Abstract
As part of a population-based study in Beaver Dam, Wisconsin, we estimated the 10-year cumulative incidence of tinnitus and its risk factors. Participants (n = 2922, aged 48–92 years) not reporting tinnitus at baseline (1993–1995) were followed for up to ten years. In addition to audiometric testing and anthropometric measures, data on tinnitus, health and other history were obtained via questionnaire. Potential risk factors were assessed with discrete-time proportional hazards models. The 10-year cumulative incidence of tinnitus was 12.7%. The risk of developing tinnitus was significantly associated with: history of arthritis (Hazard ratio (HR) = 1.37), history of head injury (HR = 1.76), history of ever smoking (HR = 1.40), and among women, hearing loss (HR = 2.59). Alcohol consumption (HR = 0.63 for ≥ 141 grams/week vs. < 15 grams/week), age (among women, HR = 0.90 for each 5-year increase in age), and among men, obesity (HR = 0.55), were associated with decreased risk. The risk of developing tinnitus was high for older adults and associated with modifiable health and behavioral factors.
Keywords: Tinnitus, hearing disorders, older adults
Tinnitus, the perception of sound in the ears or head without an audible external source, is common among older adults (Nondahl et al, 2002). It can range from barely noticeable to debilitating. While there is no single agreed upon definition of tinnitus for research purposes, a number of population-based studies have attempted to estimate the prevalence of this condition. Prevalence estimates generally range from 7 to 20% (Hoffman & Reed, 2004).
Prevalence estimates are useful for gauging how common a condition is within a population at a point in time and capture cases with recent onset and those of long duration. Incidence estimates assess the risk of developing a condition over a period of time among those without the condition at the beginning of the study period. Longitudinal incidence studies are essential for identifying factors associated with the development of disorders. In a population-based study of older adults in Beaver Dam, Wisconsin, the 5-year incidence of tinnitus among those free of tinnitus at baseline was 5.7% (Nondahl et al, 2002). Risk factors for the 5-year incidence of tinnitus included hearing loss, higher serum total cholesterol, history of head injury, and otosclerosis. To our knowledge this is the only published study of tinnitus incidence in older adults. The purpose of the current study was to estimate the cumulative 10-year incidence of tinnitus in a population of older adults and to identify risk factors for developing tinnitus during the 10-year interval. The identification of risk factors that, if modified, may reduce the incidence or severity of tinnitus, might facilitate the efficient allocation of public health resources aimed at reducing the negative effects of tinnitus in everyday life.
METHODS
Subjects
The Epidemiology of Hearing Loss Study (EHLS) is a population-based study of hearing loss in adults 48 to 92 years of age at baseline (Cruickshanks et al, 1998). During 1987 to 1988, residents of the city or township of Beaver Dam, Wisconsin, who were 43 to 84 years of age (N = 5924) were identified through a private census and invited to participate in a study of age-related ocular disorders (The Beaver Dam Eye Study, 1988–1990, N = 4926)(Klein et al, 1991). All who participated in the baseline eye examination and were alive as of March 1, 1993 were eligible to participate in the baseline examination of the hearing study (EHLS, N = 4541). Of those eligible, 3753 (82.6%) participated (1993–1995).
Among the baseline participants, 3429 reported no tinnitus; 42.1% of these were male and their mean age was 65.8 years (range 48–92 years). Among these 3429 participants considered at risk for developing tinnitus, 2922 provided information on their tinnitus status one to four additional times over the next ten years. Follow-up studies were conducted in 1995–1997, 1998–2000, 2000–2002 and 2003–2005. The tinnitus status of those not participating in an intermediate follow-up examination was considered unchanged until the next examination in which they participated. Study approval was granted by the Human Subjects Committee of the University of Wisconsin-Madison. Informed consent was obtained from each participant examined.
Procedure
Audiologic tests included pure-tone air- and bone-conduction audiometry. Audiometric equipment and procedures have been described in detail elsewhere (Cruickshanks et al, 2003). All testing was conducted by examiners trained and monitored by a certified audiologist (TST).
A questionnaire about health history (including tinnitus) and occupational and leisure time noise exposure was administered as an interview. At the baseline and subsequent examinations, participants were asked, “In the past year have you had buzzing, ringing, or noise in your ears?” (No/Yes/Unknown). Examiners were instructed to record “no” if a participant reported hearing an odd or unusual noise on a single occasion in the past year. Participants responding positively to this question were then asked, “How severe is this noise in its worst form?” (Mild/Moderate/Severe/Unknown), and “Does this noise cause you to have problems getting to sleep?” (No/Yes/Unknown). A person was classified as having tinnitus if he/she reported having “buzzing, ringing, or noise” in the ears in the past year that was at least moderate in severity or that caused problems getting to sleep (Nondahl et al, 2002).
Potential risk factors from the baseline examination were evaluated for their association with the incidence of tinnitus. These included age, sex, hearing loss (average of air-conduction hearing thresholds at .5, 1, 2 and 4 kHz > 25 dB in the worse ear), history of arthritis, regular use of aspirin (at least twice a week for more than three months), history of cardiovascular disease (myocardial infarction, stroke, or angina), alcohol consumption (grams of ethanol per week), history of heavy drinking (ever drinking four or more alcoholic beverages per day), recent (past year) exposure to firearms through hunting or target shooting, history of head injury (skull fracture; concussion; broken nose; loss of consciousness due to a head injury; whiplash or other serious neck injury), recent (past year) regular participation in loud hobbies (woodworking, metalworking, chain sawing, driving loud recreational vehicles, or doing yard work with power tools), recent (past year) current occupational noise exposure (had to speak in a loud voice in order to be heard by another person two feet away), hypertension (systolic ≥ 140 mmHg, diastolic ≥ 90 mmHg, or physician-diagnosis of hypertension with current use of antihypertensive medication), obesity (body mass index ≥ 30), smoking status (never/ever), history of otosclerosis, and serum total and non-HDL cholesterol.
Statistical analyses were completed with SAS (SAS Institute Inc., Gary, NC). Comparisons of baseline characteristics between those who had a follow-up examination and those who did not were made with the chi-square test for general association for categorical variables. When adjusted for age, these comparisons were made with the Cochran-Mantel-Haenszel test for general association (Landis et al, 1978). For continuous variables, these comparisons were made with F-tests from Analysis of Covariance (ANCOVA) models. Cumulative incidence of tinnitus was estimated accounting for the competing risk of death (Gooley et al, 1999; Satagopan et al, 2004), yielding estimates of the probability of simultaneously remaining alive over the follow-up period and having developed tinnitus by its end. In accordance with this method, drop-outs were treated as censored data. The discrete hazards of developing tinnitus were compared with the Cox proportional hazards models (Cox, 1972) using its discrete-time version because of the relatively small number of follow-up intervals.
RESULTS
The 10-year cumulative incidence of tinnitus was 12.7% (95% confidence interval [CI] 11.4–14.0). Incidence rates by age and gender are shown in Table 1 for the 2922 participants at risk for whom tinnitus status was determined at follow-up. The incidence of tinnitus was higher for men (14.8%, 95% CI 12.7,16.9) than women (11.2%, 95% CI 9.7, 12.7).
Table 1.
Cumulative 10-Year Incidence of Tinnitus by Gender and Baseline Age Group: Epidemiology of Hearing Loss Study
| Age Group (yr) | Number at Risk | Incidence | 95% CI |
|---|---|---|---|
| Men | 1188 | 14.8 | 12.7, 16.9 |
| 48–59 | 485 | 14.0 | 10.9, 17.1 |
| 60–69 | 361 | 14.8 | 11.1, 18.5 |
| 70–79 | 264 | 17.5 | 12.5, 22.5 |
| 80–92 | 78 | 11.0 | 2.0, 20.0 |
| Women | 1734 | 11.2 | 9.7, 12.7 |
| 48–59 | 606 | 13.0 | 10.3, 15.7 |
| 60–69 | 473 | 10.5 | 7.7, 13.3 |
| 70–79 | 444 | 9.7 | 6.7, 12.7 |
| 80–92 | 211 | 8.5 | 4.0, 13.0 |
| Both sexes | 2922 | 12.7 | 11.4, 14.0 |
| 48–59 | 1091 | 13.4 | 11.4, 15.4 |
| 60–69 | 834 | 12.3 | 10.1, 14.5 |
| 70–79 | 708 | 12.6 | 9.9, 15.3 |
| 80–92 | 289 | 9.1 | 5.0, 13.2 |
Follow-up status was compared by self-reported baseline tinnitus status. There was no significant relation between baseline self-reported tinnitus severity (None; Mild; Moderate/Severe) and subsequent participation in the Epidemiology of Hearing Loss Study (p = .76). Table 2 shows baseline characteristics of 3385 participants without tinnitus at baseline, by subsequent participation status. After adjusting for age, the following baseline characteristics differed by participation status: smoking status, hypertension, having arthritis, having hearing loss, sex, history of heavy drinking, history of cardiovascular disease, having a loud job, ethanol consumption, and serum total and non-HDL cholesterol. The predominant reason for not participating in follow-up was death.
Table 2.
Baseline Characteristics of Participants at Risk for Incident Tinnitus, by Follow-up Status.
| Characteristic | Percentage with Characteristic | p-value | |||
|---|---|---|---|---|---|
| Participated (n = 2922)1 | Did Not Participate (n = 114)2 | Deceased (n = 349)3 | Unadjusted | Age- adjusted | |
| History of ever smoking | 52.1 | 62.6 | 66.0 | < .0001 | < .0001 |
| Hypertension present | 49.8 | 43.3 | 63.6 | < .0001 | .0394 |
| Any loud hobbies (past year) | 49.7 | 52.6 | 40.4 | .0031 | .33 |
| Arthritis present | 45.8 | 30.8 | 46.9 | .0154 | .0212 |
| Obese | 41.8 | 50.0 | 38.2 | .14 | .39 |
| Hearing loss present | 41.3 | 36.3 | 63.9 | < .0001 | .0006 |
| Male | 40.7 | 43.9 | 53.6 | < .0001 | < .0001 |
| History of head injury | 29.8 | 21.9 | 27.2 | .13 | .09 |
| Regular aspirin use | 27.5 | 24.2 | 33.1 | .07 | .84 |
| History of heavy drinking | 14.8 | 24.2 | 27.6 | < .0001 | < .0001 |
| History of cardiovascular disease | 12.2 | 7.7 | 31.1 | < .0001 | < .0001 |
| Gunfire exposure in past year | 9.5 | 10.5 | 8.6 | .79 | .47 |
| Loud job in past year | 8.2 | 6.1 | 7.5 | .69 | .0325 |
| History of otosclerosis | 0.3 | 0.0 | 0.0 | .53 | .52 |
| Mean | p-value | ||||
| Age (years) | 65.2 | 60.6 | 70.8 | < .0001 | -- |
| Ethanol consumption (g/wk) | 43.1 | 41.5 | 53.5 | .17 | .0047 |
| Serum non-HDL cholesterol (mg/dL) | 187.2 | 191.4 | 178.4 | .0042 | .0017 |
| Serum total cholesterol (mg/dL) | 240.0 | 243.4 | 226.6 | < .0001 | < .0001 |
Those who participated in any of the four follow-up studies. Excludes 44 participants who did not provide sufficient information to classify tinnitus status upon follow-up.
Those without follow-up, but living at the end of the follow-up period.
Those without follow-up who had died by the end of the follow-up period.
Discrete-time proportional hazards modeling was used to identify baseline factors that were associated with developing tinnitus over the 10-year follow-up period. In a model adjusted for only age and sex, age was not significantly associated with the incidence of tinnitus (HR = 1.03 for every 5 years, 95% CI = 0.97, 1.09), but male sex was associated with an increased incidence of tinnitus (HR = 1.38, 95% CI 1.10, 1.71). There was no age/sex interaction with this model. Additional baseline factors described in the Methods section were also evaluated. The results of the final model are shown in Table 3. History of arthritis (HR = 1.37, 95% CI 1.08, 1.73), history of head injury (HR = 1.76, 95% CI 1.40, 2.22), hearing loss (in women, HR = 2.59, 95% CI 1.79, 3.74), and having ever smoked (HR = 1.40, 95% CI 1.10, 1,79) were associated with an increased hazard of developing tinnitus. Age (in women, HR = 0.90 for every 5-year increase in age, 95% CI 0.81, 0.99), higher ethanol consumption (HR = 0.63, 95% CI 0.41, 0.96 for ≥ 141 g/week vs. < 15 g/week, where 141 g/week is roughly equivalent to 12 light beers per week) and obesity (in men, HR = 0.55, 95% CI 0.39, 0.78), were associated with a decreased hazard. Other factors, including regular aspirin use, history of cardiovascular disease, history of heavy drinking, recent exposure to firearms, participation in loud hobbies, occupational noise exposure, hypertension, history of otosclerosis, and serum total and non-HDL cholesterol were not associated with the incidence of tinnitus and are not shown in the final model. Due to the three interaction terms with sex (sex/age, sex/hearing loss and sex/obesity; p-values of 0.0278, 0.0036, and 0.0044, respectively), the main effect of sex could not be determined from the final model.
Table 3.
Risk Factors for Incidence of Tinnitus from Final Discrete-Time Proportional Hazards Model: Epidemiology of Hearing Loss Study
| Risk Factor | Hazard ratio | 95% CI |
|---|---|---|
| Age (per 5 years) | ||
| Women | 0.90 | 0.81, 0.99 |
| Men | 1.05 | 0.94, 1.16 |
| Arthritis | 1.37 | 1.08, 1.73 |
| Ethanol consumption (g/wk) | ||
| None | 0.85 | 0.62, 1.16 |
| < 15 | 1.00 | -- |
| 15–74 | 0.91 | 0.66, 1.26 |
| 75–140 | 0.82 | 0.55, 1.22 |
| 141+ | 0.63 | 0.41, 0.96 |
| History of head injury | 1.76 | 1.40, 2.22 |
| Hearing loss | ||
| Women | 2.59 | 1.79, 3.74 |
| Men | 1.19 | 0.82, 1.72 |
| Obesity | ||
| Women | 1.08 | 0.79, 1.49 |
| Men | 0.55 | 0.39, 0.78 |
| Ever smoked | 1.40 | 1.10, 1.79 |
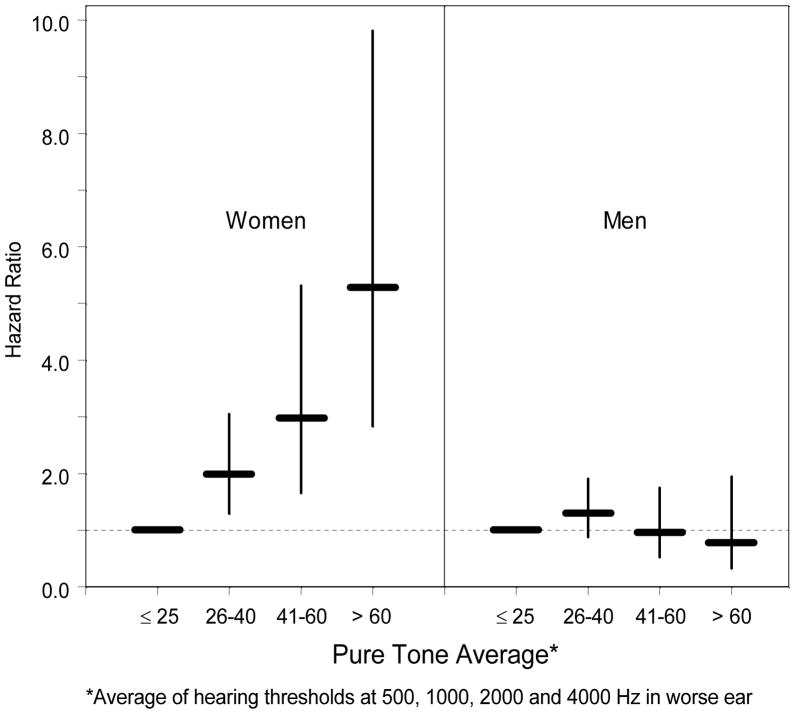
The relation between hearing loss and the risk of developing tinnitus is illustrated further in Figure 1. After adjusting for age, arthritis, ethanol consumption, head injury, obesity, and smoking, for women, the more severe the hearing loss, the higher the risk of developing tinnitus (trend test: p <.0001). No such relation was seen for men.
Figure 1.
Effect of Hearing Loss on Risk of Developing Tinnitus (hazard ratio and 95% confidence interval).
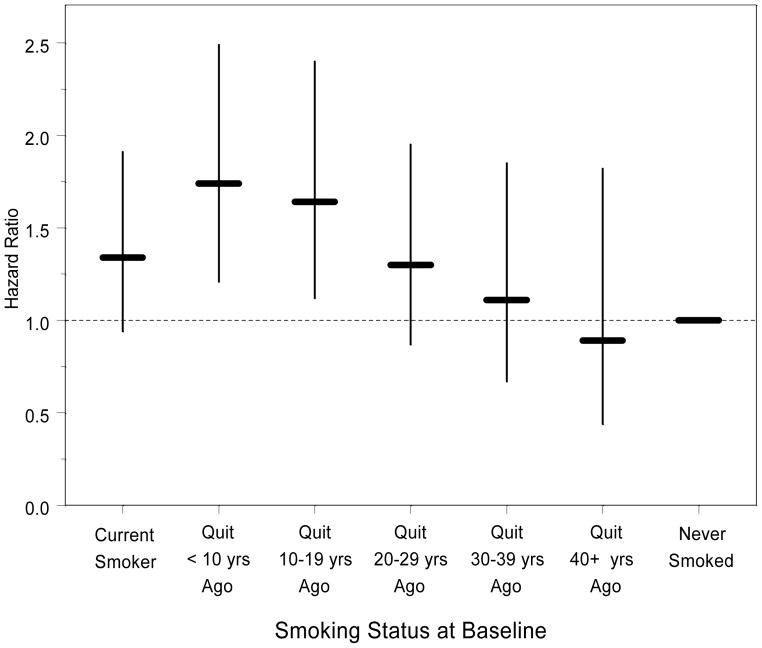
Those who had quit smoking were asked how many years it had been since they quit. We examined the effect of smoking cessation on the relative hazard of developing tinnitus (Figure 2). After adjusting for age, sex, arthritis, ethanol consumption, head injury, hearing loss and obesity, the hazard ratio peaked among those who had quit smoking less than 10 years earlier, and gradually declined to the level of never smokers the longer it had been since smoking ceased. This pattern roughly corresponded to the pattern of the average number of packs of cigarettes that participants reported having smoked per day: 0.86 for current smokers, and 1.14, 1.05, 1.01, 1.02 and 0.66 for those who had quit 1–9 years ago, 10–19 years ago, 20–29 years ago, 30–39 years ago, and 40 or more years ago, respectively.
Figure 2.
Effect of Smoking Cessation on Risk of Developing Tinnitus (hazard ratio and 95% confidence interval).
DISCUSSION
The 10-year cumulative incidence of tinnitus was 12.7%, a little more than twice the incidence reported after 5-years (5.7%; Nondahl et al., 2002). We know of no other studies that have reported on the long-term incidence of tinnitus. The risk of developing tinnitus was significantly associated with: history of head injury, history of ever smoking, history of arthritis, and among women, hearing loss. Alcohol consumption, age (among women), and obesity (among men) were associated with decreased risk. These results suggest that modifiable factors may contribute to the development of tinnitus.
Our results are consistent with our earlier report with shorter follow-up (5 years) in which we also found that hearing loss and head injury were associated with the incidence of tinnitus. The consistency of these findings suggests that auditory and/or some traumatic brain changes may be associated with the development of tinnitus. Other long-term studies in other populations are needed to confirm these findings. Among men, the lack of an association between hearing loss and 10-year incidence of tinnitus may reflect the longer duration and greater severity of hearing loss present in some men at the baseline. This could lead to an earlier onset of hearing loss-related tinnitus (eg., during the first 5-years of follow-up). The second 5 years of follow-up could have added a disproportionate number men with onset of tinnitus due to other factors, thus making it more difficult to demonstrate an association among men between hearing loss and tinnitus over the entire follow-up period.
Tinnitus and auditory system pathology are known to be related for some conditions (Nuttall, Meikle and Trune, 2004), but these data are the first population-based data to demonstrate that hearing loss may increase the risk of developing tinnitus over the next 10 years. Reducing the risk of head injury by preventive measures such as proper use of helmets on bicycles, motorcycles, and other recreational vehicles; safe driving habits; and reducing the risk of falls could have the added benefit of reducing the risk of developing tinnitus.
Some associations differed between the 5-year incidence and the 10-year incidence findings. Total cholesterol was associated with 5-year but not 10-year incidence. With 5 years of follow-up the strength of the association was very modest (OR=1.04 per 10 mg/dL (0.26 mmol/L)). The lack of a statistically significant association with the 10-year incidence may be due to chance, loss to follow-up (higher 10-year mortality among those with the highest cholesterol levels), or be due to changing cholesterol levels (Newschaffer et al, 1992). During this time period, 1993–2005, there were significant changes in clinical treatment of hypercholesterolemia, with the introduction and widespread adoption of statin medications (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) (Klein et al, 2007), which are usually prescribed to lower cholesterol levels to reduce or prevent atherosclerosis and cardiovascular disease. Individuals who began treatment after the baseline in 1993 would be expected to have experienced lower cholesterol levels during the follow-up period. We have previously shown that statin users in 1998 (the 5-year follow-up) had lower cholesterol levels than non-users (Carlsson et al, 2009). Successful treatment would have lowered the duration of exposure to hypercholesterolemia, and contributed to the loss of an effect over time. This pattern also is similar to that seen in studies of heart disease. Some studies have reported that there is a diminishing effect of hypercholesterolemia on risk of cardiovascular disease in older adults but the mechanism is still poorly understood (Iverson et al, 2009).
A history of otosclerosis was associated with the 5-year incidence of tinnitus (OR = 8.85, 95% CI = 1.42, 55.14), but was not significantly associated with the 10-year incidence (HR = 2.90, 95% CI = 0.63, 13.38). Given the wide range of the CI, it is likely these unstable estimates reflect the small number of participants at risk for incident tinnitus who reported a history of otosclerosis at baseline (n=8). The low prevalence of otosclerosis prevents this condition from being considered a major risk factor for the development of tinnitus.
A history of smoking was associated with an increased 10-year risk of developing tinnitus. Although no association was detected at 5 years, some risks become apparent with longer duration of exposure or longer time since exposure. The fact that increased years since smoking cessation was associated with a lower risk of developing tinnitus strengthens the evidence that smoking may be a modifiable risk factor for developing tinnitus. For those who had quit smoking 1–9 years previously, a higher mean number of packs of cigarettes smoked per day may account for some of the increased risk of developing tinnitus shown in Figure 2. Smoking may be associated with tinnitus through damage to the microvasculature in the cochlea, either through direct toxicity or inflammation or may result in neural damage through similar mechanisms. Smoking also can result in increased susceptibility to infection (Arcavi & Benowitz, 2004), which in turn may lead to an increased risk of developing tinnitus. Alternatively, smoking has been associated with higher levels of anxiety (Morissette et al, 2007), which may increase awareness of tinnitus (Welch and Dawes, 2008).
Tinnitus has been linked with many diseases, including autoimmune diseases. In this study we found those with a history of arthritis had an increased risk of developing tinnitus. In the EHLS we could not reliably determine whether the reported arthritis was rheumatoid (an autoimmune condition), or osteoarthritis (a non-autoimmune degenerative condition). While it is possible that medications (especially salicylates) used for arthritis could exacerbate tinnitus, in this study we found no association between regular aspirin use and incidence of tinnitus. In an earlier cross-sectional study, data from the Supplement on Aging from the 1984 Health Interview Survey (Brown, 1990) showed those reporting “arthritis or rheumatism” had a 48% higher odds of reporting tinnitus.
Another new finding with 10 years of follow-up was a protective effect of moderate alcohol consumption (alcohol consumption was associated with a decreased risk of developing tinnitus). Light to moderate daily alcohol consumption has been shown to be associated with cardiovascular benefits, primarily through improvements in insulin sensitivity and high-density lipoprotein cholesterol (O’Keefe, Bybee, and Lavie, 2007), and therefore may also help to preserve microvascular health in the cochlea. We found the risk of developing tinnitus to be lower among those who consumed 141 grams of ethanol per week or more, which is roughly equivalent to 12 or more 12-ounce cans of light beer per week. One or two drinks per day has been shown to be associated with a reduction in all-cause mortality, as well as the risk of myocardial infarction, stroke, congestive heart failure and diabetes. However, consumption of more than two drinks per day is associated with numerous negative health effects (O’Keefe, Bybee, and Lavie, 2007). We found no association between history of heavy alcohol consumption and risk of tinnitus in this older population but this study may have been limited by the low rates of reported past heavy drinking in these older adults and the effects of mortality among heavy drinkers.
Obesity (Body Mass Index ≥ 30 kg/m2) was associated with a reduced risk of developing tinnitus in men, but not women. We know of no other studies that would help to explain a beneficial effect of obesity, and suspect that this may be a chance finding. Body Mass Index modeled continuously was not associated with incidence of tinnitus, nor were Body Mass Index quartiles. Future follow-up of this cohort will help to clarify this relation.
Age was not associated with the 5-year incidence of tinnitus (Nondahl et al., 2002), nor was it associated with 10-year incidence among men. There was a borderline negative association between age and the 10-year incidence of tinnitus for women. Older women may under-report tinnitus onset because they are attending to more serious health problems or they are less aware of the symptoms, or the pool of genetically susceptible women may be depleted over time. In any case, there is no evidence, in either women or men, of a positive statistically significant association between age and incidence of tinnitus among older adults. Although some earlier population-based cross-sectional epidemiologic studies have demonstrated an increasing prevalence of tinnitus with age, prevalence estimates appear to level off or even decline at the oldest ages included in these studies (Davis and Rafaie, 2000; Hoffman and Reed, 2004). While tinnitus may be more common among older adults than young adults, our results suggest that this pattern may represent accumulating exposures rather than biological aging.
After adjusting for age and sex, noise exposure (from firearms, noisy hobbies, or noisy occupations) was not associated with the 10-year incidence of tinnitus. This is consistent with results from our previous study (Nondahl et al, 2002). One reason for the lack of association between noise exposure and incidence of tinnitus may be the low exposure to loud noise among this older population and the fact that noise exposure tends to decrease with age.
Since there is no universally accepted definition of tinnitus for research purposes, the definition used in this study may have led to some misclassification of tinnitus status. In addition, tinnitus may represent a heterogeneous disorder that can be caused by or exacerbated by many factors (Tyler et al, 2008), and those classified as incident cases in the current study only represent a segment of participants who have developed tinnitus at one time or another. Nonetheless, using the same standard definition over time (Nondahl et al, 2002) has allowed a meaningful assessment of the long-term incidence of tinnitus and its risk factors that has heretofore not been available.
This is the first long-term study of the incidence of tinnitus. Our findings suggest that the 10-year incidence of tinnitus remains high among older adults, but is not increasing at older ages. The pattern of risk factors (hearing loss, head injury, smoking, arthritis, and a protective effect of moderate alcohol consumption) suggest that tinnitus may be associated with vascular or neural damage perhaps through inflammatory processes. Although studies of tinnitus are hampered by relying on reported symptoms and the likely heterogeneity of pathways, this study provides some important evidence that modifiable factors may contribute to risk of developing tinnitus. It appears that avoiding or stopping smoking and preventing head injuries may lead to a reduction in the long-term risk of developing tinnitus.
Acknowledgments
This research was supported by National Institutes of Health grants AG11099 (KJC) and EY06594 (RK, BEKK).
Abbreviations
- EHLS
Epidemiology of Hearing Loss Study
- CI
Confidence Interval
- HR
Hazard ratio
Footnotes
Declaration of Interest
The authors have no conflicts of interest pertaining to this work.
Presented in part at the American Auditory Society Annual Meeting, Scottsdale, Arizona, March 6–8, 2008.
References
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- Brown SC. GRI monograph series A, number 2. Washington (DC): Gallaudet University; 1990. Older Americans and tinnitus: a demographic study and chartbook. [Google Scholar]
- Carlsson CM, Nondahl DM, Klein BEK, McBride PE, Sager MA, et al. Increased atherogenic lipoproteins are associated with cognitive impairment. Alzheimer Dis Assoc Disord. 2009;23(1):11–17. doi: 10.1097/wad.0b013e3181850188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;B34:187–220. [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. Am J Epidemiol. 1998;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BEK, Klein R, et al. The 5-year incidence and progression of hearing loss: the Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- Davis A, Refaie AE. Chapter 1: Epidemiology of Tinnitus. In: Tyler RS, editor. Tinnitus Handbook. Clifton, NY: Delmar Learning; 2000. pp. 1–23. [Google Scholar]
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Reed GW. Chapter 3: Epidemiology of Tinnitus. In: Snow J Jr, editor. Tinnitus: Theory and Management. Hamilton, Canada: BC Decker; 2004. pp. 16–42. [Google Scholar]
- Iversen A, Jensen JS, Scharling H, Schnohr P. Hypercholesterolaemia and risk of coronary heart disease in the elderly: impact of age: the Copenhagen City Heart Study. Eur J Intern Med. 2009 Mar;20(2):139–44. doi: 10.1016/j.ejim.2008.06.003. 2008. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Linton KLP, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Klein BEK. Statin use and the five-year incidence and progression of age-related macular degeneration. Am J Ophthalmol. 2007;144:1–6. doi: 10.1016/j.ajo.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis RJ, Heyman ER, Koch GG. Average partial association in three-way contingency tables: A review and discussion of alternative tests. Int Stat Rev. 1978;46:237–254. [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychol Bull. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Bush TL, Hale WE. Aging and total cholesterol levels: cohort, period, and survivorship effects. Am J Epidemiol. 1992 Jul 1;136(1):23–34. doi: 10.1093/oxfordjournals.aje.a116417. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BEK, et al. Prevalence and 5-year incidence of tinnitus among older adults: The Epidemiology of Hearing Loss Study. J Am Acad Audiol. 2002;13:323–331. [PubMed] [Google Scholar]
- Nuttall AL, Meikle MB, Trune DR. Chapter 5: Peripheral Processes Involved in Tinnitus. In: Snow J Jr, editor. Tinnitus: Theory and Management. Hamilton, Canada: BC Decker; 2004. pp. 52–68. [Google Scholar]
- O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R, Coelho C, Tao P, Ji H, Noble W, et al. Identifying tinnitus subgroups with cluster analysis. Am J Audiol. 2008;17:S176–S184. doi: 10.1044/1059-0889(2008/07-0044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D, Dawes PJD. Personality and perception of tinnitus. Ear Hear. 2008;29:684–692. doi: 10.1097/AUD.0b013e318177d9ac. [DOI] [PubMed] [Google Scholar]