Abstract
Toll/Interleukin-1 like receptors are evolutionarily conserved proteins in eukaryotes that play crucial role in pathogen recognition and innate immune responses. Brucella are facultative intracellular bacterial pathogens causing brucellosis in animal and human hosts. Brucella behaves as a stealthy pathogen by evading the immune recognition or suppressing the TLR signaling cascades. Brucella encode a TIR domain containing protein, TcpB, which suppresses NF-κB activation as well as proinflammatory cytokine secretion mediated by TLR2 and TLR4 receptors. TcpB targets the TIRAP mediated pathway to suppress TLR signaling. With the objective of detailed characterization, we have over expressed and purified TcpB from Brucella melitensis in native condition. The purified protein exhibited lipid binding properties and cell permeability. NF-κB inhibition property of endogenous TcpB has also been demonstrated. The data provide insight into the mechanism of action of TcpB in the intracellular niche of Brucella.
Keywords: Brucella, TcpB, TIR domain, NF-κB, Phosphoinositide, Inflammation
Introduction
Innate immunity is the first level of defense against an invading pathogen and pathogen recognition is mediated by a family of trans-membrane receptors called Toll/interleukin-1 like receptors (TLR) [1; 2; 3]. TLRs are characterized by an extra cellular leucine-rich repeat (LRR) domain and an intracellular Toll/IL-1 receptor-like (TIR) domain [3; 4]. The TIR domain is the conserved intracellular domain of TLRs and the downstream adapter molecules MyD88, TIRAP, TRAM, TRIF and SARM [5]. Recognition of Pathogen Associated Molecular Patterns by the LRR domain of TLRs leads to the formation of a signaling complex at the cytoplasmic face which culminates into the activation of transcription factors including NF-κB and the up regulation of several pro-inflammatory cytokines [1; 6].
Microbes have evolved diverse range of strategies to subvert the innate and adaptive immune responses [7; 8]. One of those strategies involves the interference of TLR signaling pathways using microbe encoded TIR domain containing proteins. Subversion of innate immune responses by TIR proteins has been described in Brucella, Salmonella, E.coli and Paracoccus [8; 9; 10; 11]. Brucella are highly infectious intracellular bacteria causing brucellosis in animal and human hosts [12]. Brucella replicate and survive in a variety of host cells including professional and non-professional phagocytes [13; 14]. Brucella minimally induce pro-inflammatory cytokines in the host, which is attributed to the capacity of this pathogen to evade or suppress innate immune responses [15]. Brucella encodes a TIR domain containing protein (TcpB) which efficiently suppresses TLR2 and TLR4 mediated NF-κB activation and pro-inflammatory cytokine secretion [8; 10; 11; 16]. B.melitensis deficient in the TcpB gene presented an attenuated phenotype in IRF−/− mice [11]. Recent studies have revealed that TcpB targets the TLR adaptor protein, TIRAP (also known as MAL) to inhibit TLR signaling but the exact mechanism is yet to be understood [11; 16].
Large scale production and purification of TIR protein from an infectious pathogen has not been previously successful hampering the detailed biochemical and structural characterization. To facilitate the in depth analysis of TcpB, we have successfully over expressed TcpB of B.melitensis in E.coli as a maltose binding protein (MBP) fusion. We have purified the protein in large quantities in non-denaturing condition and the activity of the purified protein has been verified by biochemical assays. We have demonstrated that TcpB is a cell permeable protein and the internalized TcpB could efficiently inhibit NF-κB activation induced by TLR4 receptor.
Materials and methods
Cloning and over expression of TcpB
Full-length TcpB gene was amplified from the chromosome of B.melitensis by PCR using TcpB.F (GAATTCATGTCTAAAGAGAAACAAGCC) and TcpB.R (CTGCAGTCAGATAAGGGAATGCAG) primers. The PCR amplicon was gel purified and cloned into pGEM-T vector (pGEMTcpB) and sequenced to verify the identity. TcpB gene was then released from pGEMTcpB with BamHI/ EcoRI and cloned into the corresponding sites of pMALc4G E.coli expression vector (NEB) to generate pMALTcpB. BL21 DE3 cells were transformed with pMALTcpB construct and the transformants were analyzed for MBP-TcpB expression. To perform this, E. coli BL21 cells harboring the pMALTcpB plasmid were grown at 37° C and induced with IPTG to a final concentration of 0.5 mM when the OD600 reached 0.6. After the induction, cells were grown at the same temperature for 3 hrs. Cells were then spun down, re-suspended in water and mixed with SDS PAGE sample buffer (BioRad) (1:1 ratio). The tubes were boiled for 5 min and a fraction of the boiled protein sample was analyzed by SDS PAGE.
To perform the western blotting, a fraction of the uninduced and induced protein samples were resolved on SDS PAGE gel. Protein bands were then electroblotted to nitrocellulose membrane (BioRad). The membrane was then blocked with 5% milk for 1 hr and probed with monoclonal anti-MBP antibody conjugated with HRP (NEB) overnight. SuperSignal West Pico Chemiluminescent Substrate (Pierce) was used to detect the reaction and the signal was captured on X-ray film.
Purification of MBP-TcpB
Overnight grown E. coli BL21 cells harboring the pMALTcpB plasmid was inoculated (0.1%) into 1L of LB medium with glucose (2g) and ampicillin (100 µg/mL). The culture was grown at 37° C and induced with IPTG to a final concentration of 0.5 mM when the OD600 reached 0.6. After the induction, cells were grown at 25° C for 5 hrs. Amylose affinity chromatography was employed for purification. Cells were collected by centrifugation and washed with phosphate buffered saline. Cells were then resuspended in sonication buffer containing 50 mM Tris-HCI [pH 8.0], 1M NaCI, 1mM EDTA and 1X protease inhibitor cocktail (Pierce). Cells were sonicated and then centrifuged at 16000 × g for 20 min to clarify the supernatant. The supernatant was collected and passed through a column harboring 5mL of amylose resin (NEB). The column was then washed with the sonication buffer followed by the same buffer containing decreasing concentrations of NaCI (750, 500, 250 and 100 mM). Protein elution was performed with elution buffer containing 50 mM Tris-HCI [pH 8.0] and 30 mM maltose. The eluted protein was passed through an SP Sepharose (Sigma) column. The column was washed with 10 column volumes of 50 mM Tris-HCI [pH 8.0] and eluted with elution buffer containing 50 mM Tris-HCI and 1M NaCI. The eluted protein was concentrated using centricon protein concentrator (Millipore) and dialyzed in a buffer containing 50 mM Tris-HCI, 100 mM NaCI and 1mM EDTA.
Separation of TcpB from MBP by Genenase I digestion
One hundred micrograms of MBP-TcpB fusion protein was incubated with 1 µg of Genenase I (NEB) at room temperature for 8 hrs. To separate the digested products, ion exchange chromatography was employed. The digested sample was diluted with column buffer (50 mM Tris-HCl [pH 8] and 1mM EDTA) and applied to a column containing 3 mL SP Sepharose (Sigma). The column was then washed with 10 column volumes of buffer. The bound protein was eluted with column buffer containing increasing concentrations of NaCI. The fractions were collected and analyzed by SDS PAGE. TcpB containing fractions were pooled and concentrated. Purified TcpB was then dialyzed against the buffer containing Tris-HCI [pH 8.0], 100 mM NaCI and 50% glycerol and stored in −20 freezer as aliquots.
Analysis of PIP binding property of purified MBP-TcpB fusion protein
Phosphoinositide phosphate (PIP) binding of refolded MBP-TcpB fusion protein was performed as described [11]. PIP strips (Echelon) were blocked with blocking buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 0.1% Tween 20 and 0.1 % ovalbumin) for 1 hr and then probed with 500 ng of purified MBP-TcpB fusion protein for 3 hrs at 25° C in the presence of anti-MBP monoclonal antibody conjugated with HRP (NEB). The bound protein was detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce). To perform liposome pull-down assays, 20 µl of 1 mM PolyPIPosomes (Echelon) were mixed with 1 µg of MBP-TcpB fusion protein in binding buffer (50 mM Tris [pH 8.0], 150 mM NaCl, and 0.05% Nonidet P-40) and rotated for 30 min at 25° C. The liposomes were washed 3 times with the binding buffer and analyzed by SDS-PAGE. The bound protein was detected by western blotting.
Analysis of cell permeable property of MBP-TcpB
RAW 264.7 cells (1 × 105) were seeded into 12 well plates and allowed to adhere over night. The medium (RPMI) was then replaced with fresh medium (0.5 ml) and increasing concentrations (5, 10, 30 & 50 µg/mL) of purified MBP-TcpB or MBP alone were added. The pates were incubated for 8 hrs at 37° C. The culture medium was then aspirated out and the wells were washed 5 times with PBS. Cells were then treated with 1X Trypsin-EDTA for 5 min. Adhered cells were scraped off and the cell suspension was transferred to 1 mL tubes and centrifuged to collect the cells. Cells were lysed with M-PER mammalian protein extraction reagent (Pierce). Cell lysates were clarified by centrifugation and mixed with SDS PAGE sample buffer. A fraction of the samples were resolved on SDS PAGE gel, and the presence of MBP-TcpB was detected by western analysis using anti-MBP antibody conjugated with HRP (NEB).
To analyze the inhibition of IκBα degradation, RAW cells were incubated with various concentrations (10, 30 & 50 µg/mL) of purified MBP-TcpB for 8 hrs. Cells were then induced with 100 ng/mL of E.coli lipopolysaccharide (Sigma) overnight. After induction, cells were washed with PBS and lyzed with M-PER mammalian protein extraction reagent (Pierce). Clarified lysates were subjected to SDS PAGE and western blotting. After blocking with 5% milk the membrane was probed with anti-IκBα (Cell Signaling Technology) and HRP conjugated anti-mouse IgG (Pierce). The membrane was reprobed with anti-actin antibody (Santa Cruz Biotechnology) to detect actin, which served as the loading control.
Results and Discussion
Over expression and purification of MBP-TcpB
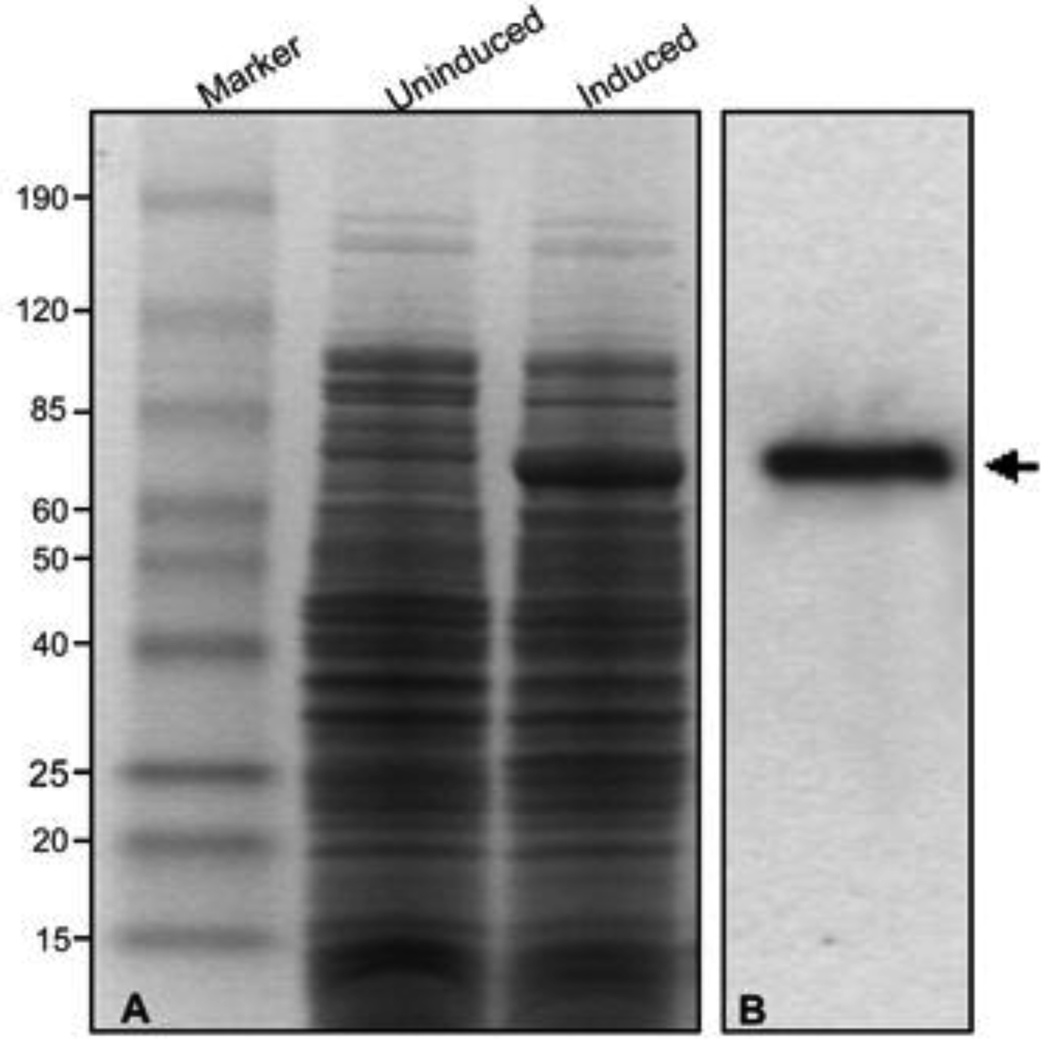
We have over expressed TcpB in E.coli as poly-histidine (HIS), glutathione-S transferase (GST) or MBP as the fusion partners. Both the HIS and GST tags generated insoluble proteins that hampered the subsequent purification of the native fusion protein in large quantities. Therefore over expression and purification was attempted using MBP as the fusion partner. MBP is a highly soluble protein and enables the native purification of the fusion protein by virtue of its affinity to amylose and maltose. pMAL-c4G vector was employed for cloning and expression of TcpB. In the pMALc4G expression system the fusion protein is expressed in the cytoplasm and the protein of interest could be cleaved from MBP with the specific protease Genenase I. TcpB gene was sub cloned into the pMalc4G vector and the construct was transformed into BL21 DE3 cells. The IPTG induced transformants expressed the fusion protein of ~ 70 kDa in sufficient quantities, and the identity of the expressed protein was confirmed as MBP-TcpB by western analysis (Fig. 1). Over expressed MBP-TcpB appeared soluble as the major fraction present in the supernatant after sonication and centrifugation of the induced cell lysate (Fig. 2A; Lane 5).
Figure 1. Over expression of MBP-TcpB.
Panel A: Uninduced and Induced E.coli (BL21) cells harboring pMal-TcpB construct; Panel B: Confirmation of the over expressed MBPTcpB by western analysis using anti-MBP antibody. Arrow indicates MBP-TcpB.
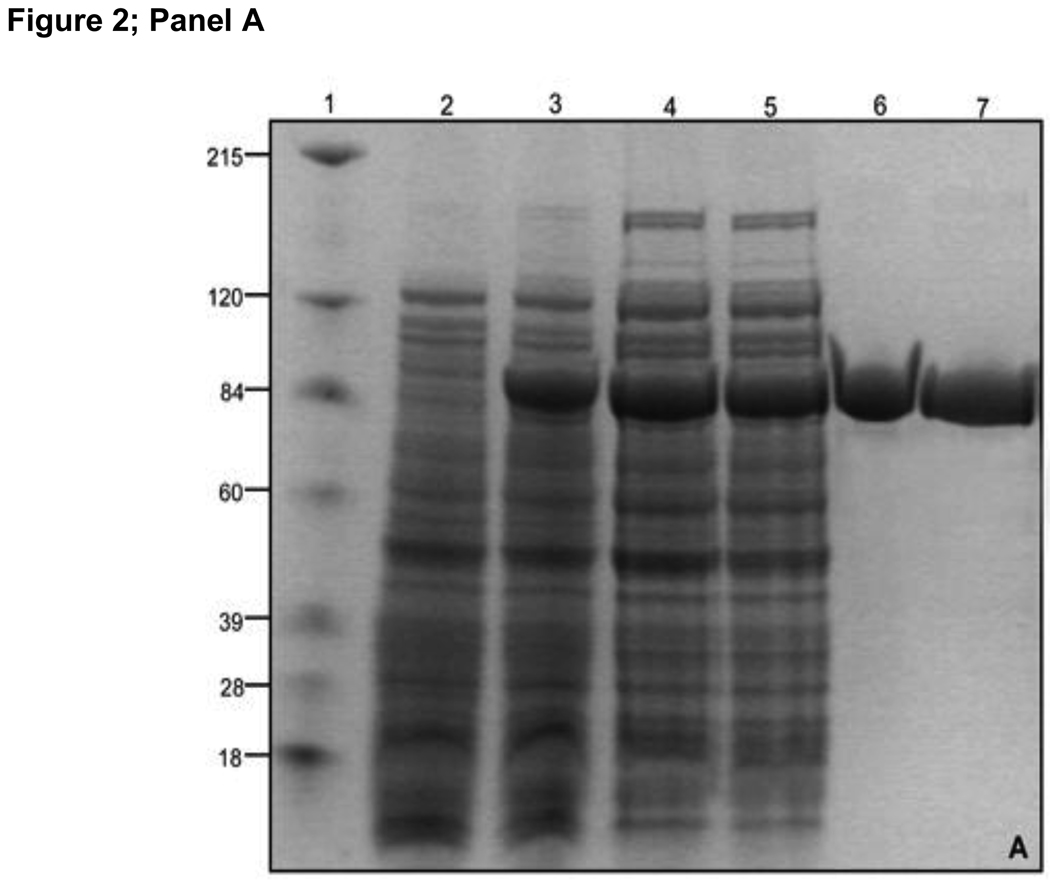
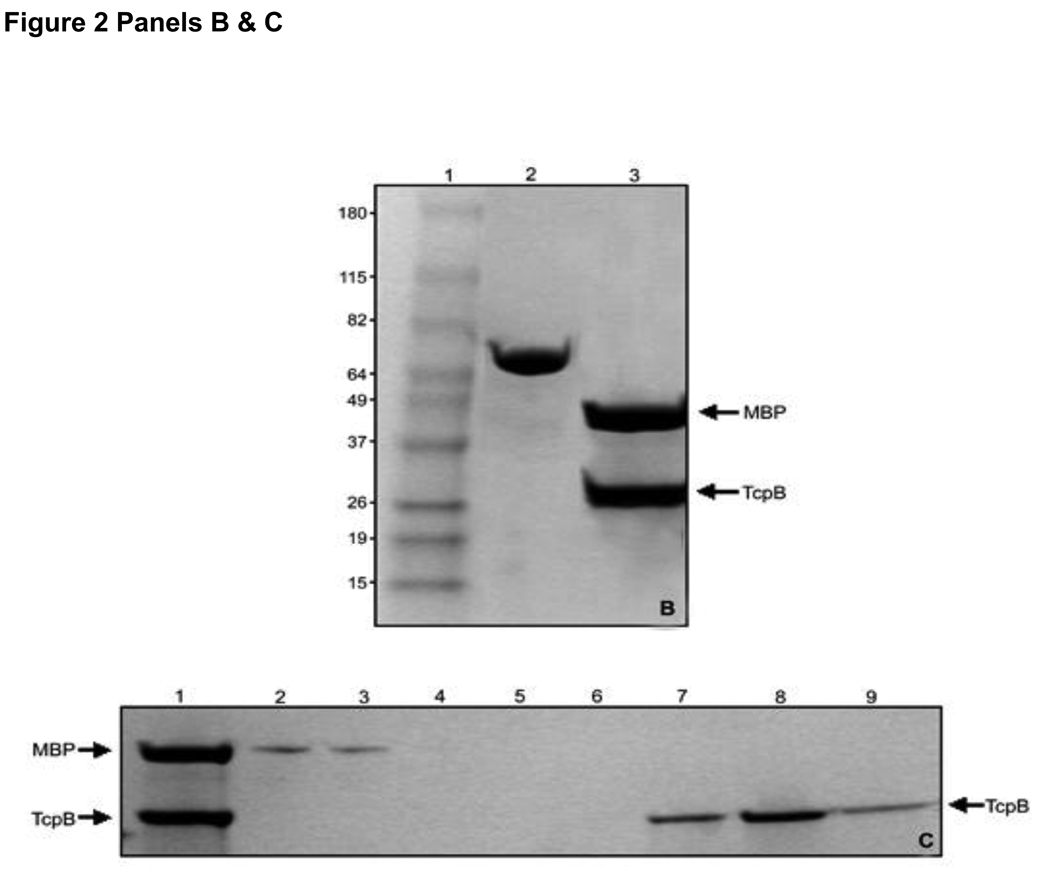
Figure 2. Purification of MBP-TcpB.
Panel A: Purification of MBP-TcpB by amylose affinity chromatography. Lanes 1) Marker; 2) Uninduced cells; 3) Induced cells; 4) Induced cells after sonication; 5) Cell supernatant after sonication and centrifugation; 6) Purified MBP-TcpB after the amylose affinity chromatography; 7) MBP-TcpB after ion exchange. Panel B: Genenase I digestion of MBP-TcpB. Lanes 1) Marker; 2 & 3) MBP-TcpB before and after Genenase I digestion, respectively. Panel C: Separation of MBP and TcpB by ion exchange chromatography. Lanes 1) MBP-TcpB digested with Genenase I; 2) Flow through fraction; 3 & 4) Washed fractions; Lanes 5 to 9 are eluted fractions with 50, 100, 150, 200 & 250 mM NaCI, respectively. The eluted TcpB is indicted by arrow.
Amylose affinity chromatography was employed for purification of the soluble fraction of MBP-TcpB. MBP-TcpB bound to the amylose column was washed with buffer containing decreasing concentrations of NaCI to achieve the maximum purity by removing unbound E.coli proteins. The eluted MBP-TcpB appeared to be nearly homogenous as indicated by Coomassie stained PAGE gel (Fig 2A; lane 6). Next we removed the bound and free maltose from the eluted protein by ion exchange chromatography as dialysis was not an efficient method to remove the MBP bound maltose. MBP-TcpB was allowed to bind the SP Sepharose resin and the maltose was washed away. After eluting the MBP-TcpB with a buffer containing 1M NaCI (Fig 2A; lane 7), the fusion protein was subjected to dialysis to decrease the NaCI concentration to 100 mM. The purified MBP-TcpB appeared to be stable and soluble and there was no aggregation when concentrated.
Separation of TcpB from MBP
Initially we purified MBP-TcpB with a Factor Xa protease site between MBP-TcpB. When we attempted the digestion of MBP-TcpB with Factor Xa, it cleaved the TcpB internally without having any obvious Factor Xa sites in the TcpB. Therefore we purified the MBP-TcpB with a Genenase I protease site to achieve the separation of TcpB from MBP. Genenase I cleaved the MBP-TcpB precisely and completely (Fig. 2B). SP Sepharose ion exchange chromatography was employed for separating TcpB from MBP. MBP did not bind to the SP Sepharose resin as indicated its presence in the flow through and the wash fractions (Fig. 2C). TcpB was eluted between the fractions containing 150–250mM NaCI concentrations (Fig. 2C).
Affinity of MBP-TcpB to phosphoinositides
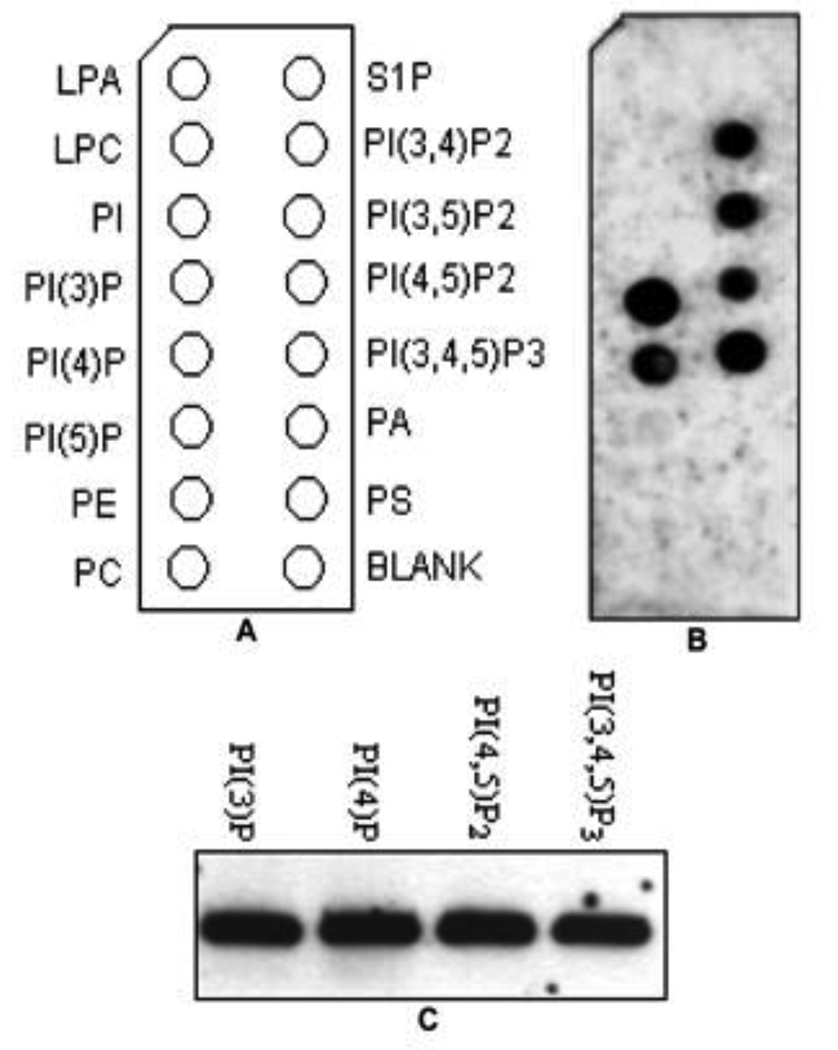
Our previous studies indicated that TcpB is a phosphoinositides-binding protein [11]. Therefore PIP binding property of TcpB was analyzed to determine the activity of purified MBP-TcpB fusion protein. PIP binding was tested by incubating MBP-TcpB with nitrocellulose strips spotted with various lipids. The bound protein was detected by western analysis using anti-MBP antibody (Fig.3B). Liposome pull-down assay was employed for the confirmation of PIP binding (Fig. 3C). MBP-TcpB could be pulled down with lyposomes, indicating the strong affinity of TcpB to the PIP. PIP binding assays clearly indicated that the purified MBP-TcpB had folded correctly and the fusion protein is biologically active.
Figure 3. Analysis of PIP binding of purified MBP-TcpB by PIP strip binding and pull-down assays.
Planel A indicates the identity of the phospholipids on the PIP strips (LPA - Lysophosphatidic Acid, LPC - Lysophosphocholine, PIP – Phosphoinositide Phosphates, PE - Phosphatidylethanolamine, PC - Phosphatidylcholine, S1P - Sphingosine-1-Phosphate, PA - Phosphatidic Acid, PS - Phosphatidyl Serine); Panel B shows the binding of purified MBP-TcpB to the phosphoinositides on the PIP strip. Panel C indicates the confirmation of PIP binding by PolyPIPosome pull-down assays.
Uptake of MBP-TcpB by macrophages
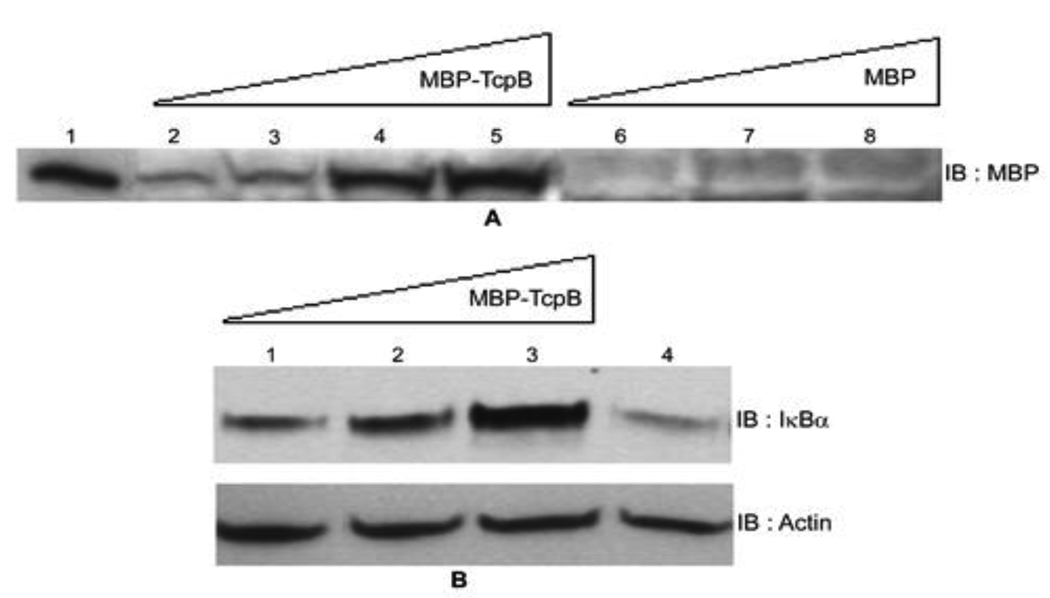
TcpB harbors a basic amino acid rich lipid-binding domain at the N-terminus and a TIR domain at the C-terminus [11]. TcpB co-localized with the plasma membrane and the cytoskeleton when expressed in eukaryotic cells. Lipid binding property of TcpB was attributed to the N-terminal cationic motif as the motif alone could bind to PIP and co-localized with the plasma membrane. Point mutations at the cationic motif of TcpB resulted diminished lipid binding [11]. The proteins or peptides that harbor the endogenous cationic motifs have been shown to exhibit different extent of cell permeable properties [17]. The cationic amino acid residues bind to the cell surface and the entrapped protein is taken up by the endocytic pathway. Since TcpB carries a cationic motif; we wished to analyze the internalization of TcpB by eukaryotic cells. We incubated murine macrophages with various concentrations of purified MBP-TcpB and analyzed the translocated MBP-TcpB by western blotting. Remarkably, MBP-TcpB could be detected inside the cells in a dose dependent manner (Fig. 4A). Internalization was not observed in cells incubated with MBP alone which indicated that the cell permeable property is attributed to TcpB (Fig. 4A).
Figure 4. TcpB is a cell permeable protein.
Panel A: Internalization of MBP-TcpB by RAW macrophages. Lanes 1) Purified MBP-TcpB; 2, 3, 4 & 5) RAW cells incubated with increasing concentrations (5, 10, 30 & 50 µg, respectively) of MBP-TcpB; 6, 7 & 8) RAW cells incubated with increasing concentrations (10, 30 & 50 µg, respectively) of MBP alone. Panel B: Inhibition of IκBα degradation by internalized TcpB. Lanes 1, 2 & 3) RAW cells incubated with 10, 30 and 50 µg of MBP-TcpB, respectively; 4) RAW cells with 30 µg of MBP alone. Actin was used as the loading control (bottom panel).
Anti-inflammatory property of internalized MBP-TcpB
Studies have indicated that TcpB inhibited TLR2 and TLR4 mediated NF-κB activation [8; 10; 11]. We analyzed the inhibition of NF-κB activation by internalized MBP-TcpB. The transcription factor NF-κB consists of homo- or heterodimers of different subunits that are sequestered in the cytosol of unstimulated cells via interactions with a class of inhibitor proteins, Inhibitory Kappa Bs (IκBs) [18]. Signals that induce NF-κB activity cause the phosphorylation of IκBs leading to their dissociation and subsequent degradation, allowing NF-κB proteins to enter the nucleus and induce gene expression. Therefore the degradation of IκB in stimulated cells would indicate the activation of NF-κB. We have analyzed the degradation of IκBα in TcpB internalized macrophages. Macrophages were incubated with increasing concentration of MBP-TcpB or MBP alone. The NF-κB activation was stimulated by induction of TLR4 using E.coli lipopolysaccharide. Enhanced degradation of IκBα was observed in cells incubated with MBP alone where as MBP-TcpB incubated cells displayed elevated levels of IκBα (Fig. 4B). The analyses indicate that internalized MBP-TcpB is capable of inhibiting the dissociation and degradation of IκBα that could lead to the inhibition of NF-κB activation. TcpB co-localizes with the plasma membrane and microtubules and targets membrane localized TIRAP to inhibit the NF-κB activation [11; 16]. Therefore TcpB may exit the endocytic vesicles after the internalization and translocate to the site of TLR signaling machinery to inhibit NF-κB activation. Brucella reside in macrophages in an infected host and their intracellular niche is composed of membranous vesicles [19]. TcpB may be secreted by Brucella in these vesicular compartments and TcpB subsequently cross the vesicular membrane to get access to the target proteins.
In conclusion we have successfully over expressed and purified TcpB in large quantities in native condition to facilitate its detailed characterization. The purified TcpB retained the biological activities as demonstrated by its affinity towards PIP. TcpB was readily internalized by macrophages and the intracellular TcpB exhibited anti-inflammatory activity. The study illustrates that the membrane affinity and permeability properties of TcpB likely facilitate its trafficking from Brucella containing vesicles to the vicinity of the TLR signaling pathway to suppress NF-κB and inflammatory responses.
Acknowledgements
This work was supported by NIH/NIAID GLRCE for Biodefense and Emerging Infectious Disease Research Program grant 1U54-AI-057153, NIH 1R01AI073558, NIH R21 AI088038 and BARD grant US-3829-06 R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takeda K, Kaisho T, Akira S. Toll-Like Receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]; Akira S. Tlr Signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 2.Pasare C, Medzhitov R. Toll-Like Receptors: Linking Innate and Adaptive Immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]; Kaisho T, Akira S. Critical Roles of Toll-Like Receptors in Host Defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-Like Receptors: Critical Proteins Linking Innate and Acquired Immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Miggin SM, O'Neill LA. New Insights into the Regulation of Tlr Signaling. J Leukoc Biol. 2006;80:220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill LA, Bowie AG. The Family of Five: Tir-Domain-Containing Adaptors in Toll-Like Receptor Signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural Basis for Signal Transduction by the Toll/Interleukin-1 Receptor Domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- 7.Bowie AG, Unterholzner L. Viral Evasion and Subversion of Pattern-Recognition Receptor Signalling. Nat Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. Subversion of Toll-Like Receptor Signaling by a Unique Family of Bacterial Toll/Interleukin-1 Receptor Domain-Containing Proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 9.Newman RM, Salunkhe P, Godzik A, Reed JC. Identification and Characterization of a Novel Bacterial Virulence Factor That Shares Homology with Mammalian Toll/Interleukin-1 Receptor Family Proteins. Infect Immun. 2006;74:594–601. doi: 10.1128/IAI.74.1.594-601.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel JP. Brucella Control of Dendritic Cell Maturation Is Dependent on the Tir-Containing Protein Btp1. PLoS Pathog. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan GK, Yu Q, Harms JS, Splitter GA. Brucella Tir Domain-Containing Protein Mimics Properties of the Toll-Like Receptor Adaptor Protein Tirap. J Biol Chem. 2009;284:9892–9898. doi: 10.1074/jbc.M805458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trujillo IZ, Zavala AN, Caceres JG, Miranda CQ. Brucellosis. Infect Dis Clin North Am. 1994;8:225–241. [PubMed] [Google Scholar]; Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 13.Gorvel JP, Moreno E. Brucella Intracellular Life: From Invasion to Intracellular Replication. Vet Microbiol. 2002;90:281–297. doi: 10.1016/s0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 14.Celli J, Salcedo SP, Gorvel JP. Brucella Coopts the Small Gtpase Sar1 for Intracellular Replication. Proc Natl Acad Sci U S A. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzman-Verri C, Chacon-Diaz C, Rucavado A, Moriyon I, Moreno E. Brucella Abortus Uses a Stealthy Strategy to Avoid Activation of the Innate Immune System During the Onset of Infection. PLoS One. 2007;2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kohler S, Michaux-Charachon S, Porte F, Ramuz M, Liautard JP. What Is the Nature of the Replicative Niche of a Stealthy Bug Named Brucella? Trends Microbiol. 2003;11:215–219. doi: 10.1016/s0966-842x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta D, Koblansky A, Gaines J, Brown T, West AP, Zhang D, Nishikawa T, Park SG, Roop RM, 2nd, Ghosh S. Subversion of Innate Immune Responses by Brucella through the Targeted Degradation of the Tlr Signaling Adapter, Mal. J Immunol. 2010;184:956–964. doi: 10.4049/jimmunol.0902008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogoyevitch MA, Kendrick TS, Ng DC, Barr RK. Taking the Cell by Stealth or Storm? Protein Transduction Domains (Ptds) as Versatile Vectors for Delivery. DNA Cell Biol. 2002;21:879–894. doi: 10.1089/104454902762053846. [DOI] [PubMed] [Google Scholar]; Low W, Mortlock A, Petrovska L, Dottorini T, Dougan G, Crisanti A. Functional Cell Permeable Motifs within Medically Relevant Proteins. J Biotechnol. 2007;129:555–564. doi: 10.1016/j.jbiotec.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vives E. Cellular Uptake [Correction of Utake] of the Tat Peptide: An Endocytosis Mechanism Following Ionic Interactions. J Mol Recognit. 2003;16:265–271. doi: 10.1002/jmr.636. [DOI] [PubMed] [Google Scholar]
- 18.Wan F, Lenardo MJ. The Nuclear Signaling of Nf-Kappab: Current Knowledge, New Insights, and Future Perspectives. Cell Res. 20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arenas GN, Staskevich AS, Aballay A, Mayorga LS. Intracellular Trafficking of Brucella Abortus in J774 Macrophages. Infect Immun. 2000;68:4255–4263. doi: 10.1128/iai.68.7.4255-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Brucella Abortus Transits through the Autophagic Pathway and Replicates in the Endoplasmic Reticulum of Nonprofessional Phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]