Abstract
Background and objective
Aging is associated with an increase in myocardial susceptibility to ischaemia/reperfusion (I/R) injury. Na+/H+ exchange (NHE) inhibition and anaesthetic preconditioning (APC) are shown to protect myocardium from I/R injury. We set out to investigate if NHE inhibition can induce protection against I/R injury and whether KATP channel inhibition can enhance this effect in aged rat myocardium.
Methods
Hearts from 24-month old rats were assigned to 4 groups: (1) Control group; (2) APC group perfused with 2.5% sevoflurane before ischemia; (3) HOE group perfused with (3-methylsulfonyl-4-piperidinobenzoyl) guanidine methanesulfonate (HOE-694) prior to ischaemia; (4) HOE+5HD group perfused with both HOE and 5-hydroxydecanoic acid before ischaemia. We measured intracellular Na+ and Ca++ to quantitate the severity of myocardial injury.
Results
Both intracellular Na+ and Ca++ were significantly increased at the end of ischaemia and both were attenuated by NHE inhibition. Intracellular Na+ was 134±12 mEq/kg/dry weight in control group and 55±7 in HOE group (p<0.05). Intracellular Ca++ was 1764±142 nM in control group and 694±213 in HOE group (p<0.05). Infarct size was measured at 28±4% in control group vs. 17±2% in HOE group (p<0.05). High-energy phosphates and myocardial function were better preserved in HOE group compared to control (p<0.05). The beneficial effects of HOE on myocardial preservation was not blocked by 5HD nor were there any differences between APC and control groups.
Conclusions
NHE inhibition was effective in protecting myocardium from I/R injury in aged rats whereas APC was not. 5HD failed to block the protective effect of NHE inhibition.
Keywords: aged, anaesthetic preconditioning, NHE inhibition, myocardium, ischaemic/reperfusion injury
Introduction
As the population ages, more and more elderly patients will present for surgical procedures. Aging is associated with an increase in myocardial susceptibility to ischaemia and a decrease in post-ischemic recovery of function. Many investigators have observed a decreased capacity of the aged myocardium to tolerate an ischaemic/hypoxic stress in both animal models and in humans [1,2]. Due to the important implications for managing patient risk from cardiac complications and for the development of specific intraoperative cardioprotective therapies that minimize the risk of cardiac injury, studies have focused on both inhibition of Na+/H+ exchange 1 (NHE1) and volatile anaesthetic preconditioning (APC) to protect myocardium from ischaemia/reperfusion (I/R) injury [3-5]. While ischaemic preconditioning (IPC) has been shown to have a strong cardioprotective effect against I/R injury, its protective effect may be significantly attenuated in aged hearts [6,7]. Anaesthetic preconditioning shares similar benefits to IPC in protecting the heart from I/R injury including the observation that APC is also less effective against I/R injury in aged hearts [8,9]. Several studies have focused on the affect of aging on the ability of NHE inhibition and APC to protect the myocardium against I/R injury and found that NHE inhibition and APC are both effective in protecting myocardium from I/R injury in young animals [8-11]. The APC has failed to protect aged myocardium from I/R injury [8]. In the present study we set out to investigate the effect of NHE1 inhibition on myocardial protection against I/R injury in aged rat myocardium and to determine if mitochondrial KATP channel inhibition can modulate this protection.
Methods
The study protocol was approved by the Animal Care Committee of the University of California, Davis (Davis, California, USA) and all experiments were conducted in accordance with “The Guide for Care and Use of Laboratory Animals” (NIH publication vol.25 no.28, revised 1996) and policies of the University of California, Davis.
Preparation of Isolated Hearts
Hearts were obtained from male Fischer 344 rats of age 22-24 months (NIA, Maryland, USA). A total of 72 rats were used in this study. Anaesthesia was first obtained with an intra-peritoneal injection of sodium thiopental (50-75mg/kg) along with 1,000 units of heparin. Thiopentone was chosen for initial anaesthesia since this drug has been shown not to influence preconditioning [8]. Once no response to tail-clamp was obtained, the heart was excised via thoracotomy and placed in an iced solution of Krebs-Henselet buffer (KHB). The aorta was quickly cannulated and aerobically perfused with KHB on a non-recirculating Langendorff apparatus at a perfusion pressure of 90±10 mmHg. The concentration of KHB reagents were (in mmoles/L): NaCl 127, KCl 4.7, MgCl2 1.25, CaCl2 2.5, NaHCO3 25, and glucose 10. The perfusate was continuously oxygenated with a 95% O2 / 5% CO2 at 37±0.5° C.
Experimental Design and Groups
Six rats from each group were randomly selected. Control group was perfused for a 45-60 minute baseline interval before initiating 25 minutes of global ischemia followed by 60 minutes of reperfusion. The HOE group was perfused with NHE1 inhibitor, HOE 694 [(3-methylsulfonyl-4-piperidinobenzoyl) guanidine methanesulfonate](10 uM), for 10 minutes prior to 25 minutes of ischemia and followed by 60 minute of reperfusion. The APC group was perfused with sevoflurane (2.5%) for 10 minutes and followed by a 10-minute washout period prior to 25 minutes of global ischaemia, and finally 60 minutes of reperfusion. The HOE+5HD group was perfused with both HOE and 100 uM 5-hydroxydecanoic acid (Sigma, St. Louis, MO) for 10 minutes prior to 25 minutes of ischaemia and followed by 60 minute of reperfusion. The sevoflurane was delivered at 2.5% to the gas mixture via a standard Sevotec5 variable bypass vaporizer (Datex-Ohmeda, Milwaukee, WI, USA) at a final concentration of 0.4±0.02 mM. Global ischaemia was induced by shutting off all flow of the KHB perfusate to the heart [8].
NMR spectroscopy
23Na, 19F, and 31P nuclear magnetic resonance (NMR) were used to measure intracellular Na+ (Na+i), intracellular Ca++ ([Ca++]i,), and intracellular pH (pHi) and high energy phosphates, respectively [8]. In order to measure Na+i, 7.5 mM dysprosium triethylenetetraminehexaacetic acid (DyTTHA) was substituted iso-osmotically for NaCl in the perfusate and Ca++ was added to reach a perfusate concentration of 2.5 mM as measured by Ca++ electrode. In order to measure [Ca++]i, hearts were perfused for 30-40 minutes prior to the control interval with perfusate containing the acetoxymethyl ester of 5F-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (FBAPTA) at 2.5 μM. FBAPTA was then washed out of the extracellular space with control solution for 15 min before measurement of [Ca++]i. 23Na, 19F and 31P experiments were conducted using a Bruker AMX400 spectrometer (Bruker, Rheinstetten, Germany). 23Na, 19F, and 31P spectra were generated from the summed free induction decays of 1000, 1500, and 148 excitation pulses (90°, 45° and 60°) using 2K, 2K, and 4K word data files and ±4000, ±5000, and ±4000 Hz sweep widths, respectively. For all nuclei, data files were collected over 5 minute intervals. Because the NMR signal intensity reflects the time average for the interval over which data are collected, data are represented in time as corresponding to the midpoint of the appropriate 5 acquisition interval. All three nuclei were measured in separate hearts.
Na+i in mEq/kg dry weight was calculated from the calibrated area under the unshifted peak of the 23Na spectra after subtracting out the extracellular peak. At the end of the experiment, hearts were weighed wet and dried to constant weight (at least 48 hr) at 65°C to determine dry weight. [Ca++]i in nmoles/liter cell water was calculated as the product of the ratio of the areas of the Ca++-bound and Ca++-free peaks in the FBAPTA spectrum and the 500 nM Ca-FBAPTA dissociation constant. Intracellular pH was determined from the chemical shift of the inorganic phosphate (Pi) resonance [with reference to control phosphocreatine (PCr)] calibrated at 37°C. High-energy phosphates are reported as percent of control peak height.
Haemodynamic measurements
To measure left ventricular pressures, a latex balloon was filled with water and connected to a pressure transducer (Medex, Dublin, CA, USA) via a piece of PE40 tubing. The balloon was inserted into the left ventricle via the left atrial appendage through the mitral valve. The balloon volume was adjusted during the equilibration period to yield a left ventricular end diastolic pressure (LVEDP) of 3-5 mmHg. Pressures were recorded onto a computer using Powerlab (ADInstruments, Colorado Springs, CO, USA). The haemodynamic measurements included LVEDP and left ventricular developed pressure (LVDP) was defined as left ventricular end systolic pressure (LVESP) – LVEDP [8].
Creatine Kinase Analysis
The run-off from the coronary sinus was collected for the first 10 minutes of reperfusion. This was placed in aliquots and stored at -80° C until analysis could take place. The amount of CK was determined using CK kit from Sigma Diagnostics (Sigma Diagnostics, St. Louis, MO, USA) and a Shimadzu UV-VIS recording photospectrometer (Shimadzu, Columbia, MD, USA). Units are expressed as IU/ gram of dry weight [8].
Determination of Infarct Size
At the end of reperfusion, the hearts were quickly taken down from the Langendorff apparatus and sliced into 2 mm sections. The sections were immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC) staining solution and placed in a 37°C incubator for 20 minutes. Non-infarcted myocardium stains a bright red that is caused by reduction of TTC by dehydrogenases present in viable tissue. After 20 minutes, the sections were rinsed off with water and placed on transparent petri dishes. They were then scanned into a computer with Adobe Photoshop software (Adobe, San Jose, CA, USA). Standard computer planometric analysis, using NIH image 1.62 (National Institutes of Health, MD, USA), was used to determine infarct area. Infarct size was expressed by dividing the necrotic area by the total slice area of myocardium to obtain the percent necrosis [8].
Statistical Analysis
The animals were randomly assigned to each group. Data presented are mean ± SEM. Differences in data between groups were analyzed using Analysis of Variance. Two-tailed Student-Neuman-Keuls post-test was used if the Analysis of Variance was significant. A value of P < 0.05 was considered statistically significant.
Results
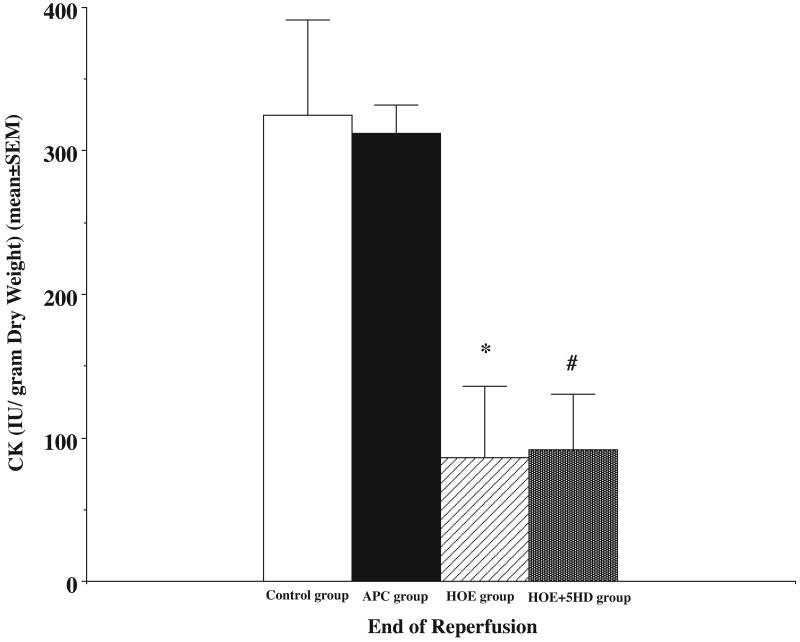
Our results in Figure 1 demonstrated that I/R caused significant myocardial injury. The infarct size was 28±4% in control group, and 31±3% in APC group which showed no statistical difference between the two groups (p>0.05). After treating the hearts with NHE inhibitor, the infarct size decreased to 17±2%. The mitochondrial KATP channel inhibitor, 5HD, did not change the myocardial protective effect of the NHE inhibition, and the infarct size in this group was 17±1% (p>0.05 compared to the HOE group). But, the infarct sizes in both groups were significantly smaller than that of the control and APC groups (p<0.05). In Figure 2, myocardial CK release (IU/gram dry weight) was measured during reperfusion. I/R caused significant CK release during reperfusion (325±66 in first 10 minutes in control group and 321±20 in APC group). There were no statistical differences between the two groups (p>0.05). After treated with HOE, the CK release was significantly decreased (86±50) compare to control (p<0.05) and 5HD did not alter the effect of NHE1 inhibition. The CK release in HOE+5HD group was 92±38 and there were no statistical differences compare to the HOE group (p>0.05).
Figure 1.
Ischaemia caused myocardial infarction (control group) and volatile anaesthetic preconditioning (APC) with sevoflurane did not decrease infarct size (APC group). Na+/H+ exchanger inhibitor, HOE 694 (HOE group), decreased the infarct size. Mitochondrial KATP channel blocker, 5-HD, did not affect the HOE effects during reperfusion in aged rat hearts. HOE+5HD: both HOE 694 and 5-HD were added to the perfusate 10 minutes prior to ischaemia. * HOE group vs. control group, P<0.05; # HOE+5HD group vs. control group, P<0.05. N = 6 in each group. Unit: % area change.
Figure 2.
Ischaemia caused a significant increase in release of myocardial creatine kinase (CK) (control group). Volatile anaesthetic preconditioning (APC) with sevoflurane did not decrease CK release (APC group). Na+/H+ exchanger inhibitor, HOE 694 (HOE group), limited the CK release. Mitochondrial KATP channel blocker, 5-HD, did not affect the HOE effects on CK during reperfusion in aged rat hearts. HOE+5HD: both HOE 694 and 5-HD were added to the perfusate 10 minutes prior to ischaemia. * HOE group vs. control group, P<0.05; # HOE+5HD group vs. control group, P<0.05. N = 6 in each group. Unit: IU/gram dry weight.
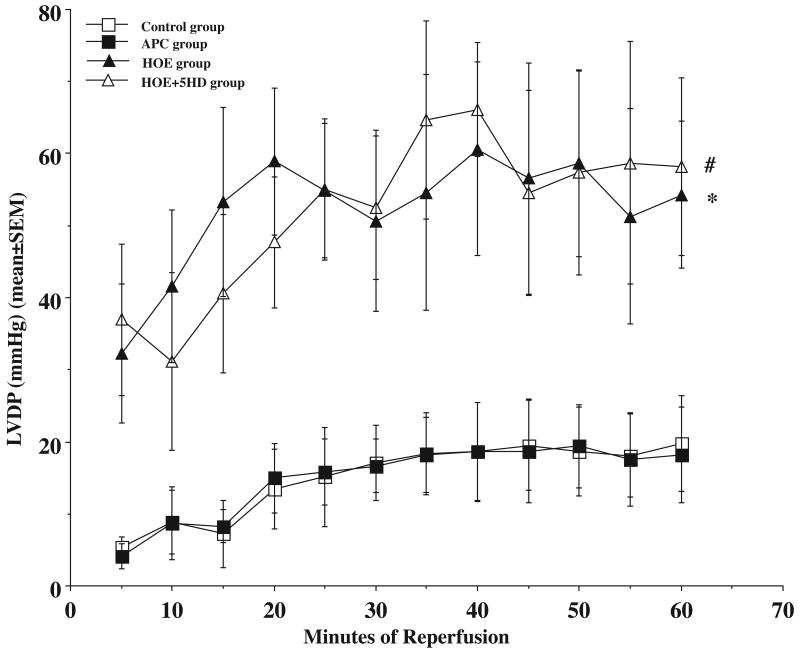
Average haemodynamic variables during the pre-ischemic period are presented in Table 1. There were no significant differences in pre-ischemic haemodynamic variables among all the 4 groups. Haemodynamic measurements following 60 minutes of reperfusion are also presented in Table 1. Figure 3 demonstrated that there were no significant differences in LVDP (mmHg) between control and APC hearts (20±6.6 vs. 18±6.7, p>0.05) during reperfusion. There was a significant difference between the HOE treated hearts (54±10) and the control hearts (p<0.05), and 5HD did not block the effect of NHE1 inhibition on myocardial protection (58±12, p>0.05) at the end of reperfusion.
Table 1. Results of LVEDP, LVDP, ATP, pH, PCr, and Pi before ischemia, at the end of ischemia, and at the end of reperfusion in four groups.
| Control | APC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEDP | LVDP | ATP | pH | PCr | Pi | LVEDP | LVDP | ATP | pH | PCr | Pi | |
| Before Ischemia | 5±1.9 | 91±15 | 103±5 | 7.11±0.02 | 99±1 | 101±1 | 4.4±1.9 | 102±17 | 98±3 | 7.12±0.04 | 102±2 | 102±2 |
| End of ischemia | N/A | N/A | 14±3 | 5.89±0.02 | 6±2 | 1082±143 | N/A | N/A | 17±7 | 5.86±0.02 | 5±1 | 832±91 |
| End of Reperfusion | 26±7 | 20±6.6 | 23±5 | 6.99±0.06 | 50±7 | 146±20 | 21±4 | 18±6.7 | 19±4 | 7.03±0.03 | 32±6 | 158±7 |
| HOE | HOE+5HD | |||||||||||
| LVEDP | LVDP | ATP | pH | PCr | Pi | LVEDP | LVDP | ATP | pH | PCr | Pi | |
| Before Ischemia | 5.6±1.9 | 94±14 | 100±3 | 7.10±0.03 | 101±2 | 103±27 | 5±0.6 | 97±12 | 103±5 | 7.08±0.04 | 100±3 | 104±6 |
| End of ischemia | N/A | N/A | 41±5* | 6.13±0.05* # | 5±4 | 744±21* | N/A | N/A | 33±3* | 5.91±0.04 | 13±1 | 630±37* |
| End of Reperfusion | 15±3* | 54±10* | 30±2* | 7.08±0.03 | 35±4 | 164±24 | 14±2* | 58±12* | 31±5* | 6.94±0.17 | 46±9 | 148±18 |
The units for LVEDP, LVDP data are mmHg. The units for ATP, PCr, and Pi expressed as % of baseline. Data are presented as mean±SEM.
APC, HOE, or HOE+5HD vs. Control,
HOE vs. HOE+5HD, P < 0.05 is considered significant. n=6 in each group.
LVEDP: left ventricular end diastolic pressure, LVDP: left ventricular develop pressure, PCr: phosphocreatine, Pi: inorganic phosphate.
Figure 3.
During reperfusion, the left ventricular develop pressure (LVDP) only recovered to about 20% of the pre-ischaemic level in control group (open square). Volatile anaesthetic preconditioning (APC) with sevoflurane did not improve the LVDP during reperfusion (closed square). Na+/H+ exchanger inhibitor, HOE 694 (HOE group), improved the LVDP (closed triangle), and mitochondrial KATP channel blocker, 5-HD, did not affect the HOE effects on LVDP during reperfusion in aged rat hearts (open triangle). * HOE group vs. control group, P<0.05; # HOE+5HD group vs. control group, P<0.05. N = 6 in each group. Unit: mmHg.
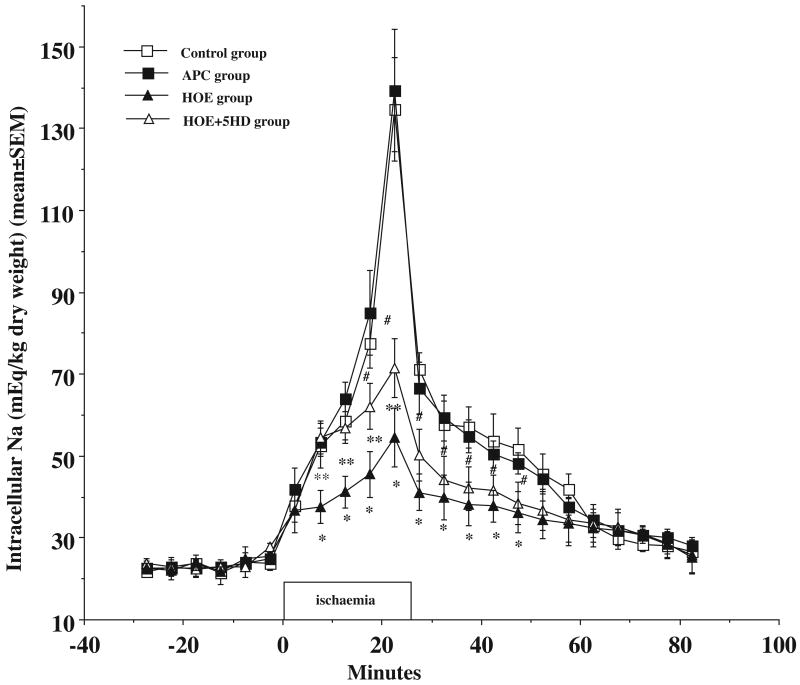
The Nai + (mEq/kg dry weight) increased from baseline 24±4 prior to ischaemia to 134±12 (p<0.05) at the end of ischaemia and recovered to 27±2 at the end of reperfusion (Fig.4). APC had no effect on the Nai+ during I/R compared to control group (P>0.05). The Nai+ rose from 25±1 to 139±14 at the end of ischaemia and returned to 28±2 at the end of reperfusion (Fig. 4). The ischaemia induced increase of Nai+ was significantly blocked by adding NHE1 inhibitor prior to ischaemia (55±7 in HOE group vs. 134±12 in control group, p<0.05) at the end of ischaemia. Although Mitochondrial KATP channel inhibitor 5HD partially blocked Na effect of HOE during ischemia (72±2), it did not block the protective effects of HOE in aged hearts during reperfusion which was significantly lower than control heart (p<0.05).
Figure 4.
Ischaemia caused an increase in intracellular Na+ (open square) and volatile anaesthetic preconditioning (APC) with sevoflurane did not limit the increase in Na+ (closed square) during ischaemia and reperfusion. Na+/H+ exchanger inhibitor, HOE 694 (closed triangle) limited the increase at the end of ischaemia and reperfusion. Mitochondrial KATP channel blocker, 5-HD (open triangle) partially blocked the HOE effect during ischaemia, but not in reperfusion in aged rat hearts. * HOE vs. control, P<0.05, #HOE+5HD vs. control, P<0.05. ** HOE group vs. HOE+5HD group, P<0.05. N = 6 in each group. Unit: mEq/kg dry weight.
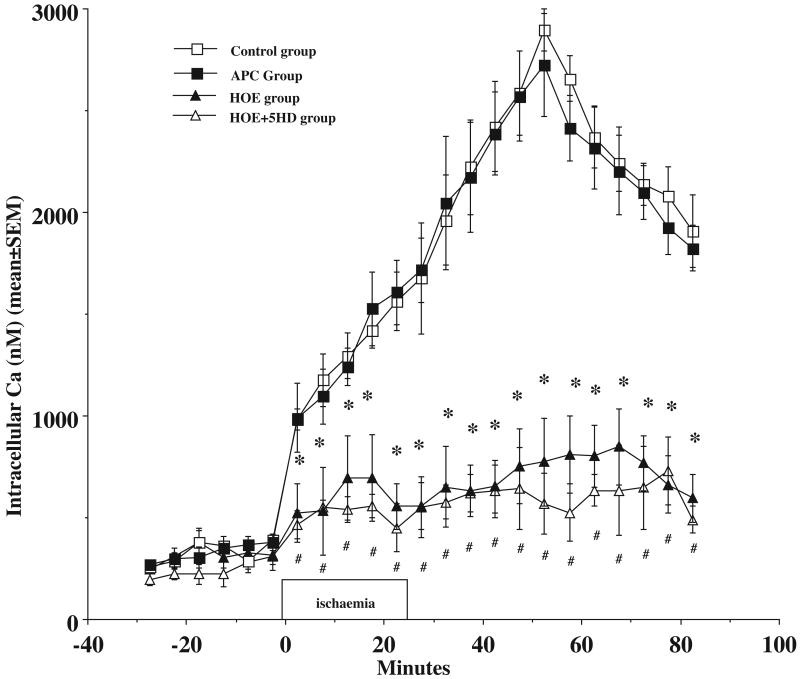
Figure 5 in this study demonstrated that ischaemia caused increases in [Ca++]i during I/R in aged myocardium and APC did not attenuate the increases in [Ca++]i. Compare to the control hearts, [Ca++]i levels were significantly lower in the HOE group at the end of ischaemia (1764±142 vs. 694±213 nM, p<0.05) and reperfusion (1905±178 vs. 595±118 nM, p<0.05). After adding 5-HD to the HOE treated hearts, the [Ca++]i levels were not different between the two groups (449±117 at the end of ischaemia and 490±67 at the end of reperfusion, p>0.05).
Figure 5.
Ischaemia caused an increase in intracellular Ca++ (open square) and volatile anaesthetic preconditioning (APC) with sevoflurane did not limit the increase in Ca++ (closed square) during ischaemia and reperfusion. Na+/H+ exchanger inhibitor, HOE 694 (closed triangle) limited the increase in of ischaemia and reperfusion. Mitochondrial KATP channel blocker, 5HD (open triangle) did not block the HOE effect during ischaemia, and reperfusion in aged rat hearts. * HOE vs. control group; # HOE+5HD vs. control group, P<0.05. N = 6 in each group. Unit: no.
In Table 1, we demonstrated that there was a significant decrease of ATP during ischaemia from baseline of 103±5% to 14±3% at the end of ischaemia (p<0.05) and there were no significant difference between control and APC hearts (17±7%, p>0.05). Pi, an ATP breakdown product, is significantly increased at the end of ischaemia (1082±143%, p<0.05) in control group and was 832±91 in APC group, but did not reach statistical difference (p>0.05). NHE1 inhibition significantly decreased the consumption of ATP to 41±5% at the end of ischaemia (p<0.05). In the HOE+5HD group, the ATP was 33±3% at the end of ischaemia which is lower than HOE group, but, it was still statistically higher than control group (p<0.05). Pi in HOE group was 744±21% (p<0.05) and was 630±37% after treated with mitochondrial KATP channel inhibitor which were significantly lower than control heart (p<0.05), but there were no statistical differences between HOE and HOE+5HD groups (p>0.05).
Ischaemia caused anaerobic metabolism resulting in a significant decrease in pHi during ischaemia period (5.89±0.02) (Table 1). Acute APC did not prevent the decrease in pHi during ischaemia (5.86±0.02, p>0.05 compared to control). After treating with NHE1 inhibitor prior to ischaemia, the decrease in pHi was significantly less (6.13±0.05, p<0.05, Table 1). As also shown in Figure 4, the mitochondrial KATP channel inhibitor 5HD did alter the pHi in NHE1 inhibitor treated hearts (5.91±0.04, p<0.05 compared to HOE group). At the end of the 60 minute reperfusion, the pHi all 4 groups were recovered to the same level.
Discussion
In the present study, NHE1 inhibition limited severity of myocardial injury and myocardium functional recovery following ischaemia and reperfusion by limiting the increase in Na+i and [Ca++] i, preserving high-energy phosphates and decreasing CK release and infarct size. However, inhibition of mitochondrial KATP channel did not modify the myocardial protective effect induced by NHE1 inhibition in aged rats. Over the past 30 years, several experimental interventions have been shown to protect the myocardium from I/R injury. The two areas that have gained most attention are NHE1 inhibition and myocardial preconditioning. NHE is the pivotal membrane protein in the maintenance of intracellular pH and ion homeostasis. The key step in NHE1 inhibition is by inhibition of Na+ entry into the cell thereby preventing subsequent cellular Ca++ overload that in turn is mediated through inhibition of Na+-coupled Ca++ influx. Intracellular Ca++ overload is identified as a major factor associated with irreversible cell damage. In contrast, mitochondrial KATP channel activation is proposed as the primary mediator for the protective mechanism of myocardial preconditioning [5,10,12-18]. Nonetheless, there is considerable interest in the possible interactions between NHE1 inhibition and KATP activation that may possibly enhance myocardial protection when both mechanisms are activated at the same time [19,20].
The selective and potent NHE1 inhibitor, HOE 694, a compound similar to cariporide (HOE 642) used in clinical trials [5], provides cardioprotective in various experimental models. It was shown to reduce infarct size, improve myocardial function, decrease the incidence of arrhythmias, and attenuate of apoptosis following ischaemia and reperfusion [15,21].
As the aging process continues, metabolic and structural differences begin to develop in senescent myocardium [11]. Several investigators have reported that the hearts of senescent animals are less tolerant to I/R injury when compared to young adult animals. These reports provided evidence of diminished anti-oxidative capability, decreased ATP content, and increased intracellular calcium in senescent hearts following I/R injury when compared to young adult hearts [8,11,22,23]. We asked the question whether NHE1 inhibition could provide the same or similar protection against I/R injury in senescent myocardium that was observed in previous studies in young adult hearts. Our results demonstrated that even in senescent myocardium, NHE1 inhibition confers cardioprotection by limiting the ischaemia induced intracellular H+ production, Na+ influx, and Ca++ overload and further leads to significant recovery of both systolic (LVDP) and diastolic (LVEDP) functions during reperfusion as demonstrated in newborn and young animal models by inhibition of NHE1 [12, 15,21,24]. There is also other evidence that Na+i overload can also independently cause mitochondrial damage [25] as increased Na+i during ischaemia induced depolarization of the mitochondrial membrane potential and depression of a mitochondrial state 3 respiration, which directly impaired the cells abilities to generate ATP. Our findings are also consistent with this observation as our results showed a limited increase in Na+i and an improvement in ATP levels at the end of ischaemia in the HOE group.
The myocardial protective effect of APC in I/R injury is well supported in several studies [4,8,26]. The proposed mechanism for this effect was activation of the KATP channel particularly in the mitochondrial membrane by the anaesthetic agent [18,27]. As a result volatile anaesthetics have been investigated as pharmacological agents for myocardial preconditioning against I/R injury in both newborn and young adults in animal studies with proven benefit [8,16]. In contrast, clinical studies conducted in patients undergoing coronary artery bypass graft (CABG) surgery yielded both positive and negative results [28-30]. As the majority of patients undergoing CABG are older, this raises the question of the efficacy of preconditioning in aged heart. Other studies, together with our current and previous studies in isolated perfused rat heart model, have shown that both APC and IPC failed to induce cardio-protection in the senescent myocardium [6-8,31]. The observed metabolic and structural differences in the aged myocardium could be one of the explanations for these results. For example, in one recent published study, the authors demonstrated that the aged myocardium had an elevated (ROS) baseline level compared to young adult hearts; the ROS level failed to further increase in response to I/R stimulation [31].
As both myocardial preconditioning and NHE1 inhibition exert potent myocardial protective effect against I/R injury [12,13,32-34], we asked the question whether there is a relationship between the NHE inhibition and the KATP channel activation that could confer additive protection to myocardium following I/R. NHE is known to exist not only in plasmatic cell membrane, but also in the inner mitochondrial membrane [35]. This may be important as during ischaemia the normal mechanism of H+ extrusion associated with electron transport stops, subsequently the mitochondrial matrix becomes overloaded with H+, which results in collapse of the H+ gradient across the mitochondrial membrane leading to the cessation of ATP synthesis [36,37]. In a recent study, the authors suggested that NHE inhibitors could exert their anti-ischaemic effect at the mitochondrial level by first favouring the opening of mitochondrial KATP channel and mitochondrial KATP channel blocker abolished the protective effect of NHE inhibition [38], and second, delaying mitochondrial matrix acidification to inhibit ATP depletion during ischaemia [37]. In a related study, the authors found that pH-mediated arteriolar dilation caused by NHE inhibition was attenuated by KATP channel blockade [39]. Our results were consistent with the previous observation as the mitochondrial KATP channel blocker, 5HD, blocked the effect of HOE 694 on intracellular pH during ischaemia when used in conjunction with the NHE1 inhibitor. However, although the intracellular acidosis was greater with 5HD blockade, we did not observe any changes in the other intracellular anion concentrations and myocardial function or infarct size changes as observed by others [38].
Although evidence indicating that the protection produced by preconditioning and NHE1 inhibition occurs via different mechanisms, controversy remains concerning the relationship between those two modes of protective interventions. Principally, controversy persists in relation to the attenuation of intracellular acidosis that occurs during ischaemia that is a hallmark of preconditioning [8,13,40-42] as the use of 5HD to block preconditioning causes intracellular pH to decrease.
There are several limitations in the present study. First, the purpose of the study was to investigate whether the mitochondrial KATP channel inhibition can affect NHE1 inhibition induced protection against I/R injury in aged rat myocardium. We only used aged animals and we made no comparisons between young and old. Second, although we are fully aware that there are other pathways involved in myocardial protection, we only investigated the pathways we outlined in this paper. Third, the NMR spectroscopy we used here can only measure one nucleus at a time; for this reason the pHi, Na+i, and [Ca++]i were measured in separated hearts.
Conclusion
In conclusion, the NHE1 inhibition reduced myocardial infarct size and CK release, and improved myocardial function recovery during reperfusion in aged rats. It prevented the myocardial overload of Na+ and Ca++ during I/R. APC with sevoflurane did not provide myocardial protection against I/R injury in this age group. Mitochondrial KATP channel antagonist, 5-HD, did not block the beneficial effects provided by NHE1 inhibition against I/R injury in aged myocardium. These findings will assist us in future research and development against myocardial I/R injury aged population.
Acknowledgments
Sources: This work was supported in part by the Foundation for Anesthesia Education Research, Mayo Clinic, Rochester, MN, USA and the UC Davis Health System Research Award, Davis, California, USA. Spectrometers were further supported by grant No. RR08206 from the NIH, Bethesda, Maryland.
References
- 1.Lakatta EC, Yin FC. Myocardial aging: functional alterations and related celluar mechanisms. Am J Physiol. 1982;242:H927–HH941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- 2.Mariani J, Ou R, Bailey M, et al. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120(4):660–7. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2002;39(4):569–75. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 4.An J, Varadarajan SG, Novalija E, et al. Ischemic and anesthetic preconditioning reduces cytosolic [Ca] and improves Ca responses in intact hearts. Am J Physiol. 2001;281:H1508–23. doi: 10.1152/ajpheart.2001.281.4.H1508. [DOI] [PubMed] [Google Scholar]
- 5.Mentzer RM, Jr, Bartels C, Bolli R, et al. EXPEDITION Study Investigators. Sodium-Hydrogen Exchange Inhibition by Cariporide to Reduce the Risk of Ischemic Cardiac Events in Patients Undergoing Coronary Artery Bypass Grafting: Results of the EXPEDITION Study. Ann Thorac Surg. 2008 Apr;85(4):1261–70. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Fenton RA, Dickson EW, Meyer TE, et al. Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–5. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- 7.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol. 2001;281:H1630–6. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 8.Sniecinski R, Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in the isolated rat myocardium. Anesthesiology. 2004;100:589–597. doi: 10.1097/00000542-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Heinen A, Huhn R, Smeele KM, et al. Helium-induced preconditioning in young and old rat heart: impact of mitochondrial Ca(2+) -sensitive potassium channel activation. Anesthesiology. 2008 Nov;109(5):830–6. doi: 10.1097/ALN.0b013e3181895aa0. [DOI] [PubMed] [Google Scholar]
- 10.Simm A, Friedrich I, Scheubel RJ, et al. Age dependency of the cariporide-mediated cardio-protection after simulated ischemia in isolated human atrial heart muscles. Exp Gerontol. 2008 Jul;43(7):691–9. doi: 10.1016/j.exger.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Besse S, Tanguy S, Boucher F, et al. Protection of endothelial-derived vasorelaxation with cariporide, a sodium-proton exchanger inhibitor, after prolonged hypoxia and hypoxia-reoxygenation: effect of age. Eur J Pharmacol. 2006 Feb 15;531(1-3):187–93. doi: 10.1016/j.ejphar.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Cala PM, Anderson SE. Ethylisopropylamiloride diminishes changes in intracellular Na, Ca and pH in ischemic newborn myocardium. J Mol Cell Cardiol. 1997;29:2077–2086. doi: 10.1006/jmcc.1997.0442. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Cala PM, Anderson SE. Ischemic preconditioning: Effects on pH, Na and Ca in newborn rabbit hearts during ischemia/reperfusion. J Mol Cell Cardiol. 1998;30:685–697. doi: 10.1006/jmcc.1997.0636. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SE, Liu H, Ho HS, et al. Age-related differences in Na+-dependent Ca2+ accumulation in rabbit hearts exposed to hypoxia and acidification. Am J Physiol. 2003;284:C1123–32. doi: 10.1152/ajpcell.00148.2002. [DOI] [PubMed] [Google Scholar]
- 15.Linz W, Albus U, Crause P, et al. Dose-dependent reduction of myocardial infarct mass in rabbits by the NHE-1 inhibitor cariporide (HOE 642) Clin Exp Hypertens. 1998;20(7):733–49. doi: 10.3109/10641969809052116. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Wang L, Schaefer S. Sevoflurane preconditioning limits intracellular/mitochondrial Ca2+ in ischemic newborn myocardium. Anesthesia and Analgesia. 2005;101(2):349–55. doi: 10.1213/01.ANE.0000154197.24763.EC. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Sato T, O'Rourke B, et al. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection. Circulation. 1998 Jun 23;97(24):2463–9. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 18.Pagel PS, Krolikowski JG, Pratt PF, Jr, et al. Reactive oxygen species and mitochondrial adenosine triphosphate-regulated potassium channels mediate helium-induced preconditioning against myocardial infarction in vivo. J Cardiothorac Vasc Anesth. 2008 Aug;22(4):554–9. doi: 10.1053/j.jvca.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugge E, Ytrehus K. Inhibition of sodium-hydrogen exchange reduces infarct size in the isolated rat heart-a protective additive to ischemic preconditioning. Cardiovasc Res. 1995;29:269–274. [PubMed] [Google Scholar]
- 20.Shipolini AR, Yokoyama H, Galiñanes M, et al. Na+/H+ exchanger activity does not contribute to protection by ischemic preconditioning in the isolated rat heart. Circulation. 1997;96:3617–3625. doi: 10.1161/01.cir.96.10.3617. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama A, Aye NN, Sawada N, et al. Cariporide, a highly selective Na+/H+ exchange inhibitor, suppresses the reperfusion-induced lethal arrhythmias and “overshoot” phenomenon of creatine phosphate in situ rat heart. J Cardiovasc Pharmacol. 1999;33:116–121. doi: 10.1097/00005344-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Tani M, Suganuma Y, Hasegawa H, et al. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29(11):3081–9. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura K, Tani M, Hasegawa H, et al. Very low dose of the Na(+)/Ca(2+) exchange inhibitor, KB-R7943, protects ischemic reperfused aged Fischer 344 rat hearts: considerable strain difference in the sensitivity to KB-R7943. Cardiovasc Res. 2001;52(3):397–406. doi: 10.1016/s0008-6363(01)00409-6. [DOI] [PubMed] [Google Scholar]
- 24.Cun L, Ronghua Z, Bin L, et al. Preconditioning with Na+/H+ exchange inhibitor HOE642 reduces calcium overload and exhibits marked protection on immature rabbit hearts. ASAIO J. 2007 Nov-Dec;53(6):762–5. doi: 10.1097/MAT.0b013e31815766e3. [DOI] [PubMed] [Google Scholar]
- 25.Iwai T, Tanonaka K, Inoue R, et al. Mitochondrial damage during ischemia determines post-ischemic contractile dysfunction in perfused rat heart. J Mol Cell Cardiol. 2002;34(7):725–38. doi: 10.1006/jmcc.2002.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kersten JR, Schmeling TJ, Pagel PS, et al. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Zaugg M, Lucchinetti E, Spahn DR, et al. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Belhomme D, Peynet J, Louzy M, et al. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation. 1999;100(suppl II):II-340–4. doi: 10.1161/01.cir.100.suppl_2.ii-340. [DOI] [PubMed] [Google Scholar]
- 29.Haroun-Bizri S, Khoury SS, Chehab IR, et al. Does isoflurane optimize myocardial protection during cardiopulmonary bypass? J Cardiothorac Vasc Anesth. 2001;15:418–21. doi: 10.1053/jcan.2001.24954. [DOI] [PubMed] [Google Scholar]
- 30.DeHert SG, Broecke PW, Mertens E, et al. Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology. 2002;97:42–9. doi: 10.1097/00000542-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Sergeev P, da Silva R, Lucchinetti E, et al. Trigger-dependent gene expression profiles in cardiac preconditioning: evidence for distinct genetic programs in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100(3):474–88. doi: 10.1097/00000542-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen LT, Rebecchi MJ, Moore LC, et al. Attenuation of isoflurane-induced preconditioning and reactive oxygen species production in the senescent rat heart. Anesth Analg. 2008;107:776–78246. doi: 10.1213/ane.0b013e318180419d. [DOI] [PubMed] [Google Scholar]
- 33.Avkiran M. Protection of the ischaemic myocardium by Na+/H+ exchange inhibitors: potential mechanisms of action. Basic Res Cardiol. 2001;96:306–311. doi: 10.1007/s003950170037. [DOI] [PubMed] [Google Scholar]
- 34.Haist JV, Hirst CN, Karmazyn M. Effective protection by NHE-1 inhibition in ischemic and reperfused heart under preconditioning blockade. Am J Physiol Heart Circ Physiol. 2003;284(3):H798–803. doi: 10.1152/ajpheart.00659.2002. [DOI] [PubMed] [Google Scholar]
- 35.Numata M, Petrecca K, Lake N, et al. Identification of a mitochondrial Na+/H+ exchanger. J Biol Chem. 1998;273(12):6951–9. doi: 10.1074/jbc.273.12.6951. [DOI] [PubMed] [Google Scholar]
- 36.Di Lisa F, Bernardi P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem. 1998;184(1-2):379–91. [PubMed] [Google Scholar]
- 37.Ruiz-Meana M, Garcia-Dorado D, Pina P, et al. Cariporide preserves mitochondrial proton gradient and delays ATP depletion in cardiomyocytes during ischemic conditions. Am J Physiol Heart Circ Physiol. 2003;285(3):H999–1006. doi: 10.1152/ajpheart.00035.2003. [DOI] [PubMed] [Google Scholar]
- 38.Miura T, Liu Y, Goto M, et al. ATP-sensitive K+ channels play a role in cardioprotection by Na+-H+ exchange inhibition against ischemia/reperfusion injury. J Am Coll Cardiol. 2001;37(3):957–63. doi: 10.1016/s0735-1097(00)01183-9. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblum WI, Wei EP, Kontos HA. Vasodilation of brain surface arterioles by blockade of Na-H+ antiport and its inhibition by inhibitors of KATP channel openers. Brain Res. 2004;1005(1-2):77–83. doi: 10.1016/j.brainres.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 40.Gabel SA, Cross HR, London RE, et al. Decreased intracellular pH is not due to increased H+ extrusion in preconditioned rat hearts. Am J Physiol. 1997;273(5 Pt 2):H2257–62. doi: 10.1152/ajpheart.1997.273.5.H2257. [DOI] [PubMed] [Google Scholar]
- 41.Lutz M, Liu H. Inhaled sevoflurane produces better delayed myocardial protection at 48 versus 24 hours after exposure. Anesthesia and Analgesia. 2006;102:984–90. doi: 10.1213/01.ane.0000198568.79079.4c. [DOI] [PubMed] [Google Scholar]
- 42.Deyhimy DI, Fleming NW, Brodkin IG, et al. Anesthetic preconditioning combined with post conditioning offers no additional benefit over preconditioning or postconditioning alone. Anesthesia and Analgesia. 2007;105(2):316–24. doi: 10.1213/01.ane.0000267524.71445.e7. [DOI] [PubMed] [Google Scholar]