Abstract
Toxic airline syndrome is assumed to be caused by exposure to tri-cresyl phosphate, an additive in engine lubricants and hydraulic fluids, which is activated to the toxic 2-(o-cresyl)-4H-1,3,2-benzodioxaphosphoran-2-one (CBDP). At present there is no laboratory evidence to support intoxication of airline crew by CBDP. Our goal was to develop methods for testing in vivo exposure by identifying and characterizing biomarkers. Mass spectrometry was used to study the reaction of CBDP with human albumin, free tyrosine, and human butyrylcholinesterase. Human albumin made a covalent bond with CBDP, adding a mass of 170 to tyrosine 411 to yield the ortho-cresyl phosphotyrosine derivative. Human butyrylcholinesterase made a covalent bond with CBDP on serine 198 to yield 5 adducts with added masses of 80, 108, 156, 170, and 186. The most abundant adduct had an added mass of 80 from phosphate (HPO3), a surprising result since no pesticide or nerve agent is known to yield phosphorylated serine with an added mass of 80. The next most abundant adduct had an added mass of 170 to form ortho-cresyl phosphoserine. It is concluded that toxic gases or oil mists in cabin air may form adducts on plasma butyrylcholinesterase and albumin, detectable by mass spectrometry.
Keywords: CBDP, butyrylcholinesterase, serum albumin, tyrosine, organophosphorus agent, mass spectrometry, toxic airline syndrome
Introduction
Over the past 20 years, flight-crew members (both commercial and military) have reported symptoms such as dizziness, nausea, disorientation, blurred vision, short term memory issues and tingling legs which have been associated with smoke or fumes from the jet engines that have entered the cabin area. A prime candidate for the causative agent in these exposures is tri-cresyl phosphate (TCP) 2, a common additive in engine lubricants and hydraulic fluids [1; 2; 3]. TCP is a mixture of various positional cresyl isomers (o, m, p-TCP). Tri-o-cresyl phosphate (TOCP) is more toxic than the meta and para forms, which are considered to be non-toxic [1; 4]. Isomers containing mono-o-cresyl phosphate are considered to be the most toxic, followed by the di-ortho and tri-ortho compounds [5].
TCP is infamously associated with “ginger jake paralysis”, a condition that afflicted 20,000-50,000 people in the United States in 1930. The paralysis was caused by the use of tri-cresyl phosphate (containing principally tri-o-cresyl phosphate) as an adulterant in Jamaica Ginger, a medicinal alcohol extract of ginger that was used for stomach problems and commonly abused as an illicit source of alcohol during prohibition [6; 7]. Another major outbreak of tri-cresyl phosphate poisoning, involving 10,000 victims, occurred in 1959 in Morocco. This one was caused by cooking oil adulterated with aircraft hydraulic oil [7; 8]. Other outbreaks have been reported [7], with the latest occurring in China in 1995 [9].
Paralysis in these cases involved the extremities, principally the legs, and appeared one to two weeks after consumption of the TCP [6]. These outbreaks all involved oral consumption of high doses of tri-o-cresyl phosphate. A high oral dose for humans is considered to be around 6.6 mg of TOCP/kg body weight [10]. As a consequence of these poisonings, manufacturers of TCP reduced the level of o-cresyl phosphate isomers in their products, from 25-40% in the 1930s-1940s to 0.1-1.0% in the 1990s [1; 2]. Reduction in tri-o-cresyl phosphate levels was the primary focus, however the content of mono-o-cresyl phosphate remains a point of concern.
Tri-cresyl phosphate currently is used as an anti-wear and extreme pressure additive in lubricants and hydraulic fluids [1; 2]. It has been used as a plasticizer in lacquers and varnishes, as a flame retardant in plastics and rubbers, as a lead scavenger in gasoline, and as a hydrophobic additive in waterproofing materials [2; 11]. Except for its use as an additive in engine lubricants and hydraulic fluids most commercial applications of TCP had been discontinued by 2002 [2].
Assuming that ortho isomers of TCP are the causative agents in the airline incidents, symptoms appear after relatively low dose exposure. Exposure is presumed to occur from breathing contaminated cabin air or by absorption through the skin from deposits of contaminated particulates coming from the cabin air [1; 2; 3]. Lack of accurate data makes it difficult to assess the actual level of exposure that may have been involved [3], but the absence of paralysis argues that exposure levels were not high. The range of potential exposure covers four orders of magnitude. High dose exposure would correspond to breathing an atmosphere containing 1300 mg of tri-o-cresyl phosphate per cubic meter, for 30 minutes, by a 70 kg individual. This corresponds to oral consumption of 6.6 mg TOCP per kg, which is sufficient to cause serious paralysis. The recommended safe exposure limit is considered to be breathing 0.1 mg of tri-o-cresyl phosphate per cubic meter, for 30 minutes, by a 70 kg individual [10]. Neither of these estimates takes into consideration the variability in P450 enzyme levels among individuals, or the higher toxicity of the mono-ortho isomer of TOCP.
The increasing number of reports from flight-crew members complaining of ill health following incidents of cabin air contamination by engine fumes [2; 3] prompted us to explore ways to test for exposure to tri-cresyl phosphate. Our plan was to use adducts formed on selected peptides from human serum albumin and human butyrylcholinesterase as biomarkers of exposure. Previous studies with other organophosphorus agents (OP) have identified tyrosine-411 as the most reactive residue on serum albumin [12], and it is generally accepted that serine-198 is the only residue in butyrylcholinesterase that reacts with OP. Peptides associated with these residues have already been successfully used for determination of in vivo exposure to other organophosphorus agents (OP) [13; 14; 15]. We anticipated that adducts formed upon exposure to TOCP would appear on these same peptides. However, to properly apply these biomarkers to TOCP exposure, we first needed to more fully understand the reactions that might be involved. To that end, we have examined the reactions of human butyrylcholinesterase and human serum albumin with the principal, toxic metabolite of TOCP: 2-(o-cresyl)-4H-1,3,2-benzodioxaphosphoran-2-one (CBDP) [16].
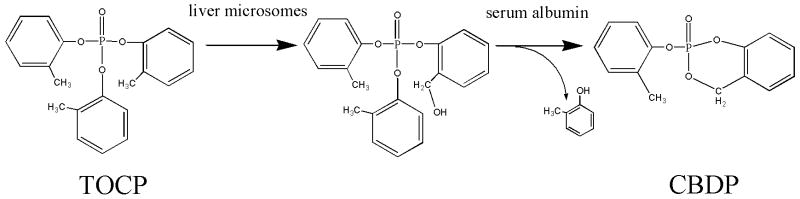
We chose to work with CBDP rather than with TOCP because it is accepted that TOCP is activated to CBDP in vivo [16; 17]. Activation involves oxidation of TOCP to di-o-cresyl-o-(alpha-hydroxy)tolyl phosphate, in the liver [16] followed by cyclization, with the aid of albumin, to yield CBDP in the serum (Scheme 1). Cyclization will occur spontaneously, but at 1/10 the rate of that catalyzed by albumin [18].
Scheme 1.
The toxic effects of TOCP are generally attributed to CBDP. CBDP is known to react with the active site serine in serine esterases, lipases and proteases, forming a covalent o-cresyl phosphate adduct, and causing inhibition of catalytic activity [16; 19]. Such a reaction with neurotoxic esterase has been proposed as the causative event in “organophosphorus ester induced delayed neuropathy” (OPIDN) [20]. OPIDN is the formal description for ginger jake paralysis.
We performed four studies in this report. 1) We examined the mass spectrum of CBDP to determine its purity and we annotated the mass spectral fragmentation pattern of CBDP so that we would have a better understanding of the mass spectral properties of the free compound. CBDP was found to be pure. 2) We used mass spectrometry to test whether or not CBDP could react with human serum albumin. It was found that an o-cresyl phosphotyrosine adduct was formed on Tyr411. 3) We reacted CBDP with free tyrosine in solution to test the general reactivity of CDBP with tyrosine. Ortho-cresyl phosphotyrosine was formed in a two step process involving a transient intermediate (a ring-opened form of the cyclic saligenin portion of CBDP-tyrosine). 4) We reacted CBDP with human butyrylcholinesterase and analyzed the tryptic peptides using mass spectrometry to confirm that the reaction of CBDP was with the active site serine in this serine hydrolase and that the reaction gave an o-cresyl-phosphate serine adduct. We found that the active site serine (Ser198) formed several CBDP related adducts, the major adduct actually being phosphoserine with o-cresyl-phosphoserine being a minor species.
Methods and Materials
2-(o-cresyl)-4H-1,3,2-benzodioxaphosphoran-2-one (CBDP) was obtained as a generous gift from Dr. W. D. Dettbarn. The CBDP (99.5% pure) had been custom synthesized by Starks Associates Inc. (Buffalo, NY). CBDP was dissolved in acetonitrile to 100 mM and stored at -80°C. Tyrosine (cat# T3754), acetonitrile (ACS reagent grade cat# A6914), and porcine pepsin (cat# P6887) were from Sigma/Aldrich (St. Louis, MO). Human serum albumin (essentially fatty acid free, cat# 05418) and formic acid (puriss p.a. for mass spectrometry cat# 94318) were from Fluka (Buchs, Switzerland). Sequencing grade trypsin (porcine, reductively methylated, TPCK treated, from Promega, Madison, WI, cat# V5113) was dissolved at 0.4 μg/μl in 50 mM acetic acid and stored at -80°C. Alpha-cyano-4-hydroxy cinnamic acid (CHCA, from Fluka cat# 70990) was recrystallized before use then suspended to 10 mg/ml (saturated solution) in 50% acetonitrile/50%water/0.1% trifluoroacetic acid. Trifluoroacetic acid (cat# 13972) was from Acros (Geel, Belgium). Methanol (HPLC grade, cat# MX0475) was from EMD (Gibbstown, NJ). Acetic acid (glacial, ACS reagent grade, cat# A38C-212) was from Fisher (Pittsburgh, PA). Butyrylcholinesterase (BChE) was purified from human plasma using ion-exchange chromatography on Q Sepharose (cat# 17-0510-04 Amersham Biosciences, Piscataway, NJ) and affinity chromatography on procainamide Sepharose 4B, as previously described [21]. BChE was stored at 4°C in the presence of 0.02% azide. All other chemicals were of ACS quality and used without further purification.
Sample preparation
Butyrylcholinesterase
Stock BChE was purified to 54% purity as described by Lockridge et al.[21]. It had an activity of 2620 units/ml, where a unit is defined as the amount of enzyme that hydrolyzes 1 micromole of butyrylthiocholine per minute. Fifty-five microliters of stock BChE (0.2 mg or 2.34 nmoles), in 10 mM ammonium bicarbonate, pH 8, plus 0.02% sodium azide, were mixed with 1 μl of 100 mM CBDP (100 nmoles) and incubated for 0.5-10 minutes at room temperature. These conditions produce a 40:1 molar ratio of CBDP to protein. Though this ratio is much higher than would be expected for an in vivo exposure, we chose it to ensure that the active site serine (serine-198) would be extensively labeled in these in vitro studies. This treatment inhibited all of the activity of BChE. The mixture was then either boiled for 10 minutes or mixed 1-to-1 with acetonitrile to denature the protein. Acetonitrile was used in place of boiling to test the effect of a milder denaturant on the nature of the labeled products. The method of denaturation had no effect on the nature of the final labeled product. When boiling was used to denature, 1 μl of 1 M ammonium bicarbonate and 8 μl of trypsin (0.4 μg/μl) were added to the reaction mixture (to give a 60:1 molar ratio of BChE to trypsin) and the mixture was incubated overnight at 37°C. When acetonitrile was used to denature, the reaction mixture was dried by vacuum centrifugation and resuspended in 50 μl water plus 1 μl of 1 M ammonium bicarbonate before adding the 8 μl of trypsin. Drying was done to remove the acetonitrile so that the trypsin would remain active. In neither procedure was the sample reduced or alkylated.
For MALDI analysis, two methods for handling the tryptic peptides were used. First, a 1 μl aliquot of the digest was diluted 1-to-10 with 50% acetonitrile plus 0.1% trifluoroacetic acid and used directly. Second, the entire 0.2 mg digest was separated via offline HPLC. Fractions were dried in a vacuum centrifuge and redissolved in 5 μl of 50% acetonitrile plus 0.1% trifluoroacetic acid. For HPLC details see the methods section entitled “Offline HPLC”.
For LCMSMS analysis with electrospray ionization, two methods for handling the tryptic peptides were used. In the first, an aliquot of the entire tryptic digest was simply dried in a vacuum centrifuge and redissolved in 5% acetonitrile plus 0.1% formic acid to a final concentration of 2 pmole/μl (assuming no losses during preparation). In the second, the entire 0.2 mg digest was separated via offline HPLC. Selected fractions were re-dried in a vacuum centrifuge and redissolved in 5% acetonitrile plus 0.1% formic acid to a final concentration of 2 pmole/μl (assuming no losses).
For mass spectral analysis using static infusion and electrospray ionization to introduce the sample into the mass spectrometer, offline HPLC was used to reduce the complexity of the sample. Selected fractions were re-dried in a vacuum centrifuge and redissolved in 50% acetonitrile, 25% methanol and 1% acetic acid.
Serum Albumin
Ten milliliters of human serum albumin (1 mg/ml or 150 nmoles) in 10 mM Tris/Cl pH 8.0 containing 0.1% sodium azide were mixed with 0.06 ml of 100 mM CBDP (6000 nmoles) and incubated at 37°C for 43 hours. These conditions produce a 40:1 molar ratio of CBDP to protein. Though this ratio is much higher than would be expected for an in vivo exposure, we chose it to ensure that the reactive residue (tyrosine-411) would be extensively labeled in these in vitro studies. A 10 μl aliquot (0.01 mg or 1.5 nmoles of albumin) was mixed with 10 μl of 1% trifluoroacetic acid to stop the reaction, denature the albumin, and prepare the mixture for peptic digestion. Two microliters of pepsin (1 mg/ml in 10 mM HCl) were added and the preparation was digested for 4 hours at 37°C. The remainder of the reaction mixture was divided into aliquots and frozen to -80°C. CBDP-treated human serum albumin was used without reduction or alkylation.
For MALDI analysis, two methods of sample preparation were used. First, an aliquot of the digest was diluted 1-to-10 with 50% acetonitrile plus 0.1% trifluoroacetic acid and used directly. In the second method, peptides from 0.01 mg of the albumin digest were separated by offline HPLC. Fractions were dried by vacuum centrifugation and redissolved in 5 μl of 50% acetonitrile plus 0.1% trifluoroacetic acid.
Tyrosine
One millimolar L-tyrosine was reacted with 1 mM CBDP in 1 ml of 25 mM ammonium bicarbonate buffer, pH 7.8, at 37°C. The reaction mixture was kept in a sealed microfuge tube. At intervals, 2 μl of the reaction mixture was mixed with 2 μl of a saturated solution of CHCA, in 50% acetonitrile plus 0.1% trifluoroacetic acid, to stop the reaction and to prepare the sample for analysis on the MALDI mass spectrometer.
Offline HPLC
Offline HPLC was performed on proteolytic digests. Each sample was centrifuged to remove particulate material before injecting it into a Phenomenex C18 column (100 mm × 4.6 mm) on a Waters 625 liquid chromatography system (Waters Corp, Milford, MA). Peptides were eluted at a flow rate of 1 ml/min with a 60 min gradient starting at 100% buffer A (0.1% trifluoroacetic acid in water) and ending with 60% buffer B (acetonitrile containing 0.09% trifluoroacetic acid) and 40% buffer A. One milliliter fractions were collected, evaporated to dryness in a vacuum centrifuge, and resuspended in 100 μl of 50% acetonitrile plus 0.1% trifluoroacetic acid. Each fraction was examined in the MALDI TOF TOF mass spectrometer to locate the peptides of interest.
MALDI TOF TOF mass spectrometry 3
One μl of sample was air-dried onto a 384 well Opti-TOF sample plate (Applied Biosystems, Foster City, CA, cat #1016491). If the sample did not already contain the CHCA matrix, it was overlaid with 1 μl CHCA. MALDI mass spectra were taken using a MALDI TOF TOF 4800 tandem time-of-flight mass spectrometer (Applied Biosystems Inc., Framingham, MA). Data collection was controlled by 4000 Series Explorer v 3.5 software. Simple mass spectra (MS) were acquired in reflector mode using delayed extraction and default calibration. MS calibration was made with Cal Mix 5 (bradykinin, 2-9 clip; angiotensin I; Glu-fibrinopeptide B; ACTH, 1-17 clip; ACTH, 18-39 clip; and ACTH, 7-38 clip from Applied Biosystems Inc., Framingham, MA). MS spectra consisted of 500 laser pulses taken with the laser energy adjusted to yield optimal signal to noise. MSMS fragmentation spectra were taken using post source decay in either positive or negative ion mode, at 1 Volt collision energy, in the absence of collision gas, and with meta-stable ion suppression on. Each spectrum consisted of 500 laser pulses taken with the laser energy adjusted to yield optimal signal to noise. MSMS calibration was made on the fragmentation spectrum of Angiotensin I.
MS spectra were examined manually for the presence of masses which were not present in unlabeled controls or theoretical digests (tryptic digest of BChE or peptic digest of serum albumin). Theoretical digests were generated by the MS-Digest algorithm from Protein Prospector v 5.3.2. from the University of California Mass Spectrometry Facility (http://prospector.ucsf.edu/prospector). The amino acid sequences of peptides were determined by manual inspection of MSMS fragmentation spectra with the aid of the MS-Product algorithm from Protein Prospector v 5.3.2, and Proteomics Toolkit from DB Systems Biology (http://db.systemsbiology.net:8080/proteomicsToolkit/FragIonServlet.html).
Tandem-quadrupole ion-trap electrospray ionization mass spectrometry 3
A QTRAP 4000 tandem-quadrupole, linear ion-trap mass spectrometer (Applied Biosystems Inc. Framingham, MA) was used to collect electrospray ionization mass spectra. Data collection was controlled by Analyst v 1.5 software.
Static infusion with electrospray ionization was performed on 5-10 μl of sample dissolved in 50% acetonitrile, 25% methanol, 1% acetic acid, using an EconoTip emitter (1 μm orifice, #Econo12 from New Objective, Woburn, MA). The mass spectrometer was run in positive mode with an ion spray voltage of 1850 volts, an interface heater temperature of 70°C, a declustering potential of 70 volts, Qo trapping on, a linear ion trap filling time of 20 msec, and a scan rate of 1000 Da/sec. Product ion fragmentation spectra were taken at a collision energy of 30 volts, with 40 μTorr of pure nitrogen in the collision cell. Five hundred spectra were summed. The mass spectrometer was calibrated against fragments of Glu-fibrinopeptide B and Agilent electrospray calibrant solution (cat # G2421-6001, Agilent Technologies, Santa Clara, CA).
Liquid chromatography tandem mass spectrometry (LCMSMS) was performed on 4 pmoles of sample in a 2 μl volume. The sample was injected onto an HPLC nanocolumn (Vydac C18 polymeric rev-phase, 75 μm i.d. × 150 mm long; cat#218MS3.07515 P.J. Cobert Assoc, St. Louis, MO). Peptides were separated with a 90 min linear gradient from 0 to 60% acetonitrile containing 0.1% formic acid at a flow rate of 0.3 μl/min and electrosprayed through a nanospray fused silica emitter (360 μm o.d., 75 μm i.d., 15 μm taper, New Objective) directly into the QTRAP 4000 mass spectrometer. Data were collected using information dependent acquisition which took a simple mass spectrum and then triggered the collection of an enhanced high resolution spectrum and four enhanced product ion spectra on the four most intense ions entering the mass spectrometer having an m/z between 200 and 1500, a charge state of +2 to +4 and an intensity greater than 500,000 counts per second. After an ion was analyzed twice, it was excluded from analysis for 60 seconds. Collision energy was determined by the mass spectrometer based on mass and charge state of the ion. Collision gas was pure nitrogen (40 μTorr), and the scan rate was 4000 Da/sec. An ion spray voltage of 1900 V was maintained between the emitter and the mass spectrometer. The mass spectrometer was calibrated using MSMS fragments of Glu-Fibrinopeptide B and Agilent electrospray calibrant solution.
MS spectra of the tryptic digest of BChE were examined manually for the presence of multiply-charged masses consistent with singly-charged peptides: 2910, 2928, 3008, 3036, 3084, 3098, and 3114 amu. These masses were identified in earlier MALDI MS analyses as forms of the CBDP-labeled BChE active site peptide. MSMS spectra were examined manually to determine peptide sequences with the aid of the MS-Product algorithm from Protein Prospector v 5.3.2 and Proteomics Toolkit.
Results
Adducts formed from the reaction of CBDP with human serum albumin
We have recently found that organophosphates react with Tyr411 on human serum albumin, as well as with tyrosines on a variety of other proteins [22]. To determine whether CBDP covalently modifies albumin, we treated human serum albumin with CBDP and digested the mixture with pepsin.
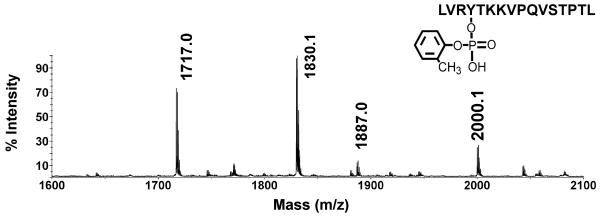
The MALDI mass spectrum of the peptic digest in Figure 1 yielded four masses of interest: the unlabeled Tyr411 peptide VRYTKKVPQVSTPTL ([M+H]+1 of 1717.0 amu), a missed-cleavage form of the same peptide, LVRYTKKVPQVSTPTL ([M+H]+1 of 1830.1 amu), a mass at 1887.0 (+170 amu from 1717.0 amu), and another at 2000.1 amu (+170 amu from 1830.1 amu). The latter two masses were not present in untreated albumin.
Figure 1.
A portion of a MALDI MS spectrum taken from a complete peptic digest of CBDP-modified human serum albumin. An aliquot of the complete digest was diluted 10-fold with 50% acetonitrile plus 0.1% trifluoroacetic acid, and 1 μl was applied to a MALDI target plate. The values shown indicate the monoisotopic masses for VRYTKKVPQVSTPTL at 1717 amu, LVRYTKKVPQVSTPTL at 1830.1 amu, VRYTKKVPQVSTPTL with an added mass of 170 on Tyr411 to give 1887 amu, and LVRYTKKVPQVSTPTL with an added mass of 170 on Tyr411 to give 2000.1 amu. The structure is for the 2000.1 amu peptide which carries o-cresyl phosphate attached to Tyr411. The accession number for human albumin in the NCBI database is gi:122920512.
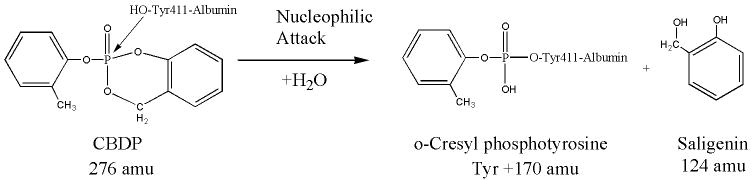
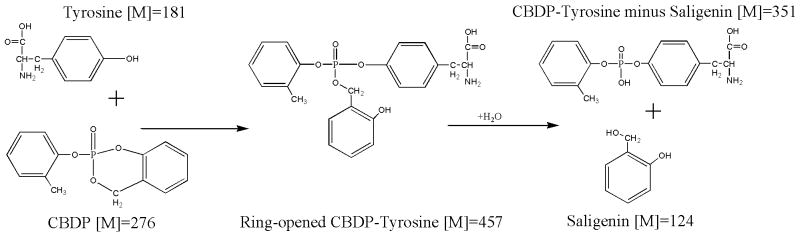
The 170 amu added mass indicates that the cyclic saligenin moiety was displaced from CBDP during this reaction. See Scheme 2. Note that the masses in Scheme 2 are for the neutral species. If the o-cresyl moiety had been eliminated from CBDP, rather than the saligenin, the added mass would have been 168 amu.
Scheme 2.
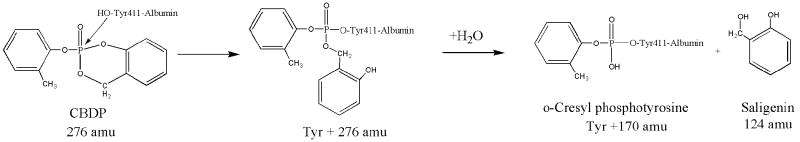
For saligenin to have been eliminated, two phosphorus-oxygen bonds must have been broken. To accomplish this, sequential reactions would be expected with an intermediate in which only one of the cyclic saligenin bonds to the phosphorus was hydrolyzed (see Scheme 3). Note that the ring-open structure depicted is only one of two possible forms. Choice of this depiction is for illustrative purposes and is not intended to indicate the actual chemical mechanism. Also note that the masses in Scheme 3 are for the neutral forms. The added mass from CBDP for such an intermediate would be 276 amu. Peptides VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL with an added mass of 276 amu would appear at 1993 amu (1717.0 + 276 amu) and 2106 amu (1830.1 + 276 amu), respectively. Careful examination of the MALDI spectrum revealed peaks at these masses, but their intensities were barely 2-fold above background. More convincing evidence for this ring-opened intermediate was found when CBDP was reacted with free tyrosine (see the section entitled “Adducts formed from the reaction of CBDP with free tyrosine”).
Scheme 3.
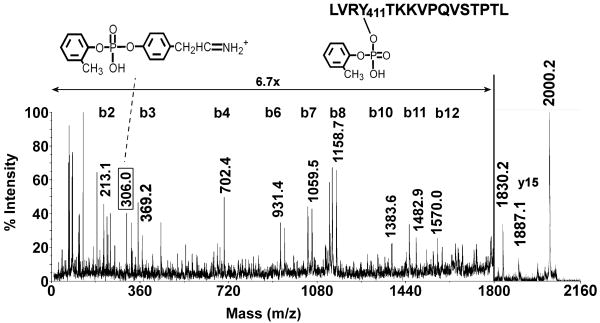
Fragmentation of the 2000 amu mass (LVRYTKKVPQVSTPTL + 170 amu) in the MALDI mass spectrometer yielded an extensive b-ion series (b2-b12) that was characteristic for this peptide, see Figure 2. The sequence included the interval for the sequence ion of CBDP-labeled tyrosine (b3 to b4 = 163 + 170 = 333 amu). The majority of the unannotated peaks correspond to a-ions, c-ions, ions that have lost NH3 or H2O, an internal fragment (Pro Gln Val), and immonium ions for amino acids Val, Leu and Arg. These peaks were left unlabeled in Figure 2 for the sake of clarity, but a complete list of the annotated masses is given in Table 1 of the Supplemental Material.
Figure 2.
MALDI post source decay fragmentation spectrum of the CBDP-labeled human serum albumin peptide LVRYTKKVPQVSTPTL ([M+H]+1 = 2000.2 amu). The spectrum was for an HPLC purified fraction of a peptic digest. The masses are centered over the peaks to which they apply. The y-axis is expanded 6.7-fold for peaks between 0 and 1800 m/z. The 306.0 amu mass, enclosed in a box, indicates the Tyr immonium ion derived from o-cresyl phosphate, whose structure is shown. The 1830.2 mass is the parent ion minus the OP.
In addition to the above mentioned masses, the MSMS spectrum contains a prominent mass at 306.0 amu that corresponds to the tyrosine immonium ion plus the 170 amu added mass from CBDP (136 + 170 = 306 amu). We have found that MSMS spectra from organophosphate-labeled tyrosine-containing peptides typically yield masses equal to the tyrosine immonium ion plus the added mass from the organophosphate [22].
The most intense fragment in the MSMS spectrum in Figure 2 appeared at 1830.2 amu, which is 170 amu less than the parent ion 2000.2 amu. This is consistent with facile elimination of the organophosphorus-adduct from the parent ion. The next most intense fragment in the MSMS appeared at 1887.1 amu (-113.0 amu) which is consistent with release of leucine from the N-terminus of the parent ion to produce the y15 ion.
Observation of an added mass of 170 amu on the parent peptide, plus release of 170 from the parent ion during MSMS is indicative of the presence of an o-cresyl phosphate adduct on the peptide. Observation of the tyrosine immonium+170 amu ion (306 amu) and the sequence ion for tyrosine + 170 amu (702.4 amu) strongly indicates that the o-cresyl phosphate is present on tyrosine (Tyr411).
A similar MALDI fragmentation spectrum was obtained from the 1887 amu peptide, VRYTKKVPQVSTPTL plus 170 amu (data not shown).
The MALDI MS spectrum from a total peptic digest of human serum albumin (36% sequence coverage) was examined manually for other peptides that might have received 170 amu added mass from CBDP. None were found. The spectrum was also examined for the presence of a phosphate adduct on the Tyr411 containing peptides (+80 amu). This was done because the +80 amu adduct was the dominant modification arising from the reaction of CBDP with BChE (see the section entitled “Adducts formed from the reaction of CBDP with human butyrylcholinesterase”). No evidence for phosphate was found. Absence of this phosphate adduct marks a noteworthy difference between the reactions of CDBP with albumin and BChE.
Adducts formed from the reaction of CBDP with free tyrosine
To further test the reaction of CBDP with tyrosine and to re-examine the issue of the chemical nature of the adducts formed, 1 mM CBDP was reacted with 1 mM free tyrosine, at pH 7.8, and samples were withdrawn at intervals. Simple MALDI mass spectra of the samples showed prominent masses consistent with the presence of the protonated forms of tyrosine ([M+H]+1 = 181.9 amu), CBDP ([M+H]+1 = 276.9 amu), a tyrosine-CBDP ring-opened adduct ([M+H]+1 = 458.0 amu), and o-cresyl phosphotyrosine ([M+H]+1 = 352.0 amu), see Scheme 4 Note that the masses given in Scheme 4 are for the neutral charge state. No phosphorylated tyrosine with an added mass of 80 from HPO3 was seen. This is noted because the +80 adduct is the major form found in the reaction of CBDP with the active site serine of butyrylcholinesterase.
Scheme 4.
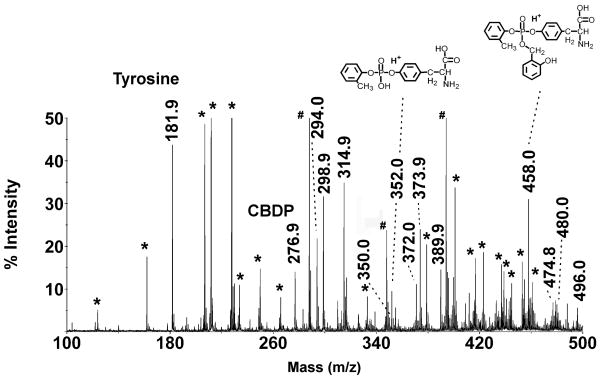
Figure 3 shows the MS spectrum for the 15.5 hour time point, at which time each of the major species was represented. In addition to the protonated form of each CBDP species, there were significant signals corresponding to the NH4+1, Na+1, and K+1 forms (plus 17, 22 and 38 amu respectively). These alternate forms created a group of four peaks for CBDP (276.9, 294.0, 298.9 and 314.9 amu) and four peaks for the tyrosine-CBDP ring-opened adduct (458.0, 474.8, 480.0 and 496.0 amu). For the o-cresyl phosphotyrosine the NH4+1 form was not seen, leaving a three peak group (352.0, 373.9 and 389.9 amu). Small signals were also detected at [M+H]+1 = 350 and 372 (see Figure 3), which are consistent with cyclic saligenin phosphotyrosine (protonated and Na+ forms). These signals suggest that a small portion of the reaction of CBDP with tyrosine proceeds through displacement of the cresyl moiety. The cyclic saligenin phosphotyrosine signals amounted to no more than 4% of the total CBDP signal. Most of the other major peaks in Figure 3 were present in the buffer/matrix blank; these are marked by asterisks. In addition, there were three prominent masses (at 288.0, 348.0 and 394.0 amu) that could not be assigned; these are marked by pound signs. They were not present in the 1 hour time point and had decayed significantly by the 72 hour time point, suggesting that they were products of the reaction.
Figure 3.
MS spectrum from a mixture of tyrosine and CBDP, after 15.5 hours of reaction. Peaks marked by masses represent the reactants and the reaction products. The inserted structures are consistent with the indicated masses. Masses found in a mass spectrum of the buffer/matrix alone are marked by an asterisk (*). Three prominent, unassigned masses (at 288.0, 348.0 and 394.0 amu) are marked by a pound sign (#).
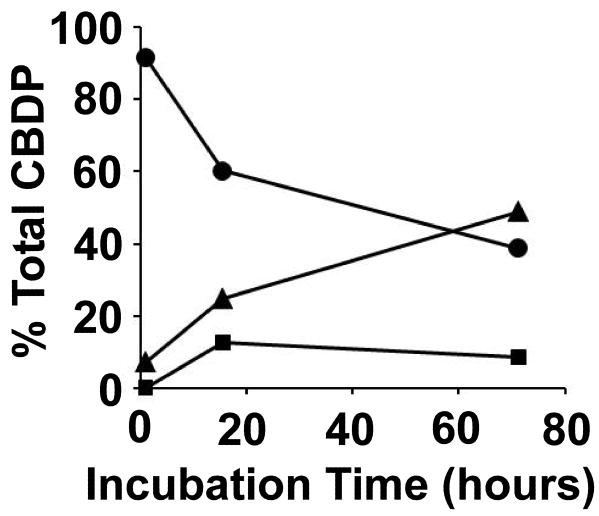
The time dependence for the reaction of CBDP with tyrosine is shown in Figure 4. There is a progressive fall in the amount of CBDP (circles) with a concomitant rise in the amount of o-cresyl phosphotyrosine (triangles). The fraction of the tyrosine-CBDP ring-opened adduct (squares) rises initially but then falls. This pattern is characteristic of a reaction sequence that proceeds through tyrosine-CBDP ring-opened adduct as an intermediate on the way to formation of o-cresyl phosphotyrosine.
Figure 4.
Time course for the reaction of CBDP with tyrosine at pH 7.8. The circles indicate CBDP, the squares a tyrosine-CBDP ring-opened adduct, and the triangles o-cresyl phosphotyrosine.
The % Total CBDP for each species at each time point was calculated by summing the cluster areas for all forms of that species (i.e. their NH4+, Na+ and K+ forms) at that time point, and then dividing that value by the sum of the cluster areas for all species, at that time point.
Adducts formed from the reaction of CBDP with human butyrylcholinesterase
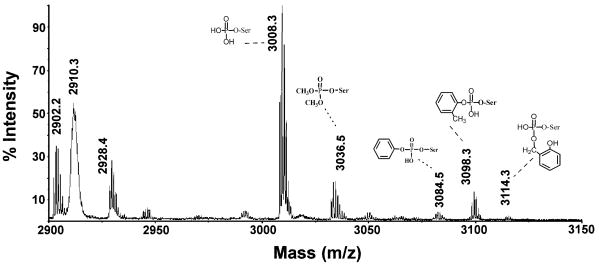
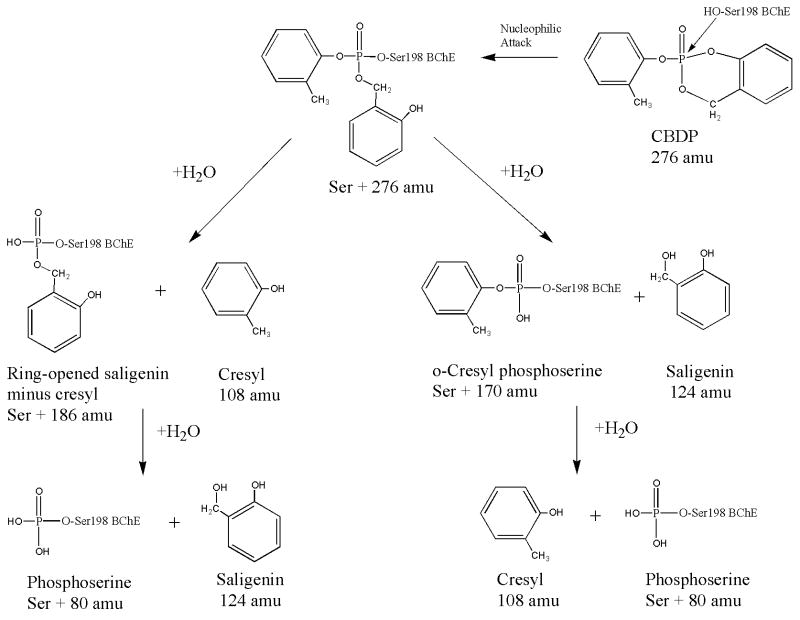
The MALDI mass spectrum in Figure 5 is from a tryptic digest of CBDP-treated BChE. The 29-residue active site peptide SVTLFGES198AGAASVSLHLLSPGSHSLFTR has a mass of 2928.4. Isotopic clusters at 3008.3, 3036.5, 3084.5, 3098.3 and 3114.3 amu (monoisotopic masses) are present in the CBDP treated BChE. These clusters were not present in the spectrum of untreated BChE. Three of these can be related to adducts of CBDP. The new masses are consistent with the active site peptide from BChE (SVTLFGESAGAASVSLHLLSPGSHSLFTR, 2928.4 amu), plus masses for phosphate (3008.3 = 2928.4 + 80 amu); o-cresyl phosphate (3098.3 = 2928.4 + 170 amu); and a ring-opened form of CBDP lacking the cresyl moiety (3114.3 = 2928.4 + 186 amu). The identities of these masses were confirmed by MSMS mass spectrometry.
Figure 5.
A portion of a MALDI MS spectrum taken on a complete tryptic digest of CBDP-inhibited human BChE. BChE was reacted with a 40-fold excess of CBDP for 30 seconds, and the reaction was stopped by addition of an equal volume of acetonitrile. The sample was digested with trypsin and the complete digest was diluted 10-fold in 50% acetonitrile plus 0.1% trifluoroacetic acid before applying 1 μl to the MALDI target plate. The values shown indicate the monoisotopic masses. The active site peptide SVTLFGES198AGAASVSLHLLSPGSHSLFTR has a mass of 2928.4. Five adducts on serine 198 (serine 8 in the peptide) were found. They have masses of 3008.3, 3036.5, 3084.5, 3098.3, and 3114.3. Adduct structures are associated with the indicated masses. The accession number for human BChE in the NCBI database is gi:116353.
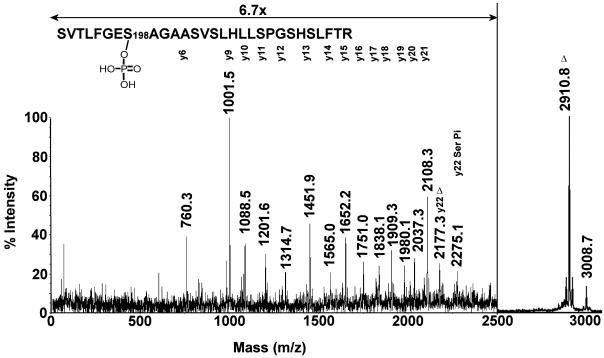
The MALDI MSMS spectrum of the 3008 amu mass (+80 amu adduct) is shown in Figure 6. A y-ion series extending from y7 to y22 defines the active site peptide for human BChE. The series encompasses the position of the active site serine (y22) which appears in two forms. The interval between 2108.3 amu (y21) and 2177.3 amu (y22) is 69.0 amu which is consistent with the presence of dehydroalanine in the position expected for Ser198. This strongly suggests that the serine was modified and that a portion of the modification was lost during the fragmentation process. The interval between 2108.3 (y21) and 2275.1 amu (y22) is 166.9 amu which is consistent with the presence of phosphoserine at position y22 (mass for dehydroserine, 87 amu plus the mass for phosphate, 80 amu). Taken together, these observations indicate that the active site serine (Ser198) was modified by a phosphate in this peptide. The most intense fragment mass was observed at 2910.8 amu which is 97.9 amu smaller than the parent ion at 3008.7 amu. We typically find that the major mass fragment in MALDI MSMS spectra of organophospho-peptides corresponds to loss of the phospho-modification from the parent ion. When the adduct is on a tyrosine, this loss is equivalent to the original added mass (170 amu in the case of the o-cresyl phosphate modified albumin peptide in Figure 2). When the modified residue is a serine, the loss is equivalent to the added mass plus loss of water, thus converting the active site serine to dehydroalanine. In Figure 6, the 98 amu mass loss from the parent ion is consistent with loss of phosphate (added mass = 80 amu, plus 18 amu for water).
Figure 6.
MALDI post-source decay fragmentation spectrum of the human BChE active site peptide labeled at Ser198 with phosphate (monoisotopic mass of 3008.7 amu). The spectrum was taken from an offline HPLC purified fraction of the tryptic digest. The masses are centered over the peaks to which they apply. The y-axis is expanded 6.7-fold for peaks between 0 and 2500 m/z. The symbol Δ indicates ions that contain dehydroalanine in place of serine 198 due to loss of phosphate plus a molecule of water. The y22 ion at 2275.1 amu retains the phosphate on serine 198.
A similar fragmentation pattern was obtained from a triply-charged peptide ([M+3H]+3 = 1003.8 m/z) in an LCMSMS experiment on the complete tryptic digest of CBDP-modified BChE. These data were taken in the QTRAP mass spectrometer using electrospray ionization and low-energy collision induced dissociation (data not shown).
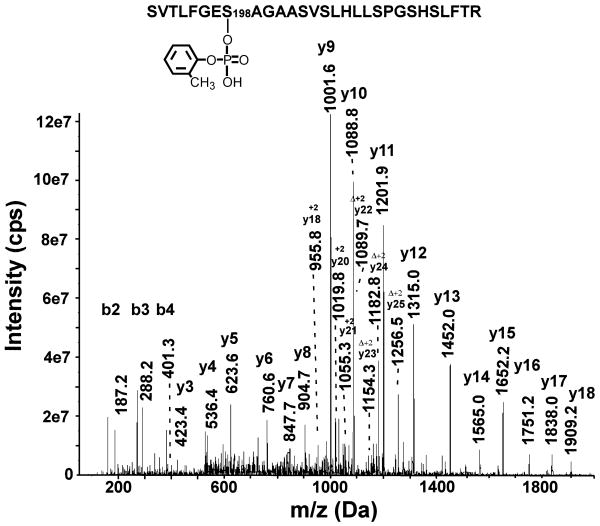
MSMS spectra were obtained by infusing the sample into the QTRAP 4000 mass spectrometer and averaging 500 scans. Figure 7 shows the collision induced fragmentation spectrum of the 3098 amu mass (observed mass for the triply-charged parent ion [M+3H]+3 = 1034.2 m/z). A singly-charged y-ion series from y3 to y18 and a singly-charged b-ion series from b2 to b4 establish this peptide to be the active-site tryptic-peptide from human BChE. A doubly-charged y-ion series from y18 to y25 defines the region of the peptide around the active site serine (Ser198). The sequence interval between 1055.3 and 1089.7 m/z (y21-y22) is 68.8/2 which identifies the presence of dehydroalanine at the position expected for Ser198. During collision induced fragmentation we routinely observe loss of the organophosphate adduct from serine to yield dehydroalanine. This observation is analogous to the well established loss of phosphate from phosphoserine during collision induced dissociation. The presence of dehydroalanine at the sequence location expected for serine strongly argues that this serine was modified by the organophosphate. The added mass on the parent ion (170 amu) strongly indicates that the organophosphate modification was o-cresyl phosphate.
Figure 7.
A low-energy, collision induced fragmentation spectrum of the active site peptide from BChE, labeled with o-cresyl phosphate (monoisotopic mass of 3098.3 amu). The tryptic digest of CBDP-modified BChE was separated offline by HPLC and the fraction containing the 3098 amu mass was infused into the QTRAP 4000 mass spectrometer, using static infusion. The spectrum is the sum of 500 scans. Annotated masses include a singly-charged b-ion series (b2-b4), a singly-charged y-ion series (y3-y18) and a doubly-charged y-ion series (y18-y25). The symbol Δ indicates ions that have dehydroalanine in place of serine due to beta-elimination of the OP (mass of OP plus mass of a molecule of water). All other, unannotated, major peaks could be accounted for as b- or y-ions minus water or minus amine, as a-ions, or as internal fragments.
The 3114.3 amu mass (+186 amu adduct) in Figure 5 is present in low yield. MALDI MSMS of an HPLC purified fraction containing this peptide showed release of the 186 amu adduct mass plus water that is expected from a modified serine. Infusion of this HPLC fraction into the QTRAP 4000 enabled the accumulation of 500 collision induced fragmentation spectra. This revealed a short y-ion series consisting of the five most intense fragments from the BChE active site peptide (data not shown), which confirmed that this modified peptide was the active-site peptide from human BChE. Taken together the parent ion mass, the MALDI MSMS spectrum, and the QTRAP fragmentation spectrum indicate that the 3114.3 amu mass consisted of the active site peptide from human BChE modified on Ser198 by an added mass of 186 amu. An added mass of 186 amu is consistent with the presence of a ring-opened phospho-saligenin without the cresyl moiety.
Two additional masses could be ascribed to modified active site peptides. One was at 3036.5 amu (added mass of 108 amu). Fragmentation of this mass using infusion and summation of 500 scans showed peaks for the six most intense fragments from the active site peptide of human BChE (data not shown), thus establishing the identity of this modified peptide. Fragmentation on the MALDI-TOF-TOF mass spectrometer yielded an intense peak with a mass of 2910.5. This mass is the dehydroalanine form of the active site peptide and supports the conclusion that the label is on serine. An added mass of 108 amu is consistent with a dimethoxyphosphate adduct or a monoethoxyphosphate adduct. It is not clear how such adducts would arise from CBDP. The second peptide was at 3084.5 amu (added mass of 156 amu). Fragmentation of this peptide was also performed after infusing the sample into the mass spectrometer. The MSMS spectrum from 500 scans showed an 8 residue y-ion series (singly-charged) stretching from y8-y16 and ions for b2, a2 and b3. These masses established the 3084.5 amu peptide to be the active site tryptic peptide from human BChE. Masses for fragments from a doubly-charged y-ion series stretching from y22-y25 all included the mass of dehydroalanine. This is consistent with conversion of Ser198 (residue y22) into dehydroalanine during fragmentation and argues that this serine was modified. The added mass of 156 is consistent with addition of O-phenyl phosphoserine to the peptide. The origin of this adduct is not clear, though it could have arisen from the presence of a minor amount of O-phenyl phospho-saligenin.
The 2910.3 amu mass in Figure 5 is another noteworthy peak that did not appear in the unlabeled spectrum. 2910 amu is the expected mass for the active site peptide minus one water. Conversion of the organophosphorus-modified active site serine into dehydroalanine would yield this mass. Because this mass appears in the MS spectrum, we initially considered it to be formed by loss of the organophosphorus modification before the preparation was introduced into the mass spectrometer. However, this MS peak lacks isotopic resolution. That argues that it is generated by fragmentation occurring in the MALDI mass spectrometer, probably after the reflector. Continuous fragmentation of labile masses in the portion of the mass spectrometer after the reflector leads to slight changes in the speed of the newly formed fragments, causing them to arrive at the detector at slightly different times, thus resulting in loss of resolution. We have observed this type of behavior for BChE modified with other OP compounds. MSMS analysis of this mass has confirmed that it was the active site peptide.
The MALDI MS spectrum and the electrospray LCMSMS spectrum from a total tryptic digest of human serum albumin (47% and 25% sequence coverage respectively) were examined manually for peptides other than the active site peptide that might have received 80 or 170 amu added mass from CBDP. None were found.
Analysis of the mass spectral fragmentation spectrum of CBDP
Understanding the nature of the fragmentation of CBDP in the mass spectrometer would be a valuable asset to the study of the mass spectrometry of CBDP. We have therefore undertaken to annotate the fragmentation pattern of CBDP from the MALDI TOFTOF mass spectrometer (obtained by post source decay, using 1 kV collision energy, in the absence of collision gas). The results are presented in the Supplemental Material.
Discussion
Reaction of CBDP with Albumin and Tyrosine
Reaction of CBDP with human serum albumin resulted in a 170 amu mass being added to Tyr411. The 170 amu added mass indicates that the saligenin moiety was displaced from CBDP leaving an o-cresyl phosphotyrosine adduct. For saligenin to have been displaced, two phosphorus-oxygen bonds must have been broken. Though it is reasonable to expect that the two bonds were broken sequentially, convincing evidence for an intermediate was not detected with albumin, but was detected with free tyrosine.
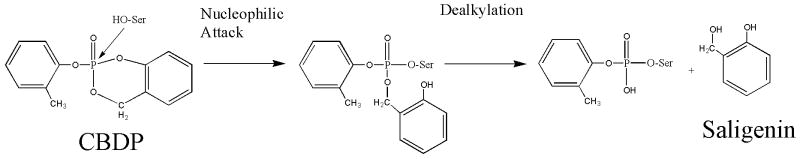
Casida and co-workers have shown that reaction of CBDP with trypsin and chymotrypsin also resulted in the displacement of saligenin. They also argued that the two bonds were broken in sequential steps, the first being displaced upon initial reaction with the nucleophilic active-site serine, and the second being hydrolyzed in a rapid “aging” reaction [16; 17; 19] (see Scheme 5). Aging is a well established phenomenon exhibited by serine hydrolases. It is defined as loss of the alcohol group from the organophosphorylated-serine adduct. It is catalyzed by groups in the active site of the OP-inhibited enzyme [23]. Aging is generally considered to be a unique function of serine hydrolases. It is noteworthy to mention here that OP-albumin does not age. Organophosphorus adducts which age rapidly on butyrylcholinesterase or acetylcholinesterase are stable on albumin [24]. Since displacement of saligenin occurs upon reaction of CBDP with Tyr411 on serum albumin, we suggest that the second step does not require enzymatic intervention such as occurs in aging, and therefore should be called dealkylation rather than aging. This conclusion is supported by the results from the reaction of CBDP with free tyrosine, where release of saligenin also occurs. That reaction can be seen to proceed via the expected two-step mechanism without any apparent assistance for the second step.
Scheme 5.
CBDP appears to be more reactive toward tyrosine than paraoxon. At pH 7.8 and 37°C, 60% of a 1 mM preparation of CBDP had reacted with 1 mM tyrosine in 60 hours. Under the same conditions, there was no reaction of paraoxon with tyrosine, in agreement with Ashbolt and Rydon [25].
Reaction of CBDP with human BChE
Reaction of CBDP with BChE yields at least five different adducts: a phosphoserine (+80 amu), an o-cresyl phosphoserine (+170 amu), a ring-opened phospho-saligenin adduct, without cresyl (+186 amu), an o-phenyl phosphoserine (+156) and a +108 amu adduct. These modifications are not artifacts generated in the mass spectrometer because all five adducts can be separated by offline HPLC before being exposed to the mass spectrometer. Though we boiled the samples in some cases to stop the reaction, these modifications are not the result of boiling because they were observed when the reaction was stopped by diluting the reaction with an equal volume of acetonitrile.
Formation of the phospho-, o-cresyl phospho-, and ring-opened saligenin minus cresyl-adducts can be rationalized by a logical sequence of reactions, as shown in Scheme 6. Initial reaction of CBDP with BChE would be expected to yield a ring-opened derivative, by analogy with the tyrosine-CBDP reaction (see Scheme 4). Subsequent hydrolysis of the ring-opened species could remove either the cresyl moiety or the saligenin moiety. Loss of the cresyl would yield the +186 amu species, and loss of the saligenin would yield the +170 amu species. The existence of both of these species strongly implies the transient existence of the ring-opened derivative. Failure to observe the ring-opened species argues that it is hydrolyzed more rapidly than it is formed. Finally, hydrolysis of either the +170 or the +186 amu adduct would yield the phosphoserine adduct (+80 amu).
Scheme 6.
On the other hand, one might argue that each of these modifications arose independently from contaminations in the CBDP preparation. However, this alternative is not supported by observation. There was no observable contamination in the MALDI mass spectrum of the CBDP preparation, or in the electrospray ionization mass spectrum. Furthermore, based on the relative intensities of the adduct masses in the MALDI mass spectrum for the tryptic digest from the CBDP-BChE reaction (Figure 5), the phosphoserine adduct is the most abundant form present. This observation makes it most unlikely that the phosphoserine adduct was formed by a contamination in the CBDP preparation.
Although there is well established precedent for BChE catalyzed hydrolysis of OP adducts via the process commonly referred to as aging [23], it is unclear whether or not any of the hydrolysis reactions proposed in Scheme 6 were promoted by groups in the active site of BChE. However, it is abundantly clear from the data on the reaction of CBDP with albumin and tyrosine that formation of the o-cresyl phosphoserine adduct could have occurred spontaneously. By analogy, formation of the ring-opened saligenin phosphoserine adduct minus cresyl also could have occurred spontaneously. Although there is no evidence for or against an aging mechanism being responsible for the formation of the phosphoserine, the fact that neither albumin nor tyrosine promote formation of the simple phospho-derivative makes it appealing to speculate that some form of aging is responsible for the formation of phosphoserine in BChE.
An interesting note is that to our knowledge a phosphoserine adduct on BChE with an added mass of 80 has never before been reported. We have re-examined our mass spectral data from reactions between BChE and a variety of OP looking for phosphoserine adducts, but have found no evidence for the presence of phosphorylated serine adducts (added mass 80) from reaction with any OP other than CBDP.
The discovery of phosphoserine as the predominant adduct formed upon reaction of BChE with CBDP validates the importance of the experiments described in this report. None of our experience on OP reactions with BChE or albumin would have induced us to predict the formation of this phosphoserine adduct a priori. Without prior knowledge of the adducts formed by reaction with CBDP, designing a diagnostic protocol would be fruitless.
Toxic airlines syndrome
Exposure to tri-cresyl phosphate isomers through leaks of engine gases into the cabin area of aircraft is currently the number one scenario for the cause of toxic airline syndrome. The active compound derived from ortho isomers of TCP is CBDP. Historically, diagnosis of incidences of poisoning by CBDP have relied upon clinical symptomatology and epidemiology. Successful diagnoses generally have been made only after severe, widespread, high-dose exposure. If toxic airline syndrome is in fact caused by CBDP, then the levels of exposure are probably relatively low because the symptomatology is different from the paralysis observed upon high dose exposure.
There are exceptions to the generalization that toxic airline syndrome produces mild symptoms. Recent incidents indicate that some individuals are significantly more sensitive than others to the cabin air oil exposures. We hypothesize that this is most likely due to the well-known inter-individual difference in OP metabolism by cytochromes P450 [26]. Cytochrome P450 catalyzes the first step in the conversion of TOCP into the toxic CBDP. Still, the levels of exposure are likely to have been relatively low, compared with the cases of TCP ingestion that caused paralysis.
Investigation into low dose exposure calls for a sensitive means of diagnosis. The experiments described in this report position our laboratories to investigate samples from candidates who may have been exposed to low doses of TCP isomers. Identification of phosphoserine adducts on Ser198 of human BChE and/or o-cresyl phosphotyrosine adducts on Tyr411 of serum albumin from airline passengers and crew would strongly support the proposal that low level exposure to TOCP occurs on aircraft. Such a diagnostic for TCP exposure would contribute greatly to epidemiological studies on toxic airline syndrome.
The neurological symptoms characteristic of the toxic airlines syndrome are not likely to be caused by reaction of CBDP with either serum albumin or butyrylcholinesterase. This suggests that other proteins must also be sensitive to CBDP. As support for this proposal, we have demonstrated that an array of proteins can be labeled by a variety of OP, in vitro [22]. In addition, we have recently found that tubulin from mouse brain can be labeled in vivo by sub-lethal doses of chlorpyrifos or chlorpyrifos-oxon [27].
Supplementary Material
Footnotes
This work was supported by the U.S. Army Medical Research and Materiel Command W81XWH-07-2-0034, the National Institutes of Health Grants U01 NS058056, P30CA36727, R01ES09883 and P42ES04696, as well as funding from Pilot Unions, Flight Attendant Unions, the Royal Australian Air Force, the Norwegian Union of Energy workers (SAFE) and NYCO S.A.
Abbreviations: BChE, butyrylcholinesterase; LCMSMS, liquid chromatography tandem mass spectrometry; TCP, Tricresyl phosphate; TOCP, Tri-ortho-cresyl phosphate; ICSC, International Chemical Safety Cards; OP, organophosphorus agent; CBDP, 2-(o-cresyl)-4H-1,3,2-benzodioxaphosphoran-2-one; OPIDN, organophosphorus ester induced delayed neuropathy; MALDI TOF, matrix assisted laser desorption ionization time of flight; MSMS, fragmentation induced in a mass spectrometer; MS, simple mass spectrum; CHCA, alpha-cyano-4-hydroxy cinnamic acid; ACTH, adrenocorticotropic hormone
Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Nola G, Kibby J, Mazurek W. Determination of ortho-cresyl phosphate isomers of tricresyl phosphate used in aircraft turbine engine oils by gas chromatography and mass spectrometry. J Chromatogr A. 2008;1200:211–6. doi: 10.1016/j.chroma.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Winder C, Balouet JC. The toxicity of commercial jet oils. Environ Res. 2002;89:146–64. doi: 10.1006/enrs.2002.4346. [DOI] [PubMed] [Google Scholar]
- 3.van Netten C, Leung V. Hydraulic fluids and jet engine oil: pyrolysis and aircraft air quality. Arch Environ Health. 2001;56:181–6. doi: 10.1080/00039890109604071. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge WN. Tricresyl phosphates and cholinesterase. Biochem J. 1954;56:185–9. doi: 10.1042/bj0560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henschler D. Tricresylphosphate poisoning; experimental clarification of problems of etiology and pathogenesis. Klin Wochenschr. 1958;36:663–74. doi: 10.1007/BF01488746. [DOI] [PubMed] [Google Scholar]
- 6.Kidd JG, Langworthy OR. Jake paralysis. Paralysis following the ingestion of Jamaica ginger extract adulterated with tri-ortho-cresyl phosphate. Bull. Johns Hopkins Hosp. 1933;52:39–65. [Google Scholar]
- 7.Morgan JP. The Jamaica ginger paralysis. JAMA. 1982;248:1864–7. [PubMed] [Google Scholar]
- 8.Smith HV, Spalding JM. Outbreak of paralysis in Morocco due to ortho-cresyl phosphate poisoning. Lancet. 1959;2:1019–21. doi: 10.1016/s0140-6736(59)91486-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Tao Y, Li Z. Toxic polyneuropathy due to flour contaminated with tricresyl phosphate in China. J Toxicol Clin Toxicol. 1995;33:373–4. doi: 10.3109/15563659509028927. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Occupational Safety and Health including the associated IDLH documentation (Immediately Dangerous to Life and Health documentation) for CAS #78-30-8. 2006. International, Chemical Safety Card #0961 validated April 05, 2006. [Google Scholar]
- 11.Casida JE. Specificity of substituted phenyl phosphorus compounds for esterase inhibition in mice. Biochem Pharmacol. 1961;5:332–42. doi: 10.1016/0006-2952(61)90024-7. [DOI] [PubMed] [Google Scholar]
- 12.Ding SJ, Carr J, Carlson JE, Tong L, Xue W, Li Y, Schopfer LM, Li B, Nachon F, Asojo O, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem Res Toxicol. 2008;21:1787–94. doi: 10.1021/tx800144z. PMCID:2646670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Masson P, Lockridge O. Dichlorvos, chlorpyrifos oxon, and aldicarb adducts of butyrylcholinesterase detected by mass spectrometry, in human plasma following deliberate overdose. J Appl Toxicol. 2010 doi: 10.1002/jat.1526. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Nachon F, Masson P, Lockridge O. Detection of adducts on tyrosine 411 of albumin in humans poisoned by dichlorvos. Tox Sci. 2010 doi: 10.1093/toxsci/kfq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–90. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- 16.Eto M, Casida JE, Eto T. Hydroxylation and cyclization reactions involved in the metabolism of tri-o-cresyl phosphate. Biochem Pharmacol. 1962;11:337–52. doi: 10.1016/0006-2952(62)90056-4. [DOI] [PubMed] [Google Scholar]
- 17.Casida JE, Eto M, Baron RL. Biological activity of a trio-cresyl phosphate metabolite. Nature. 1961;191:1396–7. doi: 10.1038/1911396a0. [DOI] [PubMed] [Google Scholar]
- 18.Eto M, Oshima Y, Casida JE. Plasma albumin as a catalyst in cyclization of diaryl o-(alpha-hydroxy)tolyl phosphates. Biochem Pharmacol. 1967;16:295–308. doi: 10.1016/0006-2952(67)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.Toia RF, Casida JE. Phosphorylation, “aging” and possible alkylation reactions of saligenin cyclic phosphorus esters with alpha-chymotrypsin. Biochem Pharmacol. 1979;28:211–6. doi: 10.1016/0006-2952(79)90506-9. [DOI] [PubMed] [Google Scholar]
- 20.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–98. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 21.Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J Med CBR Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: Characteristic mass fragments and a new binding motif for organophosphates. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 doi: 10.1016/j.jchromb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovach IM. Stereochemistry and secondary reactions in the irreversible inhibition of serine hydrolases by organophosphorus compounds. J Phys Org Chem. 2004;17:602–614. [Google Scholar]
- 24.Li B, Nachon F, Froment MT, Verdier L, Debouzy JC, Brasme B, Gillon E, Schopfer LM, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem Res Toxicol. 2008;21:421–431. doi: 10.1021/tx700339m. [DOI] [PubMed] [Google Scholar]
- 25.Ashbolt RF, Rydon HN. The action of diisopropyl phosphorofluoridate and other anticholinesterases on amino acids. Biochem J. 1957;66:237–42. doi: 10.1042/bj0660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang J, Cao Y, Rose RL, Brimfield AA, Dai D, Goldstein JA, Hodgson E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos. 2001;29:1201–4. [PubMed] [Google Scholar]
- 27.Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.