Abstract
Background
Observational studies, including recent large cohort studies which were unavailable for prior meta-analysis, have suggested an association between migraine headache and ischemic stroke. We performed an updated meta-analysis to quantitatively summarize the strength of association between migraine and ischemic stroke risk.
Methods
We systematically searched electronic databases, including MEDLINE and EMBASE, through February 2009 for studies of human subjects in the English language. Study selection using a priori selection criteria, data extraction, and assessment of study quality were conducted independently by reviewer pairs using standardized forms.
Results
Twenty-one (60%) of 35 studies met the selection criteria, for a total of 622,381 participants (13 case-control, 8 cohort studies) included in the meta-analysis. The pooled adjusted odds ratio of ischemic stroke comparing migraineurs to non-migraineurs using a random effects model was 2.30 (95% confidence interval [CI], 1.91-2.76). The pooled adjusted effect estimates for studies that reported relative risks and hazard ratios, respectively, were 2.41 (95% CI, 1.81-3.20) and 1.52 (95% CI, 0.99-2.35). The overall pooled effect estimate was 2.04 (95% CI, 1.72-2.43). Results were robust to sensitivity analyses excluding lower quality studies.
Conclusions
Migraine is associated with increased ischemic stroke risk. These findings underscore the importance of identifying high-risk migraineurs with other modifiable stroke risk factors. Future studies of the effect of migraine treatment and modifiable risk factor reduction on stroke risk in migraineurs are warranted.
Keywords: meta-analysis, stroke, cerebral ischemia, risk factors, epidemiology
BACKGROUND
Stroke is the second highest cause of disability in developed countries and the second most common cause of death globally, surpassed only by coronary heart disease.1 A recent review of population-based studies of stroke incidence and mortality indicates that the burden of stroke is likely to rise, primarily due to the advancing age of the population and disease-promoting lifestyle factors.2 Migraine headache is also associated with significant morbidity. Migraine occurs in seventeen percent of women and six percent of men each year and can be incapacitating.3,4
Migraine has been proposed as an ischemic stroke risk factor in addition to in addition to traditional risk factors such as atherosclerosis and atrial fibrillation.5 Several prior systematic reviews have reported an increased risk of stroke in certain migraineur populations.6,7 Ischemic stroke accounts for over eighty percent of all strokes,8 and migraine is a potentially modifiable risk factor. Therefore, better understanding of the association between migraine and ischemic stroke is important.
Since the reporting of two prior systematic reviews of predominantly case-control studies,6,7 four large cohort studies9-12 containing a combined total of more than 300,000 patients have been published. Here we provide an updated systematic review and meta-analysis, utilizing the most recent Cochrane Collaboration guidelines for systematic reviews,13 to determine the strength of the association of migraine and ischemic stroke.
METHODS
Search Strategy
We performed a systematic literature search of MEDLINE (using PubMed) and EMBASE for relevant published reports from the beginning of indexing for each database through February 2009. We also searched the National Library of Medicine's Health Services Research Projects in Progress, National Institute of Health's clinical trials registry, World Health Organization's International Clinical Trials Registry Platform, Cochrane Central Register of Controlled Trials, Open System for Information on Grey Literature in Europe, and the New York Academy of Medicine Grey Literature through February 2009 for unpublished reports. PubMed was searched using the following combination of exploded Medical Subject Heading (MeSH) terms and text words: [“migraine disorders” or “migrain*” in all search fields] and [“cerebrovascular disorders”, within which the term “stroke” is fully embedded]. The EMBASE search was conducted using the following combination of exploded terms and synonyms for terms: [“migraine” or “migraine*”] and [“stroke” or “brain infarction” or “brain ischemia” or “cerebrovascular accident”]. The PubMed and EMBASE searches were limited to English language studies in human subjects, and the EMBASE search was additionally limited to studies that had available abstracts. As we focused on original studies, review articles in both searches were excluded. Databases of unpublished studies were searched using simple keywords “migraine” and “stroke”. After retrieval of articles from the search, the reference lists of selected articles were checked for other potentially relevant articles.
Study Selection
Pairs of reviewers independently evaluated articles for selection criteria using article titles, abstracts, and full texts. Pre-specified selection criteria included: 1) inclusion of studies with case-control or cohort study design; 2) inclusion of studies with reported or extractable adjusted quantitative estimates of the risk of ischemic stroke in migraineurs compared to non-migraineurs; 3) exclusion of studies of transient stroke-like syndromes only, concurrent ischemic stroke and migraine (migrainous infarctions), or silent infarcts, in which the temporal relationship between migraine and stroke is difficult to determine; 4) exclusion of studies in which stroke outcomes were defined as mixed (e.g. hemorrhagic and ischemic stroke); and 5) exclusion of studies of rare genetic syndromes characterized by both migraine and stroke14,15 or of pregnant patients; and 6) exclusion of studies not in the English language. In cases where an article was based on overlapping data from the same cohort and reported the same type of effect estimate, we selected the largest and most complete article from each cohort to avoid duplicate inclusion of data. Articles that did not have available full-text (for example, meeting abstracts with no existing full article) were excluded.
Based on the pre-specified selection criteria, all studies that were included in the prior meta-analysis by Etminan et al7 were included in the present study with the exception of two studies that did not meet our inclusion criteria.16,17 Nine additional studies,9-12,18-22 which were either not captured in the Etminan study7 or were subsequently published, were included in the present study.
Data Extraction
Pairs of reviewers independently abstracted data and information on study quality from eligible articles using standardized abstraction tables. Study quality was assessed according to published guidelines for assessing bias in observational studies.13,23-25 Valid definitions of migraine and stroke included use of the International Headache Society's Headache Classification26 and Neurological Disorders and Stroke (NINDS) classification27 or the Acute Ischemic Cerebrovascular Syndrome (AICS) classification,28 respectively, or reasonable variations on these accepted definitions. Although infrequent, disagreement during the abstraction process was resolved by consensus discussion between all study authors.
Data Synthesis
Odds ratios (OR), relative risks (RR), hazard ratios (HR), and incidence rate ratios (IRR) were used to estimate effect sizes. To estimate overall effect sizes, each natural log effect was weighted by the inverse of its variance, and the weighted natural log effect estimate summed across samples and then divided by the sum of the weights.13
In accordance with the Cochrane Collaboration guidelines for systematic reviews, clinical, methodological, and statistical heterogeneity of included studies was assessed.13 Clinical heterogeneity was examined by determining whether studies addressed similar populations, exposures, and outcomes, and methodological heterogeneity was addressed by comparing methodology and quality across studies. Statistical heterogeneity was assessed using the I2 statistic to quantify the proportion of variability in effect estimates due to heterogeneity between studies versus sampling error within studies. I2 values greater than 50% were considered to denote substantial heterogeneity.13
For each effect type of estimate, studies without substantial heterogeneity were pooled using a random effects model. A random effects model was chosen because of the high likelihood of between-study variance in observational studies. An overall pooled effect estimate across different effect estimate types was also computed for comparison. A priori subgroup analyses by type of migraine (with aura versus without aura) and gender, factors reported to be associated with ischemic stroke risk,7 were performed. These subgroup analyses were also used to investigate heterogeneity, if present.
The study team chose to pool adjusted rather than crude measures of effect given the significant threat of confounding to the validity of unadjusted results of observational studies. However, recognizing that different observational studies may address confounding and other sources of bias differently, a sensitivity analysis was performed to quantify the effect on summary results of including only studies with a low risk of bias. Low risk of bias studies were defined as those with poor methodological quality in fewer than three areas in the standardized study quality abstraction tables. Biases whose existences were deemed by consensus to be uncertain in particular studies were not included in the assessment of low risk of bias studies. We also examined the degree to which excluding single studies, one by one, influenced summary results. Finally, the possibility of publication bias was assessed by inspecting funnel plots. All statistical analyses were performed using Stata version 9.2 (StataCorp, College Station, Texas).
RESULTS
Literature Search
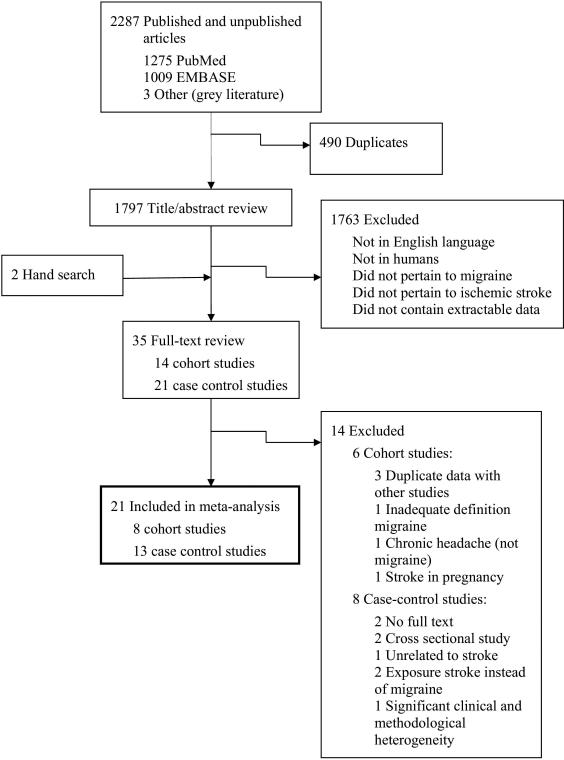
The search strategy retrieved 2,287 citations: 1,275 from PubMed, 1,009 from EMBASE, and 3 from the grey literature (Figure 1). Hand searching of bibliographic references identified 2 additional articles, leaving 1,799 unique articles for screening of titles or abstracts. Of 35 articles evaluated by full-text review, 21 studies were eligible for final inclusion in the meta-analysis.
Figure 1.
Selection Process for Study Inclusion in the Meta-Analysis.
Study Characteristics
Characteristics of the 21 selected studies are shown in Tables 1A and 1B.9-12,18-22,29-40 There were 13 case-control (Table 1A) and 8 cohort studies (Table 1B). The studies were drawn from developed countries and were published between 1975 and 2007. Sample sizes of case-control studies ranged from about 250 to 4,500, and sample sizes of cohort studies ranged from about 12,000 to 260,000, for a total of 622,381 participants in the meta-analysis. Two studies included only men,11,30 one study included both men and women but only reported a measure of association for men,34 and nine studies included only women.10,18,20,29,32,33,37,38,40 Most studies were of middle-aged adults, with average ages of participants in the range of 30-50 years.
Table 1A.
Summary of 13 Case-control Studies in the Meta-analysis
| Effect Estimate of Migraine (95% CI) |
Total number: Number with migraine |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Country | Age, mean (range), y | Female, % | Sources of Cases/Controls | Type of Effect Estimate | Any | With Aura | Without Aura | Participants with stroke | Participants without stroke |
| Carolei et al,31 1996 | Italy | Cases: 36 Controls: 36 | 47 | Hospital Cases/Hospital and Population Controls | OR | 1.7 (1.1,2.8)† 1.9 (1.1,3.1)* | 8.60 (1.0,75.0)* | 1.0 (0.5,2.0)* | 308:46 | 591:54 |
| Chang et al,32 1999 | Five European Countries (WHO Collaboration) | Cases: 36 Controls: 36 (20-44) | 100 | Hospital Cases/Hospital Controls | OR | 3.54 (1.30,9.61)* | 3.81 (1.26,11.5)* | 2.97 (0.66,13.5)* | 86:26 | 220:26 |
| Collaborative Group,29 1975 | US | N/A(15-44) | 100 | Hospital Cases/Hospital, Neighborhood Controls | OR | 2.0 (1.2,3.3)* | N/A | N/A | 140:48 | 451:106 |
| Donaghy et al,33 2002 | Five European Countries (WHO Collaboration) | Cases: 36 Controls: 36 (20-44) | 100 | Hospital Cases/Hospital Controls | OR | 1.9 (0.48,7.43)* | N/A | N/A | 86:26 | 214:26 |
| Haapaniemi et al,34 1997 | Finland | Male cases: 49 Female cases: 45 Male and female controls: 43 (16-60) | 31 | Hospital Cases/Hospital Controls | RR | Men: 2.12 (1.05,2.95)* | N/A | N/A | Total: 506:86 Men: 366:43 | Total: 345:42 Men: 219:14 |
| Henrich et al,35 1989 | US | Cases: 58 Controls: 56 | 37 | Hospital Cases/Hospital Controls | OR | 1.8 (0.9,3.6)* | 2.6 (1.1,6.6)* | 1.30 (0.90,3.6)* | 89:17 | 178:20 |
| Lidegaard et al,37 1995 | Denmark | N/A (15-44) | 100 | Registry of Hospitals/National Register | OR | 2.9 (N/A, p<0.01)† 2.8 (N/A, P<0.01)* | N/A | N/A | 497:64 | 1370:66 |
| Lidegaard et al,20 2002 | Denmark | N/A (15-44) | 100 | Registry of Hospitals/National Register | OR | 3.2 (2.5,4.2)* | N/A | N/A | 626:107 | 4054:258 |
| McLellan et al,18 2007 | US | 38 (15-49) | 100 | Hospital Cases/Community Controls | OR | N/A | 1.3 (0.9,1.8)* | N/A | 386:145 with aura (35 without aura) | 614:175 with aura (79 without aura) |
| Naess et al,19 2004 | Norway | N/A (15-49) | 41 | Hospital Cases/County Controls | OR | 1.7 (0.9,3.2)* | N/A | N/A | 187:33 | 217:25 |
| Nightingale et al,38 2004 | UK | N/A (15-49) | 100 | General Practitioner Database Cases/General Practitioner Database Controls | OR | 2.35 (1.29,4.30)† 2.33 (1.04,5.21)* | N/A | N/A | 190:16 | 1129:44 |
| Tzourio et al,39 1993 | France | Male cases: 56 Female cases: 57 Male and female controls: 56 (18-80) | 35 | Hospital Cases/Hospital Controls | OR | 1.3 (0.8,2.3)* | 1.3 (0.5,3.8)* | 0.8 (0.4,1.5)* | 212:41 (9 with aura) | 212:34 (7 with aura) |
| Tzourio et al,40 1995 | France | Cases: 36 Controls: 35 | 100 | Hospital Cases/Hospital Controls | OR | 3.5 (1.8,6.4)* | 6.2 (2.1,18)* | 3.0 (1.5,5.8)* | 72:43 (10 with aura, 33 without aura) | 173:52 (10 with aura, 42 without aura) |
Abbreviations: CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio; MI, myocardial infarction; N/A, not available; OR, odds ratio; RR, relative risk; UK, United Kingdom; US, United States; WHO, World Health Organization.
Adjusted
Unadjusted
Table 1B.
Summary of 8 Cohort Studies in the Meta-analysis
| Effect Estimate of Migraine (95% CI) |
Total No.: No. with stroke |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Country/Cohort | Age, mean (range), y | Female, % | Follow-up, mean (max), mo. unless specified | Type of Effect Estimate | Any | With Aura | Without Aura | With migraine | Without migraine |
| Becker et al,12 2007 | UK/General Practice Research Database | N/A (<79) | 72 | N/A | RR | 2.85 (1.88,4.30)* | N/A | N/A | 51688:N/A | 51688: N/A |
| Buring et al,30 1995 | US/Physicians’ Health Study | 53 (40-84) | 0 | 60 (77) | RR | 1.98 (1.20,3.28)† 2.00 (1.10,3.64)* | N/A | N/A | 1479:17 | 20481:154 |
| Hall et al,9 2004 | UK/General Practice Research Database | N/A (0 – over 70) | 74 | Migraine: 36 No migraine: 33 | HR | 2.49 (1.62,3.83)* | N/A | N/A | 63575:71 | 77239:31 |
| Kurth et al,10 2005 | US/Women's Health Study | No migraine: 55 Migraine with and without aura: 53 | 100 | 9 years; 353,170 person-years | HR | 1.31 (0.94,1.83)† 1.36 (0.97,1.92)* | 1.72 (1.11,2.67)† 1.73 (1.10,2.71)* | 1.02 (0.64,1.63)† 1.11 (0.69,1.78)* | 5173:41 (2059:22 with aura, 3114:19 without aura) | 32425:252 |
| Kurth et al,11 2007 | US/Physicians’ Health Study | No migraine: 58 Migraine: 57 (40-84) | 0 | 15.7 years; 316,076 person-years | HR | 1.09 (0.82,1.44)† 1.12 (0.84,1.50)* | N/A | N/A | 1449:51 | 18635:699 |
| Merikangas et al,36 1997 | US/National Health and Nutrition Examination Survey | N/A (25-74) | 60 | N/A | OR | 2.1 (1.5,2.9)‡ | N/A | N/A | 1108:46 | 10982:375 |
| Stang et al,21 2005 | US/Atherosclerosis Risk In Communities Study | 60 (45-64) | 56 | N/A | OR | N/A | 2.68 (1.58,4.57)§ 2.81 (1.60,4.92)* | 0.79 (0.40,1.55)§ 0.82 (0.39,1.69)* | 1015:N/A (345:N/A with aura, 670:N/A without aura) | 11735 |
| Velentgas et al,22 2004 | US/United Health Care | Migraine: 38 No migraine: 38 | 76 | 1.4 years, 353, 190 total person-years | IRR | 1.67 (1.31-2.13)* | N/A | N/A | 130,411: 216 | 130,411: 98 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio; N/A, not available; OR, odds ratio; RR, relative risk; UK, United Kingdom; US, United States.
Adjusted
Unadjusted
Adjusted for age and gender only
Adjusted for age, gender, and race only
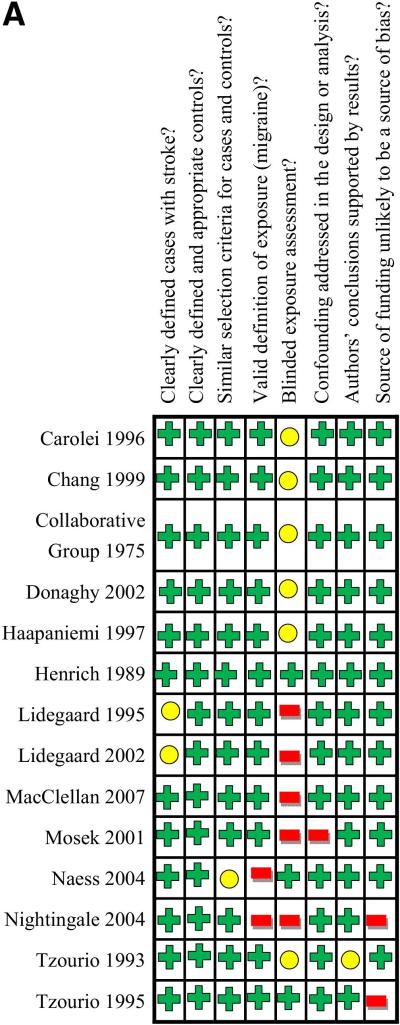
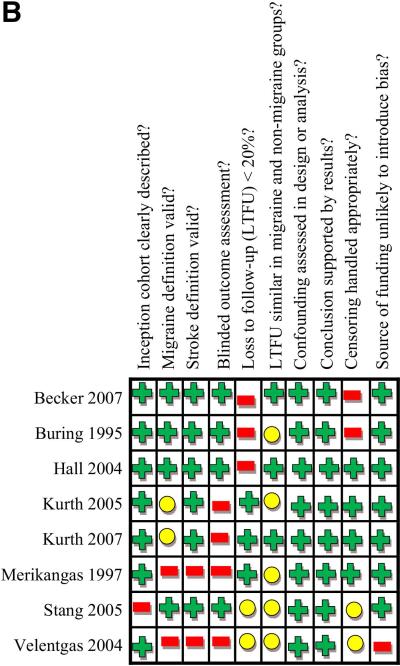
Study quality is summarized in Figures 2A and 2B. Study quality was generally good in case-control studies (Figure 2A) and moderate in cohort studies (Figure 2B). All studies addressed the potential confounder of age in effect estimates, and all studies except the Mosek et al41 study addressed gender. Some studies addressed potential confounders of hypertension (19 studies), smoking (16 studies), oral contraceptive use (10 studies), cholesterol (9 studies), cardiac disease (8 studies), family history of migraine or stroke (3 studies), and postmenopausal hormone therapy (2 studies) (Table 2).
Figure 2A. Methodological Quality Summary for 14 Case-Control Studies.
Colors in table correspond to reviewers’ consensus answers to questions at the top of the figure for each study, with green indciating “yes”, yellow indicating “uncertain”, and red indicating “no”.
Figure 2B. Methodological Quality Summary for 8 Cohort Studies.
Colors in table correspond to reviewers’ consensus answers to questions at the top of the figure for each study, with green indciating “yes”, yellow indicating “uncertain”, and red indicating “no”. LTFU indicates loss to follow-up.
Table 2.
Confounding Factors and Methods for Addressing Confounders
| Source | Study Design | Confounders Assessed | Method of Addressing Confounders |
|---|---|---|---|
| Carolei et al,31 1996 | Case-Control | Age, gender, hypertension, smoking, cholesterol, diabetes mellitus, obesity, OC use, alcohol, residence | Matching (age, gender, residence), Conditional Logistic Regression |
| Chang et al,32 1999 | Case-Control | Age, hypertension, education, smoking, family history of migraine, alcohol consumption, social class, admission time | Matching (age, admission time), Conditional Logistic Regression |
| Collaborative Group,29 1975 | Case-Control | Age, OC use, race, source of control group | Matching (age, race) Stratification (OC use) |
| Donaghy et al,33 2002 | Case-Control | Age, smoking, hypertension, family history, alcohol use, education, social class, OC use, hospital, date of hospital admission | Matching (age, hospital, date of hospital admission), Conditional Logistic Regression |
| Haapaniemi et al,34 1997 | Case-Control | Age, gender, smoking, hypertension, cardiac disease, diabetes mellitus, alcohol, BMI, cholesterol, day of onset of symptoms, acuity of disease | Matching (day of onset of symptoms, acuity of disease), Stratification (gender), Multiple Logistic Regression |
| Henrich et al,35 1989 | Case-Control | Age, gender, race, hypertension, diabetes mellitus, smoking, date of hospital discharge | Matching (gender, race, age, date of hospital discharge), Multiple Logistic Regression |
| Lidegaard et al,37 1995 | Case-Control | Age, hypertension, diabetes mellitus, pregnancy, prior thromboembolic disease, OC use | Matching (age), Block Recursive Graphical Log Linear Regression |
| Lidegaard et al,20 2002 | Case-Control | Age, hypertension, diabetes mellitus, cardiac disease, family history of VTE/stroke/cardiac disease, smoking, education, cholesterol, hypercoagulable state, year, OC use | Matching (age, year), Conditional Logistic Regression |
| McLellan et al,18 2007 | Case-Control | Age, race, hypertension, diabetes mellitus, geographic region, smoking, cardiac disease, OC use, and study period | Matching (age, geographic region, race), Multiple Logistic Regression |
| Mosek et al,41 2001 | Case-Control | Age | Matching (age) |
| Naess et al,19 2004 | Case-Control | Age, gender, hypertension, cardiac disease, smoking | Matching (age, gender), Multiple Logistic Regression |
| Nightingale et al,38 2004 | Case-Control | Age, hypertension, alcohol intake, smoking status, cardiac disease, history of VTE, OC use, diabetes mellitus, location | Matching (age, location), Conditional Logistic Regression |
| Tzourio et al,39 1993 | Case-Control | Age, gender, hypertension | Matching (age, gender, hypertension), Multiple Logistic Regression |
| Tzourio et al,40 1995 | Case-Control | Age, hypertension, OC use, smoking, year | Matching (hospital, year), Multiple Logistic Regression |
| Becker et al,12 2007 | Cohort | Age, gender, smoking, BMI, diabetes mellitus, hypertension, cholesterol, location, year | Matching (age, gender, location, year), Conditional Logistic Regression |
| Buring et al,30 1995 | Cohort | Age, treatment, smoking, hypertension, cholesterol, diabetes mellitus, cardiac disease (angina), BMI, parental history of MI, alcohol, exercise frequency | Cox Regression |
| Hall et al,9 2004 | Cohort | Age, gender, hypertension, diabetes mellitus, cardiac disease, obesity, cholesterol, OC use, smoking status | Stratification (age, gender), Cox Proportional Hazards Regression |
| Kurth et al,10 2005 | Cohort | Age, hypertension, menopausal status, history of OC, alcohol, randomized aspirin assignment, exercise, BMI, smoking, postmenopausal hormone therapy, diabetes mellitus, cholesterol | Cox Proportional Hazards Regression |
| Kurth et al,11 2007 | Cohort | Age, hypertension, diabetes mellitus, smoking, exercise, BMI, alcohol, cholesterol, parental history of premature MI, randomized treatment assignments | Cox Proportional Hazards Regression |
| Merikangas et al,36 1997 | Cohort | Age, gender | Multiple Logistic Regression |
| Stang et al,21 2005 | Cohort | Age, gender, race, parental history of migraine, smoking status, pack-years of smoking, diabetes mellitus, regular aspirin and NSAID use, hypertension medication use, systolic blood pressure, cholesterol | Multiple Logistic Regression |
| Velentgas et al,22 2004 | Cohort | Age, gender, year of cohort entry, cardiac disease, cerebrovascular disease, peripheral arterial disease, diabetes mellitus, hypertension, lipids, OC use, postmenopausal hormone therapy, health plan | Matching (age, gender, health plan), Poisson Regression |
Abbreviations: BMI, body mass index; MI, myocardial infarction; N/A, not available; NSAID, non-steroidal anti-inflammatory drug; OC, oral contraceptives; UK, United Kingdom; US, United States; VTE, venous thromboembolism
The case-control study by Mosek et al41 was not included in the meta-analysis because it was determined to have significant clinical and methodological heterogeneity. The Mosek et al study focused on a substantially older population than the other studies and, although age-matched, failed to adjust for important potential confounders, including gender and co-morbidities associated with stroke that are known to be prevalent in older age groups.
Risk of Ischemic Stroke in Migraineurs Compared With Non-Migraineurs
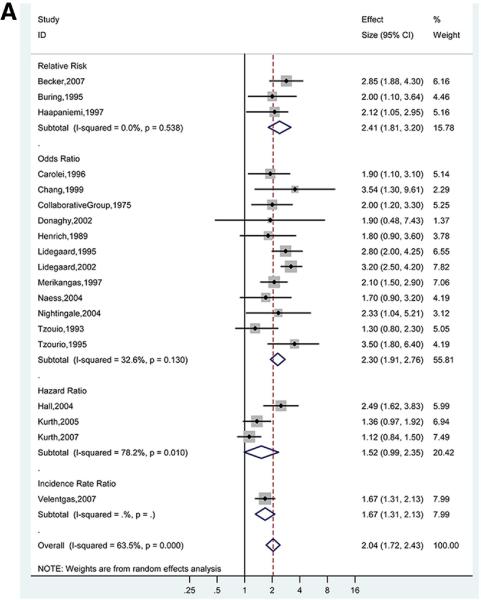
For the association between any migraine and ischemic stroke, the pooled adjusted OR (12 studies) was 2.30 (95% CI, 1.91-2.76), with evidence of low to moderate statistical heterogeneity (I2 =32.6%; p for χ2 test of heterogeneity =0.13) (Figure 3A).19,20,29,31-33,35-40 For the 3 studies that presented RRs, the pooled adjusted RR was 2.41 (95% CI, 1.81-3.20), with evidence of low statistical heterogeneity (I2=0.0%; p=0.54).12,30,34 The pooled adjusted HR (3 studies) was 1.52 (95% CI, 0.99-2.35), with high statistical heterogeneity (I2 =78.2%; p =0.01).9-11 The high degree of heterogeneity in studies reporting HRs was likely driven by differences in study population (100% men11 versus 100% women10 in studies by Kurth et al versus 74% women in the Hall et al study9 ). The overall pooled adjusted effect estimate was 2.04 (95% CI, 1.72-2.43).
Figures 3A. Adjusted Effect Estimates of Ischemic Stroke in Participants With Any Migraine Versus No Migraine.
Size of data markers indicates weight of study.
Subgroup Analyses
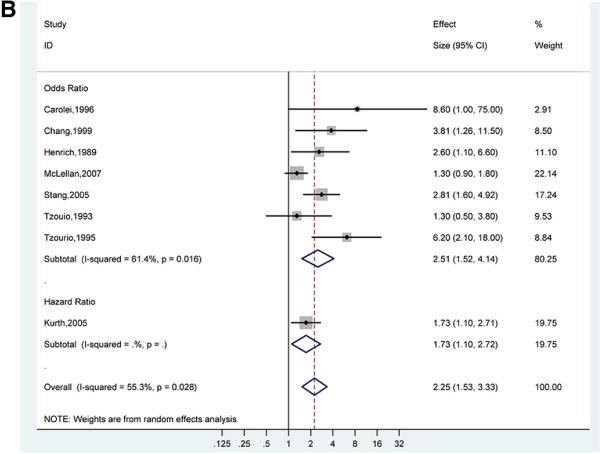
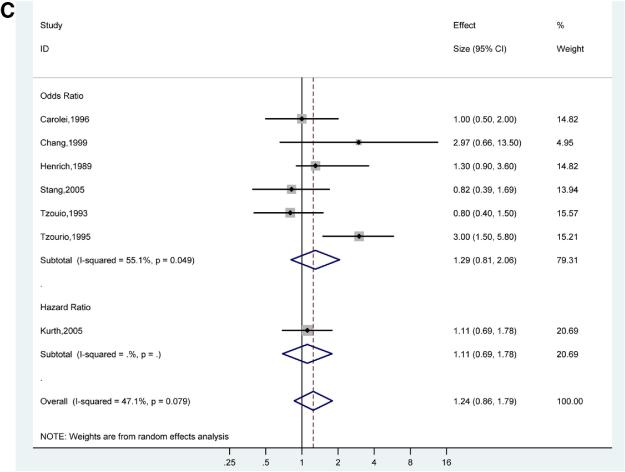
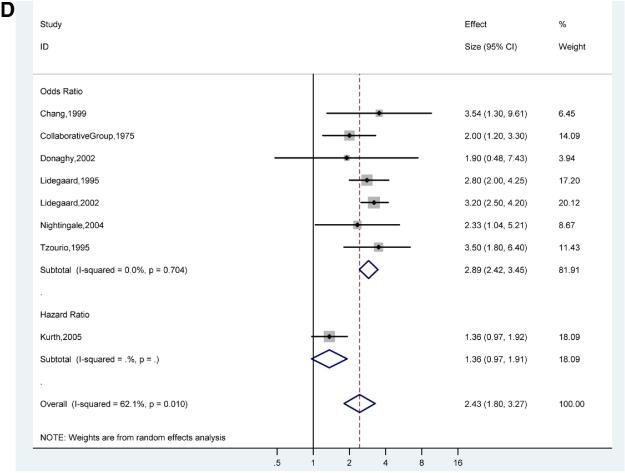
There was a stronger association of ischemic stroke and migraine with aura (pooled adjusted OR for 7 studies 2.51; 95% CI, 1.52-4.14)18,21,31,32,35,39,40 (Figure 3B) compared to the association of ischemic stroke and migraine without aura (pooled adjusted OR for 6 studies 1.29; 95% CI, 0.81-2.06)21,31,32,35,39,40 (Figure 3C). However, the confidence intervals for the pooled adjusted ORs of ischemic stroke in migraine with aura and migraine without aura overlap, suggesting that there is no statistically significant difference between these subgroups. The pooled adjusted OR for ischemic stroke in studies of only women migraineurs versus non-migraineurs (7 studies) was 2.89 (95%, CI 2.42-3.45) with evidence of low statistical heterogeneity (I2 0.0%, p=0.70)20,29,32,33,37,38,40 (Figure 3D).
Figures 3B. Adjusted Effect Estimates of Ischemic Stroke in Participants with Migraine With Aura Versus No Migraine.
Size of data markers indicates weight of study.
Figures 3C. Adjusted Effect Estimates of Ischemic Stroke in Participants with Migraine Without Aura Versus No Migraine.
Size of data markers indicates weight of study.
Figures 3D. Adjusted Effect Estimates of Ischemic Stroke in Studies of Only Women Participants with Any Migraine Versus No Migraine.
Size of data markers indicates weight of study.
Sensitivity Analyses
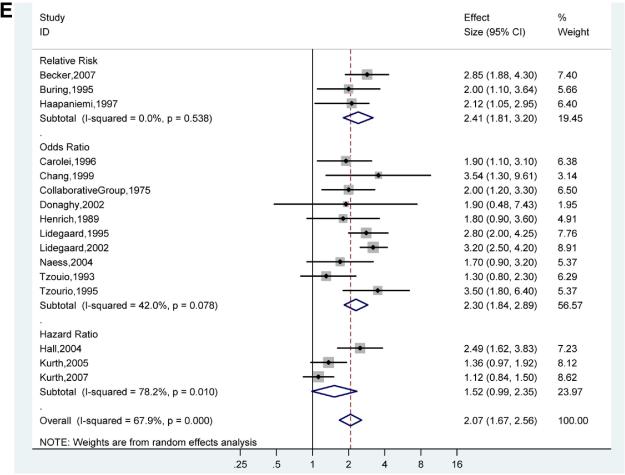
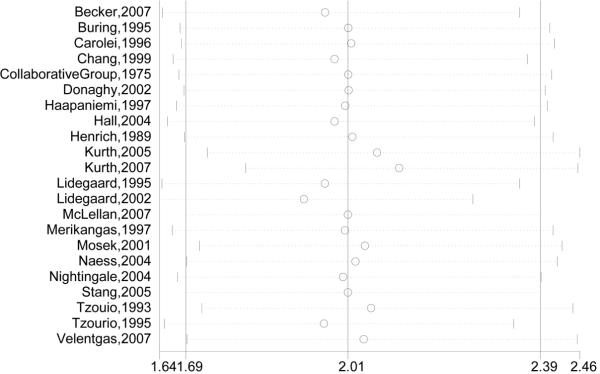
In a sensitivity analysis of study quality, three studies that were not at low risk of bias (low risk of bias defined as fewer than 3 negatives in the standardized study quality abstraction tables, Figure 2) were removed from the analysis.22,36,38 The effect on pooled adjusted RRs, ORs, and HRs was minimal (Figure 3E). In the influence analysis, there was minimal change in the quantitative summary measure of effect or 95% CI, and there was no change in the direction of effect, when any one study was excluded (Figure 4).
Figures 3E. Adjusted Effect Estimates of Ischemic Stroke in Low Bias Studies in Participants With Any Migraine Versus No Migraine.
Size of data markers indicates weight of study.
Figure 4. Influence of Removing Studies One By One on Adjusted Effect Estimates of Ischemic Stroke.
Circles are effect estimates and horizontal dotted lines 95% confidence intervals for meta-analysis of the studies listed, excluding the study indicated by the circle. The vertical line in the center is the summary effect estimate including all listed studies.
Publication Bias
Visual inspection of funnel plots revealed no significant publication bias for studies that provided ORs (Figure 5). There were not enough studies to produce interpretable funnel plots for studies providing RRs, HRs, or IRRs.
Figure 5. Funnel Plot of Studies Reporting Adjusted Odds Ratios.
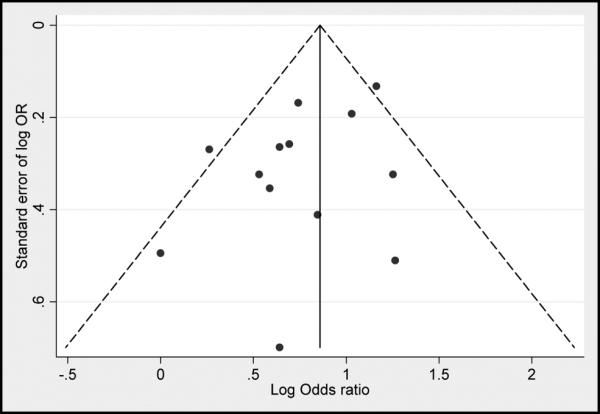
Plots are log standard error of effect estimate by adjusted effect estimate, centered on the pooled adjusted effect estimate. The pseudo 95% confidence interval corresponds to the expected 95% confidence interval for a given standard error. OR indicates odds ratio.
DISCUSSION
We report the largest meta-analysis to date of the association between migraine and stroke. In this meta-analysis of 21 observational studies of the association of migraine headache and ischemic stroke, migraine was independently associated with a 2-fold increased risk of ischemic stroke.
There are several potential mechanisms for the increased risk of ischemic stroke in migraineurs. Migraine may increase ischemic stroke risk via vasospasm-induced cerebrovascular hypoperfusion,42 platelet activation,43 increased platelet aggregation,44 and increased concentrations and activity of vascular pro-coagulant factors such as endothelin 1,45 von Willebrand factor,46 prothrombin factor 1.2,47 homocysteine (MTHFR C677T genetic variant),48 and antiphospholipid antibody.49 An increased prevalence of patent foramen ovale (PFO) in patients with migraine may also predispose to embolic stroke via transit of a blood clot from the right to left-sided circulation through the PFO.50
Comparison to Prior Meta-Analysis
Our results expand on those of a prior smaller systematic review and meta-analysis by Etminan et al, which reported a similar magnitude of ischemic stroke risk in participants with migraine with aura and in women.7 Important differences among our and Etminan et al's study include temporal inclusion of studies from 1996 to 2004 (versus through 2009 in the current study). Also, Etminan et al assumed ORs approximate RRs.7 This assumption is tenable given the low prevalence of stroke in migraineurs and is supported by the similar magnitudes of pooled ORs and RRs in our meta-analysis. However, newer studies reporting hazard ratios and incidence rate ratios were also included in our study. We therefore preferred not to pool across all effect estimates because of the potential for significant methodological heterogeneity, particularly differences in biases, by type of observational study design. However, we did provide the overall pooled effect estimate for comparison.
Stroke Risk in Migraineurs With or Without Aura
We found a greater risk of ischemic stroke in migraine with aura than migraine without aura, although this difference was unlikely to be significant. Migraine with aura is characterized by cortical spreading depression, oligemia, and changes in vascular perfusion.42 Changes in vascular perfusion may be associated with vasospasm, which could lead to cerebral hypo-perfusion and ischemic stroke.51 In comparison to our study, the Etminan et al study also reported a statistically significant, albeit reduced, risk of stroke for migraine without aura (pooled RR 1.83; 95% CI 1.06, 3.15). This discrepancy is likely the result of differential inclusion in our meta-analysis of a large study by Stang et al, which reported a negative association with ischemic stroke in migraine patients without aura.21
Stroke Risk in Female Migraineurs
The association between migraine and stroke was strongest in studies of women. However, no direct comparison of effect estimates between men and women could be made as no studies of both men and women presented data separately by gender. This finding could represent a true increased risk, or it could be the consequence of residual or unmeasured confounding. Important potential confounders include those that reflect hormone status in women, including pregnancy52 and oral contraceptive53 and post-menopausal hormone use,54 and factors such as smoking that may interact with these risk factors to further increase the risk of ischemic stroke.53 Increased estrogen levels may increase the risk of ischemic stroke via their affect on endothelial function, coagulation factors, and inflammation.55
Although we excluded studies solely of pregnant participants, few studies in our meta-analysis that included women adjusted for pregnancy status. In addition, not all studies adjusted for oral contraceptive or post-menopausal hormone use, which is likely to confound the relationship between migraine in women and ischemic stroke, as estrogen-containing therapies have been used to treat certain types of migraines.56,57 Finally, migraine is more common in women,3,4 and vasoactive medications used to treat migraines, such as triptans, may predispose to ischemic stroke.42,51
Limitations
Potential limitations of this meta-analysis must be considered. First, our review was subject to language bias, as we only included articles in the English language. However, a secondary search of PubMed and EMBASE using the same strategy, but without the English language limitation, yielded no additional articles that would have met our selection criteria. Second, the meta-analysis was limited by limitations in its included studies. Certain data from individual studies, for example subgroup data or information about potential confounders such as PFO prevalence or vasoactive medication use, were often not available or not reported. We did not attempt to procure this information or impute data. Third, this meta-analysis may not be generalizable to all populations. Included studies were from the United States, United Kingdom, and Europe and consisted of largely white populations. Finally, while our results strongly suggest that migraine and stroke are associated, they do not shed light on whether this represents a true etiological association or rather an epiphenomenon whereby migraine and stroke are both manifestations of a shared, underlying propensity to cerebral vascular dysfunction.
Conclusion
Migraines appear to be independently associated with a two-fold increased risk of ischemic stroke. Migraine is a potentially modifiable risk factor that can be treated,58 and stroke risk can be reduced through reduction of other risk factors.5 Therefore, further study is warranted to assess the effects of migraine control and stroke risk factor reduction on the risk of ischemic stroke in migraineurs.
Funding Sources
Dr. Kahn is a recipient of a National Career Scientist Award from the Fonds de la Recherche en Santé du Québec. Dr. Nazarian is funded by the Johns Hopkins Richard S. Ross Clinician Scientist Award, the PJ Schafer Memorial Research Award, and the National Institutes of Health Clinical Research Scholars Program (1KL2RR025006-01). We did not receive any additional funding, including pharmaceutical industry funds, for the preparation of this manuscript or any related research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors report no conflicts of interest.
Authorship Criteria: All authors had access to the data and a role in writing the manuscript.
FUNDING SOURCES
Dr. Kahn is a recipient of a National Career Scientist Award from the Fonds de la Recherche en Santé du Québec. Dr. Nazarian is funded by the Johns Hopkins Richard S. Ross Clinician Scientist Award, the PJ Schafer Memorial Research Award, and the National Institutes of Health Clinical Research Scholars Program (1KL2RR025006-01).
DISCLOSURES
None.
REFERENCES
- 1.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology. 1994;44:S17–23. [PubMed] [Google Scholar]
- 4.Lipton RB, Bigal ME, Diamond M, et al. AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LB, Adams R, Alberts MJ, et al. American Heart Association/American Stroke Association Stroke Council, Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group, Cardiovascular Nursing Council, Clinical Cardiology Council, Nutrition, Physical Activity, and Metabolism Council, Quality of Care and Outcomes Research Interdisciplinary Working Group, American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 6.Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73:189–194. doi: 10.1016/j.contraception.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 9.Hall GC, Brown MM, Mo J, MacRae KD. Triptans in migraine: the risks of stroke, cardiovascular disease, and death in practice. Neurology. 2004;62:563–568. doi: 10.1212/01.wnl.0000110312.36809.7f. [DOI] [PubMed] [Google Scholar]
- 10.Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64:1020–1026. doi: 10.1212/01.WNL.0000154528.21485.3A. [DOI] [PubMed] [Google Scholar]
- 11.Kurth T, Gaziano JM, Cook NR, et al. Migraine and risk of cardiovascular disease in men. Arch Intern Med. 2007;167:795–801. doi: 10.1001/archinte.167.8.795. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Brobert GP, Almqvist PM, et al. Migraine and the risk of stroke, TIA, or death in the UK (CME). Headache. 2007;47:1374–1384. doi: 10.1111/j.1526-4610.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 13.GS, Higgins JPT. [March, 2009];Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Available at: www.cochrane-handbook.org.
- 14.Pavlakis SG, Phillips PC, DiMauro S, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16:481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- 15.Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol. 1998;44:731–739. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- 16.Marini C, Carolei A, Roberts RS, et al. Focal cerebral ischemia in young adults: A collaborative case-control study. Neuroepidemiology. 1993;12:70–81. doi: 10.1159/000110303. [DOI] [PubMed] [Google Scholar]
- 17.Schwaag S, Nabavi DG, Frese A, Ingo-W. Husstedt, Evers S. The association between migraine and juvenile stroke: A case-control study. Headache. 2003;43:90–95. doi: 10.1046/j.1526-4610.2003.03023.x. [DOI] [PubMed] [Google Scholar]
- 18.MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 19.Naess H, Nyland HI, Thomassen L, et al. Etiology of and risk factors for cerebral infarction in young adults in western Norway: a population-based case-control study. Eur J Neurol. 2004;11:25–30. doi: 10.1046/j.1351-5101.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Lidegaard O, Kreiner S. Contraceptives and cerebral thrombosis: A five-year national case-control study. Contraception. 2002;65:197–205. doi: 10.1016/s0010-7824(01)00306-7. [DOI] [PubMed] [Google Scholar]
- 21.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64:1573–1577. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- 22.Velentgas P, Cole JA, Mo J, et al. Severe vascular events in migraine patients. Headache. 2004;44:642–651. doi: 10.1111/j.1526-4610.2004.04122.x. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 26.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 27.Special report from the National Institute of Neurological Disorders and Stroke Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 28.Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome: diagnostic criteria. Stroke. 2003;34:2995–2998. doi: 10.1161/01.STR.0000098902.69855.A9. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative Group Oral contraceptives and stroke in young women. Associated risk factors. JAMA. 1975;231:718–722. doi: 10.1001/jama.1975.03240190022010. [DOI] [PubMed] [Google Scholar]
- 30.Buring JE, Hebert P, Romero J, et al. Migraine and subsequent risk of stroke in the Physicians’ Health Study. Arch Neurol. 1995;52:129–134. doi: 10.1001/archneur.1995.00540260031012. [DOI] [PubMed] [Google Scholar]
- 31.Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. The Italian National Research Council Study Group on Stroke in the Young. Lancet. 1996;347:1503–1506. doi: 10.1016/s0140-6736(96)90669-8. [DOI] [PubMed] [Google Scholar]
- 32.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13–18. doi: 10.1136/bmj.318.7175.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donaghy M, Chang CL, Poulter N. European Collaborators of The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Duration, frequency, recency, and type of migraine and the risk of ischaemic stroke in women of childbearing age. J Neurol Neurosurg Psychiatr. 2002;73:747–750. doi: 10.1136/jnnp.73.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haapaniemi H, Hillbom M, Juvela S. Lifestyle-associated risk factors for acute brain infarction among persons of working age. Stroke. 1997;28:26–30. doi: 10.1161/01.str.28.1.26. [DOI] [PubMed] [Google Scholar]
- 35.Henrich JB, Horwitz RI. A controlled study of ischemic stroke risk in migraine patients. J Clin Epidemiol. 1989;42:773–780. doi: 10.1016/0895-4356(89)90075-9. [DOI] [PubMed] [Google Scholar]
- 36.Merikangas KR, Fenton BT, Cheng SH, et al. Association between migraine and stroke in a large-scale epidemiological study of the United States. Arch Neurol. 1997;54:362–368. doi: 10.1001/archneur.1997.00550160012009. [DOI] [PubMed] [Google Scholar]
- 37.Lidegaard O. Oral contraceptives, pregnancy and the risk of cerebral thromboembolism: the influence of diabetes, hypertension, migraine and previous thrombotic disease. Br J Obstet Gynaecol. 1995;102:153–159. doi: 10.1111/j.1471-0528.1995.tb09070.x. [DOI] [PubMed] [Google Scholar]
- 38.Nightingale AL, Farmer RD. Ischemic stroke in young women: a nested case-control study using the UK General Practice Research Database. Stroke. 2004;35:1574–1578. doi: 10.1161/01.STR.0000129789.58837.e4. [DOI] [PubMed] [Google Scholar]
- 39.Tzourio C, Iglesias S, Hubert J, et al. Migraine and risk of ischaemic stroke: A case control study. Br Med J. 1993;307:289–292. doi: 10.1136/bmj.307.6899.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830–833. doi: 10.1136/bmj.310.6983.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosek A, Marom R, Korczyn AD, Bornstein N. A history of migraine is not a risk factor to develop an ischemic stroke in the elderly. Headache. 2001;41:399–401. doi: 10.1046/j.1526-4610.2001.111006399.x. [DOI] [PubMed] [Google Scholar]
- 42.Elliott D. Migraine and stroke: current perspectives. Neurol Res. 2008;30:801–812. doi: 10.1179/174313208X341049. [DOI] [PubMed] [Google Scholar]
- 43.DAndrea G, Hasselmark L, Alecci M, et al. Platelet secretion from dense and alpha-granules in vitro in migraine with or without aura. J Neurol Neurosurg Psychiatry. 1994;57:557–561. doi: 10.1136/jnnp.57.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeller JA, Frahm K, Baron R, et al. Platelet-leukocyte interaction and platelet activation in migraine: a link to ischemic stroke? J Neurol Neurosurg Psychiatry. 2004;75:984–987. doi: 10.1136/jnnp.2003.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallai V, Sarchielli P, Firenze C, et al. Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand. 1994;89:47–55. doi: 10.1111/j.1600-0404.1994.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 46.Tietjen GE, Al-Qasmi MM, Athanas K, et al. Increased von Willebrand factor in migraine. Neurology. 2001;57:334–336. doi: 10.1212/wnl.57.2.334. [DOI] [PubMed] [Google Scholar]
- 47.Hering-Hanit R, Friedman Z, Schlesinger I, Ellis M. Evidence for activation of the coagulation system in migraine with aura. Cephalalgia. 2001;21:137–139. doi: 10.1046/j.1468-2982.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 48.Scher AI, Terwindt GM, Verschuren WM, et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol. 2006;59:372–375. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 49.Tanasescu R, Nicolau A, Caraiola S, et al. Antiphospholipid antibodies and migraine: a retrospective study of 428 patients with inflammatory connective tissue diseases. Rom J Intern Med. 2007;45:355–363. [PubMed] [Google Scholar]
- 50.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 51.Katsarava Z, Rabe K, Diener HC. From migraine to stroke. Intern.Emerg.Med. 2008;3(Suppl 1):S9–16. doi: 10.1007/s11739-008-0190-7. [DOI] [PubMed] [Google Scholar]
- 52.Feske SK. Stroke in pregnancy. Semin Neurol. 2007;27:442–452. doi: 10.1055/s-2007-991126. [DOI] [PubMed] [Google Scholar]
- 53.Allais G, Gabellari IC, Mana O, et al. Migraine and stroke: The role of oral contraceptives. Neurol Sci. 2008;29:S12–4. doi: 10.1007/s10072-008-0877-6. [DOI] [PubMed] [Google Scholar]
- 54.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: A meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis PH. Use of oral contraceptives and postmenopausal hormone replacement: Evidence on risk of stroke. Curr Treat Options Neurol. 2008;10(6):468–474. doi: 10.1007/s11940-008-0049-2. [DOI] [PubMed] [Google Scholar]
- 56.Nappi RE, Sances G, Detaddei S, et al. Hormonal management of migraine at menopause. Menopause Int. 2009;15:82–86. doi: 10.1258/mi.2009.009022. [DOI] [PubMed] [Google Scholar]
- 57.Lay CL, Payne R. Recognition and treatment of menstrual migraine. Neurologist. 2007;13:197–204. doi: 10.1097/NRL.0b013e31805c746f. [DOI] [PubMed] [Google Scholar]
- 58.Pryse-Phillips WE, Dodick DW, Edmeads JG, et al. Guidelines for the diagnosis and management of migraine in clinical practice. Canadian headache society. CMAJ. 1997;156:1273–1287. [PMC free article] [PubMed] [Google Scholar]