Abstract
Mechanisms regulating sexual differentiation of the zebra finch song system appear to include both genetic and hormonal factors. Sorting Nexin 2 (SNX2), which is involved in trafficking proteins between cellular membranes, and androgen receptor (AR) mRNA are both increased in song control nuclei of juvenile males compared to females. Here, in situ hybridization for SNX2 and immunohistochemistry for AR were used to evaluate these sexual dimorphisms in more detail. Estimates of the total number of HVC cells expressing SNX2 and AR, individually as well as together, were greater in 25-day-old males compared to females. The densities of these types of cells were generally also increased in males compared to females in HVC and Area X (or the equivalent portion of the medial striatum in females). On average, more than half of the AR+ cells co-expressed SNX2 in both brain regions. The potential, therefore, exists for both AR and SNX2 to be involved in masculinization of these two brain regions. One possibility is that they, either separately or in conjunction, enhance the action of trophic factors within the brain.
Keywords: sex difference, sexual dimorphism, steroid hormone, development
1. Introduction
Sex differences in brain and behavior have been identified in a wide variety of vertebrate species (e.g., Balthazart and Adkins-Regan, 2002; Cooke et al., 1998; Simerly, 2002). Early exposure to gonadal hormones, testosterone or its metabolite estradiol in particular, commonly initiates the cascade of events that eventually creates a masculine adult phenotype. While the role of steroid hormones has long been established for a number of model systems, the ensuing cellular and molecular events are just now being identified (Forger, 2009; McCarthy, 2009; McCarthy et al., 2009)
Zebra finches are particularly useful for investigating this topic, as the neural circuit controlling their courtship song is well-defined and highly sexually dimorphic. Only males sing, and forebrain regions involved in learning and production of song, including the robust nucleus of the arcopallium (RA) and HVC (used as a proper name; Reiner et al., 2004), are larger in volume and contain more and larger cells in males compared to females. Another region, Area X of the striatum, cannot be detected in females (reviewed in Wade, 2001; Wade and Arnold, 2004).
These sexual dimorphisms in both structure and the capacity for function are permanently established in the first few weeks after hatching. Results from a variety of initial experiments were consistent with the idea that mechanisms are similar to rodents, in which testosterone secreted by the testes is metabolized in the brain to estradiol (via the aromatase enzyme), and this hormone is directly responsible for masculinization (reviewed in De Vries and Simerly, 2002). For example, systemic estradiol treatment of zebra finch females shortly after hatching masculinizes the morphology of the brain regions, including increasing the volume of HVC by enhancing cell number and size and inducing a visible Area X, and it allows females to sing in adulthood (Adkins-Regan and Ascenzi, 1987; Arnold, 1997; Grisham and Arnold, 1995; Gurney and Konishi, 1980; Gurney, 1981; Konishi and Akutagawa, 1988; Simpson and Vicario, 1991). A variety of results, however, have called the role of aromatization and estradiol into question. For example, the masculinization of estradiol-treated females is not complete. Brain regions in these individuals do not reach the size of normal males (Grisham and Arnold, 1995). Treating males with estrogen synthesis inhibitors or estrogen receptor blockers during the post-hatching critical period has not inhibited masculine development (Balthazart et al., 1995; Mathews et al., 1988; Mathews and Arnold, 1990; Mathews and Arnold, 1991; Wade and Arnold, 1994). Additionally, song system morphology and singing behavior are not masculinized in females that develop with substantial amounts of functional testicular tissue, even in the absence of ovarian tissue (Springer and Wade, 1997; Wade and Arnold, 1996; Wade et al., 1996; Wade et al., 1999). While one characteristic – the projection of axons from HVC to RA – does seem to depend mainly on neural estradiol (Holloway and Clayton, 2001), data are consistent with the idea that steroid hormones are insufficient to induce full masculine development.
We conducted a microarray study to indentify other potential, molecular, players in the masculinization process. Sorting nexin 2 (SNX2) was among the genes that exhibited increased expression in males. On both the cDNA arrays, and using real-time qPCR on RNA extracted from a different set of juveniles, expression in the whole forebrain was approximately two times greater in males compared to females. In situ hybridization in 25-day-old birds, which is an age when males are beginning to learn their songs and is in the heart of sexual differentiation of song system morphology, revealed even larger sex differences in mRNA levels in HVC and Area X (or the equivalent portion of the medial striatum in females). These effects appeared quite specific, as expression of this gene was not sexually dimorphic in another song control nucleus (lateral magnocellular nucleus of the anterior nidopallium, lMAN) and was not detected above background in RA (Tomaszycki et al., 2009).
No work on SNX2 has been conducted in birds; available reports are from mammalian and yeast systems. At least 25 sorting nexins have been identified in humans; the proteins are involved in trafficking through various cellular compartments. Members of this family of proteins each contain a PX domain. These sequences of around 100 amino acids bind phosphatidylinositol phosphates, thus targeting the SNXs to cellular membranes, such as endosomes, that are enriched in these phospholipids (reviewed in Worby and Dixon, 2002). While some of the data are not fully consistent, SNX2 specifically is thought to be part of the retromer, a complex that mediates transport of transmembrane proteins between endosomes and the trans-Golgi network. Thus, it is involved in recycling of membrane proteins. The retromer, which contains a dimer of either SNX1, SNX2, or one of each protein, has been implicated in a variety of physiological and developmental processes, and defects can produce pathology including some forms of Alzheimer's disease (Bonifacino and Hurley, 2008).
In zebra finches, SNX2 is located on a sex chromosome. Male birds are homogametic (ZZ) and females are heterogametic (ZW). SNX2 is on the Z-chromosome, and as dosage compensation in birds is limited (Itoh et al., 2007), the potential for expression of Z-genes to facilitate masculinization is high. In addition, sexually dimorphic expression of SNX2 occurs in regions with androgen receptors (AR). Males have more AR than females in HVC and Area X by post-hatching days 9–11 (Kim et al., 2004). As with morphological features regions, exogenous estradiol in females increases the expression of AR in HVC and Area X, as well as lMAN (Kim et al., 2004; Nordeen et al., 1986). Somewhat surprisingly, few estrogen receptors α are expressed in the song control nuclei, particularly in zebra finches, and the little expression there is appears equivalent in the two sexes (Gahr et al., 1987; Gahr et al., 1993; Gahr and Metzdorf, 1997; Jacobs et al., 1999). Also, estrogen receptor β has not been detected in these areas (information on song nuclei is only available from starlings; Bernard et al., 1999). While treatment of females with androgen alone has only modest and somewhat inconsistent effects on masculinization of the forebrain song circuit (reviewed in Wade and Arnold, 2004), at least one study (Grisham et al., 2002) suggests that an estrogen-induced increase in AR is a critical step in morphological differentiation. In that experiment, the masculinizing effects of estradiol in developing females were almost completely prevented by concurrent administration with the androgen receptor blocker, Flutamide.
One explanation for the collective results from hormone manipulations is that estrogen triggers a cascade of cellular events leading to masculinization of the song control regions, and that one or more of these events requires the action of androgen. If so, females have sufficient endogenous androgen to allow substantial masculinization when they are treated only with estradiol (in fact, two studies suggest they have plasma levels equal to or higher than males during development; Adkins-Regan et al., 1990; Hutchison et al., 1984). However, a lack of complete masculinization after even combined androgen and estrogen exposure (Jacobs et al., 1995) suggests that additional molecular mechanisms are involved.
The present study was designed to address the potential for interactions between SNX2 and AR by determining whether they are co-expressed in individual cells within the song circuit. A combination of in situ hybridization for SNX2 and immunohistochemistry for AR was used. If substantial co-localization exists, the genes/proteins could be involved in either the same or different pathways facilitating masculinization within a cell. In addition, if the pattern of co-expression differs in males and females, it would provide additional evidence that the interaction between SNX2 and AR could be important for the masculinization process.
2. Results
In HVC, estimates of the total number of cells labeled for AR protein (t=6.12, p<0.0001), SNX2 mRNA (t=5.76, p<0.0001), and double-labeled cells (t=5.43, p<0.0001) were all significantly greater in males than females (Figures 1 and 2). In HVC, the densities of SNX2-(t=3.65, p=0.004), and double-labeled cells (t=4.26, p=0.002) were increased in males compared to females. The pattern was the same for AR+ cells, but this value did not reach statistical significance (Figure 3A; t=2.80; p=0.019) In Area X, the densities of cells containing AR, SNX2 and AR+SNX2 were all greater in males than females (all t>5.45, p<0.0001; Figures 3B and 4). Sex differences in the percentages of double-labeled AR+ cells and double-labeled SNX2+ cells did not differ between the sexes in either brain region (all t<1.81, p<0.103; Table 1).
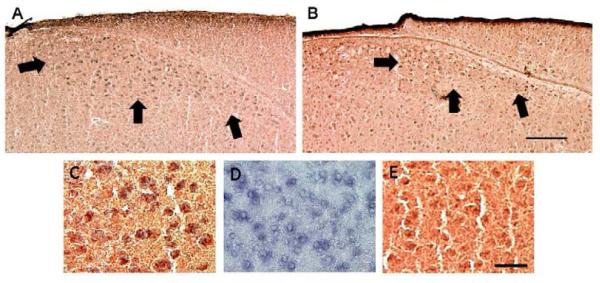
Figure 1.
Double-labeling of SNX (blue) and AR (reddish brown) in the HVC of male (A) and female (B) zebra finches. In A and B, arrows indicate the ventral and lateral borders of the brain region. Panel C depicts double-labeling in the male at higher power. Panels D and E show single-labeld in situ hybridization for SNX2 and immunohistochemistry for AR, respectively, from a different male. Note that labeling, especially for SNX2, tended to be lighter in females. Scale bars = 200 μm for the upper and 25 μm for the bottom panels.
Figure 2.
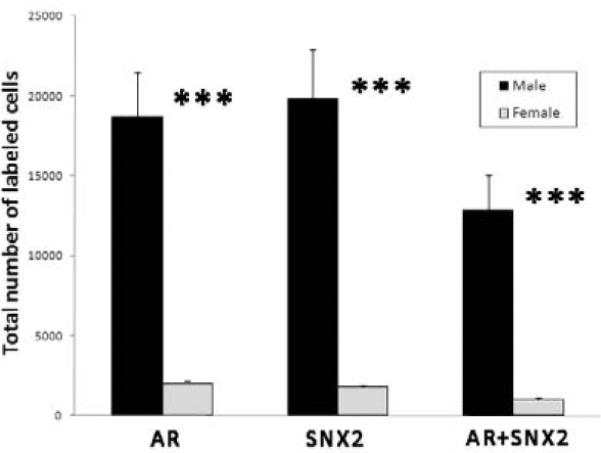
Stereologic analysis of AR-, SNX2- and AR+SNX2-labeled cell numbers in HVC. Estimates of these total cell numbers were all greater in males than females (***p < 0.0001).
Figure 3.
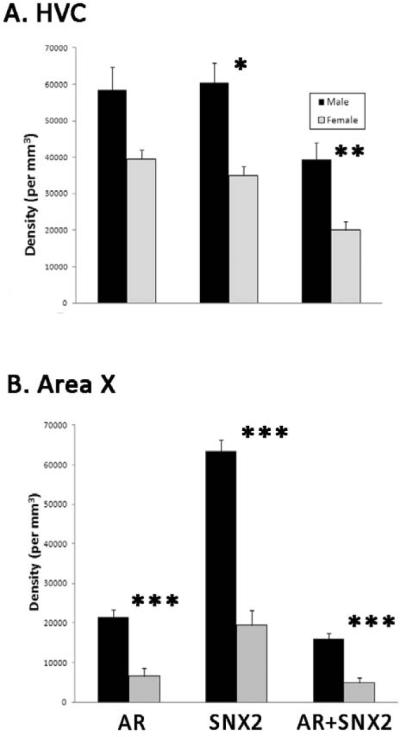
Densities of AR-, SNX2- and AR+SNX2-labeled cells in HVC (A) and Area X (B). *p=0.004, **p=0.002, ***p<0.0001.
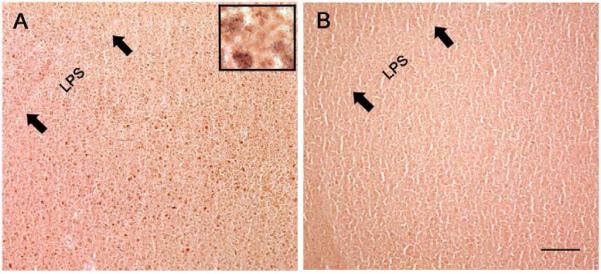
Figure 4.
Photomicrographs of representative sections of the medial striatum of male (A) and female (B) juvenile zebra finches. The inset depicts a small portion from the male image in more detail; it includes a few double-labeled cells, as well as some single-labeled for AR (nuclei). Area X, which is ventromedial to the lamina pallio-subpallialis (LPS), is visible in the male, but cannot be detected from background in the female. While small numbers of positive cells were present in the medial striatum of females, as in HVC the labeling generally appeared lighter than in males. Scale bar = 200μm.
Table 1.
Proportions of SNX2 and AR co-localization in the HVC and Area X of zebra finches
| (SNX2 + AR)/AR % co-localization | (SNX2 + AR)/SNX2 % co-localization | |||
|---|---|---|---|---|
| HVC | Area X | HVC | Area X | |
| Male | 66.7 ± 3.9 | 76.8±5.2 | 64.9 ± 3.4 | 25.4±2.2 |
| Female | 52.6 ± 6.8 | 66.3±13.9 | 56.0 ± 6.3 | 21.4±4.8 |
3. Discussion
The present data document substantial sex differences in both SNX2 and AR in the HVC and Area X of juvenile zebra finches. In HVC, the estimated total number of cells expressing each individually, as well as those expressing both AR and SNX2, was greater in 25-day-old males than females. These values in males ranged from 9 to 13 times those in females, sex differences that are substantially larger than the approximately 2.25-fold difference in total number of cells previously reported for HVC at this age (Kirn and DeVoogd, 1989). Thus, it seems reasonable to conclude that the sex differences in the number of HVC cells expressing AR, SNX2, as well as those positive for both markers, are not simply due to a general sexual dimorphism in cell number.
The densities of these cell types in HVC were also on average greater in males than females. Ratios ranged from 1.5 for cells that were only AR+ (which did not quite reach statistical significance) to 2.0 for AR+SNX2-positive cells. To address the question of whether this result simply reflected a dimorphism in cell density overall, we evaluated Nissl-stained brain sections from a previous study, as tissue was not available from the animals used here. Cells were counted in 20μm sections from 25-day old control males and females stained with cresyl violet (Tang and Wade, 2009). Boxes of 100μm2 × 100μm2 were used – four placed randomly within each brain region in five individuals of each sex, and the data were averaged within each individual. The pattern was reversed. That is, the average cell density in the HVC of females was 1.87 times that of males (t=13.95, p<0.0001). Similarly, in Area X, the densities of AR+, SNX2+ and double-labeled cells were uniformly 3.3 times greater in males than females, and the estimated density of Nissl-stained cells was 1.51-fold greater in females (t=3.26, p=0.012). The dimorphisms in AR- and SXN2-expressing cells in both regions therefore cannot be due to general sex differences in cell density.
Collectively, the present data are consistent with the idea that both AR and SNX2 may influence masculinization of these brain regions within the song circuit. As the percentage of AR+ cells co-expressing SNX2 was over 50% in both HVC and Area X (and more than 20% of SNX2+ cells also contained AR), considerable opportunity for interaction between these genes/proteins also exists. However, as the sexes did not differ on the percentages of double-labeled cells in either brain region, the degree of any potential interaction between these factors is likely similar in males and females. Thus, the increase in overall expression of SNX and AR in males, as opposed to a sex difference in the specific nature of the relationship between them, may facilitate masculinization.
The present data on AR-like immunoreactivity are consistent with previous research on developing zebra finches. Kim et al. (2004) reported expression of AR mRNA in HVC and Area X by post-hatching days 9–11. Labeling is increased in males in these brain regions, and it can be modulated by estradiol availability. AR mRNA was increased in females treated with estradiol and decreased in males given an estrogen synthesis inhibitior. Gahr and Metzdorf (1999), however, report that the initial sex difference in HVC AR mRNA detected on day 9 is independent of the direct action of gonadal steroids. Thus, the data collectively suggest that ARs are increased in the HVC and Area X of juvenile male zebra finches compared to their female counterparts. The sexual dimorphism, which at least for HVC extends into adulthood (hormone binding study: Arnold and Saltiel, 1979), can clearly be modulated by post-hatching estradiol availability. However, the initial difference in HVC AR between males and females might be regulated by estrogen synthesized in the brain and/or another factor, perhaps as expression of a sex chromosome gene such as SNX2.
As indicated in the Introduction, no information is available about SNX2 (or any member of the SNX family) in birds other than the male-biased sex difference in expression that we reported at post-hatching day 25. Species-specific cDNA microarray and qPCR detected the dimorphism in the whole telencephalon, and 33P-labeled in situ hybridization indicated specificity for HVC and Area X (Tomaszycki et al., 2009). The magnitudes of the sex difference in SNX2 mRNA detected in that previous assessment of silver grain density in cross sections of Area X and in the present analysis of the density of digoxigenin-labeled cells the region are identical (3.2-fold). In HVC, the present study revealed 1.7 times the density of SNX2 expressing cells in males compared to females, less than the 7.4-fold difference detected for silver grain density over the region. However, such a difference is not necessarily surprising. The two techniques address different issues – distribution of labeled cells versus mRNA signal without regard to the position of individual cells in the brain region. The estimate of total number of SNX2 expressing cells in the HVC of juvenile males in the current experiment is 11 times that in females. Thus, overall, the present data are highly consistent with previous results and indicate relatively large sex differences in synthesis of SNX2 in the HVC of juvenile zebra finches.
Based on information from mammalian systems on SNX2, and related information from yeast, this protein is involved in the trafficking of membrane proteins. It is a component of the retromer, which functions at least in part to facilitate recycling of receptors to the cell membrane instead of to the lysosome for degredation (Bonifacino and Hurley, 2008). While this area of research is relatively new and more interactions remain to be discovered, existing data indicate that SNX2 is involved in the sorting of tyrosine kinase receptors, including those for epidermal growth factor (EGF) and platelet-derived growth factor (PDGF). It therefore seems reasonable to speculate that SNX2 might mediate activity associated with tyrosine kinase B (trkB), the high affinity receptor for brain derived neurotrophic factor (BDNF), and/or vascular endothelial growth factor receptor 2 (VEGFR2), both of which have been implicated in song system plasticity.
TrkB mRNA (Dittrich et al., 1999) and protein (Wade, 2000) are present in the HVC of juvenile male and female zebra finches. BDNF mRNA has also been detected in juvenile males, and is upregulated by estradiol (Dittrich et al., 1999). In adult canaries, which unlike zebra finches exhibit substantial seasonal changes in their songs and the morphology of song control regions, trkB is also present in the HVC of both sexes, and BNDF is detected in the HVC of males only (Rasika et al., 1999). BDNF is involved in the regulation of neuronal replacement in this region, and in adult testosterone-treated females (which sing) HVC BDNF is increased compared to controls. In male canaries, HVC BDNF is upregulated by singing, and in parallel, the survival of new neurons is increased in singing birds (Li et al., 2000). Similar results are seen in white-crowned sparrows (Wissman and Brenowitz, 2009), such that BDNF is upregulated in HVC by the long days typical of spring breeding conditions. While the trophic factor is not detected in Area X, trkB is detected widely in the telencephalon, including HVC and Area X. Data from infusion and inhibition of BDNF in RA suggest that BDNF is important for seasonal plasticity of the song system in this seasonal species as well. T-treatment of adult female canaries also increases VEGF and its VEGFR2 receptor in HVC. This increase leads to increases in production of BDNF (Louissaint et al., 2002). Song in T-treated female canaries is blocked with inhibition of VEGFR2, an effect that is reversed by increased expression of BDNF (Hartog et al., 2009). This inhibition also decreases the recruitment of HVC neurons (Hartog et al., 2009).
In sum, while at present no evidence directly implicates SNX2 in the recruitment or survival of neurons in the song system, it is worth testing the hypothesis, and specifically the idea that this protein enhances the function of BDNF and/or VEGF by increasing the availability of their membrane receptors. As the present data indicate extensive co-localization of AR and SNX2 in both HVC and Area X, the association between the two should also be directly tested. If SNX2 is involved in masculinization, it may be that it and androgen independently affect particular aspects of song system development. However, it also seems reasonable to hypothesize that SNX2 modulates the brain's response to androgen, at least in part by potentiating the effect of steroid-modulated neurotrophins. Finally, as estradiol can at least partially masculinize numerous features of song system structure, neurochemistry and function (perhaps via some AR-mediated pathway; see above), the potential for more complex interactions among SNX2, steroid hormones and neurotrophins should be considered.
4. Experimental Procedure
4.1 Animals
Zebra finches were housed on a 12:12 light cycle in walk-in colony cages, each containing approximately 5–7 breeding pairs and their offspring. They were fed with standard finch seeds (Kaytee Supreme Fortified Daily Blend Bird Food, Chilton, Wisconsin) and had water available ad libitum. Spinach, orange juice, and bread mixed with hard-boiled chicken eggs were supplied weekly. Each aviary contained five nests (diameter 14 cm, height 13 cm) filled with wood chips and shredded paper. They were checked daily. When each hatchling was found was considered post-hatching day 1. Birds were toe-clipped at that time to provide unique identification, and then banded before fledging, which occurs within two weeks.
All procedures were approved by the Michigan State University IACUC and followed conditions outlined in the NIH Guide for the Care and Use of Experimental Laboratory Animals.
4.2 Tissue Collection
Birds were euthanized on post-hatching day 25 by rapid decapitation. Gonads were examined to determine the sex of each bird. The brains were removed from the skull and frozen immediately in cold methylbutane and then wrapped with foil and stored at −80 ºC. Tissue sections (20 μm-thick) were cut on a cryostat (CM3050 S, Leica Microsystems, Nussloch, Germany) and thaw-mounted onto SuperFrost Plus slides (Fisher Scientific, Hampton, NH). Six sets of slides containing alterate series of sections from the whole telencephalon were collected. Slides were stored at −80 ºC with desiccant until further processing.
4.3 In situ hybridization and immunohistochemistry
The plasmid containing SNX2 (Tomaszycki et al., 2009) was isolated using Qiagen plasmid Maxi prep kit (Valencia, CA) and the template was linearized using XhoI and NotI (New England Biolabs, Ipswich, MA). T3 (antisense) and T7 (sense) probes were labeled and purified using digoxygenin (DIG) RNA labeling kit (Roche, Indianapolis, IN) per manufacturer's instructions. Probes were stored at −80 ºC until further use.
Brain section sets from female and male birds were process in a single run in coplin jars. They were gradually warmed to room temperature and then fixed with 4% paraformaldehyde for 10 min. In situ hybridization for SNX2 was conducted prior to immunohistochemistry for AR. Slides were prehybridized in TEA/HCl buffer (0.1M Triethanolamine/HCL, 0.9% NaCl, pH=8.0) with 0.25% acetic anhydrate for 10 min and rinsed with phosphate buffered saline (PBS). Slides were incubated overnight at 55 ºC in the hybridization buffer (50% formamide, 4X SSC, 1X Denhardt's solution, 200 μg/ml herring sperm DNA, 10% dextran sulfate, 20mM dithiothreitol, 250μg/ml, 2mM EDTA, 0.1% Tween-20) containing 200ng/ml antisense digoxigenin-labeled probe. The tissue was washed in 2 × SSC for 2×5 min at 60 ºC, in 0.2 × SSC for 3×5 min at 60 ºC, in MABT (100mM maleic acid, 150mM NaCl, 0.5% Tween 20, pH=7.5) for 3×5 min at room temperature. Slides were then immersed in MABT with 0.9% H2O2 at room temperature for 30 min and rinsed in MABT for 3×5 min. After blocking in MABT with 10% normal sheep serum, sections were incubated with anti-DIG-AP antibody (1μl/ml) (Roche) in MABT overnight at 4ºC. For development of colorimetric signal, slides were washed with MABT for 2×5min and equilibrated with detection buffer (0.1 M Tris-HCl, 0.1 M NaCl, pH=9.5) before incubating in NBT/BCIP solution (4.5 μl/ml NBT and 3.5 μl/ml BCIP in detection buffer, both from Roche) for 1.5 h. The color reaction was stopped by rinsing slides with TE (1mM EDTA, 10 mM Tris-HCl, pH=7.5) for 3×5min. One set of slides was treated with sense probe as a negative control; no labeling was detected.
After in situ hybridization, slides were washed with PBS for 3×5 min, blocked in PBST (PBS with 0.03% triton-x) with 10% normal goat serum for 30 min and incubated in the rabbit polyclonal AR antibody N-20 (0.5μg/ml; Santa Cruz Biotechnology, Inc) at 4ºC for two days. This antibody was developed against amino acids 299–315 of the human AR and has been validated extensively by preadsorption of the antibody with the antigen, western blotting, and other controls (Chlenski et al., 2001; McKinnell et al., 2001; Wu and Gore, 2009). In zebra finch brain tissue, no labeling was observed when the primary antibody was omitted.
The slides were then washed in PBS and incubated in biotinylated anti-rabbit immunoglobulin G (IgG, 1:600, Vector Laboratories, Burlingame, CA) for 1 hr followed by rinsing in PBS. The tissue was then incubated in ABC (Elite kit, Vector Laboratories) for 1 hr. The slides were rinsed in PBS and developed using NovaRED chromogen (Vector). Slides were rinsed, dehydrated with 70%, 95% and 100% ethanol and then cleared with Citrisolv (Fisher Scientific), and coverslipped with VectaMount (Vector).
4.4 Stereological analysis
Slides were analyzed by an individual blind to sex of the birds. HVC was analyzed in six males and six females. Quantitative analyses were done using Stereo Investigator® software (version 8, MicroBrightField, Williston, VT). To obtain a total estimate of cells (single labels for AR and SNX2, and double-labeled) HVC was traced on one side of the brain (randomly chosen) in one series of sections (120 μm apart) throughout the rostro-caudal extend of the brain region. Closed contours were drawn around HVC at low magnification (4X objective) using an Olympus BX-51 microscope. Section mount thickness was measured within the contour. A buffer zone at the top of the tissue, where the slice thickness is most likely to have been affected by tissue processing and is not representative of the tissue specimen from the volume, was set at 2 μm in all cases. The Stereo Investigator software randomly placed 200 μm × 125 μm grids (“dissector frames”) within each contour. The DIG-SNX2 and NovaRed- AR nuclei were individually counted within 40 μm × 40 μm counting frames (“optical dissectors”). Only cells that came into focus and fell entirely within or touched the top or right lines of the frame were counted. These criteria insured that the same cells would not be included twice. The coefficients of error and variation of the estimates were calculated as per Schmitz and Hof (2000). They never exceeded 0.10. Densities of labeled cells of each of the three types were calculated by dividing the estimated total number by the estimated HVC volume for each animal. Percentages of double labeled (AR + SNX2) cells were also calculated for each individual.
Labeling in Area X was quantified in seven birds of each sex. The procedure was identical to that described above for HVC with the following exceptions. Area X is not detectable in female zebra finches, so contours could not be drawn and estimates of total cells could not be obtained for them. Thus, only sex differences in densities and percentages of labeled cells were analyzed. Densities of labeled cells were quantified using a 103,479 μm2 box without knowledge of the sex of each animal. For animals in which an Area X could be identified, the box was placed near its center on one side of the brain in three consecutive sections near the middle of the rostro-caudal extent of the brain region. For birds in which Area X could not be detected, the same size of rectangle was put in the equivalent portion of the striatum, based on external landmarks. The Stereo Investigator software randomly placed 100 μm × 100 μm grids within each rectangle. Within these dissectors, the DIG-stained SNX2 or NovaRed-labeled AR nuclei were counted within a 50 μm × 50 μm counting frame. In this brain region, densities were calculated by dividing the estimated number of each of the cell types by the estimated volume across the three sections quantified (in all cases 0.0020696 mm3).
4.5 Statistical Analysis
Statistical analyses were done using SPSS (17.0; SPSS Inc., Chicago, Illinois). Sex differences were evaluated for estimates of total numbers of AR, SNX2 and AR+SNX2 labeled cell in HVC, densities of cells containing each of these labels in HVC and Area X, and percentages of double-labeled cells in both brain regions. Bonferroni corrections were used. Based on eight tests in HVC, α = 0.0063; for the five tests in Area X, α = 0.01.
Acknowledgments
This work was supported by NIH grant MH55488. We thank Camilla Peabody for technical support, and are also greatful to members of the Wade lab for help with animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E, Ascenzi M. Social and sexual behaviour of male and female zebra finches treated with oestradiol during the nestling period. Anim. Behav. 1987;35:1100–1112. [Google Scholar]
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen. Comp. Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Saltiel A. Sexual difference in pattern of hormone accumulation in the brain of a songbird. Science. 1979;205:702–704. doi: 10.1126/science.205.4407.702. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypothesis, and a paradigm shift. J. Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Fiasse V, Ball GF. Effects of the aromatase inhibitor R76713 on sexual differentiation of brain and behavior in zebra finches. Behaviour. 1995;120:225–260. [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Academic Press; San Diego: 2002. pp. 223–301. [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor α, and estrogen receptor β show distinct patterns of expression in forebrain song control nuclei of european starlings. Endocrinol. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr. Op. Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlenski A, Nakashiro K, Ketels KV, Korovaitseva GI, Oyasu R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate. 2001;47:66–75. doi: 10.1002/pros.1048. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: Principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and functions of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Academic Press; New York: 2002. pp. 137–191. [Google Scholar]
- Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc. Natl. Acad. Sci. USA. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J. Neuroendocrinol. 2009;24:393–399. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Flügge G, Guttinger H-R. Immunocytochemical localization of estrogen-binding neurons in the songbird brain. Brain Res. 1987;402:173–177. doi: 10.1016/0006-8993(87)91063-8. [DOI] [PubMed] [Google Scholar]
- Gahr M, Guttinger H-R, Kroodsma DE. Estrogen receptors in the avian brain: Survey reveals general distribution and forebrain areas unique to songbirds. J. of Comp. Neurol. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res. Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. The sexually dimorphic expression of androgen receptors in the song nucleus hyperstriatalis ventrale pars caudale of the zebra finch develops independently of gonadal steroids. J. Neurosci. 1999;19:2628–2636. doi: 10.1523/JNEUROSCI.19-07-02628.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham W, Arnold AP. A direct comparison of the masculinizing effects of testosterone, androstenedione, estrogen, and progesterone on the development of the zebra finch song system. J. Neurobiol. 1995;26:163–170. doi: 10.1002/neu.480260202. [DOI] [PubMed] [Google Scholar]
- Grisham W, Lee J, McCormack ME, Yang-Stayner K, Arnold AP. Antiandrogen blocks estrogen-induced masculinization of the song system in female zebra finches. J. Neurobiol. 2002;51:1–8. doi: 10.1002/neu.10028. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Gurney ME. Hormonal control of cell form and number in the zebra finch song system. J. Neurosci. 1981;1:658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog TE, Dittrich F, Pieneman AW, Jansen RF, Frankl-Vilches C, Lessmann V, Lilliehook C, Goldman SA, Gahr M. Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song in female canaries. J. Neurosci. 2009;29:15511–15519. doi: 10.1523/JNEUROSCI.2564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nature Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J. Endocrinol. 1984;103:363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, Schadt EE, Lusis AJ, Arnold AP. Dosage compensation is less effective in birds than in mammals. J. Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Grisham W, Arnold AP. Lack of a synergistic effect between estradiol and dihydrotestosterone in the masculinization of the zebra finch song system. J. Neurobiol. 1995;27:513–519. doi: 10.1002/neu.480270406. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Arnold AP, Campagnoni AT. Developmental regulation of the distribution of aromatase- and estrogen-receptor-mRNA-expressing cells in the zebra finch brain. Dev. Neurosci. 1999;21:453–472. doi: 10.1159/000017413. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: Developmental regulation by estrogen. J. Comp. Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Kirn JR, DeVoogd TJ. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J. Neurosci. 1989;9:3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. A critical period for estrogen action on neurons of the song control system in the zebra finch. Proc. Natl. Acad. Sci. USA. 1988;85:7006–7007. doi: 10.1073/pnas.85.18.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-C, Jarvis ED, Alvarez-Borda B, Lim DA, nottebohm F. A relationship between behavior, neurotrophin expression and new neuron survival. Proc. Natl. Acad. Sci. USA. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Mathews GA, Brenowitz EA, Arnold AP. Paradoxical hypermasculinization of the zebra finch song system by an antiestrogen. Horm. Behav. 1988;22:540–551. doi: 10.1016/0018-506x(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Mathews GA, Arnold AP. Antiestrogens fail to prevent the masculine ontogeny of the zebra finch song system. Gen. Comp. Endocrinol. 1990;80:48–58. doi: 10.1016/0016-6480(90)90147-e. [DOI] [PubMed] [Google Scholar]
- Mathews GA, Arnold AP. Tamoxifen's effects on the zebra finch song system are estrogenic, not antiestrogenic. J. Neurobiol. 1991;22:957–969. doi: 10.1002/neu.480220907. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. The two faces of estradiol: Effects on the developing brain. The Neuroscientist. 2009;15:599–610. doi: 10.1177/1073858409340924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwartz JM. New tricks by an old dogma: Mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm. Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: Low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reprod. 2001;122:419–29. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ, Arnold AP. Estrogen establishes sex differences in androgen accumulation in zebra finch brain. J. Neurosci. 1986;6:734–738. doi: 10.1523/JNEUROSCI.06-03-00734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gütürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Early estrogen treatment alone causes female zebra finches to produce learned, male-like vocalizations. J. Neurobiol. 1991;22:755–776. doi: 10.1002/neu.480220710. [DOI] [PubMed] [Google Scholar]
- Springer ML, Wade J. The effects of testicular tissue and prehatching inhibition of estrogen synthesis on the development of courtship and copulatory behavior in zebra finches. Horm. Behav. 1997;32:46–59. doi: 10.1006/hbeh.1997.1406. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wade J. Sexual differentiation of the zebra finc h song system: Potential roles for estradiol and ribosomal proteins L17 and L37. Dev. Neurobiol. 2009;69:462–75. doi: 10.1002/dneu.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki ML, Peabody C, Replogle K, Clayton DF, Tempelman RJ, Wade J. Sexual differentiation of the zebra finch song system: Potential roles for sex chromosome genes. BMC Neurosci. 2009;10:24. doi: 10.1186/1471-2202-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Post-hatching inhibition of aromatase activity does not alter sexual differentiation of the zebra finch song system. Brain Res. 1994;639:347–350. doi: 10.1016/0006-8993(94)91752-3. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc. Natl. Acad. Sci., USA. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Springer ML, Wingfield JC, Arnold AP. Neither testicular androgens nor embryonic aromatase activity alters morphology of the neural song system in zebra finches. Biol. Reprod. 1996;55:1126–1132. doi: 10.1095/biolreprod55.5.1126. [DOI] [PubMed] [Google Scholar]
- Wade J, Swender DA, McElhinny TL. Sexual differentiation of the zebra finch song system parallels genetic, not gonadal, sex. Horm. Behav. 1999;36:141–152. doi: 10.1006/hbeh.1999.1537. [DOI] [PubMed] [Google Scholar]
- Wade J. TrkB-like immunoreactivity in the song system of developing zebra finches. J. Chem. Neuroanat. 2000;19:33–39. doi: 10.1016/s0891-0618(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Wade J. Zebra finch sexual differentiation: The aromatization hypothesis revisited. Microsc. Res. Tech. 2001;54:354–363. doi: 10.1002/jemt.1148. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann. Rev. N.Y. Acad. Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J. Neurosci. 2009;29:6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- Wu D, Gore AC. Sexual experience change sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged rats. Horm. Behav. 2009;56:299–308. doi: 10.1016/j.yhbeh.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]