Abstract
Objective:
To describe provocative testing and alternative imaging strategies used to localize an androgen-producing tumor in a 58 year old woman with severe hirsutism.
Design:
Case report.
Setting:
Clinical Research Center.
Patient(s):
A 58 year old woman who presented for evaluation of severe hirsutism.
Intervention(s):
Serum androgens were drawn at baseline, 4 hours after administration of 2000 IU of hCG and 11 days following administration of 3.75 mg of leuprolide. MRI and FDG-PET/CT were performed.
Main Outcome Measure(s):
Description of pre-operative provocative testing and imaging.
Results:
In response to hCG, testosterone rose from 243 to 288 ng/dL then decreased to 233 ng/dL following leuprolide administration. FDG-PET/CT scan demonstrated focal hypermetabolism in the right pelvis, corresponding to a soft tissue density on the non-contrast CT. MRI images were correlated with the PET/CT and the right ovary was identified. Right salpingo-oophorectomy was performed and final pathology revealed a hilar cell tumor with ovarian cortical hyperplasia.
Conclusion:
This case demonstrates the utility of provocative testing in the evaluation of a patient with severe hirsutism and illustrates the value of FDG-PET/CT when traditional imaging is non-diagnostic.
Keywords: hirsutism, testosterone, hilar cell tumor, FDG-PET/CT
Introduction
The most common causes of hirsutism are polycystic ovarian syndrome and idiopathic hirsutism (1-4). Androgen-secreting ovarian tumors are a rare cause of hirsutism, representing less than 1% of all cases (1-3), but should be suspected in cases of rapidly progressing hirsutism or frank virilization (4). Androgen-secreting ovarian tumors, which represent less than 5% of all ovarian neoplasms, are typically sex cord-stromal tumors (5, 6).
Identifying the etiology of androgen excess can be challenging. Recommended tests include serum androgens, imaging of the ovaries and adrenal glands, and stimulation or suppression testing based on the suspected origin of androgen excess (4). Virilizing ovarian tumors are typically solid and non-calcified on imaging; however it is possible to have normal ovarian imaging in the presence of small androgen secreting tumors (7). In such cases, surgical resection with bilateral salpingo-oophorectomy can be both diagnostic and therapeutic (8-11). Ovarian vein catheterization with or without hCG stimulation, may be useful to localize the source of excess androgen production prior to surgical resection (12-16).
Here we report on a 58 year old postmenopausal female referred for seven years of progressive and severe hirsutism. This is the first case to combine ovarian stimulation and suppression testing and F18-fluoro-D-glucose-positron emission tomography/computed tomography (FDG-PET/CT), when previous imaging was inconclusive, in the evaluation of a patient with a suspected androgen producing ovarian tumor.
Case
A 58 year old gravida 4 para 4 woman was referred to our clinic for evaluation of severe hirsutism. She noticed progressive hair growth over her face, abdomen and back approximately seven years ago, which had significantly worsened in the last 6 months. She reported temporal balding, but denied deepening of her voice, clitoral enlargement or change in libido.
Her gynecologic history was significant for longstanding menstrual irregularities. Following three years of infertility, she underwent bilateral ovarian wedge resection, after which her menses became regular and she had four spontaneous conceptions and four uncomplicated deliveries. She underwent a total abdominal hysterectomy and left salpingo-oophorectomy for menometrorrhagia at age 36. She was diagnosed with hypertension and type II diabetes five years ago.
Testing at the referring clinic revealed total testosterone 192 ng/dL (normal 7-40 ng/dL), free testosterone 3.8 ng/dL (normal 0.1-0.6 ng/dL) and sex-hormone binding globulin (SHBG) 15 nmol/L (normal 28-112 nmol/L). Dehydroepiandrosterone sulfate (DHEA-S), 17-hydroxy progesterone (17-OHP) and 24-hour urinary free cortisol were normal. The right ovary could not be visualized by ultrasound or pelvic magnetic resonance imaging (MRI). She was then referred to the gynecology consultation service at the National Institutes of Health for further evaluation.
On examination, she was obese (body mass index = 34.4 kg/m2) and hirsute with stubble visible over the upper lip, chin and lower jaw areas. She had temporal balding, consistent with a male pattern of hair loss. Diffuse terminal hair growth was present over the chest, lower abdomen, back and inner thighs. The self-rated Ferriman-Gallwey score was 32, with scores in the maximal range for 5 out of 9 body locations. On pelvic exam, the external genitalia appeared normal and there was no clitoromegaly. The remainder of the examination was unremarkable. The right ovary could not be visualized by ultrasound in the clinic or in the radiology department. Initial laboratory evaluation at the National Institutes of Health revealed total testosterone 248 ng/dL (normal 8-60 ng/dL), free testosterone 8.7 ng/dL (normal 0.3-1.9 ng/dL), follicle stimulating hormone (FSH) 2.4 IU/L, luteinizing hormone (LH) 8.9 IU/L, estradiol 44.7 pg/mL and inhibin B < 16 pg/mL. Serum values for DHEA (170 ng/dL) and 17-OHP (< 40 ng/dL) were normal. Hemoglobin A1C was 7.0%.
To localize the source of androgen production, 2000 IU of human chorionic gonadotropin (hCG) were administered intramuscularly and serum samples were drawn at baseline and 4 hours later. Three weeks following hCG provocative testing, 3.75 mg of leuprolide acetate were administered intramuscularly. Serum samples were drawn 11 days later. Laboratory values at baseline, 4 hours following hCG stimulation and 11 days following leuprolide suppression are shown in the Table.
Table 1.
Serum laboratory values following stimulation and suppression testing.
| Test | HCG dosage: 2000 IU |
Leuprolide dosage: 3.75 mg |
||
|---|---|---|---|---|
| baseline | 4 hours post | 11 days post | 10 days post-op | |
| Testosterone (ng/dL) |
243 | 288 | 233 | <20 |
| Free testosterone (ng/dL) |
7 | 9.5 | 6.7 | - |
| SHBG (nmol/L) | 16 | 14 | 15 | 14 |
| DHEA-S (mcg/mL) |
0.55 | 0.5 | 0.59 | 0.56 |
| Androstenedione (ng/dL) |
60 | 36 | 55 | 41 |
| 17-OH progesterone (ng/dL) |
<40 | <40 | <40 | <40 |
| FSH (IU/L) | 23 | 19.8 | 2.8 | 36.7 |
| LH (IU/L) | 9.2 | 17.1 | 0.7 | 10.4 |
| Estradiol (pg/mL) |
63.7 | 59.6 | 47.5 | 46.5 |
| Inhibin B (pg/mL) |
<16 | - | <16 | - |
| Urinary free cortisol (mcg/24hr) |
3 | - | - | - |
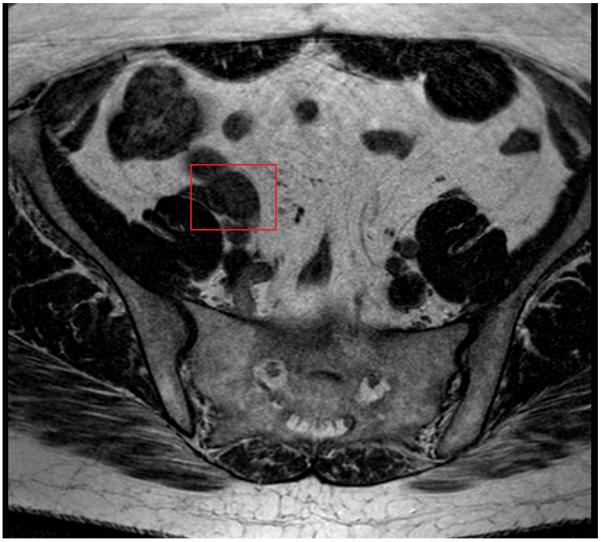
FDG-PET/CT scan demonstrated focal hypermetabolism in the right pelvis (Figure 1a), adjacent to the psoas muscle and corresponding to a suspected soft tissue density indistinguishable from bowel loops on the non-contrast CT images (Figure 1b). The MRI images were correlated with the PET/CT and the right ovarian tissue became detectable on this modality with a high degree of confidence (Figure 1c).
Figure 1a.
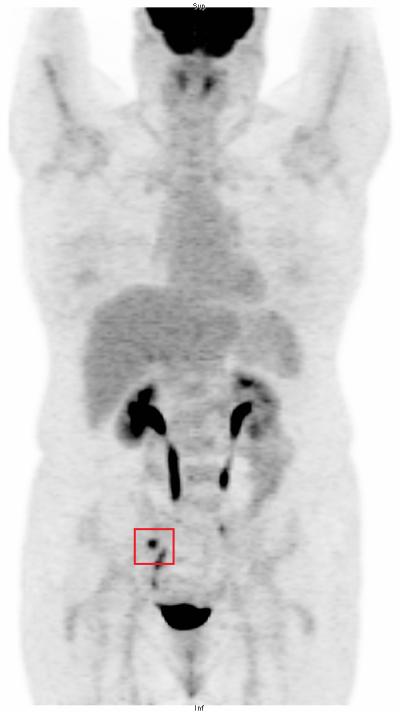
F18-FDG-PET maximal intensity projection (MIP) image demonstrating abnormal hypermetabolic focus in the right pelvis, lateral to the ureter. Remaining tracer biodistribution is physiologic.
Figure 1b.
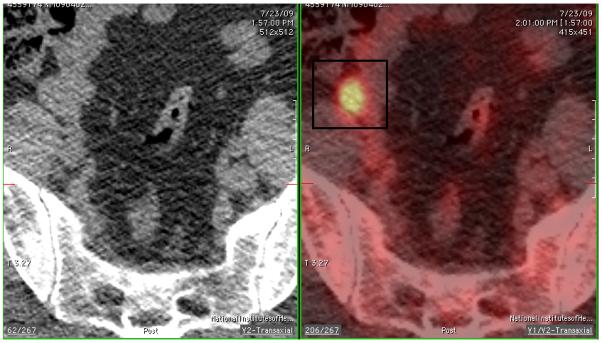
Fused axial PET/CT images show the right pelvic focus residing anterior to the psoas muscle in an area that appears inseparable from bowel loops on non-contrast CT image.
Figure 1c.
T2 weighted MRI images demonstrating right pelvic structure adjacent to the psoas muscle, compatible with ovarian tissue.
Laparoscopic right salpingo-oophorectomy was performed without complications. The right ovary measured 2 cm and the cortex was smooth. There were no peri-ovarian adhesions. Abdominopelvic adhesions were present on the left side, distant from the right ovary. Final pathology of the right fallopian tube and ovary revealed a hilar cell tumor associated with ovarian cortical hyperplasia. Corpora albicans and multiple small simple cysts were also noted. Reinke crystals were not identified. On postoperative day 10, the serum testosterone level had fallen dramatically and as expected, gonadotropin levels were elevated (Table).
Discussion
Hilar cell tumor is a rare ovarian neoplasm accounting for less than 1% of all ovarian tumors. Hyperthecosis is a similarly uncommon condition, characterized by excess ovarian androgen production and associated with polycystic ovary syndrome and metabolic syndrome. Preoperatively, we suspected hyperthecosis in our patient based on her reproductive and medical history as well as the gradual onset of symptoms and moderate elevation in serum androgens. Although patients with androgen producing ovarian pathology often report a gradual onset of hirsutism over several years, other signs of virilization may or may not be present (16, 17, 18). Classic laboratory findings for androgen secreting ovarian tumors include markedly elevated testosterone with normal DHEA-S, androstenedione, 17-OHP and urinary free cortisol levels. Androgen-producing tumors of the ovary are often difficult to localize, thus additional screening techniques are frequently necessary to evaluate the patient with androgen excess of suspected ovarian origin.
Provocative testing in the evaluation of patients with suspected androgen-producing ovarian pathology can assist in the identification of the source of androgen production. In a case series of five patients with pathologically confirmed hyperthecosis, serum testosterone levels fell in 4 out of 5 patients following treatment with dexamethasone, and serum testosterone levels rose in all five patients following hCG (19). In another study of two postmenopausal patients with hyperthecosis, a single intramuscular dose of GnRH agonist suppressed serum gonadotropins and reduced testosterone and androstenedione levels to those of normal controls in both women, demonstrating the diagnostic utility of GnRH agonist in these patients (20).
There is also evidence of a therapeutic role for GnRH agonists. A post-menopausal woman with suspected hyperthecosis based on ultrasound findings was treated successfully with a synthetic GnRH agonist after she did not tolerate side-effects of the antiandrogen cyproterone (21) and another postmenopausal patient had a dramatic fall in testosterone levels when she received leuprolide while awaiting surgery (22).
Stimulation with hCG has also been shown to be useful in the diagnostic evaluation of virilized patients. In a series of four patients with androgen excess, Levens et al. identified two patients with hilar cell tumors. Both patients demonstrated a rise in testosterone following hCG, and no change in testosterone values following dexamethasone (16). In the current case, a prompt ovarian response to hCG stimulation was observed with a substantial rise in testosterone by four hours after administration. After a single dose of leuprolide, an expected suppression of gonadotropin levels occurred with a decline in total and free testosterone to baseline, albeit above-normal, levels. The modest response to leuprolide suggested autonomous, non-suppressible ovarian androgen production in this patient. This report is the first to combine hCG stimulation with GnRH suppression testing in the evaluation of a patient with hilar cell tumor.
When the presence of ovarian pathology is not established with provocative testing, selective venous sampling may help localize excess androgen production to the ovary. Levens et al. described their experience with four hyperandrogenemic women and reviewed an additional 132 previously reported cases that underwent selective ovarian effluent sampling to diagnose and localize an androgen-producing tumor. Ovarian venous sampling correctly localized 66% of androgen-producing tumors (16). Intraoperative sampling of the ovarian veins is an option, when selective catheterization of the ovarian veins under fluoroscopy is unsuccessful (23).
Imaging is frequently a critical part of the diagnostic work-up of the patient with androgen excess. In this case, the FDG-PET/CT scan allowed localization to the right ovary preoperatively, although initial imaging studies were non-diagnostic. F18-FDG is a glucose analog which is taken up by tissues with high metabolic activity. Variability in F18-FDG ovarian uptake related to the menstrual cycle in premenopausal women is well documented in the literature (24). However, considering our postmenopausal patient, the unequivocal focal finding seen in the right adnexal region was considered highly suspicious for an ovarian abnormality. Of note, pathologic hypermetabolic foci may sometimes be detected by FDG-PET in the absence of identifiable corresponding morphologic changes on conventional imaging. Functional metabolic changes may occur prior to development of structural changes, as in the case of metastatic liver lesions, or occult tiny lesions which are unapparent morphologically but express intense “hot” metabolic activity that renders them detectable on functional imaging. In such cases, navigation with electromagnetic tracking may be considered (25).
To our knowledge only one other case has utilized F18-FDG-PET to identify an androgen producing ovarian tumor that was not visualized on ultrasound or CT. Matuszczyk and colleagues described a patient with an elevated testosterone who underwent selective venous sampling, followed by unilateral salpingo-oophorectomy and who was noted to have a Leydig cell ovarian tumor (26).
Here we report a case of severe hirsutism where preoperative provocative hormonal testing in conjunction with FDG-PET/CT localized the functionally abnormal androgen-secreting ovarian hilar cell tumor. Preoperative localization of the tumor by FDG-PET represented the crucial step in the surgical management of this patient.
Acknowledgments
This work was supported in part by the Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development
This work will be presented, in part, at the Society for Gynecologic Investigation Annual Scientific Meeting, March 24-27, 2010 in Orlando, FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement: No competing financial interests exist.
Capsule: This case describes the use of provocative testing and PET/CT to localize a hilar cell tumor of the ovary in a postmenopausal woman.
References
- 1.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–62. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 2.Moran C, Tapia MC, Hernandez E, Vazquez G, Garcia-Hernandez E, Bermudez JA. Etiological review of hirsutism in 250 patients. Arch Med Res. 1994;25:311–4. [PubMed] [Google Scholar]
- 3.O'Driscoll JB, Mamtora H, Higginson J, Pollock A, Kane J, Anderson DC. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol. 1994;41:231–6. doi: 10.1111/j.1365-2265.1994.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 4.The Practice Committee of the American Society for Reproductive Medicine The evaluation and treatment of androgen excess. Fertil Steril. 2006;86:s241–7. doi: 10.1016/j.fertnstert.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Cronje HS, Nieman I, Bam RH, Woodruff JD. Review of the granulose-thecal cell tumors from the Emil Novak ovarian tumor registry. Am J Obstet Gynecol. 1999;180(2 Pt 1):323–7. doi: 10.1016/s0002-9378(99)70207-3. [DOI] [PubMed] [Google Scholar]
- 6.Gorver V, Babu A. Hilar leydig cell tumor presenting as hirsuitism in a 51 year-old woman. Hospital Physician. 2007;43:33–8. [Google Scholar]
- 7.Outwater EK, Marchetto B, Wagner BJ. Virilizing tumors of the ovary: imaging features. Ultrasound Obstet Gynecol. 2000;15:365–71. doi: 10.1046/j.1469-0705.2000.00123.x. [DOI] [PubMed] [Google Scholar]
- 8.Siekierska-Hellmann M, Sworczak K, Babinska A, Wojtylak S. Ovarian thecoma with androgenic manifestations in a postmenopausal woman. Gynecol Endocrinol. 2006;22:405–8. doi: 10.1080/09513590600842539. [DOI] [PubMed] [Google Scholar]
- 9.Silva PD, Sorensen ML, Reynertson R, Virata RL, Mahairas GH. Laparoscopic removal of virilizing hilar cell tumor in a postmenopausal patient. J Am Assoc Gynecol Laparosc. 1997;4:499–502. doi: 10.1016/s1074-3804(05)80047-7. [DOI] [PubMed] [Google Scholar]
- 10.Baiocchi G, Manci N, Angletti G, Celleno R, Fratini D, Gilardi G. Pure Leydig cell tumor (hilus cell) of the ovary: a rare cause of virilization after menopause. Gynecol Obstet Invest. 1997;44:141–44. doi: 10.1159/000291506. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MC. Hilar cell tumor of the ovary. J Clin Path. 1972;25:106–10. doi: 10.1136/jcp.25.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh KC, Lo JC, Zaloudek CJ, Fitzgerald PA. Occult virilizing ovarian tumours in postmenopausal women: problems in evaluation with reference to a case. Ann Acad Med Singapore. 1998;27:712–716. [PubMed] [Google Scholar]
- 13.Cohen I, Cuperman S, Altaras MM, Ben-Nun I, Goldberg E, Beyth Y. Combined ovarian vein catheterization with ovarian stimulation in the diagnosis of androgen overproduction. Acta Obstet Gynecol Scand. 1992;71:245–248. doi: 10.3109/00016349209009929. [DOI] [PubMed] [Google Scholar]
- 14.Cserepes E, Szucs N, Patkos P, Csapo Z, Molnar F, Toth M, et al. Ovarian steroid cell tumor and a contralateral ovarian thecoma in a postmenopausal woman with severe hyperandrogenism. Gynecol Endocrinol. 2002;16:213–216. [PubMed] [Google Scholar]
- 15.Dickerson RD, Putman MJ, Black ME, Pinto KR, Diamond NG, Marynick S, et al. Selective ovarian vein sampling to localize a Leydig cell tumor. Fertil Steril. 2005;84:218.e19–22. doi: 10.1016/j.fertnstert.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 16.Levens ED, Whitcomb BW, Csokmay JM, Nieman LK. Selective venous sampling for androgen-producing ovarian pathology. Clin Endocrinol. 2009;70:606–14. doi: 10.1111/j.1365-2265.2008.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorgojo J, Almodovar F, Lopez E, Vicente del Cerro J, Tejerina E, Donnay S. Coincidental diagnosis of an occult hilar steroid cell tumor of the ovary and a cortisol-secreting adrenal adenoma in a 49-year-old woman with severe hyperandrogenism. Fertil Steril. 2003;80:1504–7. doi: 10.1016/j.fertnstert.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Aleem F, Spenillo, Oberlander S, Surks M. Hilar cell tumor of the ovary: preoperative localization by selective retrograde venous sampling. Obstet Gynecol. 1980;56:99–102. [PubMed] [Google Scholar]
- 19.Karam K, Hajj s. Hyperthecosis syndrome. Acta Obstet Gynecol Scand. 1979;58:73–79. doi: 10.3109/00016347909154919. [DOI] [PubMed] [Google Scholar]
- 20.Pascale M, Pugeat M, Roberts M, Rousset, Dechaud H, Dutrieux-Berger N, et al. Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumours. Clin Endocrinol. 1994;41:571–76. doi: 10.1111/j.1365-2265.1994.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 21.Parr J, Abraham R, Seed M, Short F, Wynn V. The treatment of a hyperandrogenic and virilizing state in an elderly female with a synthetic LHRH agonist. J Endocrinol Invest. 1988;11:433–6. doi: 10.1007/BF03349077. [DOI] [PubMed] [Google Scholar]
- 22.Krug E, Berga S. Postmenopausal hyperthecosis: functional dysregulation of androgenesis in climacteric ovary. Obstet Gynecol. 2002;99:893–7. doi: 10.1016/s0029-7844(01)01588-5. [DOI] [PubMed] [Google Scholar]
- 23.Regnier C, Bennet A, Malet D, Guez M, Plantavid M, Rochaix P, et al. Intraoperative testosterone assay for virilizing ovarian tumor topographic assessment: Report of a Leydig cell tumor of the ovary in a premenopausal woman with an adrenal incidentaloma. J Clin Endocrinol Metab. 2002;87:3074–77. doi: 10.1210/jcem.87.7.8583. [DOI] [PubMed] [Google Scholar]
- 24.Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266–271. [PubMed] [Google Scholar]
- 25.Wood BJ, Zhang H, Durrani A, Glossop N, Ranjan S, Lindisch D, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc IntervRadiol. 2005;16:493–505. doi: 10.1097/01.RVI.0000148827.62296.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matuszczyk A, Petersenn S, Lahner H, Haude M, Veit P, Becker J, et al. Leydig cell tumor as a cause of hirsutism in a postmenopausal woman. Med Klin (Munich) 2007;15:259–62. doi: 10.1007/s00063-007-1032-5. [DOI] [PubMed] [Google Scholar]