Abstract
Consumption of sugar-sweetened beverages may be one of the dietary causes of metabolic disorders, such as obesity. Therefore, substituting sugar with low-calorie sweeteners may be an efficacious weight management strategy. We tested the effect of preloads containing stevia, aspartame, or sucrose on food intake, satiety, and postprandial glucose and insulin levels. Design: 19 healthy lean (BMI = 20.0 – 24.9) and 12 obese (BMI = 30.0 – 39.9) individuals 18 to 50 years old completed three separate food test days during which they received preloads containing stevia (290 kcal), aspartame (290 kcal), or sucrose (493 kcal) before the lunch and dinner meal. The preload order was balanced, and food intake (kcal) was directly calculated. Hunger and satiety levels were reported before and after meals, and every hour throughout the afternoon. Participants provided blood samples immediately before and 20 minutes after the lunch preload. Despite the caloric difference in preloads (290 vs. 493 kcals), participants did not compensate by eating more at their lunch and dinner meals when they consumed stevia and aspartame versus sucrose in preloads (mean differences in food intake over entire day between sucrose and stevia = 301 kcal, p < .01; aspartame = 330 kcal, p < .01). Self-reported hunger and satiety levels did not differ by condition. Stevia preloads significantly lowered postprandial glucose levels compared to sucrose preloads (p < .01), and postprandial insulin levels compared to both aspartame and sucrose preloads (p < .05). When consuming stevia and aspartame preloads, participants did not compensate by eating more at either their lunch or dinner meal and reported similar levels of satiety compared to when they consumed the higher calorie sucrose preload.
Keywords: Stevia, Aspartame, Sucrose, Food Intake, Satiety, Hunger, Insulinogenic Index, Insulin Sensitivity
Introduction
The twin epidemics of obesity and Type 2 diabetes continue to increase in industrialized nations. Approximately two thirds of adult Americans are currently overweight or obese and therefore at increased risk for a number of deleterious health conditions including Type 2 diabetes, heart disease, and cancer (Roth, Qiang, Marban, Redelt, & Lowell, 2004). Although there is not specific evidence that sucrose, a disaccharide that consists of 50% glucose and 50% fructose, consumption affects the development of diabetes (Laville & Nazare, 2009), diets consisting of high amounts of sucrose have been found to cause weight gain (Raben, Vasilaras, Moller, & Astrup, 2002) and to have adverse effects on glucose tolerance in healthy volunteers (Cohen, Teitelbaum, Balogh, & Groen, 1966). Overconsumption of fructose has also been found to cause dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes (Le et al., 2009), as well as increase visceral adiposity and decrease insulin sensitivity in overweight individuals (Stanhope et al., 2009). In animal models, high glycemic diets and high consumption of the natural sugar fructose have been shown to induce a number of metabolic complications including hyperinsulinemia, hyperglycemia, hypertension, and insulin resistance (Barros et al., 2007). Moreover, recent human studies demonstrate that fructose infusions can induce hepatic insulin resistance (Wei, Wang, Topczewski, & Pagliassotti, 2007).
The consumption of added sugars in the United States has increased by almost 20% over the past few decades with current consumption estimated to be 142 lbs per person per year (Wells & Buzby, 2008). Consumption of sugar-sweetened foods and beverages can significantly influence the glycemic index of each meal, as well as the diet as a whole (Willett, Manson, & Liu, 2002). Moreover, excessive intake of high calorie, high glycemic food can result in exaggerated postprandial glucose and insulin levels and potentially lead to metabolic and hormonal changes that stimulate hunger levels and promote fat deposition (O’Keefe & Bell, 2007). In line with this, studies to date suggest that the consumption of sugar-sweetened beverages promotes positive energy balance, weight gain, and increases risk for Type 2 diabetes (Malik, Schulze, & Hu, 2006; Schulze et al., 2004). Based on accumulating evidence suggesting sucrose-sweetened beverages and high sucrose diets have adverse effects on body weight (e.g., Johnson et al., 2007) and are associated with other medical complications, such as diabetes, cardiovascular disease, and cancer, the American Heart Association recently released a statement recommending discretionary sugar intake be limited to just over 30 grams (100 calories) per day for average-sized women and just over 45 grams (150 calories) for average-sized men (Mitka, 2009).
The consumption of foods and beverages containing nonnutritive sweeteners has dramatically increased over the past few decades, and approximately 15% of the U.S. population are estimated to consume nonnutritive sweeteners (Mattes & Popkin, 2009). Findings have been mixed regarding the effects that nonnutritive sweeteners, particularly aspartame, have on energy intake and body weight. Most studies indicate that aspartame reduces food intake and may assist with weight control (Dela Hunty, Gibson, & Ashwell, 2006). Other studies, however, suggest that aspartame may paradoxically stimulate appetite (Blundell & Hill, 1986) and thereby lead to weight gain (Swithers & Davidson, 2008). A recent review of the effect of nonnutritive sweeteners on appetite concluded that “If nonnutritive sweeteners are used as substitutes for higher energy yielding sweeteners, they have the potential to aid in weight management, but whether they will be used in this way is uncertain (Mattes & Popkin, 2009),” however, given the mixed findings at present, there is currently no official recommendation regarding the use of nonnutritive sweeteners for weight control.
Stevia, the common name for the extract stevioside from the leaves of Stevia rebaudiana Bertoni, is a natural, sweet-tasting calorie free botanical that may also be used as a sugar substitute or as an alternative to artificial sweeteners. Stevia has been found to increase insulin sensitivity in rodent models (Chang, Wu, Liu, & Cheng, 2005) and to have beneficial effects on blood glucose and insulin levels in human studies (Curi 1986; Gregersen, Jeppesen, Holst, & Hermansen, 2004), which suggests it may have a role in food intake regulation. In safety studies, no negative side effects were reported Barriocanal, 2008). Stevia was recently approved for use as a sweetener by the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (Joint Food and Agriculture Organization/World Health Expert Committee on Food Additives, 2005), and has also recently received GRAS approval from the Food and Drug Administration. Stevia is inexpensive and available to most consumers; thus, it has the potential to be widely used and may assist individuals in regulating their weight if it has a positive effect on caloric substitution. However, no study to date has examined the effect stevia has on food intake and satiety levels.
Given the high consumption of sucrose and sucrose-sweetened soft drinks, as well as the increasing consumption of food and beverages sweetened with NNS, studies are needed to examine the effects different sweeteners have on food intake, satiety, and blood glucose/insulin levels. Thus, the present study tested the effects of preloads containing stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels in both lean and obese individuals.
Subjects and Methods
All studies were approved by the Institutional Review Board of the Pennington Biomedical Research Center (PBRC), Baton Rouge, LA. Two sets of participants were recruited for the present study: 1)19 lean individuals (BMI = 20.0 – 24.9 kg/m2) and 2) 12 obese individuals (BMI = 30 – 39.9 kg/m2) with waist circumferences of at least 36 inches for females and 40 inches for males.
Screening procedures
Potential participants attended a screening visit, during which they completed screening questionnaires, provided a blood sample (19 mL), and received a brief medical evaluation to identify any physical or psychological contraindications to participation in the study. Participants were also asked about potential obstacles for completing the study. Participants were required to meet the following inclusion criteria: 1) be a healthy man or woman with a BMI ≥ 20 kg/m2 and ≤ 24.9 kg/m2 or BMI ≥ 30 kg/m2 and ≤ 39.9 kg/m2 with waist circumference ≥ 36 for women and ≥ 40 for men, 2) be ≥18 years of age and <50 years of age, 3) be a nonsmoker, and 4) for females, premenopausal.
Participants were excluded for any of the following reasons: 1) history of diabetes, cardiovascular disease, or other chronic illnesses, 2) taking medications other than monophasic birth control or monophasic hormone replacement therapy, 3) dislike of or allergy to foods/sweeteners (stevia, aspartame, or sucrose) used in preloads and test meals, 4) high scores on the Dietary Restraint (>14), Disinhibition (>14), or Perceived Hunger (>12) scales of the Eating Inventory (Stunkard, 1985), and 5) high score (≥ 30) on the Beck Depression Inventory (Beck, 1996). Participants were also excluded if they had a diagnosable eating disorder or were taking any medications or dietary supplements that could influence appetite, hunger, or satiety.
Study design and procedure
For each of the three test meal days, qualified participants arrived at the Center in the morning after a 12-hour fast and consumed a standard 469 kcal breakfast consisting of cereal, milk, toast with butter, and orange juice. Based on the results of a pilot study, a 400g preload of tea and crackers with cream cheese sweetened with stevia (Whole Foods 365 brand), aspartame (Equal sweetener), or sucrose was used in the present study. Participants consumed this preload twenty minutes before their test lunch and dinner meals. The order in which the preloads were provided to participants was balanced, and participants were blinded to the type of sweetener used in the preloads throughout the study. Since dietary factors, such as sucrose and low calorie sweeteners, may have direct, as well as indirect effects on caloric intake, a “preload-to-test meal” paradigm is needed to better understand the potential mechanisms through which sucrose and low calorie sweeteners may affect food intake. The paradigm used in the present study, which involved a high sucrose versus low calorie sweetener preload meal followed by a test meal after a predefined short interval, is particularly useful in controlling for variables related to energy density, caloric content, and caloric intake. Therefore, the “preload-to-test meal” paradigm was chosen because it represents a useful tool to identify potential mechanisms through which dietary factors, such as sucrose and low calorie sweeteners, may affect food intake.
The test lunch meal consisted of sandwiches, potato chips, and cookies, and the test dinner meal was a self-selected buffet-type meal (i.e., Macronutrient Self-Selection Paradigm) (Geiselman et al., 1998). For both the test lunch and dinner meals, participants were informed that they could eat as much or as little food as they liked. Participants reported their hunger and satiety levels on visual analog scales (VAS) before and after each meal, as well as 30 minutes and every hour after lunch throughout the afternoon. Participants also provided blood samples immediately before consuming the first preload and lunch meal, and at 30 minutes, one hour, and two hours after the test lunch meal. All participants completed three separate food test days, which were no fewer than two days apart. For females, all test meal days occurred during the luteal phase of their menstrual cycle.
Eating behavior measures
Food intake
Food intake was directly measured in the Ingestive Behavior Laboratory using Mettler (Columbus, Ohio) Toledo ISO 9001 scales.
Visual Analogue Scales (VAS)
Computerized VAS were used to assess subjective ratings of hunger, satiety, fullness, as well as hedonic ratings of food (i.e., appearance, aroma, flavor, texture and palatability). When completing the VAS, participants rate the intensity of these subjective states on a 100-unit line from “not at all” to “extremely.” Studies support the reliability and validity of VAS for measuring subjective states related to food intake (Geiselman et al, 1998; Flint, Raben, Blundell, & Astrup, 2000).
Menstrual Cycle Interview
The phase of female participants’ menstrual cycle was determined using standardized interviews assessing menstrual patterns developed by Dr. Paula Geiselman.
Macronutrient Self-Selection Paradigm (MSSP)
The MSSP (Geiselman et al., 1998) consists of a buffet-type meal composed of foods that vary in fat (high and low) and simple sugar, complex carbohydrate, and protein composition. In the MSSP, participants are presented with large portions of foods varying in macronutrient content. The food choices are prepared as a 2 (Fat factor: High Fat and Low Fat) X 3 (Other macronutrient factor: High Simple Sugar, High Complex carbohydrate [CCHO], and High Protein) design. This design yields the following six cells: High Fat/High Simple Sugar (HF/HS), High Fat/High Complex Carbohydrate (HF/HCCHO), High Fat/High Protein (HF/HP), Low Fat/High Simple Sugar (LF/HS), Low Fat/High Complex Carbohydrate (LF/HCCHO), and Low Fat/High Protein (LF/HP). Each food item in each of the three high-fat cells is ≥ 45% fat (expressed as percent of the total kJ in a given food). Foods in the HF/HS cell are ≥45% fat and ≥ 30% sugar, and foods in the HF/HCCO are ≥ 45% fat and ≥ 30% complex CHO. Foods in the HF/HP cell are ≥ 45% fat and ≥ 13% protein; however, most foods in this cell are 20–35% protein. Each food in each of the three low-fat cells is < 20% fat. Subjects are given three foods from each cell according to the 2 × 3 design; therefore, a total of 18 foods are provided to them. The 18 foods are selected based on each individual’s hedonic responses to a pretest list of 92 foods (Food Selection Questionnaire), each of which fit into one of the six cells of the MSSP. The MSSP has been found to be a reliable and valid method to assess macronutrient selection and intake {Geiselman, 1998 55/id}.
Physiological measures
Weight
Metabolic weights, the weight taken from patients in hospital gowns during a fasting state and following voiding in the morning, were taken at the baseline screening visit.
Glucose
Glucose was measured on the Beckman Coulter (Fullerton, CA) Synchron CX7 using a glucose oxidase electrode.
Insulin
Insulin was measured on the Diagnostic Products Corp. (Los Angeles, CA) 2000 using an immunoassay with chemiluminescent detection.
Psychological Questionnaires
Beck Depression Inventory-II
Symptoms of depression were measured using the Beck Depression Inventory II (BDI-II), which has established reliability and validity (Beck, Steer, & Brown, 1996).
Eating Inventory
The Eating Inventory has established reliability and validity (Stunkard & Messick, 1985). It consists of three subscales: Dietary Restraint, Disinhibition, and Perceived Hunger.
Eating Disorder Diagnostic Scale (EDDS)
The EDDS is a 22-item valid and reliable self-report scale for diagnosing anorexia nervosa, bulimia, and binge eating disorder (Stice, Telch, & Rizvi, 2000).
Statistical Analysis
The required sample size for this study was determined based on a power analysis using data from similar studies conducted in the Ingestive Behavior Laboratory of the PBRC. Based on previous studies, it was determined a sample size of 30 participants would allow the following clinically meaningful group differences to be detected with greater than 80% power: 1) a mean difference between the conditions of 60 kcals of food consumed at the test lunch meal, 2) a mean difference of 83 kcal at the dinner meal, 3) a mean difference of 6 rating points for VAS ratings of hunger, and 4) a mean difference of 15 rating points for VAS ratings of satiety. A repeated measures design was used to test if food intake, hunger and satiety, or postprandial glucose and insulin levels differed as a function of the three different conditions (aspartame, stevia, and sucrose). We also tested whether the insulinogenic index, the ratio obtained by dividing increments of plasma insulin levels above fasting values by the relative net increase of plasma glucose levels (i.e., Δ insulin/Δ glucose at 30 minutes), varied according to the three conditions. We did not examine potential differences between lean and obese individuals since this study was not powered to detect subgroup differences. All analyses were conducted using SAS Version 9.12 software package. A Tukey-Kramer test was used to adjust for the multiple comparisons among three conditions.
Results
Descriptive characteristics of the study sample
The descriptive characteristics of the entire sample are summarized in Table 1. The sample was comprised of 19 (61%) lean individuals (BMI range = 18.5 – 25) and 12 (39%) obese individuals (BMI range = 30 – 39.9). As expected, participants in the lean and obese groups differed on BMI, waist circumference, body weight, and blood pressure (all p-values < .05). No other group differences were found. No adverse events were reported during this trial.
Table 1.
Baseline characteristics of all participants.
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age | 27.6 | 7.7 | 18 | 45 |
| Weight (kg) | 76.5 | 18 | 49.9 | 113.5 |
| Body Mass Index (kg/m2) | 27.5 | 7.1 | 19.6 | 39.9 |
| Systolic Blood Pressure | 107.9 | 7.1 | 93.0 | 125.0 |
| Diastolic Blood Pressure | 70.8 | 7.9 | 57.0 | 92.0 |
| Waist Circumference | 85.2 | 17.2 | 61.9 | 120.6 |
| Eating Inventory Subscales | ||||
| Hunger | 3.7 | 3.5 | 0.0 | 11.0 |
| Restraint | 6.8 | 3.9 | 0.0 | 14.0 |
| Disinhibition | 3.1 | 1.5 | 1.0 | 7.0 |
Food Intake
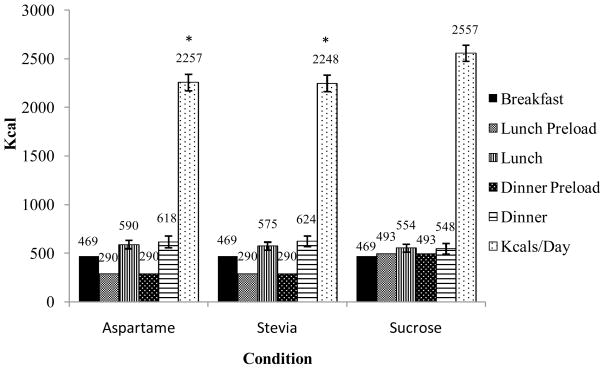
As presented in Figure 1, participants consumed significantly less food over the entire day (including preloads) in the stevia and aspartame conditions compared to the sucrose condition (mean difference between sucrose and stevia condition = 300 kcal, p < .001; aspartame condition = 334 kcal, p < .001). There were no differences in food intake between the stevia and aspartame test meal days. There was not a significant difference in food intake at either the test lunch or dinner meals between the conditions when the preload calories were removed from the analyses. This indicates that discretionary food intake did not differ between the conditions, and that the significant difference in total caloric intake was solely due to the difference in the caloric amounts of the preloads used in this study. Macronutrient consumption in terms of percent kcal did not differ between conditions.
Figure 1.
Food intake over the entire day in the aspartame, stevia, and sucrose conditions.
The lunch and dinner meals do not include the energy provided from the preloads.
Hedonic Ratings of Food
Participants rated the preloads containing aspartame as having a more pleasant taste than the preloads containing stevia and sucrose (mean VAS rating for Aspartame = 62.5; Stevia; 52.2; and Sucrose = 55.4; p-values < .01). Group assignment accounted for 15% of the variance in this rating. Participants did not differ in their hedonic ratings of the three preloads in terms of appearance, aroma, sweetness, or texture (all p-values > .10) with group assignment accounting for no more than 8% of the variance.
Hunger and Satiety
Reported hunger and satiety levels did not differ by condition at any time point (all p-values > .10). Moreover, effect sizes (Generalized eta squared) were small with group assignment accounting for no more than 3% of the variance in appetite changes. Despite eating significantly fewer kcal over the test meal day, participants consuming preloads containing stevia and aspartame reported similar levels of satiety as participants consuming the sucrose preload.
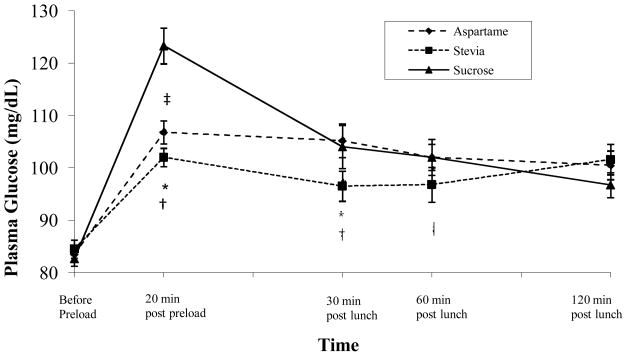
Postprandial Glucose Levels
Based on Area Under the Curve (AUC) analyses, there was a significant main effect for type of sweetener consumed on postprandial blood glucose levels, F(2, 60) = 5.13, p = .009. Post-hoc comparisons revealed that postprandial glucose levels were significantly lower in the stevia condition compared to the sucrose condition (p < .01; see Figure 2). Specifically, postprandial glucose levels were significantly lower at 20 minutes after consumption of the preload, as well as at 30 minutes after the test lunch meal, in the stevia condition compared to the sucrose condition (all ps < .05). Postprandial glucose levels were also lower in the stevia condition compared to the aspartame condition at 20 minutes after consumption of the preload, as well as at 30 and 60 minutes after the test lunch meal, (all ps < .05). Postprandial glucose levels at 20 minutes after consumption of the preload were significantly lower in the aspartame condition compared to the sucrose condition (p < .0001).
Figure 2.
Changes in postprandial glucose levels for each condition.
* indicates significant difference between stevia and sucrose conditions.
† indicates significant difference between stevia and aspartame conditions.
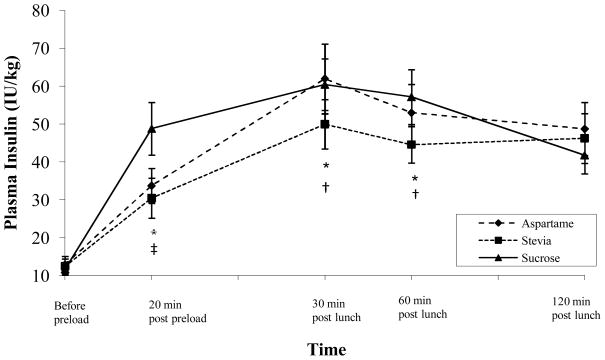
Postprandial Insulin Levels
AUC analyses also indicated that there was a significant main effect for type of sweetener consumed on postprandial blood insulin levels, F(2, 60) = 6.48, p = .003. Post-hoc comparisons revealed that postprandial insulin levels were significantly lower in the stevia condition compared to both the aspartame (p = .04) and sucrose conditions (p = .003; see Figure 3). Specifically, postprandial insulin levels were significantly reduced at 30 and 60 minutes after the test lunch meal in the stevia condition compared to the aspartame condition (all ps < .05). Postprandial insulin levels were also significantly lower at 20 minutes following consumption of the preload, as well as 30 and 60 minutes after the test lunch meal, in the stevia condition compared to the sucrose condition (all ps < .05). Postprandial insulin levels at 20 minutes after consumption of the preload were significantly lower in the aspartame condition compared to the sucrose condition (p < .01).
Figure 3.
Changes in postprandial insulin levels for each condition.
* indicates significant difference between stevia and sucrose conditions.
† indicates significant difference between stevia and aspartame conditions.
‡ indicates significant difference between aspartame and sucrose conditions.
Incremental Response
The AUC for the glucose-insulin index was also determined based on the area of incrementsabove baseline, with the baseline being the participant’s glucose or insulin value obtained prior to consuming the lunch preload. Based on Area Under the Curve (AUC) analyses, there was a significant main effect for type of sweetener consumed on the postprandial glucose-insulin index, F(2, 60) = 6.56, p = .003. For these analyses, the postprandial glucose-insulin index was lower in the stevia condition compared to sucrose condition (p < .01), as well as the aspartame condition (p = .08).
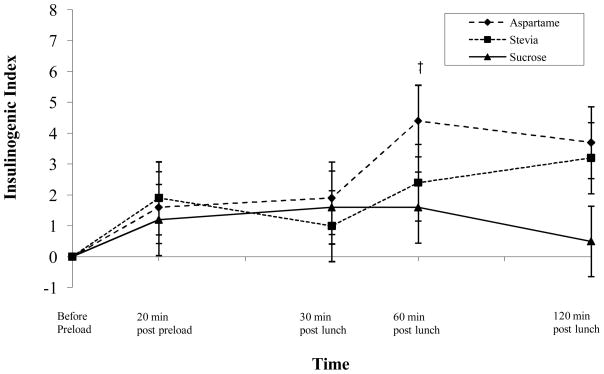
Insulingenic Index
At 60 minutes post-lunch, there was a significant difference in the insulinogenic index, the ratio obtained by dividing increments of plasma insulin levels above fasting values by the relative net increase of plasma glucose levels (i.e., Δ insulin/Δ glucose at 30 minutes), between the aspartame and sucrose conditions (p < .05; see Figure 4). No other between group differences were observed
Figure 4.
Changes in the insulinogenic index for each condition.
‡ indicates significant difference between aspartame and sucrose conditions.
Discussion
This is the first study to directly test the effects of the natural sweetener, stevia, on food intake, satiety, and postprandial glucose and insulin levels in humans. The key finding was that participants did not compensate by eating more at either their lunch or dinner meal when they consumed lower calorie preloads containing stevia or aspartame compared to when they consumed higher calorie preloads containing sucrose. In other words, even after a lower calorie preload, food intake at subsequent lunch and dinner meals was not increased and discretionary food intake did not differ between the conditions. Thus, participants’ total caloric intake was lower in the stevia and aspartame conditions, compared to the sucrose condition, solely due to the difference in caloric amounts of the preloads used in this study. Our findings are consistent with previous studies, which have found that changing the energy density of a food does not result in an accurate compensation in energy intake at subsequent meals (Levitsy, 2001; Rolls, Hetherington, & Laster, 1988; Rolls, Laster, & Summerfelt, 1989). Other studies have also found that consumption of preloads an hour and a half before testing did not influence the amount consumed in the following meal (Rolls et al., 1991). Findings such as these suggest that the eating behavior of humans may not be strongly related to previous caloric intake, at least in the short-term. Other studies suggest that compensation may not occur even over relatively long time periods. For example, sucrose-sweetened food and beverages resulted in a 1.6 kg weight gain in overweight individuals whereas artificially sweetened foods and beverages resulted in a 1.0 kg weight loss over a 10 week period (Raben, Vasilaras, Moller, & Astrup, 2002).
Consumption of stevia in preloads significantly lowered postprandial insulin levels compared to both aspartame and sucrose, as well as postprandial glucose levels compared to sucrose. Consumption of aspartame in preloads also reduced postprandial glucose compared to sucrose at twenty minutes following consumption of the preload. These effects on postprandial glucose levels are likely due in large part to the lower caloric and carbohydrate intake in the aspartame and stevia preloads compared to the sucrose preloads. However, these effects do not appear to be solely due to the lower calorie preloads in the stevia condition, as participants consumed identical calorie amounts in the preloads used in both the stevia and aspartame conditions. If future studies confirm these findings, then stevia may be helpful in managing postprandial hyperglycemia, which recent studies indicate is an important contributor to the development of insulin resistance and Type 2 diabetes (Viswanathan, Clementina, Nair, & Satyavani, 2007).
Despite consuming significantly fewer calories when provided preloads sweetened with stevia or aspartame (as compared to sucrose) in a blinded condition, participants reported similar levels of satiety in all three conditions. Since each of the preloads sweetened with sucrose contained 203 more kcal than the preloads sweetened with stevia or aspartame, this finding suggests that the additional calories provided from sucrose did not increase satiety levels, at least in the short-term. However, future studies are needed to examine this hypothesis since the caloric content of the preloads in this study was not equivalent in all three conditions.
In either case, our findings suggest that using stevia or aspartame in place of sucrose (i.e., sugar) in the diet may be an effective strategy to manage food intake since hunger and satiety levels were similar in all three conditions. In terms of hedonic ratings, participants rated the preloads containing aspartame as having a more pleasant taste than the preloads containing stevia or sucrose. There were no differences, however, in the hedonic ratings of the stevia and sucrose preloads in terms of appearance, aroma, sweetness, or texture. This suggests the observed difference in food intake were not related to the hedonic value of the three different preloads.
One limitation of the present study is that eating behavior was measured in a laboratory setting rather than the participant’s natural environment. Similarly, the design of the present study was not in line with typical eating patterns, which may limit the generalizabilty of our findings. Although some studies suggest that participants may increase their food intake in laboratory settings due to the availability of free food (Gosnell, 2001), other studies have found eating behavior in the laboratory to be consistent with eating behavior in the natural environment (Kissileff, Thornton, & Becker, 1982) and to be stable over time (Martin et al., 2005). Another potential limitation of the present study is that food intake was only measured over the course of a single day; thus, we were unable to evaluate whether compensation in food intake occurs over the long-term. We also did not collect information about the participant’s dinner the night before coming to the laboratory, which has been shown to influence the glycemic response the next day (Wolever, Jenkins, Ocana, Rao, & Collier, 1988). Finally, a control condition without a sweetener was not included in this study. Although this condition would allow for further testing of the effects of sweetness on food intake, this was not the primary purpose of the present study. Rather, the present study was designed to test the effects of the natural sweetener, stevia on food intake, satiety, and postprandial glucose and insulin levels in humans as compared to both asparatame (positive caloric control) and sucrose .
This study also had a number of strengths. First, both lean and obese individuals were included, increasing the generalizability of these findings. Second, food intake was directly measured, and satiety measurements were taken at identical time intervals as blood glucose and insulin levels. Third, a pilot study was initially conducted to determine the appropriate gram and calorie amounts to provide in the preloads. Moreover, all preloads were matched for gram weight, and the aspartame and stevia preloads were matched for caloric content.
In conclusion, participants did not compensate by eating more at either their lunch or dinner meal and reported similar levels of satiety when they consumed lower calorie preloads containing stevia or aspartame than when they consumed higher calorie preloads containing sucrose. Additionally, stevia preloads reduced postprandial blood glucose and insulin levels, suggesting stevia may assist with glucose regulation. These effects appear to be independent of reductions in caloric intake, as participants consumed similar calorie amounts in both the stevia and aspartame conditions.
Table 2.
Caloric and macronutrient content of the foods served during breakfast, lunch, and dinner meals.
| Amount | Calories | Protein (g) | Fat (g) | Carbohydrates (g) | |
|---|---|---|---|---|---|
| Breakfast Test Meal | |||||
| Milk, 2 % | 4 oz | 60 | 4 | 3 | 6 |
| Cheerios cereal | 20 | 75 | 2 | 2 | 15 |
| Toast, white | 52 | 130 | 6 | 2 | 25 |
| Butter | 14 | 105 | 0 | 11 | 0 |
| Jelly | 14 | 35 | 0 | 0 | 9 |
| Orange juice | 6 oz | 85 | 1 | 0 | 19 |
| Breakfast Total | 490 | 14 (11%) | 17 (32%) | 75 (51%) | |
| Lunch Test Meal | |||||
| Turkey Sandwich | 1447 | 2272 | 182 | 57 | 246 |
| Chips | 236 | 1168 | 17 | 50 | 158 |
| Cookies | 336 | 1735 | 23 | 81 | 232 |
| Lunch Test Meal Total | 5175 | 222 (17%) | 189 (33%) | 636 (49%) | |
| Supper Test Meal (MSSP) | |||||
| Blueberry muffins (Otis Spunkmeyer) | 501 | 1760 | 26 | 97 | 211 |
| Hershey’s Kisses | 243 | 1366 | 18 | 77 | 143 |
| Almond Joy candy bar | 322 | 1525 | 0 | 85 | 186 |
| Snack-ens snack Mix (Gardetto’s Original) | 413 | 2063 | 52 | 90 | 258 |
| Cheese crackers (Cheeze-It) | 399 | 2128 | 53 | 107 | 240 |
| Croissants | 866 | 3344 | 61 | 198 | 319 |
| Cheddar cheese (Kraft) | 298 | 1276 | 64 | 106 | 0 |
| Chopped ham | 864 | 2470 | 154 | 185 | 62 |
| Peanuts (Planter’s dry roasted unsalted) | 267 | 1619 | 76 | 133 | 48 |
| Angel Food cake | 384 | 102 | 19 | 10 | 217 |
| Chocolate mint patties | 350 | 1365 | 0 | 26 | 282 |
| Pineapple chunks (Dole, in it’s own juice) | 655 | 321 | 0 | 0 | 81 |
| Pretzels (Rold Gold sticks) | 392 | 1401 | 28 | 0 | 322 |
| Whole wheat bread (Nature’s Own 100%) | 513 | 984 | 79 | 20 | 197 |
| Fat-Free Saltine crackers | 401 | 1603 | 27 | 0 | 321 |
| Cottage cheese | 59 | 421 | 68 | 5 | 26 |
| Fat-Free mozzarella cheese | 296 | 423 | 84 | 0 | 11 |
| Oven roasted turkey breast | 836 | 744 | 149 | 15 | 0 |
| Mayonnaise (Kraft fat free) | 225 | 141 | 0 | 0 | 28 |
| Mustard (French’s) | 225 | 0 | 0 | 0 | 0 |
| Supper Test Meal Total* | 25976 | 959 (15%) | 1153 (40%) | 295 (45%) | |
For the MSSP, the foods listed represent an example meal served to participants. The actual foods served were based on each individual’s hedonic responses to a pretest list of 92 foods (Food Selection Questionnaire).
Acknowledgments
Stephen Anton is supported by a K23 AT004251-01A2, and the Claude D. Pepper Center P30AG028740.
Corby Martin is supported through a K23 DK068052-01A2.
The authors would like to express their appreciation to the participants and research associates who made it possible to complete this research project. This research was supported by the Clinical Obesity Division of the Pennington Biomedical Research Center, as well as by the P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center.
Footnotes
Reprints will not be available from the author.
Disclosure
The authors have no conflicts of interest to disclose.
References
- Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul Toxicol Pharmaco. 2008;51:37–41. doi: 10.1016/j.yrtph.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Barros CM, Lessa RQ, Grechi MP, Mouco TL, Souza MG, Wiernsperger N, et al. Substitution of drinking water by fructose solution induces hyperinsulinemia and hyperglycemia in hamsters. Clinics. 2007;62:327–334. doi: 10.1590/s1807-59322007000300019. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Invenstory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet. 1986;1:1092–1093. doi: 10.1016/s0140-6736(86)91352-8. [DOI] [PubMed] [Google Scholar]
- Chang JC, Wu MC, Liu IM, Cheng JT. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm Metab Res. 2005;37:610–616. doi: 10.1055/s-2005-870528. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Teitelbaum A, Balogh M, Groen JJ. Effect of interchanging bread and sucrose as main source of carbohydrate in a low fat diet on the glucose tolerance curve of healthy volunteer subjects. Am J Clin Nutr. 1966;19:59–62. doi: 10.1093/ajcn/19.1.59. [DOI] [PubMed] [Google Scholar]
- Curi R, Alvarez M, Bazotte RB, Botion LM, Godoy JL, Bracht A. Effect of Stevia rebaudiana on glucose tolerance in normal adult humans. Braz J Med Biol Res. 1986;19:771–774. [PubMed] [Google Scholar]
- De la Hunty A, Gibson S, Ashwell M. A review of the effectiveness of aspartame in helping with weight control. British Nutr Found Nutr Bull. 2006;31:115–128. [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53:73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Mitchell JE, Lancaster KL, Burgard MA, Wonderlich SA, Crosby RD. Food presentation and energy intake in a feeding laboratory study of subjects with binge eating disorder. Int J Eat Disord. 2001;30:441–446. doi: 10.1002/eat.1105. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- Joint Food and Agriculture Organization/World Health Expert Committee on Food Additives. Evaluation of Certain Food Additives (Rep. No. 63) Geneva: World Health Organization; 2005. [Google Scholar]
- Kissileff HR, Thornton J, Becker E. A quadratic equation adequately describes the cumulative food intake curve in man. Appetite. 1982;3:255–272. doi: 10.1016/s0195-6663(82)80022-6. [DOI] [PubMed] [Google Scholar]
- Laville M, Nazare JA. Diabetes, insulin resistance and sugars. Obes res. 2009;10(supl 1):24–33. doi: 10.1111/j.1467-789X.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89(6):1760–5. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- Levitsky D. Macronutrient intake and the control of body weight. In: Coulston A, Rock C, Monsen E, editors. Nutrition in the prevention and treatment of disease. Sand Diego: Academic Press; 2001. pp. 499–516. [Google Scholar]
- Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Williamson DA, Geiselman PJ, Walden H, Smeets M, Morales S, et al. Consistency of food intake over four eating sessions in the laboratory. Eat Behav. 2005;6:365–372. doi: 10.1016/j.eatbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. AHA: Added sugar not so sweet. JAMA. 2009;302:1741–1742. doi: 10.1001/jama.2009.1534. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. American Journal of Cardiology. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Hetherington M, Laster LJ. Comparison of the effects of aspartame and sucrose on appetite and food intake. Appetite. 1988;11(Suppl 1):62–7. [PubMed] [Google Scholar]
- Rolls BJ, Kim S, McNelis AL, Fischman MW, Foltin RW, Moran TH. Time course of effects of preloads high in fat or carbohydrate on food intake and hunger ratings in humans. Am J Physiol. 1991;260:R756–63. doi: 10.1152/ajpregu.1991.260.4.R756. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Laster LJ, Summerfelt A. Hunger and food intake following consumption of low calorie foods. Appetite. 1989;13:115–127. doi: 10.1016/0195-6663(89)90109-8. [DOI] [PubMed] [Google Scholar]
- Roth J, Qiang X, Marban SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res. 2004;12(Suppl 2):88S–101S. doi: 10.1038/oby.2004.273. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NJ, Griffen SC, Bremer AA, Graham JL. Consuming fructose-sweetened, not glucose sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of Clinical Investigation. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12:123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Viswanathan V, Clementina M, Nair BM, Satyavani K. Risk of future diabetes is as high with abnormal intermediate post-glucose response as with impaired glucose tolerance. J Assoc Physicians India. 2007;55:833–837. [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem. 2007;18:1–9. doi: 10.1016/j.jnutbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Wells HF, Buzby JC. Dietary Assessment of Major Trends in U.S. Food Consumption, 1970–2005. (Rep. No. 33) Washington DC: U.S. Department of Agriculture; 2008. [Google Scholar]
- Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76:274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. The American Journal of Clinical Nutrition. 1988;48(4):1041–1047. doi: 10.1093/ajcn/48.4.1041. [DOI] [PubMed] [Google Scholar]