Abstract
Organophosphorus pesticides used most commonly in Turkey include methamidophos, dichlorvos, O-methoate, and diazinon. These toxic chemicals or their metabolites make a covalent bond with the active site serine of butyrylcholinesterase. Our goal was to identify the adducts that result from the reaction of human butyrylcholinesterase with these pesticides. Highly purified human butyrylcholinesterase was treated with a 20-fold molar excess of pesticide. The protein was denatured by boiling and digested with trypsin. MS and MSMS spectra of HPLC-purified peptides were acquired on a MALDI-TOF-TOF 4800 mass spectrometer. It was found that methamidophos added a mass of +93, consistent with addition of methoxy aminophosphate. A minor amount of adduct with an added mass of +109 was also found. Dichlorvos and O-methoate both made dimethoxyphosphate (+108) and monomethoxyphosphate adducts (+94). Diazinon gave a novel adduct with an added mass of +152 consistent with diethoxythiophosphate. Inhibition of enzyme activity in the presence of diazinon developed slowly (15 h), concomitant with isomerization of diazinon via a thiono-thiolo rearrangement. The isomer of diazinon yielded diethoxyphosphate and monoethoxyphosphate adducts with added masses of +136 and +108. MSMS spectra confirmed that each of the pesticides studied made a covalent bond with serine 198 of butyrylcholinesterase. These results can be used to identify the class of pesticides to which a patient was exposed.
Keywords: Human butyrylcholinesterase, organophosphorus pesticides, mass spectrometry, methamidophos, diazinon, O-methoate, dichlorvos, thiono-thiolo
INTRODUCTION
Turkey is a major producer and exporter of agricultural products. Pesticides are used in farms, greenhouses, and grape vineyards. Pesticide consumption is lower than in European countries probably because of economic constraints. However, ignorance of protective cautions, and use of prohibited pesticides cause health problems in Turkey. The ministry of Agriculture provides financial support for certain pesticides, but since July 1999, the use of very poisonous types of pesticides has been limited. According to the 2008 annual report of the National Poison Center (Refik Saydam Hygiene Center), 8.34 % of the 77988 people who were diagnosed as poisoned, were poisoned by pesticides. Organophosphorus pesticide (OP) poisonings constituted 20.98 % of all pesticide poisonings in Turkey (Ergonen et al., 2005; Turgut et al., 2009).
The OP pesticides most frequently used in Turkey are methamidophos (Tamaron), dichlorvos, O-methoate, and diazinon (Ergonen et al., 2005) (Figure 1). These toxic chemicals or their metabolites make a covalent bond with the active site serine of butyrylcholinesterase (BChE, EC 3.1.1.8), resulting in inhibition of BChE activity (Main, 1976; Whittaker, 1986). The inhibited butyrylcholinesterase in plasma serves as a biomarker of OP exposure (Altintop et al., 2005; Yardan et al., 2007; Yurumez et al., 2007; Kavalci et al., 2009).
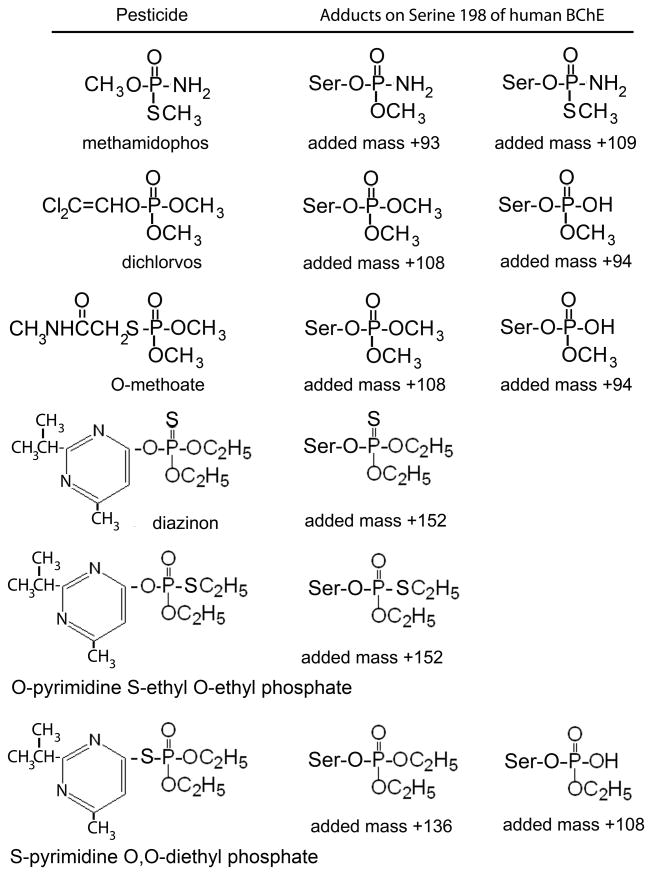
Figure 1.
Structures of pesticides and the adducts formed by covalent binding to Serine 198 of human BChE. Isomerization of diazinon via a thiono-thiolo rearrangement occurred in solution. The unlabeled active site peptide of BChE (accession number gi:116353) produced by digestion with trypsin has a monoisotopic mass of 2928.5 amu.
In Turkey, exposure to OP pesticides is inferred by measuring BChE activity in plasma (Altintop et al., 2005; Yardan et al., 2007; Yurumez et al., 2007; Kavalci et al., 2009). A limitation of this method is that it does not identify the poison. A variety of agents inhibit BChE including the Alzheimer drugs tacrine and rivastigmine (Darvesh et al., 2003), OP nerve agents (Fidder et al., 2002; Nicolet et al., 2003), carbamate pesticides (Li et al., 2009), plant poisons such as physostigmine (eserine) from the Calabar bean (Easson and Stedman, 1936), a naturally occurring OP in the blue-green algae Anabaena flos-aquea (Mahmood and Carmichael, 1987; Matsunaga et al., 1989) and OP pesticides. A method that can directly measure the OP-adducted form of the enzyme would distinguish between classes of poisons. Information of this type could be useful for identifying the poison, and for proving exposure.
Materials and Methods
Materials
Seventy liters of outdated human plasma were obtained from the University of Nebraska Hospital blood bank. Human BChE was purified from the outdated plasma by ion exchange chromatography at pH 4, followed by procainamide affinity chromatography and ion exchange chromatography at pH 7.4 (Lockridge et al., 2005). Purified BChE (54% pure) had an activity of 2620 U/ml assayed with 1 mM butyrylthiocholine at pH 7.0 and 25°C, and a BChE protein concentration of 3.64 mg/ml. Butyrylthiocholine iodide and 5,5′-dithiobis(2-nitrobenzoic acid) were from Sigma-Aldrich, St. Louis, MO. Methamidophos 98.4% pure (PS-676), dichlorvos 98.0% pure (PS-89), and O-methoate 97.3% pure (PS-2017) were from ChemService, West Chester, PA. Diazinon 96.7% pure (S-87-1185) was from Ciba Crop Protection, Greensboro, NC. OP stock solutions (50 mM) were prepared in methanol or acetonitrile. Sequencing grade modified porcine trypsin (V5113) was from Promega, Madison, WI. α-cyano-4-hydroxycinnamic acid (CHCA) was from Applied Biosystems, Foster City, CA.
Inhibition of BChE by OP
Highly purified human BChE (0.2 mg in 0.055 ml of 10 mM NH4HCO3 pH 8.3; 42 μM) was reacted with 1 μl of 50 mM OP at 22°C to give a 20-fold molar excess of OP over BChE active sites. The reaction was stopped by heating the sample in a boiling water bath.
The BChE concentration in human plasma is 0.050 microMolar. Humans are unlikely to survive a pesticide dose 20-fold higher than the plasma BChE concentration. Our studies treated purified human BChE with a 20-fold molar excess of pesticide to achieve maximum labeling of BChE. The purpose of our study is to determine the mass of the adducts on human butyrylcholinesterase after butyrylcholinesterase has been modified by organophosphorus pesticides. The adduct mass must be known before one can set up a multiple reaction monitoring experiment in the mass spectrometer to search for adducts in human plasma. Our study provides the background information that is needed for analyzing real life blood samples from exposed humans.
Assay of BChE activity
BChE activitywas assayed at 25°C by the Ellman method (Ellman et al., 1961). The assay mixture (2 ml) contained 100 mM potassium phosphate buffer (pH 7.0), 1 mM butyrylthiocholine, and 0.5 mM 5,5′-dithiobis(2-nitrobenzoic acid). The reactions were initiated by adding enzyme. The rate of butyrylthiocholine hydrolysis was monitored by the increase in absorbance at 412 nm on a Gilford spectrophotometer. BChE activity was calculated in the initial 60-sec period using the extinction coefficient (E412 = 13.6 mM−1 cm−1).
Trypsin Digestion and HPLC
OP-treated BChE was boiled for 10 min in a water bath to denaturate and unfold the protein thereby allowing trypsin access to cleavage sites. BChE (200 μg in 55 μl of 10 mM ammonium bicarbonate pH 8.3) was digested overnight at 37 °C with 20 μl of 0.4 μg/μl trypsin (8 μg in 50 mM acetic acid). A 1 μl aliquot of 1 M ammonium bicarbonate was added to adjust the pH to 8.3.
Trypsinized BChE was injected into a Phenomenex C18 column (100 × 4.6 mm) on a Waters 625 LC system to purify the labeled active site peptide. Peptides were eluted with a 60 min gradient starting with 100% buffer A (0.1% trifluoroacetic acid in water) and ending with 60% buffer B (acetonitrile containing 0.09% trifluoroacetic acid) at a flow rate of 1 ml/min. One ml fractions were collected.
Matrix-Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF-TOF) Mass Spectrometry
HPLC fractions were analyzed in the MS and MSMS modes of the MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems, Foster City, CA). Salt free HPLC fractions (0.5 μl) were spotted on a MALDI plate (Opti-TOF 384 well Insert from Applied Biosystems), air dried and overlaid with 0.5 μl of α-cyano-4-hydroxycinnamic acid (10 mg/ml in 50% acetonitrile, 0.1% trifuoroacetic acid). MS spectra were acquired using positive reflector mode with laser intensity at 4000 V. Each MS spectrum was the sum of 500 laser shots. Parent ion masses corresponding to labeled BChE active site peptides were fragmented in the MSMS mode to determine the peptide sequence and to identify the modified amino acid. Each MSMS spectrum was the sum of 2500 laser shots. The y ions and b ions in the MSMS spectra were assigned with the aid of the Proteomics Toolkit, a free online fragment ion calculator (http://db.systemsbiology.net).
RESULTS
Inhibition of BChE by methamidophos, dichlorvos, O-methoate, and diazinon
After 1 h reaction of BChE with a 20-fold molar excess of OP, enzyme inhibition levels were 97–100 % for methamidophos, dichlorvos, and O-methoate. As expected, diazinon did not significantly inhibit BChE in 1 h; phosphorothioates become good inhibitors only after oxidative desulfuration by cytochrome P450 (Sultatos, 1994). After 7 h the diazinon treated BChE was inhibited 20%. The incubations with methamidophos, dichlorvos, and O-methoate were stopped after 7 h. The incubation with diazinon was continued for a total of 15 h, at which time the BChE activity was inhibited 52%.
Analysis of methamidophos-BChE adducts by mass spectrometry
Digestion of human BChE with trypsin generates a 29 amino acid active site peptide extending from Ser 191 to Arg 219 (Lockridge et al., 1987). The amino acid sequence of this peptide is 191SerValThrLeuPheGlyGluSERAlaGlyAlaAlaSerValSerLeuHisLeuLeuSerProGly SerHisSerLeuPheThrArg219 (gi:116353 is the accession number in the NCBI nonredundant database). The active site serine is shown in bold uppercase letters. The monoisotopic mass of the unlabeled peptide is 2928.5 amu.
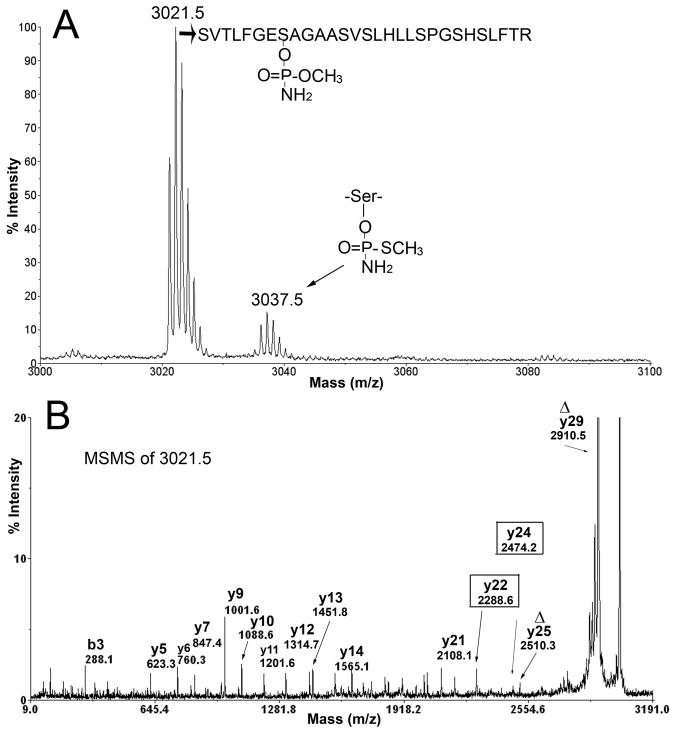
When human BChE was reacted with methamidophos, two new masses appeared in the MS spectrum (Figure 2A). The mass at 3021.5 amu is consistent with addition of methoxy aminophosphate (+93 amu) and release of thiomethyl upon covalent bond formation with BChE (Figure 1). The small peak at 3037.5 amu is consistent with addition of thiomethyl aminophosphate (+109 amu) and release of the methoxy group. Thus, methamidophos yields two types of adducts. The peak height of the adduct at 3037.5 amu was approximately 5% of that of the adduct at 3021.5 amu. This means thiomethyl is a better leaving group than methoxy, a result supported by the literature (Thompson and Fukuto, 1982). No aging was observed after 7 h at pH 8.3, 22°C. If aging had occurred, the added mass from methamidophos would have been +79, regardless of which type of adduct was formed initially. No peptides with an added mass of +79 were observed.
Figure 2.
Methamidophos adducts of BChE. A) MS spectrum showing masses of the two adducts produced by covalent binding of methamidophos to BChE. The thiomethyl group is displaced to make the 3021.5 amu adduct. A less favored reaction results in release of the methoxy group to make the 3037.5 amu adduct. B) MSMS spectrum of the 3021.5 amu parent ion. The Δy29 ion at 2910.5 amu has lost the OP and a molecule of water during the fragmentation process. The intensity of Δy29 is about 20-fold greater than that of other fragment ions. Loss of OP and water converts the OP-labeled active site serine to dehydroalanine, symbolized by Δ. A second dehydroalanine ion is at Δy25. The y22 and y24 ions enclosed in boxes carry the OP on Serine 198.
Figure 2B shows the MSMS spectrum for the singly charged parent ion of mass 3021.5. The labeled masses correspond to fragments of the BChE active site peptide. The most prominent peak, at 2910.5 amu, is the dehydroalanine containing parent ion, designated Δy29. This mass is consistent with loss of the organophosphorus agent together with a molecule of water from the 3021.5 parent ion to yield dehydroalanine in place of the OP-modified active site serine. Facile loss of phosphate from serine to yield dehydroalanine is a commonly observed fragmentation. The y22 ion at 2288.6 amu and the y24 ion at 2474.2 amu are consistent with fragments that retain the organophosphorus agent on serine 198. Their presence provides proof that the modified amino acid is Serine 198. Evidence from Figure 2B establishes that the 3021.5 amu ion is the active site peptide of BChE wherein the active site serine is labeled with methoxy aminophosphate.
O-methoate and dichlorvos adducts
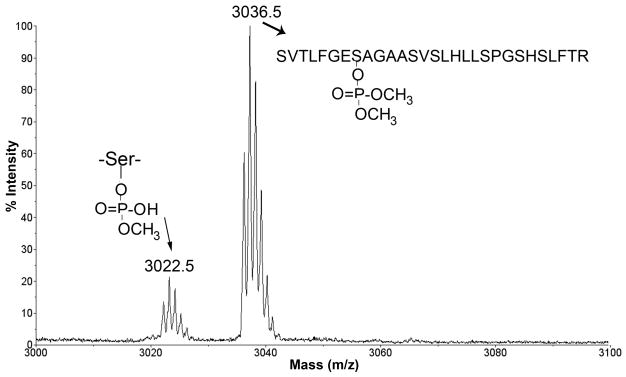
O-methoate and dichlorvos both made a dimethoxyphosphate adduct with the BChE active site peptide, to give a singly charged tryptic peptide with a mass of 3036.5 amu. In addition, the aged monomethoxyphosphate adduct with a mass of 3022.5 amu was observed with both reagents. Figure 3 shows the MS spectrum of the BChE peptide labeled with O-methoate, for the sample in HPLC fraction 36. The MS spectrum for dichlorvos labeled BChE was indistinguishable from the MS spectrum of O-methoate labeled BChE. The relative heights of the peaks in Figure 3 are not representative of the relative ratio of the aged and unaged peptides because the aged peptide also eluted in fraction 35. MSMS spectra of the 3036.5 and 3022.5 amu parent ions fully supported the assignment of the labeled amino acid as Serine 198 of BChE (data not shown).
Figure 3.
O-methoate adduct of BChE, MS spectrum. O-methoate and dichlorvos make the same adducts with BChE. The peptide with mass 3036.5 is the dimethoxyphosphate adduct. The peptide with mass 3022.5 is the monomethoxyphosphate adduct, which has lost a methoxy group as a consequence of aging.
Aging is defined as the loss of an alkyl group from the OP-modified enzyme. Aging is catalyzed by Glu 197 and His 438 (Nachon et al., 2005); aging is halted by denaturing the BChE enzyme (Li et al., 2009).
Diazinon makes a covalent bond without prior desulfuration; the 3080.5 adduct
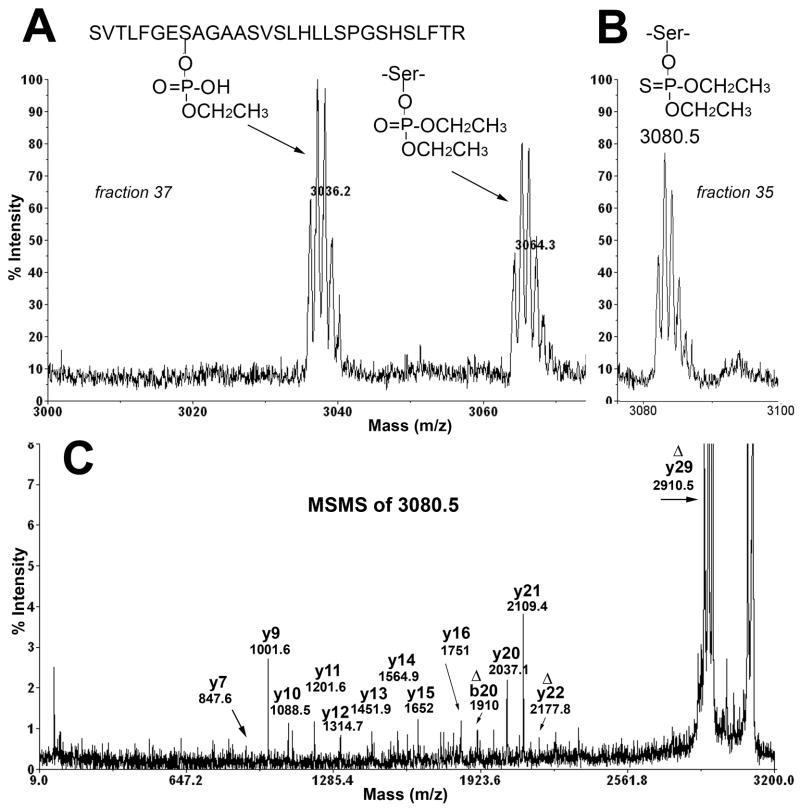
The reaction of diazinon with BChE yielded three BChE adducts (Figure 4A). The adduct with a monoisotopic mass of 3080.5 amu (Figure 4B) appeared in HPLC fraction 35. The 3080.5 mass is consistent with covalent binding of diethoxythiophosphate to the active site serine. The structure of the adduct indicated in Figure 4B is the product of the reaction with diazinon and requires no rearrangement of atoms in diazinon. The 3080.5 mass is also consistent with binding of thioethyl ethoxyphosphate. This alternative adduct would be produced from a rearranged diazinon in which the sulfur atom exchanged with an oxygen atom to yield O-pyrimidine S-ethyl O-ethylphosphate (Figure 1).
Figure 4.
Diazinon makes an unusual adduct with BChE. A) MS spectrum shows adducts with masses of 3036.2 and 3064.3 in fraction 37 from the HPLC. These adducts are from the reaction of an isomer of diazinon with BChE. B) MS spectrum shows an adduct with a mass of 3080.5 in fraction 35. The peak at 3080.5 is modified on Serine 198 by diethoxythiophosphate. Alternatively, the 3080.5 mass could come from reaction with a rearranged diazinon to yield thioethyl ethoxyphosphate. C) The MSMS spectrum of the singly charged parent ion m/z 3080.5 demonstrates that the 3080.5 amu peptide is the active site BChE peptide modified on Serine 198 by OP.
Proof that the ion at 3080.5 amu is an OP-modified BChE active site peptide is provided in the MSMS spectrum in Figure 4C. The singly charged parent ion of mass 3080.5 yielded a prominent peak at 2910.5 amu, designated Δy29 in the figure. An intense fragment ion at 2910 amu is commonly seen when the OP-labeled, BChE active-site peptide is subjected to fragmentation in the MALDI-TOF-TOF 4800. It represents loss of the organophosphorus agent and a molecule of water, with conversion of the OP-labeled serine to dehydroalanine. The intensity of the Delta;y29 ion is 25-fold greater than the intensity of other ions. To visualize the other ions it was necessary to cut off most of the signal from the Delta;y29 ion in Figure 4C. The other annotated peaks are consistent with the fragmentation pattern of the BChE active site peptide. In addition to the 2910.5 fragment, the b20 ion at 1910 and the Δy22 ion at 2177.8 amu are consistent with loss of the organophosphorus agent and a molecule of water, and the appearance of dehydroalanine.
Phosphorothioates including diazinon, parathion, and malathion are poor inhibitors of acetylcholinesterase and butyrylcholinesterase. Our mass spectrometry results suggest the inhibition rates observed in kinetic experiments can be attributed in part to the covalent bond formed between the phosphorothioate and BChE. However, a second factor must also be considered and that is the isomerization of phosphorothioates, converting them to oxons. Oxons react rapidly with the cholinesterases, so the rate limiting step in the inhibition reaction would be the rate of isomerization.
Isomerization of diazinon to explain the 3064.5 and 3036.5 adducts
The slow inhibition of BChE by diazinon was accompanied by formation of diethoxyphosphate and monoethoxyphosphate adducts with masses of 3064.5 and 3036.5 amu (Figure 4A). The sulfur originally present in diazinon was lost. The source of the diethoxyphosphate adduct at 3064.5 amu can be rationalized by invoking a thiono-thiolo rearrangement in which the double bonded sulfur on the phosphorus atom is exchanged with the oxygen on the pyrimidinol ring (Thompson et al., 1989; Barr et al., 2005). The rearranged isomer structure, S-pyrimidine O,O-diethylphosphate, is shown in Figure 1. The thiono-thiolo rearrangement is an established, facile isomerization that can be induced by heat, light or chemicals (Thompson et al., 1989; Barr et al., 2005). Subsequent reaction of the thiopyrimidine isomer with BChE would eliminate the thiopyrimidine moiety leaving the diethoxyphosphate. Aging with loss of one ethoxy group explains the monoethoxyphosphate adduct at 3036.5 amu.
The 3064.5 and 3036.5 adducts from the reaction of diazinon with BChE are the same as those produced by reaction with diazoxon. The possibility that diazoxon was present as a contaminant was ruled out by the observation that inhibition of BChE occurred slowly over a 15 h incubation period. If the diazoxon had been present as a contaminant in the diazinon solution, inhibition would have been nearly instantaneous on mixing diazinon with BChE. Absence of a diazoxon contamination in the diazinon preparation eliminates diazoxon as a source of adducts with masses of 3064.5 and 3036.5 amu.
MSMS spectra of the 3064.5 and 3036.5 parent ions (data not shown) confirmed that these were BChE peptides and that the OP was covalently bound to the active site serine.
DISCUSSION
The majority of pesticide poisoning cases in Turkey are suicide attempts (Yurumez et al., 2007). The most frequent clinical signs are miosis, respiratory distress, tachycardia, loss of consciousness, and hypertension. Out of 220 cases treated in hospital emergency rooms in Afyonkarahisar and Kayseri, Turkey, 20 patients died. Sometimes the diagnosis of poisoning is difficult. Cases have been misdiagnosed initially as brainstem stroke (Aygun, 2004), opioid overdose (Baydin et al., 2008), and foreign body aspiration (Caksen et al., 2005). Laboratory assays showing that plasma butyrylcholinesterase activity was below normal, were helpful for achieving the correct diagnosis of OP poisoning. Treatment strategies depend on a correct diagnosis.
The acute toxicity of organophosphorus pesticides is due to inhibition of acetylcholinesterase in nerve synapses. Inhibition of butyrylcholinesterase has no clinical sequelae. Butyrylcholinesterase is a good marker for OP exposure because BChE reacts rapidly with OP to form covalent adducts that have no enzyme activity, and BChE is 3000-fold more abundant in human plasma than acetylcholinesterase (Brimijoin and Hammond, 1988). It is standard practice to look for inhibition of plasma BChE activity to aid in diagnosis of OP pesticide intoxication.
Though BChE activity assays are helpful for diagnosis, they do not identify the poison. Mass spectrometry can distinguish between classes of poison. Our mass spectrometry study of pure human BChE modified by four OP pesticides will serve as an aid for future work that aims to analyze plasma samples from poisoned individuals.
The pesticides in the present report have not previously been tested by mass spectrometry of adducts with human BChE. The reaction of methamidophos with human and Torpedo californica acetylcholinesterase has been studied by mass spectrometry, and has been found to yield adducts on the active site serine similar to the major adduct we found for BChE (Elhanany et al., 2001). A study with radiolabeled methamidophos showed that thiomethyl was the leaving group in the reaction of methamidophos with electric eel acetylcholinesterase (Thompson and Fukuto, 1982), a result consistent with our result for human BChE. Aging of the acetylcholinesterase adducts was not reported, consistent with our result for BChE where aging was not observed for samples treated with methamidophos for 7 h.
Diazinon is widely used as a pesticide in sheep dip formulations, spray pesticides for household use, and cat flea collars (de Blaquiere et al., 2000; Garfitt et al., 2002). Diazinon is a poor inhibitor of acetylcholinesterase and BChE until it is activated to the oxon. Activation to the oxon is mediated by liver microsomal cytochrome P450 (Mutch and Williams, 2006). A second route to the oxon is a thiono-thiolo rearrangement. In the present work the thiono-thiolo rearrangement occurred slowly in aqueous solution to produce an oxon that inhibited the activity of BChE. A full description of the rearrangement products of diazinon is provided by Barr et al. (Barr et al., 2005).
Limitations
A mass spectrometry method for detection of OP exposure is not expected to be useful to the clinician, who will treat patients based on their symptoms. Knowing the identity of the poison is, however, likely to be useful to forensic toxicologists. The method distinguishes classes of poisons, but does not distinguish between poisons that add an identical mass. For example, dichlorvos and O-methoate both add a mass of 108 for dimethoxyphosphate, or a mass of 94 for monomethoxyphosphate. Another limitation of the method is the requirement for highly purified BChE. Purifying 4 micrograms of BChE from a milliliter of plasma that contains 50,000 micrograms of other proteins is a difficult task at this time. A one step method is needed that selectively extracts BChE from human serum or plasma.
Application
The information provided in the present work provides the masses of possible adducts and how they fragment in the mass spectrometer. This information is needed to set up sensitive multiple reaction monitoring mass spectrometry methods to analyze exposure in real life samples. As of this date, the major application will be for forensic cases, where it is important to distinguish between classes of pesticides, and between nerve agents and pesticides.
Sensitivity
It has been estimated that mass spectrometry could potentially detect OP-BChE adducts in human plasma where the BChE was inhibited as little 1% (Tsuge and Seto, 2006). The multiple reaction monitoring method would require purification of BChE from 5 ml of plasma.
CONCLUSIONS
Mass spectrometry has identified unexpected modifications on the active site serine of human butyrylcholinesterase after highly purified butyrylcholinesterase was treated with selected pesticides. Two types of adducts were produced by reaction of butyrylcholinesterase with methamidophos, and three types of adducts by reaction with diazinon. Knowing what to expect prepares one for analysis of plasma samples from poisoned patients.
Acknowledgments
Supported by NIH grant U01 NS058056, NIH Cancer Center Support grant CA036727, and US Army Medical Research & Materiel Command W81XWH-07-2-0034. Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
Abbreviations
- amu
atomic mass units
- BChE
butyrylcholinesterase
- MALDI-TOF
matrix-assisted laser desorption ionization time of flight
- OP
organophosphorus agent
- MS
mass spectrum
- MSMS
fragmentation spectrum of a selected ion acquired in the mass spectrometer
Contributor Information
Ozden Tacal, Email: tacal@hacettepe.edu.tr.
Oksana Lockridge, Email: olockrid@unmc.edu.
References
- Altintop L, Aygun D, Sahin H, Doganay Z, Guven H, Bek Y, Akpolat T. In acute organophosphate poisoning, the efficacy of hemoperfusion on clinical status and mortality. J Intensive Care Med. 2005;20:346–350. doi: 10.1177/0885066605279834. [DOI] [PubMed] [Google Scholar]
- Aygun D. Diagnosis in an acute organophosphate poisoning: report of three interesting cases and review of the literature. Eur J Emerg Med. 2004;11:55–58. doi: 10.1097/01.mej.0000114335.25496.08. [DOI] [PubMed] [Google Scholar]
- Barr JD, Bell AJ, Bird M, Mundy JL, Murrell J, Timperley CM, Watts P, Ferrante F. Fragmentations and reactions of the organophosphate insecticide Diazinon and its oxygen analog Diazoxon studied by electrospray ionization ion trap mass spectrometry. J Am Soc Mass Spectrom. 2005;16:515–523. doi: 10.1016/j.jasms.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Baydin A, Aygun D, Aydin M, Akdemir HU, Ulger F. Acute organophosphate poisoning mimicking opioid intoxication. Eur J Emerg Med. 2008;15:245–246. doi: 10.1097/MEJ.0b013e3282f3ca72. [DOI] [PubMed] [Google Scholar]
- Brimijoin S, Hammond P. Butyrylcholinesterase in human brain and acetylcholinesterase in human plasma: trace enzymes measured by two-site immunoassay. J Neurochem. 1988;51:1227–1231. doi: 10.1111/j.1471-4159.1988.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Caksen H, Demirtas M, Tuncer O, Odabas D, Ceylan N, Kati I, Koseoglu B. A boy with organophosphate poisoning mimicking a foreign body aspiration. J Emerg Med. 2005;29:217–219. doi: 10.1016/j.jemermed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D, Rockwood K, Martin E. Inhibition of human cholinesterases by drugs used to treat Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:117–126. doi: 10.1097/00002093-200304000-00011. [DOI] [PubMed] [Google Scholar]
- de Blaquiere GE, Waters L, Blain PG, Williams FM. Electrophysiological and biochemical effects of single and multiple doses of the organophosphate diazinon in the mouse. Toxicol Appl Pharmacol. 2000;166:81–91. doi: 10.1006/taap.2000.8960. [DOI] [PubMed] [Google Scholar]
- Easson LH, Stedman E. The absolute activity of choline-esterase. Proc R Soc Lond B. 1936;121:142–164. doi: 10.1098/rspb.1936.0055. [DOI] [Google Scholar]
- Elhanany E, Ordentlich A, Dgany O, Kaplan D, Segall Y, Barak R, Velan B, Shafferman A. Resolving pathways of interaction of covalent inhibitors with the active site of acetylcholinesterases: MALDI-TOF/MS analysis of various nerve agent phosphyl adducts. Chem Res Toxicol. 2001;14:912–918. doi: 10.1021/tx0100542. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ergonen AT, Salacin S, Ozdemir MH. Pesticide use among greenhouse workers in Turkey. J Clin Forensic Med. 2005;12:205–208. doi: 10.1016/j.jcfm.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Garfitt SJ, Jones K, Mason HJ, Cocker J. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol Lett. 2002;134:105–113. doi: 10.1016/S0378427402001789. [DOI] [PubMed] [Google Scholar]
- Kavalci C, Durukan P, Ozer M, Cevik Y, Kavalci G. Organophosphate poisoning due to a wheat bagel. Intern Med. 2009;48:85–88. doi: 10.2169/internalmedicine.48.1559. [DOI] [PubMed] [Google Scholar]
- Li H, Ricordel I, Tong L, Schopfer LM, Baud F, Megarbane B, Maury E, Masson P, Lockridge O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J Appl Toxicol. 2009;29:149–155. doi: 10.1002/jat.1392. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Bartels CF, Vaughan TA, Wong CK, Norton SE, Johnson LL. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987;262:549–557. [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, Winger G, Woods JH. Large Scale Purification of Butyrylcholinesterase from Human Plasma Suitable for Injection into Monkeys; a Potential New Therapeutic for Protection against Cocaine and Nerve Agent Toxicity. J Med Chem Biol Radiol Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. nihms5095.10.1901/jaba.2005.3-nihms5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood NA, Carmichael WW. Anatoxin-a(s), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon. 1987;25:1221–1227. doi: 10.1016/0041-0101(87)90140-1. DOI: 0041-0101(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Main AR. Structure and Inhibitors of Cholinesterase. In: Goldberg AM, Hanin I, editors. Biology of Cholinergic Function. Raven Press; New York: 1976. pp. 269–353. [Google Scholar]
- Matsunaga S, Moore RE, Niemczura WP, Carmichael WW. Anatoxin-a(s), a potent anticholinesterase from Anabaena flos-aquae. J Am Chem Soc. 1989;111:8021–8023. doi: 10.1021/ja00202a057. [DOI] [Google Scholar]
- Mutch E, Williams FM. Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology. 2006;224:22–32. doi: 10.1016/j.tox.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Nachon F, Asojo OA, Borgstahl GE, Masson P, Lockridge O. Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging. Biochemistry. 2005;44:1154–1162. doi: 10.1021/bi048238d. [DOI] [PubMed] [Google Scholar]
- Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- Sultatos LG. Mammalian toxicology of organophosphorus pesticides. J Toxicol Environ Health. 1994;43:271–289. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Frick JA, Natke BC, Hansen LK. Preparation, analysis, and anticholinesterase properties of O,O-dimethyl phosphorothioate isomerides. Chem Res Toxicol. 1989;2:386–391. doi: 10.1021/tx00012a006. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Fukuto TR. Mechanism of cholinesterase inhibition by methamidophos. J Agric Food Chem. 1982;30:282–285. doi: 10.1021/jf00110a016. [DOI] [Google Scholar]
- Tsuge K, Seto Y. Detection of human butyrylcholinesterase-nerve gas adducts by liquid chromatography-mass spectrometric analysis after in gel chymotryptic digestion. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:21–30. doi: 10.1016/j.jchromb.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Turgut C, Ornek H, Cutright TJ. Pesticide residues in dried table grapes from the Aegean region of Turkey. Environ Monit Assess. 2009 doi: 10.1007/s10661-009-1037-z. [DOI] [PubMed] [Google Scholar]
- Whittaker M. Cholinesterase. Karger; Basel: 1986. [Google Scholar]
- Yardan T, Baydin A, Aygun D, Karatas AD, Deniz T, Doganay Z. Late-onset intermediate syndrome due to organophosphate poisoning. Clin Toxicol (Phila) 2007;45:733–734. doi: 10.1080/15563650701502733. [DOI] [PubMed] [Google Scholar]
- Yurumez Y, Durukan P, Yavuz Y, Ikizceli I, Avsarogullari L, Ozkan S, Akdur O, Ozdemir C. Acute organophosphate poisoning in university hospital emergency room patients. Intern Med. 2007;46:965–969. doi: 10.2169/internalmedicine.46.6304. [DOI] [PubMed] [Google Scholar]