Abstract
The effects of nitric oxide in biological systems depend on its steady-state concentration and where it is being produced. The organ where nitric oxide is produced is relevant, and within the organ, which types of cells are actually contributing to this production seem to play a major determinant of its effect. Subcellular compartmentalization of specific nitric-oxide synthase enzymes has been shown to play a major role in health and disease. Pathophysiological conditions affect the cellular expression and localization of nitric oxide synthases, which in turn alter organ cross talk. In this study, we described the compartmentalization of nitric oxide in organs, cells and subcellular organelles, and how its localization relates to several relevant clinical conditions. Understanding the complexity of the compartmentalization of nitric oxide production and the implications of this compartmentalization in terms of cellular targets and downstream effects will eventually contribute toward the development of better strategies for treating or preventing pathological events associated with the increase, inhibition or mislocalization of nitric oxide production.
Keywords: nitric oxide, nitric-oxide synthase, isoforms, location, organelles, Golgi, mitochondria, diabetes, endotoxic shock, asthma, vasodilation
Introduction
Since 1987, when the endothelium-derived relaxing factor was identified as nitric oxide [1, 2], numerous reports have indicated that this small gaseous molecule, nitric oxide, is a ubiquitous mediator of many different biological processes, such as vasodilation [3], neurotransmission [4, 5], macrophage-mediated cytotoxicity [6], gastrointestinal smooth muscle relaxation [5] and bronchodilation [7], through a variety of downstream pathways (Figure 1). According to Fick’s laws of diffusion, the diffusion coefficient of nitric oxide is 4.8 × 10−5 cm2 in water at 37°C [8, 9], similar to that of oxygen under comparable conditions [10]. It has been estimated that the half-life of nitric oxide varies from about 1 s in blood-free perfused guinea pig heart to 30 s in physiological buffers [8]. Based on these half-life values, the diffusion distances are expected to be in the 120- to 700-μm range [8]. Nitric oxide has been detected at a distance of 100 to 500 μm from RAW 264.7 macrophages stimulated with interferon, yielding a diffusion radius of 10 to 50 cells (assuming an average macrophage diameter of 10 μm) [11]. Thus, theoretically, based on its diffusion coefficient and the assumption that cells in culture are a representative model for the diffusion of nitric oxide in vivo, the effects of this gaseous molecule could extend to many cells beyond its production site.
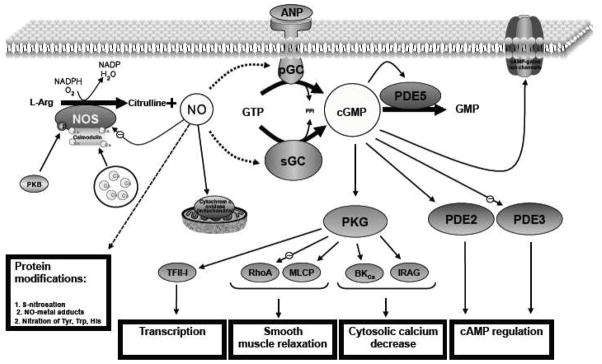
Figure 1. Nitric oxide generation and signaling.
Nitric oxide, generated by NOS, activates soluble guanylate cyclase (sGC) and particulate guanylate cyclase (pGC), and inhibits cytochrome c oxidase. cGMP activates cGMP-dependent protein kinases (PKG). As shown in the figure, some downstream pathways and cellular functions (grey boxes) are involved in the effects of endogenous cGMP. The concentration of cGMP can be controlled by the action of phosphodiesterases (PDE). In addition, nitric oxide can affect other pathways through protein modifications (nitric oxide-metal adduct formation, S-nitrosation, nitration). For instance, the nitration of specific Tyrosine residues in the beta-subunit of Complex V results in lower ATPase activity during nitrative stress or aging [137, 138]. ANP, atrial natriuretic peptide; PK, protein kinases (letter indicates the type of kinase); PDEs, phosphodiesterases; sGC, soluble guanylyl cyclase; IRAG, IP3 receptor-associated cGKIβ substrate; MLC phosphatase (MLCP); RhoA, a substrate for cGMP-dependent protein kinases (PKG); large-conductance Ca2+-activated K+ (BKCa) channels. Other details were previously described by Hofmann et al. [139].
However, estimations of the half-life of nitric oxide based on its diffusion radius do not apply to complex biological systems. Factors that limit nitric oxide diffusion and therefore its half-life in biological systems include its interactions with soluble guanylyl cyclase and other proteins (e.g., hemoglobin), lipids and free radicals [11–13]. When measured in isolated rat aorta, for example, its diffusion was shown to be four-fold smaller in an aortic wall than that in a homogeneous medium such as water [14]. It has also recently been reported that the cholesterol content in membranes decreases nitric oxide diffusion by 20 to 40% [12]. This decrease was attributed to changes in membrane fluidity caused by cholesterol. Nitric oxide efflux produced by activated macrophages was also reduced by 41% in the presence of albumin and by 53 to 70% in the presence of liposomes, indicating that intracellular structures or biomolecules could also limit nitric oxide diffusion [11], thereby establishing compartmentalized effects of nitric oxide within the cells.
Depending on the environment, other factors can affect the half-life of nitric oxide and therefore its diffusion. When high concentrations of nitric oxide are produced by activated NOS2, and superoxide anion is present, the formation of peroxynitrite will limit the diffusion of nitric oxide [8]. In addition, depending on the oxygen gradient near mitochondria, cytochrome c oxidase can become a target of nitric oxide, resulting in the inhibition of mitochondrial respiration [13].
In addition to the diffusion of nitric oxide from its production site, the partitioning of nitric oxide between polar and apolar media could play a major role in terms of localized effects [15]. Nitric oxide and oxygen have similar partition coefficients in apolar media, being 70-times more soluble in hydrophobic than in hydrophilic media [16]. Therefore, both molecules are more concentrated in hydrophobic milieu, such as liposomes, lipoproteins, biomembranes or within the hydrophobic pockets of proteins, than in polar-based environments [16, 17]. Higher concentrations of nitric oxide and oxygen in an apolar environment may result in chemical reactions favoring the formation of nitrogen oxides with chemical properties different from those of nitric oxide [18, 19].
The fact that nitric oxide encounters diffusion barriers throughout the body to find its targets and that nitric oxide-mediated responses are cell/tissue specific, the existence of NOS isoforms and their fine regulation at the pre and post translational levels constitute a sine qua non condition to accomplish its specific yet diverse functions.
In view of these arguments, we propose that the systemic effects of nitric oxide derive from the cumulative effects of the autocrine and paracrine levels in specific organs.
Isoforms of nitric oxide synthase and their cell specificity
Nitric oxide is synthesized by nitric oxide synthase (NOS). The enzymatic synthesis of nitric oxide is accomplished by three NOS isoforms: the neuronal NOS (NOS1), the endothelial NOS (NOS3) and the inducible NOS (NOS2) (see Table 1). The activation of the first two enzymes depends on calcium, whereas NOS2 is independent of calcium [20]. It has been reported that NOS1 and NOS3 are constitutively expressed, whereas NOS2 is induced only during the immune response [21, 22]. However, more recently, it has been shown that NOS2 is constitutively expressed in neurons [23, 24], kidney [25], liver [26], lung [27], colon [28] and keratinocytes [29], whereas NOS3 can be expressed at a level higher than the constitutive one under various conditions such as exercise [30], estrogen stimulation [31], hyperthermia [32] and shear stress [33, 34]. Thus, in lieu of the most current research reports, the expression and activity of the NOS isoforms appear to be cell-specific. In the case of vasodilation, nitric oxide produced by endothelial cells diffuses to the smooth muscle cells to activate soluble guanylate cyclase, and by doing so it causes vascular relaxation [20], even when both cell types have the capability of expressing the same isoform, NOS3. This difference has been attributed to the heavy methylation of the NOS3 promoter that results in the inhibition of NOS3 transcription and consequently the nitric oxide production in vascular smooth muscle cells (but not endothelial cells) from murine aorta [35].
Table I.
Cellular distribution of NOS isoforms
| NOS isoform | Cell and Expression type | References | |
|---|---|---|---|
| NOS1 | Neurons, cardiomyocytes, gastrointestinal smooth muscle, keratinocytes, macula densa, neutrophils, skeletal muscle, tubular epithelium, vascular smooth muscle cells, hepatocytes | [4, 20, 26, 80, 82, 94, 124, 129–135] | |
| NOS2 | Inducible expression Macrophages and airway smooth muscle cells, alveolar macrophages, chondrocytes, endothelial cells, Kupffer cells, lung fibroblasts, mast cells, neutrophils, skeletal muscle, Type II epithelial cells, vascular smooth muscle cells Constitutive expression Airway epithelium, colon mucosae, cortical tubules, neurons, hepatocytes, keratinocytes |
[20, 23–29, 52, 82] | |
| NOS3 | Endothelial cells, bronchial epithelial cells, eosinophils, epithelial cells of human nasal mucosa, fibroblasts, gastrointestinal mucosae, hepatocytes, lymphocytes, neutrophils, skeletal muscle, syncytiotrophoblasts of human placenta, Type II alveolar cells | [20, 26, 51, 52, 82, 134, 136] | |
Nitric oxide synthesis and its ensuing effects depend not only on the types of cells in which nitric oxide is produced but also on the particular conditions experienced by the cells, the organ and the whole organism at the time of its production. For example, nitric oxide production by vascular endothelial cells is usually continuous and in relatively small amount, contributing to the maintenance of normal blood pressure and blood homeostasis [20]. During septic shock, however, NOS2 expression is induced in vascular endothelial cells, which in turn release high concentrations of nitric oxide, a process associated to vasoplegia, persistent hypotension and decompensation [20, 36, 37]. In another example, metabolically controlled production of nitric oxide can be affected by the distribution and expression of arginases (enzymes that catalyze the production of L-ornithine and urea from L-arginine) and dimethylargininases [38–40], limited substrate concentration (L-arginine) or local concentrations of citrulline (a competitive inhibitor of dimethylarginine dimethylaminohydrolase [41]) and asymmetric dimethylarginine (a noncompetitive inhibitor of NOS1 with a Ki = 0.4 μM [42]; Figure 2). Interactions of NOS isoforms with other trafficking proteins at the cellular and subcellular levels may also alter the fate of nitric oxide [43–46].
Figure 2. Metabolite-controlled production of NO.
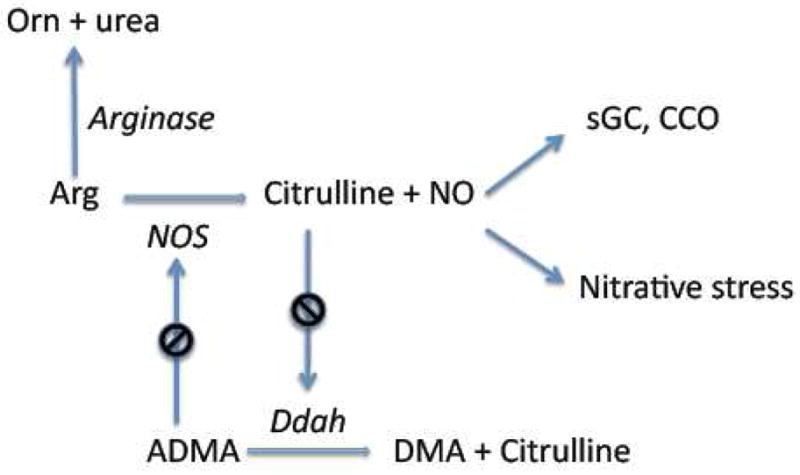
Nitric oxide (NO) is produced by nitric oxide synthases (NOS) from L-arginine. Nitric oxide can interact with specific targets, such as soluble guanylate cyclase (sGC) and cytochrome c oxidase (CCO), or with other molecules, such as superoxide anion, to trigger nitrative stress. High levels of citrulline (mM) inhibit NG,NG-dimethylarginine dimethylaminohydrolase (Ddah), resulting in an increase of NG,NG-dimethyl-L-arginine (ADMA). ADMA, in turn, is a potent NOS inhibitor. Arginine concentrations can be modulated by the activity of arginases, which catalyze the formation of L-ornithine (Orn) and urea from L-arginine. Another abbreviation: DMA, dimethylarginine. Enzyme names are in italic.
Our hypothesis is that the compartmentalized production and effects of nitric oxide define its role in pathophysiology, and therefore modulating its localized production might be the key for effective pharmacological interventions and for understanding genetic differences in pathophysiology. This hypothesis is based on the fact that compartmentalization of nitric oxide production within the cells explains its different functions and roles in different clinical conditions (Figure 3).
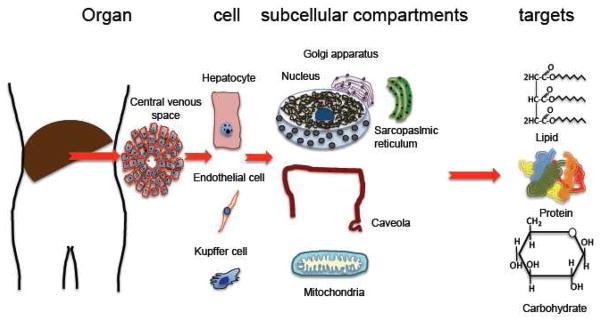
Figure 3. Location of NOS in liver, liver cells and subcellular compartments.
NOS1, NOS2 and NOS3 are present in liver. NOS1 has been shown in rat hepatocytes ([140] and Villanueva et al., 2010, submitted manuscript). NOS2 and NOS3 have been demonstrated in normal human hepatocytes [26]. NOS2 is present in Kupffer cells, whereas NO3 is present at the endothelial cells of the hepatic sinusoids [26, 141]. Within the cells, the NOS isoforms are located in different subcellular compartments such as the Golgi apparatus, caveolae or mitochondria [94]. NOS1 and NOS2 can be translocated from cytoplasm to nuclei in pathological conditions such as diabetes (see Figure 4) and cirrhosis [26], respectively. Within the cell, nitric oxide produced by NOS can interact with various specific targets (soluble guanylate cyclase, cytochrome c oxidase) and other biomolecules such as lipids, proteins and carbohydrates.
Compartmentalization of nitric oxide synthases in different organs
In this section, we present and discuss how differential compartmentalization of the NOS isoforms in different organs relates to clinical examples based on the current literature and some recent results from our group.
Lung
The lung is composed of at least forty different cell types including vascular smooth muscle cells, endothelial cells, bronchial smooth muscle cells, neurons, pneumocytes, epithelial cells and macrophages [47]. Each one has the ability to produce nitric oxide via one or more NOS isoforms [48–52]. Blood vessels irrigate all the structures in the lung including every alveolus [53]. Endothelial cells, essential components of blood vessels, express NOS3 [3, 20]. Nitric oxide in the lung is involved in many different processes originating in different cell types: vasodilation at the endothelium, bronchodilation at the inhibitory non-adrenergic/non-cholinergic nerve terminals, participation of macrophages in phagocytosis and production of mucin by the bronchial epithelial cells [48, 51, 54]. These diverse functions have to be coordinated and controlled to support adequate blood flow and air flow under normal conditions as well as when allergens or immunogens trigger an inflammatory response [51].
Nitric oxide and reactive species derived from it (known as nitrogen reactive species) play an important role in diverse inflammatory pulmonary diseases [55]. In asthma patients, elevated levels of nitric oxide have been found in their exhaled air [52, 56], consistent with the increased expression of the NOS2 gene [52, 57]. In chronic obstructive pulmonary disease (COPD) [52], a pathological lung condition associated with pulmonary hypertension [58], the nitric oxide level in patients’ exhaled air has also been reported to be increased, two-fold higher than normal but one third lower than those observed in asthma patients [59]. In COPD patients, exhaled nitric oxide has been directly associated with the exacerbation and severity of the disease [59], and as in the case of asthma, COPD has been associated with increased oxidative stress in lung [60, 61], although little is known about cell-specific expression of the NOS isoforms.
The fact that nitric oxide is known to dilate bronchia (reference), and that yet asthma patients with increased exhaled nitric oxide in their airways are afflicted with bronchoconstriction (reference) seems paradoxical but can be explained by the role of oxidative/nitrative stress [61, 62]. The strong induction of pulmonary NOS2, simultaneously with the development of bronchoconstriction, could be due to the formation of unwanted byproducts that can overcome the expected bronchodilator effect, such as peroxynitrite [62–64]. In support of this possibility, nitrotyrosine, a marker of nitrative stress, was found at increased levels in the lungs of asthmatic patients [62, 63, 65]. However, the differential expression of the NOS isoforms in different lung cells of healthy and asthmatic subjects provides an alternative scenario. Increased NOS2 specifically in bronchial epithelia and alveolar macrophages would result in increased lung mucus secretion, whereas the decreased expression of both NOS3 and NOS1 specifically in pulmonary vessels and bronchial smooth muscle would lead to pulmonary hypertension and bronchoconstriction. Thus, an effective pharmacological intervention might be to increase the expression of NOS1 and NOS3 in specifically targeted cells to limit pulmonary hypertension and bronchoconstriction, while repressing NOS2 induction to minimize nitrative stress and excessive mucus secretion.
Interestingly, genetic background differences in terms of NOS polymorphisms have been reported in asthma [66]. For example, an association between a less active polymorphic variant of NOS1 and the presence of asthma has been documented [66]. Also, one polymorphic variant of NOS3 has been associated with asthma in Caucasians [67], while another has been predicted to confer protection against asthma [68]. Differential cell-specific expression of the NOS isoforms in the lungs of asthma and COPD patients could explain the inconsistent pharmacological results obtained from NOS inhibitors [56, 69]. NOS inhibitors have been proposed as potential therapeutic agents [70, 71], but they only reduce the activity of NOS2. It is possible, however, that they could aggravate bronchial and vascular smooth muscle contractility when NOS1 and NOS3 were already reduced in those tissues.
Another clinical condition that could be explained by the compartmentalization of NOS isoforms is endotoxic shock. During endotoxic shock, pulmonary blood flow changes in conjunction with different stages of the condition [72]. Nitric oxide could be one of the major molecular mediators involved in these changes in pulmonary blood distribution. On one hand, at early stages and independently of the etiology of shock, pulmonary blood flow increases, raising the volume of oxygenated blood in vital organs. During the late stages of shock, on the other hand, massive pulmonary vasodilation is elicited in response to dramatic NOS2 induction. This vasodilation should be prevented because the pulmonary vessels could act as a sink for a large proportion of the systemic blood volume, thereby compromising the oxygen supply to other organs. NOS inhibitors have been administered as a therapy to prevent extensive systemic vasodilation [36, 37]; however, the patient mortality was higher in those treated with NOS inhibitors than that in patients without the treatment [36], suggesting that uncontrolled vasoconstriction could result in ischemia of major organs. Unfortunately, there is little experimental evidence available on the cell-specific expression of the NOS isoforms during endotoxic shock.
A study of cell-specific changes in the NOS expression in lung tissue was carried out using an isolated lung model. In this study, it was found that NOS2 expression increased in bronchial epithelial cells, bronchia-associated lymphoid tissue, alveolar macrophages, and vascular smooth muscle cells, whereas NOS3 expression decreased in the same cell types within 2 h after lipopolysaccharide (LPS) treatment [51]. Expression levels were not measured at later stages or in whole animals; therefore, the effect(s) of these changes at the systemic level were not addressed.
There are obviously multiple variables that contribute to inflammatory diseases like asthma and COPD, among which differences in the genetic background of patients in terms of NOS polymorphisms and the spatial- and temporal-specific expression of NOS genes in the lungs of these patients are important. In order to develop better strategies for treatment and prevention, further investigation is needed to identify these variables and clarify their contributions to these diseases.
Liver
In hepatocytes, nitric oxide can be synthesized by any of the NOS isoforms [26, 73]. Various cell-cell interactions, oxygen availability and differential exposure to metabolic byproducts and substances/substrates supplied by the mesenteric or cardiac circulation could modulate the production and effects of nitric oxide [26, 74] in different hepatocytes depending on their localization in liver. In fact, in liver, which is primarily comprised of hepatocytes, the metabolic functions and gene profiles of each hepatocyte are known to depend on its location [74–77]. We have therefore hypothesized that the expression of the NOS isoforms and the effects of nitric oxide depend on the compartmentalization of the enzymes within a cell, the localization of the cells within an organ and also the developmental timing of the enzyme expression. We also believe that it is appropriate to test and assess periportal, periarteriolar, perivenous or periductal expression of the NOS isoforms to better understand their different roles and functions in liver. In addition, the effects of nitric oxide derived from NOS1 produced in periportal hepatocytes, for example, could be different depending on the stage and type of a clinical condition. Concerning NOS1 expression, we have observed that it increases primarily around perivenous hepatocytes in the late stages of experimental endotoxic shock, whereas NOS1 is translocated to the nucleus in all types of hepatocytes regardless of their location within the organ in Type 1 diabetes (Figure 4).
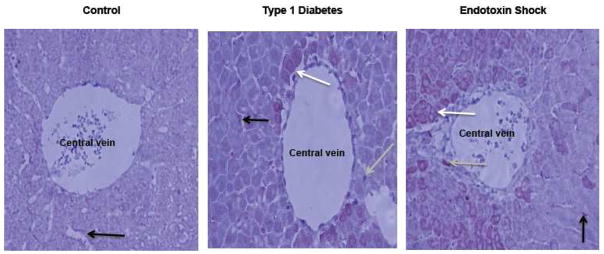
Figure 4. Immunohistochemistry of NOS1 in liver from control, Type-1 diabetic and endotoxin-treated rats.
NOS1 distribution was evaluated in the perivenous area, as shown by immunohistochemistry (at 20X; shown in a red-brown color) (Villanueva et al., 2010, submitted manuscript). Arrows show the distribution of NOS1 in endothelium (black), hepatocytes (white) and Kupffer cells (grey). A rabbit NOS1 polyclonal primary antibody (from Cayman Chemical Co.) was used in the immunostaining procedure that is followed by a diaminobenzidine-based development. In control animals, NOS1 expression was visible only at the endothelium (Control). Using the same conditions in Type-1 diabetic rats, NOS1 was present in hepatocytes, endothelium and Kupffer cells. Nuclear localization of NOS1 was only seen in hepatocytes of Type-1 diabetic rats (compare cells from the three groups). In endotoxin animals (5 h after lipopolysaccharide injection), the number of NOS1-positive cells was higher than that in control animals.
The fact that changes in the hepatic blood circulation during endotoxic shock are the opposite of those observed in lung [72] serves as another example of changes that could be resulted from the differential compartmentalization of NOS in liver. In the early stages of shock, hepatosplanchnic circulation (portal vein) is restricted to divert blood flow to organs other than liver. During late phases, the vasoplegia that characterizes multiple organ failure includes hepatosplanchnic circulation [72, 78]. It has been assumed that this vasoplegia during decompensatory endotoxic shock is mainly due to the increased NOS2 activity [20, 36, 79]. In a rat model of endotoxic shock, we have observed increased NOS2 expression, in agreement with these reports; however, the most relevant changes were attributed to the increased expression of NOS1 (Figure 4) and NOS2 (not shown) specifically in periportal and perivenous hepatocytes.
Skeletal muscle
Nitric oxide plays an important role in skeletal muscle contraction [80, 81]. All three NOS isoforms have been detected in rat quadriceps [82] and cardiomyocytes [34]. In skeletal muscle, NOS2 and NOS3 are predominantly located in the sarcoplasm, whereas NOS1 is present only in sarcolemma [82] anchored by dystrophin and syntrophin, two proteins that form the dystrophin protein complex [83]. In Duchene Muscular Dystrophy (DMD), the gene encoding dystrophin is mutated, and as a consequence, NOS1 remains localized in the sarcoplasm. This NOS1 mislocalization contributes to muscular ischemia [83]. It has also been shown that sarcolemmal NOS1 is either absent or decreased in patients with diverse myopathies (including DMD) suffering from excessive fatigue after mild exercise [84]. NOS1-null mice also exhibit the same symptoms after mild exercise [84], suggesting that NOS1 might be involved specifically in increasing blood flow to the muscle, but not necessarily in muscle contraction [84]. This hypothesis is reinforced by the findings that NOS3−/− mice have less endurance to exercise than wild-type mice, and they (similar with the NOS1−/−) have systemic blood flow problems [85], suggesting overlapping functions of NOSs.
Central nervous system
The role of nitric oxide in the control of central sympathetic outflow has been studied through the local administration of nitric oxide donors or inhibitors in different brain regions and nuclei associated with sympathetic tone. One of these regions is the rostral ventrolateral medulla (RVLM). The RVLM has glutamatergic neurons that participate in the increased sympathetic tone occurring after cardiac stimulation [86]. When NOS1 is available, the RVLM receives projections from hypothalamic nuclei related to the control of sympathetic nervous activity [87, 88]. Even though the three NOS isoforms are all present in the RVLM (NOS3 in vessels, NOS1 and NOS2 in neurons [89]), only NOS1 co-localizes with the vesicular glutamate transporter 3 and c-fos (a marker of neuron activation) in glutamatergic neurons after the epicardial application of bradykinin in anesthetized cats. These findings suggest that NOS1 in RVLM plays a role in the central sympathetic responses generated after cardiac stimulation [86]. Interestingly, when NOS3 was overexpressed in rat RVLM, the mean arterial blood pressure, heart rate and urinary excretion of norepinephrine decreased, indicating that NOS3 lowers the central sympathetic outflow [90]. NOS2 overexpression in RVLM had the opposite effect: it increased the central sympathetic tone, and this effect was related to oxidative stress [91]. These different outcomes could be explained by the differential localization of the NOS isoforms and the subsequent “local” effects of nitric oxide on downstream targets, whereas a “generalized” production of nitric oxide does not agree with these observations.
Sub-cellular compartmentalization of nitric oxide synthases
Given the short half-life of nitric oxide in biological systems (see Introduction) and the need for nitric oxide at specific sites in the cell, intracellular compartmentalization of this compound is crucial for its signal transduction activities [92, 93]. In addition, as previously mentioned, nitric oxide diffusion is limited by its interaction with different molecules within the cells, and therefore the sub-cellular location(s) of the NOS isoforms affects nitric oxide diffusion. Spatially regulating nitric oxide production additionally facilitates its specific targeting while minimizing side reactions such as the formation of peroxynitrite [94].
The expression of each NOS isoform is cell-specific and, within each cell, each isoform appears to be present in a particular subcellular compartment. Modifications of the NOS isoforms can affect their sub-cellular compartmentalization, the most well-studied example being NOS3. Co-translational N-myristoylation and subsequent post-translational Cys palmitoylation of NOS3 determine its location in the Golgi apparatus and caveolae [95].
The expression of three NOS isoforms in heart cardiomyocytes from various species has been shown by immunohistochemistry [96, 97]. The protein expression of NOS1 and NOS3 is constitutive, whereas that of NOS2 is inducible by inflammatory mediators [97]. Recently, the importance of the compartmentalization of NOS1 and NOS3 in cardiomyocytes was demonstrated in NOS1−/− and NOS3−/− mice. Both groups of mice developed age-related cardiac hypertrophy, but only the NOS1−/− mice were hypertensive [98]. NOS3, beta-adrenergic receptor and L-Type calcium channel are located in cardiomyocyte caveolae, whereas NOS1 is close to the sarcoplasmic reticulum [98]. Thus, the different clinical outcomes of the two groups of mice were linked to the NOS1 and NOS3 compartmentalization and protein-protein interactions within cardiomyocytes [98]. NOS1 and NOS3 are also known to have opposing effects on the intracellular concentration of calcium and therefore on the control of contractility. NOS3 inhibits the calcium influx produced by beta-adrenergic agonists (mediated by L-Type calcium channels), whereas NOS1 facilitates the calcium outflow from the sarcoplasmic reticulum. These opposite effects appear to be related to the different subcellular locations of the enzymes, which facilitate the protein-protein interaction of NOS3 with caveolin-3 and that of NOS1 with ryanodine receptors [98].
NOS1 regulation in mitochondria is affected by calcium levels [94]. Nitric oxide regulates mitochondrial respiration, depending on the ratio of oxygen to nitric oxide [94], through both noncompetitive and competitive mechanisms [92]. It could be conceivable that the different cholesterol content of the outer and inner membranes might explain the differences observed when nitric oxide levels are evaluated by a nitric oxide electrode (which evaluates nitric oxide diffusing away from mitochondria) or by the oxidation of oxymyoglobin or other hemoproteins (which works as a “nitric oxide trap” due to the relatively high concentrations used), effectively competing with cholesterol and possibly with other biomolecules such as cytochrome c oxidase.
Translocation of nitric oxide synthases among cellular compartments
As indicated above, the NOS isoforms are localized in different subcellular compartments. However, after a given stimulus, some of them have been known to change location, suggesting the occurrence of posttranslational modifications such as phosphorylation or the activation of translocation, a more complex process that occurs with the aid of specific protein-protein interactions, such as what has been described for NOS3 [43, 44]. If nitric oxide were as diffusible in a biological setting (read cell) as in water, then NOS translocation would not be required unless a “localized” effect is sought. The three NOS isoforms have all been observed in cell nuclei under pathophysiological conditions [99–103]. Guanylyl cyclase [104], subunits of the sGC [105], cGMP production [105], calmodulin [106] and tetrahydrobiopterin biosynthetic enzymes [107] have also been detected in nuclei, suggesting that nuclear production of nitric oxide and the subsequent activation of its downstream targets is possible.
It has been proposed that the translocation of NOS isoforms in response to various stimuli can affect cells in different ways, including changes in the regulation of gene transcription, activation/inhibition of signal transduction pathways and modulation of enzymatic activity via protein-protein interactions [43, 44]. For example, the nitric oxide synthase-interacting protein (NOSIP) negatively regulates nitric oxide production by inducing translocation of NOS1 and NOS3 to the actin present in the cytoskeleton and inhibiting the activity of these enzymes [108–110].
Regulation of gene transcription
Several lines of evidence indicate that the regulation of nitric oxide production can also occur at the level of gene transcription. In one study, for example, NOS1 was localized in the cytosol of rat cortical astrocytes during the first 6 d of culture; however, at the 7th d, NOS1 was mainly present in the nuclei of these cells, concomitant with the nuclear production of nitric oxide and the decrease of NOS2 protein expression [104]. These results suggest that the presence of NOS1 in nuclei represses the transcription of the NOS2 gene [102].
In our own studies, we have seen that while all three NOS isoforms were localized in the cytosol of the hepatocytes in the livers of Type 1 diabetic rats, only NOS1 was present in the nuclei of these cells (Figure 4). In the parallel experiments undertaken in a different biological model (endotoxic shock), NOS1 was always detected only in the cytoplasm of hepatocytes (Figure 4); the absence of insulin, presence of hyperglycemia or a response to increased oxidative stress in the Type 1 diabetic rats could account for the differences detected between their hepatocytes and those of the rats undergoing endotoxic shock. Interestingly, in the endotoxic shock model, NOS1 primarily increases in perivenous hepatocytes from 1 to 6 h after LPS administration (Figure 4).
Another interesting study was carried out in Zucker fa/fa rats. One of the main functions of brown adipocytes is to generate heat by uncoupling mitochondria in a process named non-shivering thermogenesis. This function is impaired in Zucker fa/fa rats [111, 112]. NOS2 and NOS3 are both localized in the cytoplasm and nuclei of brown adipocytes in both control and Zucker rats [103]. The in vivo and in vitro sympathetic stimulation of non-shivering thermogenesis increased the expression and activity of both enzymes in the nuclei and cytoplasm of adipocytes in both control and wild-type Zucker rats. However, nuclear-localized NOS2 protein, but not nuclear-localized NOS3, was decreased in brown adipocytes of Zucker fa/fa rats after sympathetic stimulation. The authors suggested that the nuclear localization of the NOS isoforms might provide a subcellular environment more suitable than the cytosol for a more specific and effective action of nitric oxide-producing systems, especially for their close control of thermogenic responses of brown adipocytes [103].
In freshly isolated rat hepatocytes stimulated with lysophosphatidic acid (a G protein-coupled receptor agonist that activates NOS3), NOS3 was translocated from the cytoplasm to the nucleus [100]. Once in the nucleus, NOS3 modulated, through nitric oxide production, the transcription of the gene encoding NOS2, which had been induced by nuclear factor kappa–B [100, 113].
Activation/inhibition of signal transduction pathways
NOS3 is primarily expressed in the Golgi apparatus and plasmalemmal caveolae of endothelial cells [114, 115]. It has been shown that serum starvation increases the perinuclear location of the NOS3/caveolin complex in cultured endothelial cells isolated from bovine aorta [116]. If insulin is added to the culture, NOS3 is phosphorylated through the Akt pathway, and in response to the palmitoylation of caveolin, the complex is translocated to the caveolae; this NOS3/caveolin association inhibits NOS3 activity [117] (Figure 5). This trafficking is independent of the phosphorylation status of NOS3, explains the shorter duration of nitric oxide production in the presence of insulin, regardless of the duration of NOS3 phosphorylation [116]. In high-fat fed mice, insulin-induced NOS3 phosphorylation in aorta was decreased, suggesting that NOS3 phosphorylation may have a role in the endothelial cell dysfunction that characterizes obesity and insulin resistance [118]. NOS3 translocation may therefore be related to the modulation of vasodilation. In another example of NOS3 translocation, incubation of endothelial cells with oxidized LDL decreased nitric oxide production, an event that has been associated with the displacement of NOS3 from caveolae to the Golgi apparatus [119]. In endothelial cells, NOS3 is more active in the cis-Golgi than in the trans-Golgi apparatus, mitochondria or nucleus [120]. Based on studies with organelle-targeted NOS2 constructs, NOS2 was also found to be more active in the cis-Golgi than in any other cellular compartment [96]. It was therefore concluded that the higher activity of NOS3 is the result of a facilitated access to calcium stores in the cis-Golgi apparatus under normal conditions [120].
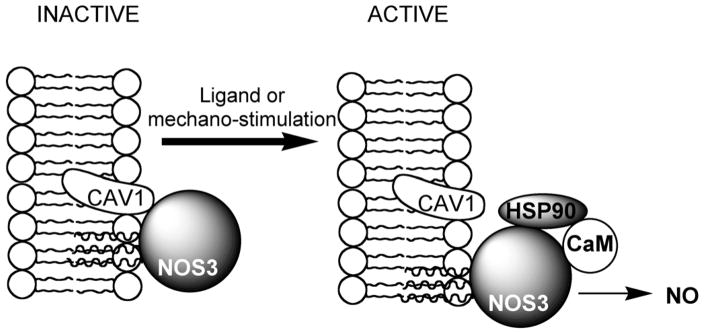
Figure 5. Role of caveolin in modulating NOS3 activity.
Caveolin-1 (CAV1), by anchoring proteins to the membrane, inhibits the activity of certain proteins, for example, NOS3. Following ligand- or mechano-stimulation, NOS3 is dissociated from CAV1, allowing its accessibility to calmodulin (CaM) and 90-kD heat-shock protein (HSP90) to produce nitric oxide (modified from Carver and Schnitzer, [142]).
Interestingly, in endothelial cells of brain lenticulostriate arterioles (pathological deterioration of which has been related to stroke), NOS3 translocation from the abluminal surface to the nucleus was associated with a decreased nitric oxide production in angiotensin-hypertensive rats, but not in control animals [121]. This result suggests that mislocalization of NOS3 plays a role in endothelial cell dysfunction in angiotensin-dependent hypertension [115]. Others have suggested that NOS3 mislocalization could be due to increased oxidative stress [121, 122].
Modulation of enzymatic activity
Here, we presented two examples from the literature on the modulation of enzymatic activity: one on the lipoxygenase activity modulation by NOS; the other on the NOS activity modulation by NOSIP. NOS3 has been detected primarily in the nuclei of human mast cells, whereas NOS1 is localized primarily in the cytosol of these cells [99]. After activation of mast cells with A23187 (a calcium ionophore) or IgE/anti-IgE, cytosolic NOS3 was phosphorylated and translocated to nuclei, and nitric oxide was detected in both the cytoplasm and nuclei of these cells. Because NOS3 co-localized with 5-lipoxygenase in the nucleus, and leukotriene formation was inhibited by a nitric oxide donor and stimulated by a nitric oxide synthesis inhibitor, the authors suggested that nuclear-localized NOS3 modulates leukotriene formation by a mechanism independent of cGMP production [99]. Whether the effects associated with any of the NOS isoforms in the nucleus occur through nitric oxide production or via a protein-protein interaction(s) needs to be further addressed.
The NOSIP (nitric oxide synthase-interacting protein) is expressed in heart, brain and lung, as well as in endothelial cells [110]. NOSIP is translocated from the nucleus to the cytoplasm in the G2 phase of the cell cycle [108, 109]. By interacting with NOS1 and NOS3 [109, 110], NOSIP negatively regulates nitric oxide production by inducing NOS1 and NOS3 translocation to the actin cytoskeleton and inhibiting their enzymatic activity [108–110].
Conclusions
Even though nitric oxide is a small molecule produced in confined compartments within different types of cells, its site-specific effects are sensed throughout the entire organism. The effects of this molecule at the organism level are not the result of a long half-life, high stability or free diffusion, but the consequence of localized effects of nitric oxide at various cellular levels and in different cell types, modulating and orchestrating complex responses requiring cross-talk among organs [25, 101, 120, 123–127]. Nitric oxide production depends on the correct localization of the enzyme isoforms involved in its synthesis, subcellular trafficking of those isoforms in response to varying conditions and proper association of regulatory proteins with the NOS isoforms to ensure correct physiological functions [43, 44, 46, 121, 128]. Understanding the complex functions of nitric oxide could someday help us to develop improved diagnostic tools and design novel preventative or treatment strategies directed to a specific NOS isoform and cellular compartment.
Acknowledgments
This study was supported by funds provided by University of California-Mexus/Consejo Nacional de Ciencia y Tecnología (Collaborative Grant 2008) to CG and CV. Dr. Villanueva was a Visitor Scholar supported by a fellowship from University of California-Mexus/Consejo Nacional de Ciencia y Tecnología. The intellectual contributions of Dr. Bulent Mutus (Visiting Professor, University of Windsor, Canada) to this study are greatly appreciated.
List of abbreviations
- ADMA
NG,NG-dimethyl-L-arginine
- CCO
cytochrome c oxidase
- COPD
chronic obstructive pulmonary disease
- Ddah
NG,NG-dimethylarginine dimethylaminohydrolase
- DMA
dimethylarginine
- DMD
Duchene Muscular Dystrophy
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- NOS
nitric oxide synthase
- NOS1
neuronal nitric oxide synthase
- NOS2
inducible nitric oxide synthase
- NOS3
endothelial nitric oxide synthase
- NOSIP
nitric oxide synthase interacting protein
- Orn
ornithine
- RVLM
rostral ventrolateral medulla
- sGC
soluble guanylyl cyclase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- 6.Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 7.Lindeman KS, Aryana A, Hirshman CA. Direct effects of inhaled nitric oxide on canine peripheral airways. J Appl Physiol. 1995;78:1898–1903. doi: 10.1152/jappl.1995.78.5.1898. [DOI] [PubMed] [Google Scholar]
- 8.Beckman JS. The physiological and pathological chemistry of nitric oxide. In: Lancaster JR Jr, editor. Nitric oxide. Principles and actions. San Diego: Academic Press; 1996. pp. 1–82. [Google Scholar]
- 9.Wise DL, Houghton G. Diffusion coefficients of neon, krypton, xenon, carbon monoxide and nitric oxide in water at 10–60∞C. Chemical Engineering Science. 1968;23:1211–1216. [Google Scholar]
- 10.Wise DL, Houghton G. Solubilities and diffusivities of oxygen in hemolyzed human blood solutions. Biophys J. 1969;9:36–53. doi: 10.1016/S0006-3495(69)86367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porterfield DM, Laskin JD, Jung SK, Malchow RP, Billack B, Smith PJ, Heck DE. Proteins and lipids define the diffusional field of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2001;281:L904–912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- 12.Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–18521. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellamy TC, Griffiths C, Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J Biol Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Srinivasan P, Collard E, Grajdeanu P, Zweier JL, Friedman A. Nitric oxide diffusion rate is reduced in the aortic wall. Biophys J. 2008;94:1880–1889. doi: 10.1529/biophysj.107.120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster JR., Jr Diffusion of free nitric oxide. Methods Enzymol. 1996;268:31–50. doi: 10.1016/s0076-6879(96)68007-0. [DOI] [PubMed] [Google Scholar]
- 17.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 18.Moller MN, Li Q, Lancaster JR, Jr, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life. 2007;59:243–248. doi: 10.1080/15216540701311147. [DOI] [PubMed] [Google Scholar]
- 19.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui M, Shimokawa H, Morishita T, Nakashima Y, Yanagihara N. Development of genetically engineered mice lacking all three nitric oxide synthases. J Pharmacol Sci. 2006;102:147–154. doi: 10.1254/jphs.cpj06015x. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui M, Shimokawa H, Otsuji Y, Ueta Y, Sasaguri Y, Yanagihara N. Nitric Oxide Synthases and Cardiovascular Diseases Insights From Genetically Modified Mice. Circulation Journal. 2009;73:986–993. doi: 10.1253/circj.cj-09-0208. [DOI] [PubMed] [Google Scholar]
- 23.Park CS, Pardhasaradhi K, Gianotti C, Villegas E, Krishna G. Human retina expresses both constitutive and inducible isoforms of nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1994;205:85–91. doi: 10.1006/bbrc.1994.2633. [DOI] [PubMed] [Google Scholar]
- 24.Park CS, Park R, Krishna G. Constitutive expression and structural diversity of inducible isoform of nitric oxide synthase in human tissues. Life Sci. 1996;59:219–225. doi: 10.1016/0024-3205(96)00287-1. [DOI] [PubMed] [Google Scholar]
- 25.Morrissey JJ, McCracken R, Kaneto H, Vehaskari M, Montani D, Klahr S. Location of an inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int. 1994;45:998–1005. doi: 10.1038/ki.1994.135. [DOI] [PubMed] [Google Scholar]
- 26.McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A. 2002;99:17161–17166. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asano K, Chee CB, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts PJ, Riley GP, Morgan K, Miller R, Hunter JO, Middleton SJ. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol. 2001;54:293–297. doi: 10.1136/jcp.54.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu Y, Sakai M, Umemura Y, Ueda H. Immunohistochemical localization of nitric oxide synthase in normal human skin: expression of endothelial-type and inducible-type nitric oxide synthase in keratinocytes. J Dermatol. 1997;24:80–87. doi: 10.1111/j.1346-8138.1997.tb02748.x. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 31.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 32.Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285:H333–340. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lam CF, Peterson TE, Richardson DM, Croatt AJ, d’Uscio LV, Nath KA, Katusic ZS. Increased blood flow causes coordinated upregulation of arterial eNOS and biosynthesis of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2006;290:H786–793. doi: 10.1152/ajpheart.00759.2005. [DOI] [PubMed] [Google Scholar]
- 34.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chan Y, Fish JE, D’Abreo C, Lin S, Robb GB, Teichert AM, Karantzoulis-Fegaras F, Keightley A, Steer BM, Marsden PA. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279:35087–35100. doi: 10.1074/jbc.M405063200. [DOI] [PubMed] [Google Scholar]
- 36.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 37.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Bratt JM, Franzi LM, Linderholm AL, O’Roark EM, Kenyon NJ, Last JA. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 242:1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene AL, Rutherford MS, Regal RR, Flickinger GH, Hendrickson JA, Giulivi C, Mohrman ME, Fraser DG, Regal JF. Arginase activity differs with allergen in the effector phase of ovalbumin- versus trimellitic anhydride-induced asthma. Toxicol Sci. 2005;88:420–433. doi: 10.1093/toxsci/kfi311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG, NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989;264:10205–10209. [PubMed] [Google Scholar]
- 42.Tsikas D, Boger RH, Sandmann J, Bode-Boger SM, Frolich JC. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000;478:1–3. doi: 10.1016/s0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- 43.Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Muller-Esterl W, Icking A. Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–3880. doi: 10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konig P, Dedio J, Oess S, Papadakis T, Fischer A, Muller-Esterl W, Kummer W. NOSIP and its interacting protein, eNOS, in the rat trachea and lung. J Histochem Cytochem. 2005;53:155–164. doi: 10.1369/jhc.4A6453.2005. [DOI] [PubMed] [Google Scholar]
- 46.Konig P, Dedio J, Muller-Esterl W, Kummer W. Distribution of the novel eNOS-interacting protein NOSIP in the liver, pancreas, and gastrointestinal tract of the rat. Gastroenterology. 2002;123:314–324. doi: 10.1053/gast.2002.34212. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn CI. The cells of the lung and their organelles. In: Lenfant C, editor. The Biochemical basis of pulmonary function (Lung biology in health and disease) New York: Dekker; 1976. pp. 3–48. [Google Scholar]
- 48.Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr, Huang PL, McMurtry IF, Rodman DM. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–478. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 49.Forstermann U, Dun N. Immunohistochemical localization of nitric oxide synthases. Methods Enzymol. 1996;268:510–515. doi: 10.1016/s0076-6879(96)68053-7. [DOI] [PubMed] [Google Scholar]
- 50.Zhan X, Li D, Johns RA. Immunohistochemical evidence for the NO cGMP signaling pathway in respiratory ciliated epithelia of rat. J Histochem Cytochem. 1999;47:1369–1374. doi: 10.1177/002215549904701103. [DOI] [PubMed] [Google Scholar]
- 51.Ermert M, Ruppert C, Gunther A, Duncker HR, Seeger W, Ermert L. Cell-specific nitric oxide synthase-isoenzyme expression and regulation in response to endotoxin in intact rat lungs. Lab Invest. 2002;82:425–441. doi: 10.1038/labinvest.3780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 53.Paes de Almeida O, Bohm CM, de Paula Carvalho M, Paes de Carvalho A. The cardiac muscle in the pulmonary vein of the rat: a morphological and electrophysiological study. J Morphol. 1975;145:409–433. doi: 10.1002/jmor.1051450403. [DOI] [PubMed] [Google Scholar]
- 54.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 55.Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med. 2006;41:515–527. doi: 10.1016/j.freeradbiomed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 57.Donnelly LE, Barnes PJ. Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:144–151. doi: 10.1165/ajrcmb.26.1.4477. [DOI] [PubMed] [Google Scholar]
- 58.Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs. 2009;69:1153–1171. doi: 10.2165/00003495-200969090-00002. [DOI] [PubMed] [Google Scholar]
- 59.Beg MF, Alzoghaibi MA, Abba AA, Habib SS. Exhaled nitric oxide in stable chronic obstructive pulmonary disease. Ann Thorac Med. 2009;4:65–70. doi: 10.4103/1817-1737.44649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy K, Borrill ZL, Starkey C, Hazel AL, Morris J, Vestbo J, Singh D. Use of different exhaled nitric oxide multiple flow rate models in COPD. Eur Respir J. 2007;29:651–659. doi: 10.1183/09031936.00149706. [DOI] [PubMed] [Google Scholar]
- 61.Langen RC, Korn SH, Wouters EF. ROS in the local and systemic pathogenesis of COPD. Free Radic Biol Med. 2003;35:226–235. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 62.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 63.Tsujino I, Nishimura M, Kamachi A, Makita H, Munakata M, Miyamoto K, Kawakami Y. Exhaled nitric oxide--is it really a good marker of airway inflammation in bronchial asthma? Respiration. 2000;67:645–651. doi: 10.1159/000056294. [DOI] [PubMed] [Google Scholar]
- 64.Kanazawa H, Shiraishi S, Hirata K, Yoshikawa J. Decreased peroxynitrite inhibitory activity in induced sputum in patients with bronchial asthma. Thorax. 2002;57:509–512. doi: 10.1136/thorax.57.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008;230:269–275. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grasemann H, Yandava CN, Storm van’s Gravesande K, Deykin A, Pillari A, Ma J, Sonna LA, Lilly C, Stampfer MJ, Israel E, Silverman EK, Drazen JM. A neuronal NO synthase (NOS1) gene polymorphism is associated with asthma. Biochem Biophys Res Commun. 2000;272:391–394. doi: 10.1006/bbrc.2000.2794. [DOI] [PubMed] [Google Scholar]
- 67.Yanamandra K, Boggs PB, Thurmon TF, Lewis D, Bocchini JA, Jr, Dhanireddy R. Novel allele of the endothelial nitric oxide synthase gene polymorphism in Caucasian asthmatics. Biochem Biophys Res Commun. 2005;335:545–549. doi: 10.1016/j.bbrc.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 68.Holla LI, Jurajda M, Pohunek P, Znojil V. Haplotype analysis of the endothelial nitric oxide synthase gene in asthma. Hum Immunol. 2008;69:306–313. doi: 10.1016/j.humimm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O’Connor BJ. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- 70.Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007;132:581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- 71.Abe M, Hayashi Y, Murai A, Shibata K, Sakata N, Igarashi R, Katsuragi T, Tanaka K. Effects of inducible nitric oxide synthase inhibitors on asthma depending on administration schedule. Free Radic Biol Med. 2006;40:1083–1095. doi: 10.1016/j.freeradbiomed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 72.Ayuse T, Brienza N, Revelly JP, O’Donnell CP, Boitnott JK, Robotham JL. Alternations in liver hemodynamics in an intact porcine model of endotoxin shock. Am J Physiol. 1995;268:H1106–1114. doi: 10.1152/ajpheart.1995.268.3.H1106. [DOI] [PubMed] [Google Scholar]
- 73.Wei CL, Khoo HE, Lee KH, Hon WM. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91–102. doi: 10.1016/s1089-8603(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 74.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 75.Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- 76.Ohno K, Maier P. Tumor necrosis factor alpha differentially modulates the cellular response of rat hepatocytes in periportal- and pericentral-equivalent cultures. Eur J Pharmacol. 1995;292:205–214. doi: 10.1016/0926-6917(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 77.Colletti M, Cicchini C, Conigliaro A, Santangelo L, Alonzi T, Pasquini E, Tripodi M, Amicone L. Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology. 2009;137:660–672. doi: 10.1053/j.gastro.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 78.Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken) 2008;291:714–720. doi: 10.1002/ar.20646. [DOI] [PubMed] [Google Scholar]
- 79.Stawicki SP, Sims C, Sarani B, Grossman MD, Gracias VH. Methylene blue and vasoplegia: who, when, and how? Mini Rev Med Chem. 2008;8:472–490. doi: 10.2174/138955708784223477. [DOI] [PubMed] [Google Scholar]
- 80.McConell GK, Wadley GD. Potential role of nitric oxide in contraction-stimulated glucose uptake and mitochondrial biogenesis in skeletal muscle. Clin Exp Pharmacol Physiol. 2008;35:1488–1492. doi: 10.1111/j.1440-1681.2008.05038.x. [DOI] [PubMed] [Google Scholar]
- 81.Steensberg A, Keller C, Hillig T, Frosig C, Wojtaszewski JF, Pedersen BK, Pilegaard H, Sander M. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007;21:2683–2694. doi: 10.1096/fj.06-7477com. [DOI] [PubMed] [Google Scholar]
- 82.Buchwalow IB, Minin EA, Samoilova VE, Boecker W, Wellner M, Schmitz W, Neumann J, Punkt K. Compartmentalization of NO signaling cascade in skeletal muscles. Biochem Biophys Res Commun. 2005;330:615–621. doi: 10.1016/j.bbrc.2005.02.182. [DOI] [PubMed] [Google Scholar]
- 83.Heydemann A, McNally E. NO more muscle fatigue. J Clin Invest. 2009;119:448–450. doi: 10.1172/JCI38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Momken I, Lechene P, Ventura-Clapier R, Veksler V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol. 2004;287:H914–920. doi: 10.1152/ajpheart.00651.2003. [DOI] [PubMed] [Google Scholar]
- 86.Guo ZL, Longhurst JC. Responses of neurons containing VGLUT3/nNOS-cGMP in the rVLM to cardiac stimulation. Neuroreport. 2006;17:255–259. doi: 10.1097/01.wnr.0000203351.06881.54. [DOI] [PubMed] [Google Scholar]
- 87.Guo ZL, Tjen ALSC, Fu LW, Longhurst JC. Nitric Oxide in Rostral Ventrolateral Medulla Regulates Cardiac-Sympathetic Reflex: Role of Synthase Isoforms. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00209.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kantzides A, Badoer E. nNOS-containing neurons in the hypothalamus and medulla project to the RVLM. Brain Res. 2005;1037:25–34. doi: 10.1016/j.brainres.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 89.Chang AY, Chan JY, Chan SH. Differential distribution of nitric oxide synthase isoforms in the rostral ventrolateral medulla of the rat. J Biomed Sci. 2003;10:285–291. doi: 10.1007/BF02256447. [DOI] [PubMed] [Google Scholar]
- 90.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. [PubMed] [Google Scholar]
- 91.Kimura Y, Hirooka Y, Sagara Y, Ito K, Kishi T, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of inducible nitric oxide synthase in rostral ventrolateral medulla causes hypertension and sympathoexcitation via an increase in oxidative stress. Circ Res. 2005;96:252–260. doi: 10.1161/01.RES.0000152965.75127.9d. [DOI] [PubMed] [Google Scholar]
- 92.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 93.Giulivi C, Oursler MJ. Role of mitochondrial oxygen and nitrogen reactive species in signaling. In: Forman HJ, Fukuto J, Torres M, editors. Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles. Dordrecht: Neth Kluwer Academic Publishers; 2003. pp. 311–332. [Google Scholar]
- 94.Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol. 2006;291:C1225–1231. doi: 10.1152/ajpcell.00307.2006. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balligand JL, Cannon PJ. Nitric oxide synthases and cardiac muscle. Autocrine and paracrine influences. Arterioscler Thromb Vasc Biol. 1997;17:1846–1858. doi: 10.1161/01.atv.17.10.1846. [DOI] [PubMed] [Google Scholar]
- 97.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 98.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 99.Gilchrist M, McCauley SD, Befus AD. Expression, localization, and regulation of NOS in human mast cell lines: effects on leukotriene production. Blood. 2004;104:462–469. doi: 10.1182/blood-2003-08-2990. [DOI] [PubMed] [Google Scholar]
- 100.Gobeil F, Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D’Orleans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem. 2006;281:16058–16067. doi: 10.1074/jbc.M602219200. [DOI] [PubMed] [Google Scholar]
- 101.Majano P, Lara-Pezzi E, Lopez-Cabrera M, Apolinario A, Moreno-Otero R, Garcia-Monzon C. Hepatitis B virus X protein transactivates inducible nitric oxide synthase gene promoter through the proximal nuclear factor kappaB-binding site: evidence that cytoplasmic location of X protein is essential for gene transactivation. Hepatology. 2001;34:1218–1224. doi: 10.1053/jhep.2001.29626. [DOI] [PubMed] [Google Scholar]
- 102.Yuan Z, Liu B, Yuan L, Zhang Y, Dong X, Lu J. Evidence of nuclear localization of neuronal nitric oxide synthase in cultured astrocytes of rats. Life Sci. 2004;74:3199–3209. doi: 10.1016/j.lfs.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 103.Giordano A, Tonello C, Bulbarelli A, Cozzi V, Cinti S, Carruba MO, Nisoli E. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 2002;514:135–140. doi: 10.1016/s0014-5793(02)02245-7. [DOI] [PubMed] [Google Scholar]
- 104.Earp HS, Smith P, Huang Ong SH, Steiner AL. Regulation of hepatic nuclear guanylate cyclase. Proc Natl Acad Sci U S A. 1977;74:946–950. doi: 10.1073/pnas.74.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pifarre P, Baltrons MA, Foldi I, Garcia A. NO-sensitive guanylyl cyclase beta1 subunit is peripherally associated to chromosomes during mitosis. Novel role in chromatin condensation and cell cycle progression. Int J Biochem Cell Biol. 2009;41:1719–1730. doi: 10.1016/j.biocel.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 106.Bachs O, Agell N, Carafoli E. Calcium and calmodulin function in the cell nucleus. Biochim Biophys Acta. 1992;1113:259–270. doi: 10.1016/0304-4157(92)90041-8. [DOI] [PubMed] [Google Scholar]
- 107.Elzaouk L, Laufs S, Heerklotz D, Leimbacher W, Blau N, Resibois A, Thony B. Nuclear localization of tetrahydrobiopterin biosynthetic enzymes. Biochim Biophys Acta. 2004;1670:56–68. doi: 10.1016/j.bbagen.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 108.Schleicher M, Brundin F, Gross S, Muller-Esterl W, Oess S. Cell cycle-regulated inactivation of endothelial NO synthase through NOSIP-dependent targeting to the cytoskeleton. Mol Cell Biol. 2005;25:8251–8258. doi: 10.1128/MCB.25.18.8251-8258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dreyer J, Hirlinger D, Muller-Esterl W, Oess S, Kuner R. Spinal upregulation of the nitric oxide synthase-interacting protein NOSIP in a rat model of inflammatory pain. Neurosci Lett. 2003;350:13–16. doi: 10.1016/s0304-3940(03)00771-7. [DOI] [PubMed] [Google Scholar]
- 110.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 111.Bing C, Pickavance L, Wang Q, Frankish H, Trayhurn P, Williams G. Role of hypothalamic neuropeptide Y neurons in the defective thermogenic response to acute cold exposure in fatty Zucker rats. Neuroscience. 1997;80:277–284. doi: 10.1016/s0306-4522(97)00121-8. [DOI] [PubMed] [Google Scholar]
- 112.Markewicz B, Kuhmichel G, Schmidt I. Onset of excess fat deposition in Zucker rats with and without decreased thermogenesis. Am J Physiol. 1993;265:E478–486. doi: 10.1152/ajpendo.1993.265.3.E478. [DOI] [PubMed] [Google Scholar]
- 113.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998;63:766–781. [PubMed] [Google Scholar]
- 114.Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The Trafficking/Interaction of eNOS and Caveolin-1 Induced by Insulin Modulates Endothelial Nitric Oxide Production. Mol Endocrinol. 2009 doi: 10.1210/me.2009-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 118.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 120.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol. 2005;289:C1024–1033. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- 121.Gerzanich V, Ivanova S, Zhou H, Simard JM. Mislocalization of eNOS and upregulation of cerebral vascular Ca2+ channel activity in angiotensin-hypertension. Hypertension. 2003;41:1124–1130. doi: 10.1161/01.HYP.0000066288.20169.21. [DOI] [PubMed] [Google Scholar]
- 122.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 123.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 124.Rothe F, Langnaese K, Wolf G. New aspects of the location of neuronal nitric oxide synthase in the skeletal muscle: a light and electron microscopic study. Nitric Oxide. 2005;13:21–35. doi: 10.1016/j.niox.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 125.Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int. 2006;97:1332–1337. doi: 10.1111/j.1464-410X.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- 126.Grant S, Lutz EM, McPhaden AR, Wadsworth RM. Location and function of VPAC1, VPAC2 and NPR-C receptors in VIP-induced vasodilation of porcine basilar arteries. J Cereb Blood Flow Metab. 2006;26:58–67. doi: 10.1038/sj.jcbfm.9600163. [DOI] [PubMed] [Google Scholar]
- 127.Barroso JB, Carreras A, Esteban FJ, Peinado MA, Martinez-Lara E, Valderrama R, Jimenez A, Rodrigo J, Lupianez JA. Molecular and kinetic characterization and cell type location of inducible nitric oxide synthase in fish. Am J Physiol Regul Integr Comp Physiol. 2000;279:R650–656. doi: 10.1152/ajpregu.2000.279.2.R650. [DOI] [PubMed] [Google Scholar]
- 128.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. Journal of Biological Chemistry. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 129.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 130.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greenberg SS, Ouyang J, Zhao X, Giles TD. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide. 1998;2:203–212. doi: 10.1006/niox.1998.0176. [DOI] [PubMed] [Google Scholar]
- 132.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52:1593–1601. doi: 10.1038/ki.1997.490. [DOI] [PubMed] [Google Scholar]
- 133.El-Yazbi AF, Cho WJ, Cena J, Schulz R, Daniel EE. Smooth muscle NOS, colocalized with caveolin-1, modulates contraction in mouse small intestine. J Cell Mol Med. 2008;12:1404–1415. doi: 10.1111/j.1582-4934.2008.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–193. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 135.Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- 136.Chen K, Inoue M, Wasa M, Fukuzawa M, Kamata S, Okada A. Expression of endothelial constitutive nitric oxide synthase mRNA in gastrointestinal mucosa and its downregulation by endotoxin. Life Sci. 1997;61:1323–1329. doi: 10.1016/s0024-3205(97)00677-2. [DOI] [PubMed] [Google Scholar]
- 137.Fujisawa Y, Kato K, Giulivi C. Nitration of tyrosine residues 368 and 345 in the beta-subunit elicits FoF1-ATPase activity loss. Biochem J. 2009;423:219–231. doi: 10.1042/BJ20090594. [DOI] [PubMed] [Google Scholar]
- 138.Haynes V, Traaseth NJ, Elfering S, Fujisawa Y, Giulivi C. Nitration of Specific Tyrosines in Fof1 Atp Synthase and Activity Loss in Aging1. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00739.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 140.Schild L, Jaroscakova I, Lendeckel U, Wolf G, Keilhoff G. Neuronal nitric oxide synthase controls enzyme activity pattern of mitochondria and lipid metabolism. FASEB J. 2006;20:145–147. doi: 10.1096/fj.05-3898fje. [DOI] [PubMed] [Google Scholar]
- 141.Rockey DC, Shah V. Nitric oxide biology and the liver: report of an AASLD research workshop. Hepatology. 2004;39:250–257. doi: 10.1002/hep.20034. [DOI] [PubMed] [Google Scholar]
- 142.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]