Abstract
Papaverine is one of the earliest opium alkaloids for which a biosynthetic hypothesis was developed on theoretical grounds. Norlaudanosoline (=tetrahydropapaveroline) was claimed as the immediate precursor alkaloid for a multitude of nitrogen containing plant metabolites. This tetrahydroxylated compound was proposed to be fully O-methylated. The resulting tetrahydropapaverine should then aromatize to papaverine. In view of new experimental data, this pathway has to be revised. Feeding of 8-day-old seedlings of Papaver followed by direct examination of the metabolic fate of stable isotope-labeled precursors in the total plant extract, without further purification of the metabolites, led to elucidation of the new papaverine pathway in vivo. The central and earliest benzylisoquinoline alkaloid is not the tetraoxygenated norlaudanosoline but the trihydroxylated norcoclaurine that is further converted into (S)-reticuline, the established precursor for poppy alkaloids. The papaverine pathway is opened by the methylation of (S)-reticuline to generate (S)-laudanine. A second methylation at the 3′ position of laudanine leads to laudanosine, both are known alkaloids from the opium poppy. Subsequent N-demethylation of laudanosine yields the known precursor of papaverine: tetrahydropapaverine. Inspection of the subsequent aromatization reaction revealed a new intermediate, 1,2-dihydropapaverine, which has been characterized. The final step to papaverine is the dehydrogenation of the 1,2-bond, yielding the target compound papaverine. We conclusively show herein that the previously claimed norreticuline does not play a role in the biosynthesis of papaverine.
1. Introduction
Papaverine is a benzylisoquinoline alkaloid (Fig. 1) that was discovered by Merck (1848) as a minor (ca. 1%) component in the latex of the opium poppy (Papaver somniferum L.). It occurs also in other members of the genus Papaver. Due to selection for morphinans in cultivars of Papaver somniferum, papaverine is either a very minor alkaloid or totally absent. The pharmacological effect of papaverine is on cerebral blood flow; it possesses spasmolytic and vasodilatory activity. The isolated natural product drug was eventually replaced by the synthetic drug for commercial use after development of an efficient chemical synthesis (Taylor and Martin, 1974). Papaverine was also used to correct male impotence, but has now been substituted by the more effective Sildenafil.
Fig. 1.

Chemical structure of papaverine consisting of the three aromatic rings A, B and C.
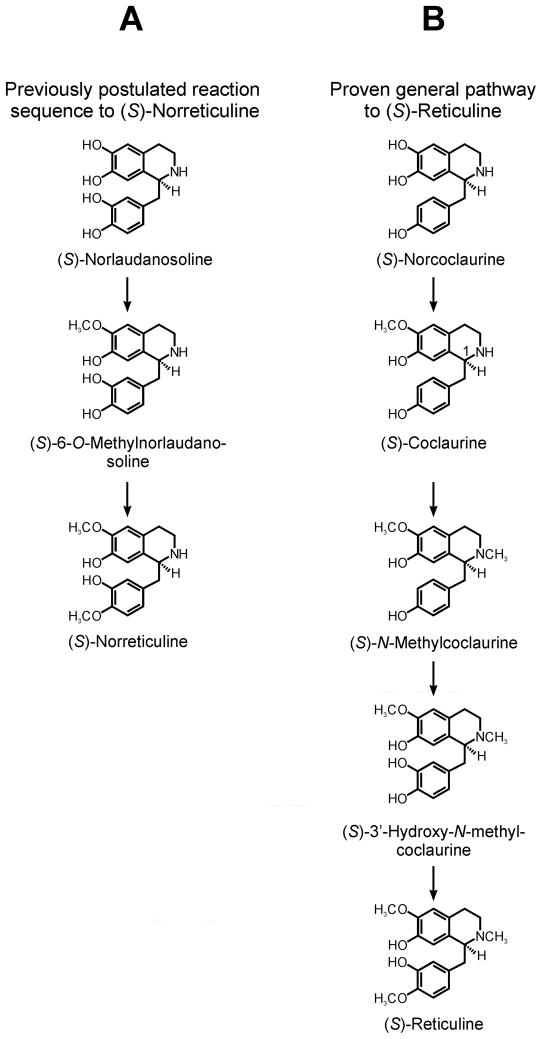
Papaverine is an interesting target for biosynthetic studies. This molecule is fully O-methylated on the hydroxyl groups in positions 6, 7, 3′, and 4′; it lacks, however, an N-methyl group and its B-ring is aromatic. Winterstein and Trier in 1910 postulated that plant-derived tetrahydrobenzylisoquinoline alkaloids are derived from the condensation of dopamine and 3,4-dihydroxyphenylacetaldehyde to form norlaudanosoline. Following this early biogenetic prediction, Battersby and co-workers conducted experiments that seemingly verified and extended the Winterstein and Trier hypothesis (Battersby and Binks, 1960; Battersby, 1963; Battersby et al., 1964). Application of position-specific labeled tyramine as precursor and tedious specific degradation of the labeled papaverine, revealed that this alkaloid is indeed formed from two C6-C2 units derived from dopamine and 3,4-dihydroxyphenylacetaldehyde (Battersby, 1963). Furthermore, it was postulated that papaverine is formed 1) from norlaudanosoline (Battersby et al., 1965), 2) from (S)-norreticuline (Battersby, 1963) as well as several other alkaloids from the nor-series such as norlaudanine and tetrahydropapaverine (possessing no N-methyl group). The latter, however, was claimed to be not significantly incorporated into papaverine due to the lack of an unmethylated phenolic hydroxyl group (Battersby et al., 1964). Independently, the group of Brochmann-Hanssen (Brochmann-Hanssen et al., 1971; Brochmann-Hanssen et al., 1975; Brochmann-Hanssen et al., 1980) extended these suggestions and claimed that norreticuline, norlaudanine and norlaudanosine are well incorporated into papaverine while reticuline is not (Uprety et al., 1975). This proposal receives support by the recent claim that a SAM-dependent O-methyltransferase exists that accepts norreticuline as the sole substrate (Pienkny et al., 2009). Our recent findings, however, are at variance to the above claims. Norlaudanosoline, norreticuline, norlaudanine or norlaudanosine have never been found in higher plants as natural products despite meticulous phytochemical work. Isotope dilution analysis showed that norlaudanosoline and norreticuline were not detected during isoquinoline alkaloid synthesis (Stadler and Zenk, 1990). Furthermore, the trihydroxylated (S)-norcoclaurine is the central precursor of benzylisoquinoline alkaloids and not the long postulated (S)-norlaudanosoline (Stadler et al., 1987; Stadler and Zenk, 1990; Frenzel and Zenk, 1990a). The sequence of enzymatically catalyzed methylation and oxidation steps of (S)-norcoclaurine excludes the existence of (S)-norreticuline, the postulated precursor of papaverine (Fig. 2). The sequence is 6-O-methylation, N-methylation, hydroxylation of 3′ position and 4′-O-methylation.
Fig. 2.
Postulated and verified sequence of reactions for the formation of benzylisoquinoline alkaloids. A, Previously postulated sequence of reactions for the biosynthesis of (S)-norreticuline, as precursor to papaverine; B, General pathway and sequence of reactions verified for the biosynthesis of benzylisoquinoline alkaloids (via (S)-reticuline).
Hence the above-described hypothesis of a pathway for the formation of papaverine (Fig. 2A), does not have experimental support. The incorporation of potential synthetic precursors such as norreticuline, and even their correct position-specific incorporation into papaverine, may be attributed to the promiscuous enzyme activities such as are found in the plant kingdom. All of these inconsistencies in the hitherto published literature and textbooks justify revisiting the biosynthesis of papaverine. We approached a solution of this problem by using high resolution mass spectrometry together with stable-isotope labeled potential precursors and 8-day-old seedlings of an opium poppy hybrid that accumulate sufficient amounts of papaverine for trace analysis of intermediates as well as end products.
2. Results and discussion
To begin our investigation, we analyzed the pertinent alkaloids in the methanolic extract of 8-day-old poppy hybrid seedlings via high resolution LC-MS. The alkaloid pattern and the molecular characteristics are shown in Table 1.
Table 1.
High resolution LC-MS/MS data and quantified amounts of pertinent alkaloids from poppy hybrid seedlings.
| Compound | [M+H]+[m/z] (experimental) | [M+H]+[m/z] (theoretical) | Retention time [min] | Amount [nmol/g dry weight] |
|---|---|---|---|---|
| Reticuline | 330.17011 | 330.16998 | 7.78 | 2.53 |
| Laudanine | 344.18542 | 344.18563 | 12.74 | 0.06 |
| Laudanosine | 358.20112 | 358.20128 | 14.79 | 0.06 |
| Tetrahydropapaverine | 344.18546 | 344.18563 | 11.59 | 0.40 |
| Papaverine | 340.15417 | 340.15433 | 16.76 | 0.98 |
| Thebaine | 312.15929 | 312.15942 | 12.61 | 3.71 |
As can be seen from the retention times in Table 1, the individual alkaloids are clearly separated from each other by high resolution LC-MS, which is a prerequisite for using isotopically labeled compounds. No prediction can be made at this stage regarding the stereochemistry of these alkaloids. Thebaine was used as an indicator for the presence of the morphine pathway. In order to establish the origin of the aromatic ring systems in the alkaloids of interest, we applied the chemically more stable 6-O-methylated derivative, [1-13C]-(S)-coclaurine (Fig. 2B), to the poppy seedlings. As a result of the feeding experiment, we found 15.08 nmol labeled and 41.86 nmol unlabeled thebaine resulting in a ratio of 0.265 for the quotient of labeled to unlabeled alkaloid. Calculation in an analogous manner for papaverine resulted in a quotient of 0.017. Based on dry weight, this calculation corresponds to 0.04% papaverine and 0.4% thebaine. The conclusion from the result of this feeding experiment is that the universal precursor (S)-coclaurine is incorporated into both papaverine and thebaine, but it cannot be excluded that coclaurine is processed further without an N-methyl group to furnish papaverine.
As quoted above (Stadler and Zenk, 1990), norreticuline has never been found in plants, therefore we applied [1-13C, N-13CH3]-(S)-reticuline to the poppy hybrid. The reason for the use of the double labeled compound was that the [1-13C, N-13CH3]-(S)-reticuline ought to be incorporated into papaverine while the N-methyl group should be lost during the transition to the target compound. (S)-Reticuline is one of the central precursors for a multitude of benzylisoquinoline alkaloids retaining the N-methyl group such as morphinans, aporphines, protoberberines and benzo[c]phenanthridines (Kutchan, 1998). The substitution pattern of the phenolic groups means that a biosynthetic relationship to papaverine could be envisaged, if it is assumed that N-demethylation is necessary in the course of the pathway to papaverine. To test this possibility, we observed the flow of [1-13C, N-13CH3]-(S)-reticuline into the hypothetical biosynthetic pathway of papaverine.
Interestingly, the majority of labeled [1-13C, N-13CH3]-(S)-reticuline was transformed into thebaine (4.35 nmol/g dry weight) while the incorporation into (S)-laudanine and (S)-laudanosine, with the two labeled positions intact, was much less (0.1 nmol/g dry weight, Table 2). This difference is due to the fact that the metabolic route to thebaine is the major pathway in the used poppy cross while the one to papaverine is only a side pathway. The detection of labeled laudanine and laudanosine is not sufficient to demonstrate that both alkaloids are precursors of papaverine rather than simply natural products occurring in the plant. However, the same reticuline feeding also resulted in single labeled tetrahydropapaverine and papaverine at the original 1-13C position, while the N-methyl group was completely lost.
Table 2.
High resolution LC-MS/MS data and quantified amounts of pertinent alkaloids from the [1-13C, N-13CH3]-(S)-reticuline feeding experiment. Note: Feeding of doubly-labeled [1-13C, N-13CH3]-(S)-reticuline to poppy plants leads to the formation of doubly-labeled (1-13C, N-13CH3) laudanine and laudanosine, but singly-labeled (1-13C) tetrahydropapaverine and singly-labeled (1-13C) papaverine while thebaine is doubly-labeled (1-13C, N-13CH3) demonstrating the branch point.
| Compound | [M+H]+[m/z] (experimental) | [M+H]+[m/z] (theoretical) | Retention time [min] | Amount [nmol/g dry weight] |
|---|---|---|---|---|
| [1-13C, N-13CH3]-Reticuline | 332.17652 | 332.17669 | 7.78 | 95.30 |
| [1-13C, N-13CH3]-Laudanine | 346.19247 | 346.19234 | 12.74 | 0.10 |
| [1-13C, N-13CH3]-Laudanosine | 360.20786 | 360.20799 | 14.79 | 0.02 |
| [1-13C]-Tetrahydropapaverine | 345.18873 | 345.18899 | 11.59 | 0.01 |
| [1-13C]-Papaverine | 341.15751 | 341.15769 | 16.77 | 0.01 |
| [1-13C, N-13CH3]-Thebaine | 314.16595 | 314.16613 | 12.61 | 4.35 |
This demonstrates that (S)-reticuline is the precursor to tetrahydropapaverine and papaverine. The question remains, however, which pathway (S)-reticuline takes on its way to these two N-demethylated compounds. As a control, we analyzed the incorporation of the doubly-labeled reticuline into thebaine, which shows an incorporation quotient of 0.54. This demonstrates the dominance of the morphine pathway over the papaverine pathway (Table 2).
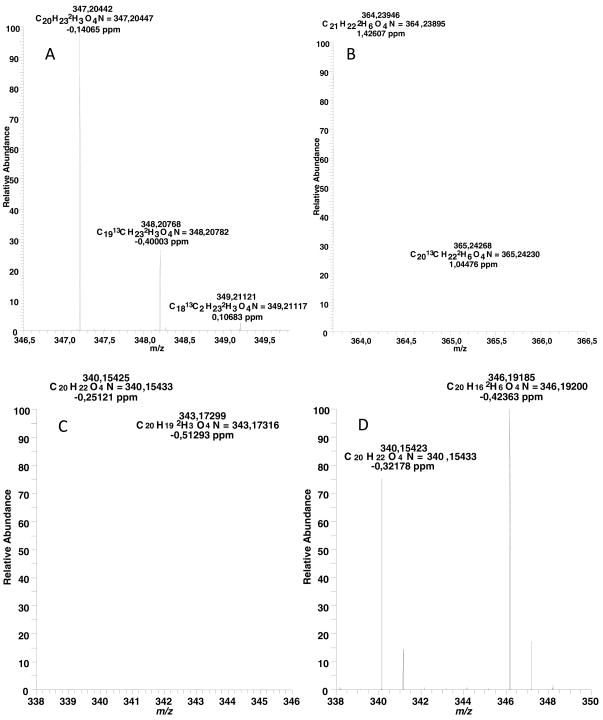
Since laudanine, the O-trimethylated, N-methylated benzylisoquinoline alkaloid, occurs in the opium poppy, and a recombinant SAM-dependent enzyme is known that methylates (S)-reticuline at the 7 position to yield laudanine (Ounaroon et al., 2003; Pienkny et al., 2009), it was tempting to test whether this precursor is incorporated into papaverine. (S)-Laudanine was synthesized by methylating (D3-methyliodide) (S)-reticuline N-oxide with subsequent deprotection (H2SO3) (Phillipson et al., 1976; Lu et al., 1987). This method yielded both [7-D3]-laudanine, as well as [3′,7-D6]-laudanosine, the former having +3 mass units due to the deuterium label while the latter has +6 mass units. The alkaloids were separated by TLC and their constitution verified by high resolution LC-MS (Fig. 3A and B). [7-D3]-laudanine and [3′,7-D6]-laudanosine were fed individually to poppy seedlings as described in the Experimental section. The result of this application experiment showed that [7-D3]-laudanine was incorporated into papaverine to 53.6% of the total papaverine present, while [3′,7-D6]-laudanosine was incorporated to 66.7% of the total target compound (Fig. 3C and D). This experiment and the high incorporations show without any doubt that the N-methylated alkaloids are excellent precursors and that in the biochemical event, the N-methyl group is removed and the resulting alkaloid is dehydrogenated to yield papaverine.
Fig. 3.
High resolution mass spectra. A, isotopically synthesized [7-D3]-laudanine; B, isotopically synthesized [3′,7-D6]-laudanosine; C, biosynthetically obtained unlabeled and [7-D3]-papaverine; D, biosynthetically obtained unlabeled and [3′,7-D6]-papaverine.
Having established the precursor function of laudanine by efficient incorporation into papaverine, we set out to explore the missing biochemical steps between laudanine and papaverine. Feeding experiments using [7-D3]-laudanine showed in the full scan the following four labeled intermediates calculated in percentage based on the total amount (labeled and unlabeled) of each alkaloid present in the seedling: laudanosine (98.6%), tetrahydropapaverine (missing the N-methyl group) (96.4%), an unknown metabolite (99.2%) and papaverine as shown before (53.6%), the obvious end product. Feeding [7-14C]-labeled laudanine to poppy seedlings, subjecting the total extract (without fractionation) to 2D TLC followed by exposure on a phosphorimager screen revealed only the four radioactive products, laudanosine, tetrahydropapaverine, the unknown metabolite and papaverine, and not any additional metabolites (data not shown).
The occurrence of stable isotope-labeled tetrahydropapaverine demonstrates that in the poppy plant the fully methylated alkaloid laudanosine is subjected to a demethylation reaction. This is the missing step between the N-methylated tetrahydrobenzylisoquinoline alkaloid and the N-demethylated papaverine skeleton. The intermediate is (S)-tetrahydropapaverine, a compound first postulated in papaverine biosynthesis (Brochmann-Hanssen et al., 1971) after it had been found as a natural product isolated from P. somniferum. This postulate was verified by Brochmann-Hanssen et al. (1975) who synthesized (−)-tetrahydropapaverine that was found to be incorporated into papaverine with an, at that time, extremely high incorporation rate in the poppy plant. However, the opinion was that (S)-norreticuline and norlaudanosoline are the precursors of norlaudanine, norlaudanosine, with the biosynthetic grid ending with tetrahydropapaverine and papaverine (Battersby, 1963; Brochmann-Hanssen et al., 1971; Uprety et al., 1975) but, as mentioned before, Brochmann-Hanssen and associates never found norreticuline, norlaudanine or norlaudanosine.
We investigated further the precursor role of tetrahydropapaverine in the biosynthesis of papaverine. The feeding of deuterated (R,S)-tetrahydropapaverine was done as usual. The plants were extracted with methanol and subjected to high resolution mass spectrometry. Quantitative mass spectrometric analysis revealed a quotient of 0.81 for the incorporation of deuterium labeled (R,S)-tetrahydropapaverine (isotopic distribution <0.1 % [2H0]/ <0.1% [2H1]/ <0.1% [2H2]/ <0.1% [2H3]/ 3% [2H4]/ 21.5% [2H5]/ 39% [2H6]/ 29.9% [2H7]/ 5.5% [2H8]/ 1% [2H9]) into papaverine and an isotopic distribution for deuterium-labeled papaverine of 11.8 % [2H0]/ 2.9% [2H1]/ 12% [2H2]/ 32.2% [2H3]/ 30% [2H4]/ 11% [2H5]/ 0.1% [2H6]. This high incorporation rate is explained by the immediate precursorship of tetrahydropapaverine to papaverine. The loss of three deuterium atoms during the aromatization process is reflected by the isotopic distribution pattern of labeled tetrahydropapaverine and labeled papaverine with the major peaks changing from D6 and D7 to D3 and D4, respectively. The feeding experiment with deuterated (R,S)-tetrahydropapaverine revealed, in addition to labeled papaverine, the presence of an intermediate m/z 342.16992 (C20H24O4N) with an isotopic distribution of <0.1 % [2H0]/ <0.1% [2H1]/ 3% [2H2]/ 1% [2H3]/ 18% [2H4]/ 32% [2H5]/ 24% [2H6]/ 21% [2H7]/ 1% [2H8]. The intermediate was identified as labeled 1,2-dihydropapaverine showing an incorporation quotient of 0.28 and, for m/z 347.20137 carrying 5 deuterium atoms, a fragment ion of m/z 195.11800 (C11H72H5O2N). The formation of labeled 1,2-dihydropapaverine from labeled tetrahydropapaverine goes along with a loss of two deuterium atoms. Indeed, the isotopic distribution patterns of both alkaloids reveal that a decrease of the number of D6- and D7-labeled isotopes by 15% and 8%, respectively, leads to an equivalent increase of the number of 1,2-dihydropapaverine isotopes carrying 4 and 5 deuterium atoms. This application experiment clearly established tetrahydropapaverine as a substrate for the aromatization process in poppy plants.
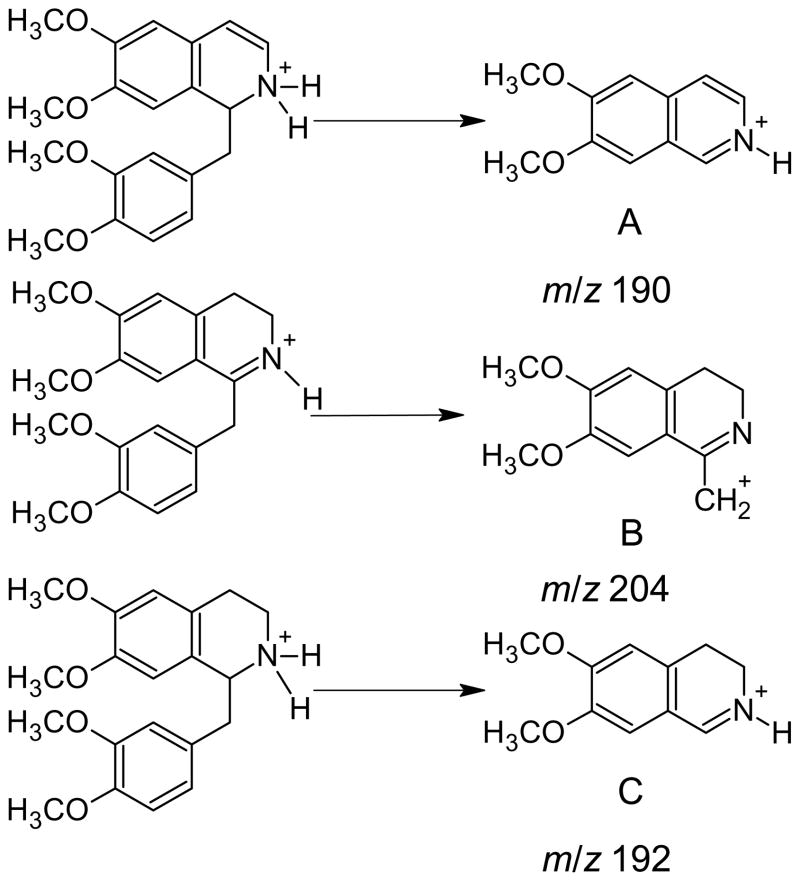
This result was confirmed by a detailed analysis of the [D6]-laudanosine feeding, which revealed an additional peak between tetrahydropapaverine and papaverine in the mass chromatograms at 13.66 min with quasimolecular ions at m/z 342.16992 (C20H24O4N) and 348.20760 (C20H182H6O4N) showing that an intermediate with 2 hydrogens less than tetrahydropapaverine was formed. The MS/MS spectrum of the new compound showed fragments at m/z 190.08629 (C11H12O2N) and 193.10512 (C11H92H3O2N), respectively. As discussed earlier (Battersby et al., 1977), the dehydrogenation of tetrahydropapaverine to papaverine may involve either a double bound formation between C1 and N or between C3 and C4. The formation of the detected product ion m/z 190 (Fig. 4A) is in good agreement with the mass fragmentation of isoquinoline alkaloids that has been shown by Wu and Huang (2006). Papaverine, containing a double bond between C1 and N and between C3 and C4, gives the major R-CH2+ (R=isoquinoline) fragment m/z 202 (Wickens et al., 2006). A compound with a double bond located between C1 and N would also reveal a R-CH2+ fragment, m/z 204 (Fig. 4B), due to its mesomeric stability. Because the dihydro-intermediate does not form a R-CH2+ fragment (Fig. 4A) it can be inferred that the additional double bond is located between C3 and C4. In addition, the MS/MS experiment of tetrahydropapaverine showed a product ion with m/z 192, which supports the structure of a 1,2-dihydro derivate (Fig. 4C).
Fig. 4.
The two possible structures for the biosynthetic intermediate between tetrahydropapaverine and papaverine and the mass fragmentation of tetrahydropapaverine. A, 1,2-Dihydropapaverine, the naturally occurring compound, and its product ion m/z 190; B, 3,4-Hydropapaverine and its predicted product ion m/z 204; C, Tetrahydropapaverine and its product ion m/z 192.
For decades, norlaudanosoline was assumed to be the first alkaloidal precursor for the major classes of plant-derived isoquinoline alkaloids (Winterstein and Trier, 1910; Robinson, 1917; Spenser, 1969). This hypothetical intermediate was seemingly verified when it was shown that the expected labeled precursors were incorporated into papaverine, the target compound (Battersby and Harper, 1959,1962; Battersby et al., 1964), despite some discrepancies in the labeling patterns (for review see Spenser, 1969) produced in poppy and other isoquinoline alkaloid accumulating plants. Extensive phytochemistry, precursor synthesis and experimentation by Brochmann-Hanssen and associates (Brochmann-Hanssen and Furuya, 1964; Brochmann-Hanssen et al., 1965; Brochmann-Hanssen et al., 1971; Brochmann-Hanssen et al., 1975) not only discovered the now recognized true precursors of papaverine, the opium poppy-derived laudanine (laudanidine) and laudanosine alkaloids, but found in labeled (S)-tetrahydropapaverine an excellent precursor for papaverine while (R)-tetrahydropapaverine is largely inactive. However, (R,S)-reticuline was very poorly incorporated into papaverine, but most importantly the highest incorporation rates into papaverine were achieved with norreticuline, which is missing the N-methyl group. These results led to the erroneous assumption that, as predicted by Winterstein and Trier (1910) and then experimentally shown by Battersby (1963), norlaudanosoline and norreticuline were precursors for papaverine. It was not yet known at that time that plant methyltransferases, and especially those from poppy, are promiscuous (Brochmann-Hanssen et al., 1980; Wat et al., 1986; Frenzel and Zenk, 1990b). Thus exogenously provided norlaudanosoline and norreticuline could easily be methylated at the nitrogen and phenolic groups by these methyltransferases. This promiscuity was demonstrated by us when poppy seedlings were fed with [1-13C]-(S)-norreticuline, which resulted in the formation of singly labeled (S)-reticuline and singly labeled thebaine, from which we found a ratio of 0.01 for the quotient of labeled to unlabeled compound. Supporting this is the fact that the unnatural alkaloid nororientaline is an excellent precursor to papaverine since presence of the 7-O-methyl group prevents funneling into the thebaine pathway (Brochmann-Hanssen et al., 1975).
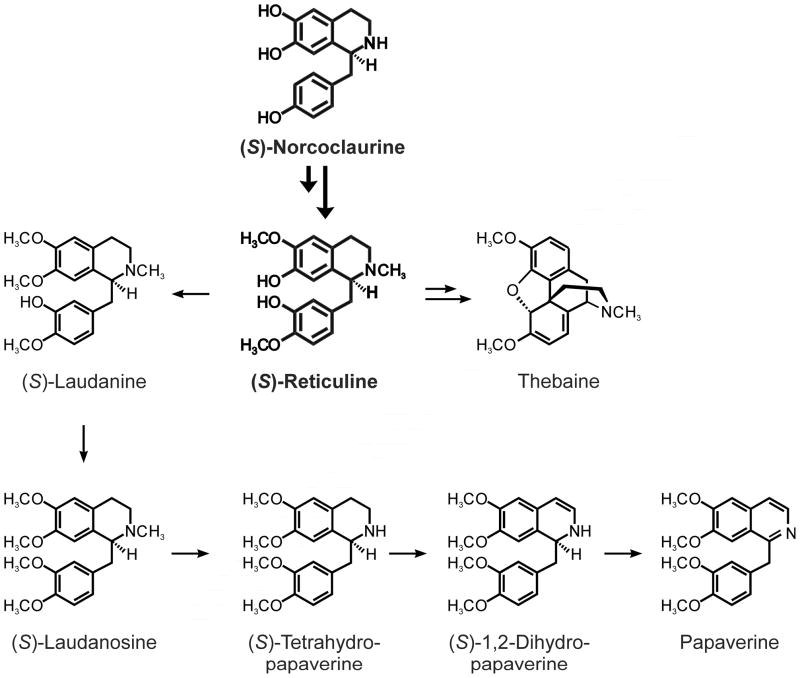
With the knowledge of the biosynthesis of isoquinoline alkaloids, the use of alkaloid producing seedlings, methanolic total plant extracts and state-of-the-art mass spectrometry, we were able to decipher the biosynthesis of papaverine, one of the oldest targets of phytochemistry. The biosynthetic pathway as it stands now is shown in Fig. 5. None of the predicted previous intermediates with the exception of (S)-tetrahydropapaverine, is present in the new scheme depicted here.
Fig. 5.
The new biosynthetic pathway to papaverine in poppy plants. Shown in bold is the common pathway leading to both papaverine and thebaine.
3. Conclusion
By administering heavy isotope labeled potential precursors to seedlings of a poppy cross that produces both thebaine and papaverine and analyzing extracts by HPLC-high resolution mass spectrometry, it was possible to resolve the controversial biosynthesis of papaverine (Fig. 5). This methodology may be applied to other biosynthetic problems in the future to resolve similar types of questions in plant and animal systems. It is advisable to use a quotient of the incorporation of diverse unlabeled and position-labeled precursors. This procedure allows the study of a biosynthetic pathway using only small amounts of seedling tissue or plant cell suspension culture and omits tedious purification steps of the target compounds.
4. Experimental
4.1 Papaver
Hybrid seeds were produced by pollinating emasculated buds of P. somniferum with pollen of P. somniferum ssp setigerum. Seeds of the F1 P. somniferum x P. somniferum ssp setigerum cross were surface sterilized with hypochlorite. 25 seeds were grown on filter paper in Petri dishes in a minimum quantity of Linsmaier Skoog mineral solution to allow uninhibited growth. The seedlings were grown in a 12 hour day/night cycle under incandescent lamps at 20°C. After a growth period of 4 days, the developing seedlings (25) were administered 350 μl of a sterile 0.5 mM solution of potential precursor which was taken up by the roots. The seedlings were supplied fresh precursor solution every 24 h for the following 3 days. On day 8, the seedlings were harvested and extracted with 20 ml of boiling 80% methanol (extracted dry weight 10.1 mg). The soluble extract was evaporated to dryness, taken up with 100 μl methanol and directly injected (10 μl) without fractionation onto the LC-MS instrument.
4.2 Mass spectrometry
High resolution mass spectra were obtained using an LTQ-Orbitrap Spectrometer (Thermo Fisher, USA). The spectrometer was operated in positive mode (1 spectrum s−1; mass range: 50–1000) with a nominal mass resolving power of 60000 at m/z 400 at a scan rate of 1 Hz using automatic gain control to provide high-accuracy mass measurements (≤ 2 ppm deviation). The internal calibration standard bis-(2-ethylhexyl)-phthalate (m/z 391.28428) was used for the determination of elemental composition. The spectrometer was equipped with a Surveyor HPLC system (Thermo Scientific, USA) consisting of LC-Pump, UV detector (λ = 254 nm) and autosampler (injection volume 10 μl). Separation of samples was achieved by using a Machery-Nagel Nucleodor Gravity column (1.8 μm, 50 × 3 mm) combined with an associated guard column (4 × 3 mm). The mobile phase total flow was set to 0.4 ml/min with binary gradient elution, using solvents A (0.1% formic acid, 10 mM ammonium acetate) and B (0.1% formic acid in acetonitrile) (all v/v). The gradient started with 5% B for 4 min and was increased to 30% B over 20 min. Elution was continued for 10 min at 100% B followed by a 7-min equilibration with the starting condition. MS/MS spectra were obtained from the corresponding parent ions ([M+H]+) with a collision energy of 35 V. The quantitation of the individual labeled compound was achieved by integration of the peak area in the extracted ion chromatogram with a mass range of 2 ppm. All calibration graphs were linear (R2> 0.99) from 20 ng ml−1 up to a concentration of 20,000 ng ml−1.
4.3 Chemical synthesis
[7-D3]-laudanine and [3′,7-D6]-laudanosine
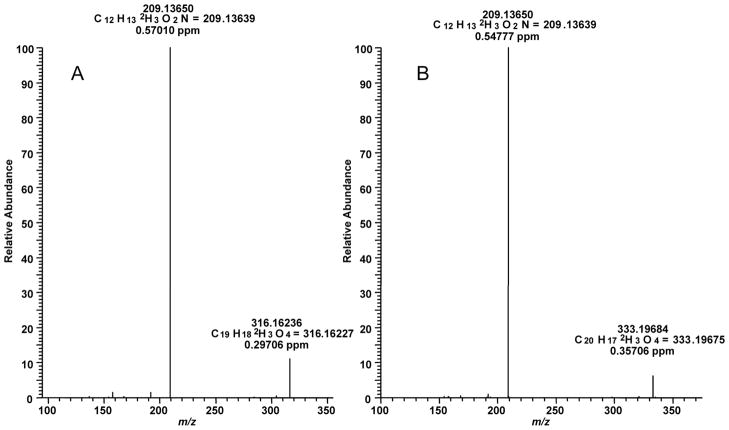
m-Chloroperbenzoic acid (100 mg) dissolved in CH2Cl2 (5 ml) were added dropwise to a stirred solution (0°) of (S)-reticuline (100 mg) in CH2Cl2 (15 ml) to yield (S)-reticuline N-oxide (66%) after 2.5 hours (Phillipson et al., 1976; Lu et al., 1987). The (S)-reticuline N-oxide was purified via thin layer chromatography (TLC) on Polygram silica G/UV254 plates (Macherey-Nagel, layer: 0.2 mm silica gel with fluorescent indicator UV254) in the solvent system acetone: H2O: NH4OH (80:15:5). The purified N-oxide was suspended in a mixture of EtOH (3 ml) and THF (9 ml) and reacted with D3-methyliodide (10 equiv) and KOH (7.5 equiv) overnight at room temperature to methylate the N-oxide. After methylation, the reaction mixture was dried and dissolved in 50% EtOH (8 ml). To remove the N-oxide protecting group, NaHSO3 (400 mg) was added and stirred for 5 hours. The reaction mixture was washed with CH2Cl2 and purified via the TLC system toluene: ethylacetate: diethylamine (7:2:1) to obtain [7-D3]-laudanine in 10% yield (Rf: 0.17) and [3′,7-D6]-laudanosine in 30% yield (Rf: 0.58). Several other compounds carrying deuterium label were seen on the chromatogram and clearly separated from each other, but their structures were not determined. Rechromatography was done on [7-D3]-laudanine using the TLC system xylene: 2-butanone: 2-propanol: diethylamine (20:20:3:1). The isotopic distribution of d3- and d6- labeled alkaloids exhibited isotopic purity > 99.5 %. [7-D3]-Laudanine and [3′,7-D6]-laudanosine were identified by comparison with unlabeled authentic standards, exhibiting the same chromatographic and spectroscopic data (Fig. 3A and B), except for their mass spectra, which show the calculated shift of their quasimolecular ion [M+H]+ either +3 or +6, respectively (Fig. 6).
Fig. 6.
High resolution MS/MS fragment spectra of synthesized [7-D3]-laudanine (A) and synthesized [3′,7-D6]-laudanosine (B).
Deuterated tetrahydropapaverine
Deuterated tetrahydropapaverine was synthesized from papaverine according to Taylor (1952) with slight modifications. To introduce a deuterium label at positions C1, C3 and C4, papaverine was refluxed in deuterium chloride and zinc dust. After 30 hours of reflux, the reaction mixture was made alkaline and extracted twice with ether. The presence of tetrahydropapaverine in the combined ether phases was confirmed by TLC in the solvent system xylene: butanone: methanol: diethylamine 20:20:3:1. Ether was removed and the residue reconstituted in methanol/acetonitrile 2:1. Deuterated tetrahydropapaverine was further purified by HPLC (Merck Hitachi) using a Hibar Pre-Packed column RT250-25 (Merck, 7 μm, LiChrosorb RP-18). The mobile phase total flow was set to 8 ml/min with binary gradient elution using 0.1% trifluoroacetic acid (solvent A) and acetonitrile (solvent B). The gradient started with 20% B and was increased to 50% B over 60 min. Elution was continued for 20 min at 100% B followed by a 20 min equilibration with the standard condition. The fraction containing deuterated tetrahydropapaverine was collected and lyophilized. Deuterated tetrahydropapaverine was obtained in 56% yield and completely free of papaverine as confirmed by TLC, HPLC and high resolution LC-MS. The isotopic distribution of deuterium-labeled tetrahydropapaverine was <0.1 % [2H0]/ <0.1% [2H1]/ <0.1% [2H2]/ <0.1% [2H3]/ 3% [2H4]/ 21.5% [2H5]/ 39% [2H6]/ 29.9% [2H7]/ 5.5% [2H8]/ 1% [2H9]. This observed isotopic distribution was largely in agreement with the expected isotopic distribution.
All other labeled or unlabeled alkaloids were from our laboratory collection.
Calculation of the quotient between labeled and unlabeled alkaloid
The following equation was used for the calculation:
Acknowledgments
This research was sponsored by NIH Grant #5R21DA024418-02 and the Mallinckrodt Foundation, St. Louis. X. Han thanks the REU program at the Donald Danforth Plant Science Center for the educational experience and the Science and Research Office at Macalester College for financial support.
This paper is dedicated to Prof. Rolf Huisgen on the occasion of his 90th birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Battersby AR, Harper BJT. Biogenesis of papaverine. Proc Chem Soc. 1959:152. [Google Scholar]
- Battersby AR, Binks R. Biosynthesis of morphine: Formation of morphine from norlaudanosoline. Proc Chem Soc. 1960:360–361. [Google Scholar]
- Battersby AR, Harper BJT. Alkaloid biosynthesis. Part I. The biosynthesis of papaverine. J Chem Soc. 1962:3526–3533. [Google Scholar]
- Battersby AR. Tilden Lecture: The biosynthesis of alkaloids. Proc Chem Soc. 1963:189–200. [Google Scholar]
- Battersby AR, Binks R, Francis RJ, McCaldin DJ, Ramuz H. Alkaloid Biosynthesis. Part IV. 1-Benzylisoquinolines as precursors of thebaine, codeine, and morphine. J Chem Soc. 1964:3600–3610. [Google Scholar]
- Battersby AR, Foulkes DM, Brinks R. Alkaloid Biosynthesis. Part VIII. Use of optically active precursors for investigations on the biosynthesis of morphine alkaloids. J Chem Soc (C) 1965:3323–3332. [PubMed] [Google Scholar]
- Battersby AR, Sheldrake PW, Staunton J, Summers MC. Stereospecificity in the biosynthesis of papaverine. Bioorg Chem. 1977;6:43–47. [Google Scholar]
- Brochmann-Hanssen E, Furuya T. New Opium Alkaloid. J Pharm Sci. 1964;53:575. doi: 10.1002/jps.2600530529. [DOI] [PubMed] [Google Scholar]
- Brochmann-Hanssen E, Nielsen B, Utzinger GE. Opium alkaloids. II Isolation and characterization of codamine. J Pharm Sci. 1965;54:1531–1532. doi: 10.1002/jps.2600541030. [DOI] [PubMed] [Google Scholar]
- Brochmann-Hanssen E, Fu CC, Leung AY, Zanati G. Opium alkaloids. X Biosynthesis of 1-benzylisoquinolines. J Pharm Sci. 1971;60:1672–1676. doi: 10.1002/jps.2600601118. [DOI] [PubMed] [Google Scholar]
- Brochmann-Hanssen E, Chen C-H, Chen CR, Chiang H-C, Leung AY, McMurtrey K. Opium alkaloids. Part XVI. The biosynthesis of 1-benzylisoquinolines in Papaver somniferum. Preferred and secondary pathways; stereochemical aspects. J Chem Soc Perkin I. 1975:1531–1537. doi: 10.1039/p19750001531. [DOI] [PubMed] [Google Scholar]
- Brochmann-Hanssen E, Chen CY, Linn EE. Biosynthesis of unnatural papaverine derivatives in Papaver somniferum. J Nat Prod. 1980;43:736–738. doi: 10.1021/np50012a007. [DOI] [PubMed] [Google Scholar]
- Frenzel T, Zenk MH. (S)-Adenosyl-L-methionine:3′-hydroxy-N-methyl-(S)-coclaurine-4′-O-methyl-transferase, a regio- and stereoselective enzyme of the (S)-reticuline pathway. Phytochemistry. 1990a;29:3505–3511. [Google Scholar]
- Frenzel T, Zenk MH. Purification and characterization of three isoforms of S-adenosyl-L-methionine: (R,S)-tetrahydrobenzylisoquinoline-N-methyltransferase from Berberis koetineana cell cultures. Phytochemistry. 1990b;29:3491–3497. [Google Scholar]
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, editor. The Alkaloids. Vol. 50. Academic Press; San Diego: 1998. pp. 257–316. [Google Scholar]
- Lu ST, Wu YC, Leou SP. The oxidation of isoquinoline alkaloids with m-chloroperbenzoic acid. J Chin Chem Soc. 1987;34:33–42. [Google Scholar]
- Merck G. Vorläufige Notiz über eine neue organische Base im Opium. Ann Chem. 1848;66:125–128. [Google Scholar]
- Ounaroon A, Decker G, Schmidt J, Lottspeich F, Kutchan TM. (R,S)-Reticuline 7-O-methyltransferase and (R,S)-norcoclaurine 6-O-methyltransferase of Papaver somniferum - cDNA cloning and characterization of methyl transfer enzymes of alkaloid biosynthesis in opium poppy. Plant J. 2003;36:808–819. doi: 10.1046/j.1365-313x.2003.01928.x. [DOI] [PubMed] [Google Scholar]
- Phillipson JD, Handa SS, El-Dabbas SW. N-Oxides of morphine, codeine and thebaine and their occurrence in Papaver species. Phytochemistry. 1976;15:1297–1301. [Google Scholar]
- Pienkny S, Brandt W, Schmidt J, Kramell R, Ziegler J. Functional characterization of a novel benzylisoquinoline O-methyltransferase suggests its involvement in papaverine biosynthesis in opium poppy (Papaver somniferum L) Plant Journal. 2009;60:56–67. doi: 10.1111/j.1365-313X.2009.03937.x. [DOI] [PubMed] [Google Scholar]
- Robinson R. A theory of the mechansim of the phytochemical synthesis of certain alkaloids. J Chem Soc Trans (London) 1917;111:876–899. [Google Scholar]
- Spenser ID. The biosynthesis of alkaloids and other nitrogenous secondary metabolites. In: Florkin M, Stotz EH, editors. Comprehensive Biochemistry. Vol. 20. Elsevier Publishing Company; Amsterdam: 1969. pp. 231–413. [Google Scholar]
- Stadler R, Kutchan TM, Loeffler S, Nagakura N, Cassels B, Zenk MH. Revision of the early steps of reticuline biosynthesis. Tetrahedron Lett. 1987;28:1251–1254. [Google Scholar]
- Stadler R, Zenk MH. A revision of the generally accepted pathway for the biosynthesis of the benzyltetrahydroisoquinoline alkaloid reticuline. Liebigs Ann Chem. 1990;1990:555–562. [Google Scholar]
- Taylor EC, Martin SF. A general method of alkylation and alkenylation heterocycles. J Am Chem Soc. 1974;96:8095–8102. [Google Scholar]
- Taylor EP. Synthetic neuromuscular blocking agents. Part II. Bis(quaternary ammonium salts) derived from laudanosine. J Chem Soc. 1952:142–145. [Google Scholar]
- Uprety H, Bhakuni DS, Kapil RS. Biosynthesis of papaverine. Phytochemistry. 1975;14:1535–1537. [Google Scholar]
- Wat CK, Steffens P, Zenk MH. Partial purification and characterization of (S)-adenosyl-L-methionine: norreticuline-N-methyltransferases from Berberis cell suspension cultures. Z Naturforsch. 1986;41c:126–134. [Google Scholar]
- Wickens JR, Sleeman R, Keely BJ. Atmospheric pressure ionisation mass spectrometric fragmentation pathways of noscapine and papaverine revealed by multistage mass spectrometry and in-source deuterium labelling. Rapid Comm Mass Spectrom. 2006;20:473–480. doi: 10.1002/rcm.2325. [DOI] [PubMed] [Google Scholar]
- Winterstein E, Trier G. Die Alkaloide. Verlag Gebrüder Bornträger; Berlin: 1910. [Google Scholar]
- Wu WN, Huang CH. Structural elucidation of isoquinoline, isoquinolone, benzylisoquinoline, aporphine, and phenanthrene alkaloids using API-ionspray tandem mass spectrometry. Chin Pharm J. 2006;58:41–55. [Google Scholar]