healthy comparison and SCZ subjects. Both BD and SCZ patients exhibited lower spatial d values and higher entropy (indicating more disorganized and less predictable activity) compared to healthy comparison subjects; however, BD patients demonstrated a unique and prominent increase in exploration (number of object interactions) compared to the other two groups. Discriminant function analyses revealed that hBPM measures (including acceleration, entropy, spatial d, and object interactions) correctly classified BD and SCZ patients better than symptom domain scores.
The human data are paralleled by animal findings describing the effect of three putative rodent models of BD in the mouse BPM: 1) mice administered an acute dose of amphetamine; 2) the DAT KD mice; 3) mice administered the selective DAT inhibitor GBR12909 (Perry et al., 2009). While all three groups of mice exhibit increased motor activity and reduced spatial d relative to control, a different picture emerges with exploration. Genetic reduction or pharmacological inhibition of DAT increases exploratory activity as assessed by holepokes and rearing; in contrast, administration of amphetamine had little effect on rearing and actually reduced holepokes compared to control animals. While GBR12909 selectively blocks DAT, amphetamine is a relatively nonselective monoamine reuptake inhibitor with the highest potency at the norepinephrine transporter (NET), exhibiting 6- to 10-fold less efficacy at DAT in rodent and human (Han and Gu, 2006; Holmes and Rutledge, 1976). These results suggest that greater NET inhibition combined with dopamine release may account for the decrease in rodent exploration observed with amphetamine (Young et al., 2010). Previous studies have demonstrated that NET inhibition, concurrent with increased norepinephrine in the synaptic cleft, reduces rat exploration in the holeboard task (Sara et al., 1995). Given that manic BD patients exhibit increased exploration in the hBPM, these findings suggest that DAT dysfunction, rather than noradrenergic activity, may mediate hyperexploratory behavior associated with BD. It is also relevant to note that the preponderance of genetic linkage studies examining NET polymorphisms have failed to establish an association between this gene and BD (Chang et al., 2007; Hadley et al., 1995; Leszczynska-Rodziewicz et al., 2002).
While several monoamines, including dopamine, norepinephrine, and serotonin, are implicated in the etiology of BD, the contribution of each of these neurotransmitters to the pathology of this disorder remains unclear (Martinowich et al., 2009). A recent report indicates that the majority of BD individuals (91% inpatient, 65% outpatient) are treated with atypical antipsychotic medications, including compounds with high affinity for the serotonin 5-HT2A receptor (Fenton and Scott, 2005; Ventimiglia et al., 2009). Widespread use of these drugs has renewed interest in dopamine-serotonin interactions relevant to symptoms associated with BD and schizophrenia (Harrison-Read, 2009). In our lab, administration of the selective 5-HT2A antagonist M100907 has been shown to decrease locomotion and minimize perseverative motor activity (increasing spatial d) in hyperdopaminergic DAT knockout (KO) mice tested in the mouse BPM (Barr et al., 2004). In addition, hyperactivity in the mBPM induced by the serotonin releaser MDMA (3,4-methylenedioxymethamphetamine) was modulated in dopamine D1 and D2 receptor KO mice (Risbrough et al., 2006); data from this study indicated that D1 but not D2 receptors modulate the structure of locomotor activity (linear vs. circumscribed movements) as quantified by spatial d. Future studies could extend this work by assessing the effect of serotonin agonists or antagonists in the hBPM. In summary, these reports illustrate the potential of the BPM model to examine relationships between multiple neurotransmitter systems that may mediate neurobiological mechanisms underlying bipolar disorder.
While genetic manipulation has been used extensively as a tool to study mouse behavior in the mBPM, recent studies have started to examine the effect of genetic variants on human behavior in the hBPM. For example, Minassian and colleagues assessed the effect of the catechol-o-methyltransferase (COMT) Val 158Met gene polymorphism on hBPM activity in a small sample of manic BD inpatients (Minassian et al., 2009). This gene for COMT, an enzyme that participates in the metabolic inactivation of dopamine in the frontal cortex, is characterized by a common functional polymorphism, the amino acid substitution of methionine (Met) for valine (Val) (Lachman et al., 1996). Expression of the Val allele, which significantly reduces prefrontal dopaminergic tone relative to Met, is associated with poor cognitive performance and perseverative behavior in schizophrenia patients (Bruder et al., 2005; Egan et al., 2001; Goldberg et al., 2003). In contrast, the Met allele has been associated with aggressive and suicidal behavior (Kotler et al., 1999). In the hBPM, BD Val homozygotes exhibited lower entropy h relative to Met homozygotes, suggesting more predictable, perseverative motor activity over time (Minassian et al., 2009). Furthermore, the Val allele “dose” was also inversely related to acceleration, such that Met homozygotes had the highest motor activity. Although these findings were preliminary and need to be replicated in a larger sample, they suggest that, in BD, the Met allele may be associated with greater liability for symptoms of overactivation, while the Val allele, as in schizophrenia, may confer liability for cognitive inflexibility, perhaps because it contributes to low dopaminergic tone in frontal systems.
5. Summary
In recent years, there has been greater awareness that alterations in motor and exploratory behavior represent a fundamental feature of many neuropsychiatric diseases, including BD. This trend has been reflected by the increasing use of motor abnormalities as diagnostic criteria. The ability to assess these behaviors has been limited by the common use of subjective and imprecise self-report and observer rating scales. In addition, the discontinuity between human actigraphy and measures of motor activity in animal models of these disorders has restricted the capacity to conduct valuable translational research. The recent development of the tools such as the hBPM thus represents one solution to these problems by providing an objective and sophisticated measure with high translational value. The pharmacological and genetic data presented in this review illustrate the capacity of the BPM paradigm to characterize endophenotypes in human psychiatric illness that can be observed in identical fashion in animal models. While many animal models of psychiatric disorders are limited by the use of a single univariate measure, such as a simple increase in general motor activity, the multivariate approach described here improves the ability to test the validity of some of these widely accepted models. While models of BD such as the DAT KD mouse are currently best supported by face validity (given the common observable behaviors in both the mouse and human BPM), the development of the human open field paradigm offers a range of opportunities for refining animal models of psychiatric disorders. Future studies could assess pharmacological predictive validity by evaluating the effect of stimulants (such as amphetamine) on both human and murine behavior in the BPM. Examining the relationship between hBPM measures and genetic polymorphisms in monoamine transporters and receptors would also elucidate how neurotransmitter dysfunction impairs motor and exploratory behavior. Finally, neuroimaging techniques such as positron emission tomography and magnetic resonance imaging could be utilized to correlate structural and functional neural abnormalities with behavioral deficits in the hBPM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LM, Geyer MA. LSD-induced alterations of locomotor patterns and exploration in rats. Psychopharmacology (Berl) 1982;77:179–185. doi: 10.1007/BF00431945. [DOI] [PubMed] [Google Scholar]
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR, Kucinski BJ, Fowler H, Gershon S, Edwards DJ, Austin MC, Stiller R, Kiss S, Kocan D. The effects of lithium on a potential cycling model of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:495–510. doi: 10.1016/s0278-5846(98)00020-7. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. third ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–132. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Aylmer CG, Steinberg H, Webster RA. Hyperactivity induced by dexamphetamine/chlordiazepoxide mixtures in rats and its attenuation by lithium pretreatment: a role for dopamine? Psychopharmacology (Berl) 1987;91:198–206. doi: 10.1007/BF00217062. [DOI] [PubMed] [Google Scholar]
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology. 2004;29:221–228. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Begley CE, Annegers JF, Swann AC, Lewis C, Coan S, Schnapp WB, Bryant-Comstock L. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics. 2001;19:483–495. doi: 10.2165/00019053-200119050-00004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Fresi F, Maccioni P, Smeraldi E. Behavioural sensitization to repeated sleep deprivation in a mice model of mania. Behav Brain Res. 2008;187:221–227. doi: 10.1016/j.bbr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Berggren U, Engel J, Liljequist S. The effect of lithium on the locomotor stimulation induced by dependence-producing drugs. J Neural Transm. 1981;50:157–164. doi: 10.1007/BF01249137. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant'anna M, Malhi GS, Bourin M, Kapczinski F, Norman T. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl. 2007:41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal and curiosity. New York: McGraw Hill; 1960. [Google Scholar]
- Berlyne DE. Curiosity and exploration. Science. 1966;153:25–33. doi: 10.1126/science.153.3731.25. [DOI] [PubMed] [Google Scholar]
- Boissier JR, Simon P. The exploration reaction in the mouse. Preliminary note. Therapie. 1962;17:1225–1232. [PubMed] [Google Scholar]
- Borcherding BG, Keysor CS, Cooper TB, Rapoport JL. Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology. 1989;2:255–263. doi: 10.1016/0893-133x(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Braff D. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Bodily changes in pain, hunger, fear, and rage; an account of recent researches into the function of emotional excitement. Boston: Branford; 1953. [Google Scholar]
- Cao BJ, Peng NA. Magnesium valproate attenuates hyperactivity induced by dexamphetamine-chlordiazepoxide mixture in rodents. Eur J Pharmacol. 1993;237:177–181. doi: 10.1016/0014-2999(93)90266-k. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lu RB, Ma KH, Chang HA, Chen CL, Huang CC, Lin WW, Huang SY. Association study of the norepinephrine transporter gene polymorphisms and bipolar disorder in Han Chinese population. World J Biol Psychiatry. 2007;8:188–195. doi: 10.1080/15622970601136195. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Colburn TR, Smith BM, Guarini JJ, Simmons NN. An ambulatory activity monitor with solid state memory. Biomed Sci Instrum. 1976;12:117–122. [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crusio WE. Genetic dissection of mouse exploratory behaviour. Behav Brain Res. 2001;125:127–132. doi: 10.1016/s0166-4328(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Bardin L, Auclair AL, Kleven MS, Prinssen E, Colpaert F, Vacher B, Newman-Tancredi A. F15063, a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. II. Activity in models of positive symptoms of schizophrenia. Br J Pharmacol. 2007;151:253–265. doi: 10.1038/sj.bjp.0707159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drai D, Golani I. SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev. 2001;25:409–426. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann J-P, Ruelle D. Ergodic theory of chaos and strange attractors. Reviews of Modern Physics. 1985;57:617–656. [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Einat H. Different behaviors and different strains: potential new ways to model bipolar disorder. Neurosci Biobehav Rev. 2007a;31:850–857. doi: 10.1016/j.neubiorev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet. 2007b;37:244–255. doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- Einat H, Manji HK, Belmaker RH. New approaches to modeling bipolar disorder. Psychopharmacol Bull. 2003;37:47–63. [PubMed] [Google Scholar]
- Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Ellison GD, Eison MS. Continuous amphetamine intoxication: an animal model of the acute psychotic episode. Psychol Med. 1983;13:751–761. doi: 10.1017/s003329170005145x. [DOI] [PubMed] [Google Scholar]
- Fenton C, Scott LJ. Risperidone: a review of its use in the treatment of bipolar mania. CNS Drugs. 2005;19:429–444. doi: 10.2165/00023210-200519050-00005. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. The reliability of the hole-board apparatus. Psychopharmacologia. 1975a;44:47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975b;44:53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Flemenbaum A. Lithium inhibition of norepinephrine and dopamine receptors. Biol Psychiatry. 1977;12:563–572. [PubMed] [Google Scholar]
- Flicker C, Geyer MA. Behavior during hippocampal microinfusions. I. Norepinephrine and diversive exploration. Brain Res. 1982;257:79–103. doi: 10.1016/0165-0173(82)90006-6. [DOI] [PubMed] [Google Scholar]
- Foster FG, McPartland R, Kupfer DJ. Motion sensors in medicine, II. J Inter Am Med. 1978;3:13–17. [Google Scholar]
- Fratta W, Collu M, Martellotta MC, Pichiri M, Muntoni F, Gessa GL. Stress-induced insomnia: opioid-dopamine interactions. Eur J Pharmacol. 1987;142:437–440. doi: 10.1016/0014-2999(87)90084-7. [DOI] [PubMed] [Google Scholar]
- Gardos G, Teicher MH, Lipinski JF, Jr., Matthews JD, Morrison L, Conley C, Cole JO. Quantitative assessment of psychomotor activity in patients with neuroleptic-induced akathisia. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:27–37. doi: 10.1016/0278-5846(92)90005-y. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5 Suppl:89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- Geyer M, Krebs-Thomson K, Braff D, Swerdlow N. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer M, Paulus M. Multivariate analyses of locomotor and investigatory behavior in rodents. In: Ossenkopp K, et al., editors. Measuring Movement and Locomotion: From Invertebrates to Humans. R.G. Landes Co; 1996. pp. 253–271. [Google Scholar]
- Geyer MA. Approaches to the Characterization of Drug Effects on Locomotor Activity in Rodents. In: Adler M, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. Modern Methods in Pharmacology. New York: Wiley-Liss; 1990. pp. 81–99. [Google Scholar]
- Geyer MA, Markou A. The role of preclinical models in the development of psychotropic drugs. In: Davis KL, et al., editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; 2002. pp. 445–455. [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford UP; 1990. [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice, Neuromethods. vol. 42. Humana Press; 2009. pp. 1–20. [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–831. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium's reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, Kelsoe JR. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–151. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hadley D, Hoff M, Holik J, Reimherr F, Wender P, Coon H, Byerley W. Manic-depression and the norepinephrine transporter gene. Hum Hered. 1995;45:165–168. doi: 10.1159/000154279. [DOI] [PubMed] [Google Scholar]
- Hall CS. Drive and emotionality factors associated with adjustment in the rat. J Comp Psychol. 1934;17:89–108. [Google Scholar]
- Hall CS, Ballachey EL. A study of the rat's behavior in a field: a contribution to method in comparative psychology. Univ Calif Publ: Psychol. 1932;6:1–12. [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, McAuliffe S, Janosky J, Feldman H, Breaux AM. A playroom observation procedure to assess children with mental retardation and ADHD. J Abnorm Child Psychol. 1998;26:269–277. doi: 10.1023/a:1022654417460. [DOI] [PubMed] [Google Scholar]
- Harrison-Read PE. Antimanic potency of typical neuroleptic drugs and affinity for dopamine D2 and serotonin 5-HT2A receptors--a new analysis of data from the archives and implications for improved antimanic treatments. J Psychopharmacol. 2009;23:899–907. doi: 10.1177/0269881108094349. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Nurnberger JI., Jr. Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5:85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Kirstein L. Effects of lithium carbonate on motor activity in mania and depression. J Nerv Ment Dis. 1977;164:168–175. doi: 10.1097/00005053-197703000-00002. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. J Psychiatr Res. 2010;44:168–176. doi: 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RA, Moore JD, Hayes C, Phillips N, Hawkins J. REM sleep deprivation increases aggressiveness in male rats. Physiol Behav. 1979;22:1097–1100. doi: 10.1016/0031-9384(79)90263-4. [DOI] [PubMed] [Google Scholar]
- Holmes JC, Rutledge CO. Effects of the d- and l-isomers of amphetamine on uptake, release and catabolism of norepinephrine, dopamine and 5-hydroxytryptamine in several regions of rat brain. Biochem Pharmacol. 1976;25:447–451. doi: 10.1016/0006-2952(76)90348-8. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Jackson S. Melancholia and depression: From Hippocratic times to modern times. New Haven, CT: Yale University Press; 1986. [Google Scholar]
- Jacobs D, Silverstone T. Dextroamphetamine-induced arousal in human subjects as a model for mania. Psychol Med. 1986;16:323–329. doi: 10.1017/s0033291700009132. [DOI] [PubMed] [Google Scholar]
- Kafkafi N, Elmer GI. Activity density in the open field: a measure for differentiating the effect of psychostimulants. Pharmacol Biochem Behav. 2005;80:239–249. doi: 10.1016/j.pbb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kafkafi N, Pagis M, Lipkind D, Mayo CL, Bemjamini Y, Golani I, Elmer GI. Darting behavior: a quantitative movement pattern designed for discrimination and replicability in mouse locomotor behavior. Behav Brain Res. 2003;142:193–205. doi: 10.1016/s0166-4328(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, Kato T. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 2006;11:577–593. doi: 10.1038/sj.mp.4001824. 523. [DOI] [PubMed] [Google Scholar]
- Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev. 2007;31:832–842. doi: 10.1016/j.neubiorev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Logue SF, Dwyer JM, Beyer CE, Majchrowski H, Cai Z, Liu Z, Adedoyin A, Rosenzweig-Lipson S, Comery TA. The supra-additive hyperactivity caused by an amphetamine-chlordiazepoxide mixture exhibits an inverted-U dose response: negative implications for the use of a model in screening for mood stabilizers. Pharmacol Biochem Behav. 2009;92:649–654. doi: 10.1016/j.pbb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Klein E, Lavie P, Meiraz R, Sadeh A, Lenox RH. Increased motor activity and recurrent manic episodes: predictors of rapid relapse in remitted bipolar disorder patients after lithium discontinuation. Biol Psychiatry. 1992;31:279–284. doi: 10.1016/0006-3223(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics. 2003;21:601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, Ebstein RP. Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am J Med Genet. 1999;88:628–633. [PubMed] [Google Scholar]
- Kraepelin E. Sechste, vollständig umgearbeitete Auflage. 2 vols. Leipzig: Barth; 1899. Psychiatrie. Ein Lehrbuch für Studirende und Aerzte. [Google Scholar]
- Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35:1333–1339. doi: 10.1001/archpsyc.1978.01770350059005. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response: role of D1 and D2 dopamine receptors. Brain Res. 1999;822:164–174. doi: 10.1016/s0006-8993(99)01149-x. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Weiss BL, Foster G, Detre TP, McPartland R. Psychomotor activity in affective states. Arch Gen Psychiatry. 1974;30:765–768. doi: 10.1001/archpsyc.1974.01760120029005. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods. 2008;171:48–52. doi: 10.1016/j.jneumeth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- LaPorte RE, Montoye HJ, Caspersen CJ. Assessment of physical activity in epidemiologic research: problems and prospects. Public Health Rep. 1985;100:131–146. [PMC free article] [PubMed]
- Lat J. The spontaneous exploratory reactions as a tool for psychopharmacological studies. A contribution towards a "theory" of contradictory results in psychopharmacology; Pharmacology of Conditioning, Learning and Retention. Proc. 2nd Intern. Pharmacol. Meeting; Prague: Pergamon Press; 1963. pp. 47–66. vol. [Google Scholar]
- Lawrence JM, Teicher MH, Finklestein SP. Quantitative assessment of locomotor activity in psychiatry and neurology. In: Joseph AB, Young R, editors. Disorders of movement in psychiatry and neurology. Boston: Blackwell Scientific; 1992. pp. 449–462. [Google Scholar]
- Lesch KP, Mossner R. Inactivation of 5HT transport in mice: modeling altered 5HT homeostasis implicated in emotional dysfunction, affective disorders, and somatic syndromes. Handb Exp Pharmacol. 2006:417–456. doi: 10.1007/3-540-29784-7_18. [DOI] [PubMed] [Google Scholar]
- Leszczynska-Rodziewicz A, Czerski PM, Kapelski P, Godlewski S, Dmitrzak-Weglarz M, Rybakowski J, Hauser J. A polymorphism of the norepinephrine transporter gene in bipolar disorder and schizophrenia: lack of association. Neuropsychobiology. 2002;45:182–185. doi: 10.1159/000063668. [DOI] [PubMed] [Google Scholar]
- Long TD, Kathol RG. Critical review of data supporting affective disorder caused by nonpsychotropic medication. Ann Clin Psychiatry. 1993;5:259–270. doi: 10.3109/10401239309148826. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:209–224. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Mamelak M. An amphetamine model of manic depressive illness. Int Pharmacopsychiatry. 1978;13:193–208. doi: 10.1159/000468341. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Schloesser RJ, Manji HK. Bipolar disorder: from genes to behavior pathways. J Clin Invest. 2009;119:726–736. doi: 10.1172/JCI37703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genet. 1995;25:557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- McPartland RJ, Foster FG, Kupfer DJ, Weiss BL. Activity sensors for use in psychiatric evaluation. IEEE Trans Biomed Eng. 1976;23:175–178. doi: 10.1109/tbme.1976.324583. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010;120:200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Kelsoe JR, Paulus MP, Geyer MA, Perry W. Associations between COMT ValMet genotype and clinical and cognitive phenotypes in manic bipolar patients. Schizophr Bull. 2009;35 S1:109–110. [Google Scholar]
- Miyauchi T, Kikuchi K, Satoh S. Further studies on the potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Jpn J Pharmacol. 1981;31:61–68. doi: 10.1254/jjp.31.61. [DOI] [PubMed] [Google Scholar]
- Morden B, Mitchell G, Dement W. Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res. 1967;5:339–349. doi: 10.1016/0006-8993(67)90042-x. [DOI] [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biol Psychiatry. 1986;21:49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Paulus M, Geyer M. Assessing the organization of motor behavior: New approaches based on the behavior of complex physical systems. In: Ossenkopp K, et al., editors. Measuring Movement and Locomotion: From Invertebrates to Humans. R.G. Landes Co; 1996. pp. 273–295. [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav Brain Res. 1993;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, Gold LH, Mandell AJ. Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proc Natl Acad Sci U S A. 1990;87:723–727. doi: 10.1073/pnas.87.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Minassian A, Masten VL, Feifel D, Geyer MA, Perry W. Human behavioral pattern monitor differentiates activity patterns of bipolar manic and attention deficit hyperactivity subjects. Biol Psychiatry. 2007;61:2275. [Google Scholar]
- Paylor R, Lindsay E. Mouse models of 22q11 deletion syndrome. Biol Psychiatry. 2006;59:1172–1179. doi: 10.1016/j.biopsych.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Knuesel I, Feldon J. Amphetamine sensitization in rats as an animal model of schizophrenia. Behav Brain Res. 2008;191:190–201. doi: 10.1016/j.bbr.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Pini S, de Queiroz V, Dell'Osso L, Abelli M, Mastrocinque C, Saettoni M, Catena M, Cassano GB. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. Eur Psychiatry. 2004;19:8–14. doi: 10.1016/j.eurpsy.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Rapoport JL, Behar D, Sceery W, Ismond DR, Bunney WE., Jr. A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Arch Gen Psychiatry. 1983;40:681–687. doi: 10.1001/archpsyc.1983.04390010091012. [DOI] [PubMed] [Google Scholar]
- Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA "ecstasy" alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl) 2004;173:310–317. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001a;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Geyer MA. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J Pharmacol Exp Ther. 2001b;298:148–155. [PubMed] [Google Scholar]
- Rechlin T, Claus D, Weis M. Heart rate variability in schizophrenic patients and changes of autonomic heart rate parameters during treatment with clozapine. Biol Psychiatry. 1994;35:888–892. doi: 10.1016/0006-3223(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Redmond D, Hegge F. Observations on the design and specifications of a wrist-worn human activity monitoring system. Behav Res Methods Instrum Comput. 1985;17:659–669. [Google Scholar]
- Reischies FM, Hartikainen J, Berghofer A. Initial lithium and valproate combination therapy in acute mania. Neuropsychobiology. 2002;46 Suppl 1:22–27. doi: 10.1159/000068020. [DOI] [PubMed] [Google Scholar]
- Reiter LW, MacPhail RC. Motor activity: a survey of methods with potential use in toxicity testing. Neurobehav Toxicol. 1979;1 Suppl 1:53–66. [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robbins T. A Critique of the Methods Available for Measurement of Spontaneous Motor Activity. In: Iversen L, et al., editors. Handbook of Psychopharmacology. New York: Plenum Press; 1979. pp. 37–82. [Google Scholar]
- Roberts MA, Ray RS, Roberts RJ. A playroom observational procedure for assessing hyperactive boys. J Pediatr Psychol. 1984;9:177–191. doi: 10.1093/jpepsy/9.2.177. [DOI] [PubMed] [Google Scholar]
- Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring) 2008;16:1946–1952. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Hagenmeyer SH, Henault MA. Automated measurement of multivariate locomotor behavior in rodents. Neurobehav Toxicol Teratol. 1985;7:87–94. [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent C, Herve A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res Cogn Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Schulman JL, Reisman JM. An objective measure of hyperactivity. Am J Ment Defic. 1959;64:455–456. [PubMed] [Google Scholar]
- Shaldubina A, Einat H, Szechtman H, Shimon H, Belmaker RH. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm. 2002;109:433–440. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Stahl Z, Furszpan M, Regenold WT, Shapiro J, Belmaker RH, Bersudsky Y. Inositol deficiency diet and lithium effects. Bipolar Disord. 2006;8:152–159. doi: 10.1111/j.1399-5618.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Sims J, Smith F, Duffy A, Hilton S. The vagaries of self-reports of physical activity: a problem revisited and addressed in a study of exercise promotion in the over 65s in general practice. Family Practice. 1999;16:152–157. doi: 10.1093/fampra/16.2.152. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Sax KW. Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry. 1998;44:1171–1177. doi: 10.1016/s0006-3223(97)00454-x. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Ornstein K, Teitelbaum P, Golani I. The morphogenesis of stereotyped behavior induced by the dopamine receptor agonist apomorphine in the laboratory rat. Neuroscience. 1985;14:783–798. doi: 10.1016/0306-4522(85)90143-5. [DOI] [PubMed] [Google Scholar]
- Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Barber NI. Objective measurement of hyperactivity and attentional problems in ADHD. J Am Acad Child Adolesc Psychiatry. 1996;35:334–342. doi: 10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Lawrence JM, Barber NI, Finklestein SP, Lieberman HR, Baldessarini RJ. Increased activity and phase delay in circadian motility rhythms in geriatric depression. Preliminary observations. Arch Gen Psychiatry. 1988;45:913–917. doi: 10.1001/archpsyc.1988.01800340039005. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, McGreenery CE. Utility of objective measures of activity and attention in the assessment of therapeutic response to stimulants in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18:265–270. doi: 10.1089/cap.2007.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Goldberg JF, Gonzalez-Pinto Arrillaga AM, Azorin JM, Vieta E, Hardy-Bayle MC, Lawson WB, Emsley RA, Zhang F, Baker RW, Risser RC, Namjoshi MA, Evans AR, Breier A. A 12-week, double-blind comparison of olanzapine vs haloperidol in the treatment of acute mania. Arch Gen Psychiatry. 2003;60:1218–1226. doi: 10.1001/archpsyc.60.12.1218. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Motor activity measurements and DSM-III. Prog Behav Modif. 1986;20:35–66. doi: 10.1016/b978-0-12-535620-6.50006-8. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Activity Measurement in Psychology and Medicine. New York: Plenum; 1991. vol. [Google Scholar]
- Valkonen-Korhonen M, Tarvainen MP, Ranta-Aho P, Karjalainen PA, Partanen J, Karhu J, Lehtonen J. Heart rate variability in acute psychosis. Psychophysiology. 2003;40:716–726. doi: 10.1111/1469-8986.00072. [DOI] [PubMed] [Google Scholar]
- Ventimiglia J, Kalali AH, McIntyre R. Treatment of bipolar disorder. Psychiatry (Edgmont) 2009;6:12–14. [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Sanchez-Moreno J. Acute and long-term treatment of mania. Dialogues Clin Neurosci. 2008;10:165–179. doi: 10.31887/DCNS.2008.10.2/evieta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivometrics . The LifeShirt System™. Ventura, CA: 2002. [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Minck DR, Butcher RE. A method for measuring locomotor behavior in rodents: contrast-sensitive computer-controlled video tracking activity assessment in rats. Neurotoxicol Teratol. 1992;14:43–49. doi: 10.1016/0892-0362(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wehr TA, Muscettola G, Goodwin FK. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm. Early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry. 1980;37:257–263. doi: 10.1001/archpsyc.1980.01780160027002. [DOI] [PubMed] [Google Scholar]
- Weiss BL, Foster FG, Reynolds CF, 3rd, Kupfer DJ. Psychomotor activity in mania. Arch Gen Psychiatry. 1974;31:379–383. doi: 10.1001/archpsyc.1974.01760150083012. [DOI] [PubMed] [Google Scholar]
- Welker WI. An analysis of exploratory and play behavior in animals. In: Fiske DW, Maddi SR, editors. Functions of varied experience. Homewood: Dorsey Press; 1961. p. 111. [Google Scholar]
- Whimbey AE, Denenberg VH. Two independent behavioral dimensions in open-field performance. J Comp Physiol Psychol. 1967;63:500–504. doi: 10.1037/h0024620. [DOI] [PubMed] [Google Scholar]
- Wielosz M. The effect of lithium chloride on the activity of some psychotropic drugs. Pol J Pharmacol Pharm. 1976;28:189–198. [PubMed] [Google Scholar]
- Wolff EA, 3rd, Putnam FW, Post RM. Motor activity and affective illness. The relationship of amplitude and temporal distribution to changes in affective state. Arch Gen Psychiatry. 1985;42:288–294. doi: 10.1001/archpsyc.1985.01790260086010. [DOI] [PubMed] [Google Scholar]
- Woods SW. The economic burden of bipolar disease. J Clin Psychiatry. 2000;61 Supp 13:38–41. [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MS, Li YC, Lin MT. A modularized infrared light matrix system with high resolution for measuring animal behaviors. Physiol Behav. 1993;53:545–551. doi: 10.1016/0031-9384(93)90152-6. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]