Abstract
Coronary revascularization by coronary artery bypass grafting (CABG) is recommended in patients with recurrent myocardial ischemia. However, the long-term results of CABG using saphenous vein (SV) graft, compared to internal mammary artery (IMA) graft, have not been satisfactory. The SV graft failure is due to development of intimal hyperplasia, a process characterized by abnormal migration and proliferation of smooth muscle cells (SMCs) in the intimal layer of the vein graft. Insulin growth factor 1 (IGF-1) is a major mitogenic growth factor released at the site of the shear stress-induced graft injury. This study, for the first time, compares the extent of IGF-1-PI3K-Akt activation in isolated human by pass graft conduits. Human SV and IMA vessels were collected and SMCs isolated and cultured. In cultured SMCs, effect of IGF-1 was examined on total and phosphorylated PI3K, Akt and IGF-1R by Western blot analysis. Cell proliferation was measured using BrdU ELISA. There was no significant difference in the basal expression of phosphorylated PI3K, Akt and IGF-1R in SV and IMA SMCs from human bypass conduits. However, we observed an upregulation of IGF-1 receptors in the SV SMCs in response to IGF-1 stimulation with no effect in IMA SMCs. Furthermore, the immunoblotting and cellular activation of signaling ELISA (CASE) assay demonstrated a significantly higher activity of both PI3K and Akt in IGF-1-stimulated SV SMCs than IMA. This was inhibited by an IGF-1R blocking antibody. IGF-1 induced proliferation in both SV and IMA SMCs was inhibited by a PI3K inhibitor, wortmannin. These data demonstrate differential activity of IGF-1-induced PI3K-Akt activation, which was quantitatively and temporally greater in SV SMCs than in the IMA. This, at least in part, could explain the greater propensity of the SV conduits than the IMA to undergo intimal hyperplasia following CABG.
Keywords: Coronary artery bypass graft, IGF-1, Internal mammary artery, Intimal hyperplasia, PI3K, Akt/PKB, Proliferation, Restenosis, Saphenous vein, Vascular smooth muscle cells
Introduction
Vascular restenosis following coronary artery bypass grafting (CABG) is a major clinical problem. The rate of restenosis due to intimal hyperplasia (IH) and secondary atheroma formation affects primarily the SV while surprisingly, the internal mammary artery (IMA) is spared from the fibroproliferative effects. Within the first year after CABG, up to 20% of SV grafts occlude. Subsequent attrition rates are as high as 4% per year, resulting in only 50% of grafts remaining patent after 10 years. In contrast with the SV, the IMA demonstrates remarkable resistance to stenosis, and is therefore the preferred alternative, with 80% of IMA grafts still patent 10 years after the procedure (Turner et al., 2007). Although many studies have investigated the histological differences between SV and IMA conduits, as well as the differences in their biochemical composition, the reason for the short-term durability of the SV graft remains obscure. Vascular smooth muscle cell (VSMC) proliferation and migration play an important role in intimal thickening in atherosclerosis and restenosis, and influence the long-term patency of venous grafts. Initiation and progression of the atherosclerotic plaque and restenosis involve complex patterns of interaction between the cells of the arterial wall, in which cytokines, such as matrix metalloproteinase (MMP), chemokines, matrix deposition, and growth factors are known to play a critical role (Jia et al., 2007). Mechanical forces also play an important role in the pathogenesis of vein graft disease. Alterations in shear stress result in the release of vasoactive mediators and localized expression of insulin-like growth factor-1 (IGF-1) (Mitra et al., 2009).
IGF-1 is involved in protein synthesis, cell migration and mitogenesis (Wu et al., 2010) and is a survival factor for VSMCs, fibroblasts, and macrophages (Furundzija et al., 2010; Wu et al., 2010; Chisalita et al., 2009), inhibiting apoptosis of these cells. Cells involved in atherogenesis secrete IGF-1, and IGF-1 receptors (IGF-1R) are upregulated in endothelial cells (ECs), VSMCs and inflammatory cells within these lesions (Bayes-Genis et al., 2000). Activation of IGF-1Rs triggers several signal pathways including phosphatidylinositol 3-kinase (PI3K) – Akt/protein kinase B (PKB) and mitogen-activated protein kinase (MAPK) (Saltiel and Kahn, 2001), which induce cell growth, differentiation and migration of VSMCs (Maile and Clemmons, 2003). “Cross talk” between IGF-1 and other growth factors makes the IGF-1-related signal pathways even more complicated. PI3K is one such family of lipid kinases, which translocates to the cell membrane and activates an intracellular signal pathway mechanism (Radcliff et al., 2005). It has the unique ability to interact with several signal-inducing molecules and is thus able to interact in multiple pathways simultaneously (Cheng and Du, 2007).
PI3K association has been documented with Ras and MAPK and focal adhesion kinase (FAK) (Campbell and Trimble, 2005). The balance between survival, apoptosis and proliferation is achieved by Akt, which plays a key role in both matrix adhesion and suppression of apoptosis (Li et al., 2010; Torella et al., 2009). Poor expression of IGF-1R in atherosclerotic plaques predisposes to apoptosis and rupture (Okura et al., 2001), while IGF1R upregulation can mediate cancer metastasis (Kucab and Dunn, 2003), which was prevented by specific inhibitors of IGF-1R (Mitsiades et al., 2004). Akt is activated by the phosphorylation of 3-phosphoinositide-dependent kinase 1 (PDK1). Sustained activation of Akt inhibits apoptosis in VSMCs, involving in part PDK1 (Jia et al., 2007) while inhibition of PDK1 by antisense oligonucleotides abolishes Akt activation by p65 in endothelial cells (Meng and D'Mello, 2003).
In the last decade, a significant body of evidence has accumulated, indicating that expression of the components of the IGF-1 system are regulated by multiple factors, including growth factors, cytokines, lipoproteins, reactive oxygen species, and hemodynamic forces (Jia et al., 2007). Thus, an in depth comparison of the underlying mechanisms of the IGF-1/PI3K/Akt axis in the pathophysiology of intimal hyperplasia in human SV and IMA could provide an opportunity to formulate better therapeutic strategies. In this study, we hypothesized that IGF-1 induces greater activation of the PI3K-Akt signaling pathway which leads to an increased proliferation of SV SMCs than IMA, and this could be responsible for intimal hyperplasia in vein grafts in an in-vivo setting.
Material and methods
Patients and tissue collection
The protocol for this study was approved by the Institutional Review Board of Creighton University. SV and IMA tissue samples were obtained anonymously from a pool of 38 patients of either sex (age 46–78 years, mean 59 ± 5 yr) undergoing CABG surgery and the excess tissue left over from the procedure was collected. We used matched samples, i.e. both SV and IMA from the same patient. Specimens were collected with minimal delay in the University of Wisconsin (UW) solution, a solution used to collect organs for transplantation, and immediately transported to the laboratory. We have previously reported that the vascular tissue and VSMCs were functionally viable for at least 36 hrs in this solution (Jia et al., 2008). Strict aseptic techniques were followed for subsequent processing of tissue samples.
Smooth muscle cell isolation and culture
VSMCs from the tissue samples were isolated by a method previously reported by our laboratory for carotid plaque VSMC culture (Jia et al., 2007) with minor modifications. Briefly, human SV and IMA samples were dissected free from the adventitia and endothelial cells were removed by gentle blunt dissection. The specimens were minced with sterile scalpels and subjected to enzymatic digestion with 1% elastase and 2% collagenase IV (Sigma, St. Louis, MO) in DMEM. The cellular digests were filtered through sterile cell strainer, centrifuged at 1,000 rpm for 10 minutes and cell pellets were washed twice in DMEM with 10% fetal bovine serum containing penicillin (100U/ml) and streptomycin (100 µg/ml). The pellets were resuspended in pre-warmed smooth muscle cell medium (ScienCell Research Laboratories, San Diego, CA) supplemented with 10% fetal bovine serum and incubated at 37°C with 5% CO2. Cells were used between the 3rd and 5th passage in order to maintain as close to normal phenotype as possible. The VSMCs were characterized by their characteristic “hill- valley” growth pattern and positive immunostaining with smooth muscle α-actin (Dako, Carpenteria, CA) and caldesmon (Biogenex, San Ramon, CA). Cells at 80–90% confluence were incubated in serum free medium for 24–48 hrs in order to render them quiescent and mitogenic stimulation was achieved using recombinant human IGF-1 (PeproTech, Rocky Hill, NJ).
For the organ culture vascular explants, two-to-three centimeter segments of the harvested veins and arteries were cut open longitudinally with the luminal surface facing upwards. These tissues were cultured in SMC medium and 30% fetal bovine serum and incubated at 37°C in 5% CO2, and the medium was changed daily. After gentle removal of the adventitia and endothelial cells, the medial smooth muscle tissues were cut into small pieces and macerated in a glass tissue homogenizer. These specimens were subsequently used for protein extraction and Western blot.
Immunoblotting
After appropriate treatment and time course cell monolayer was washed with ice cold PBS and trypsinized and the cell suspension was centrifuged at 300g for 5 minutes and supernatant aspirated. The cells were lysed by the addition of 50 µl of ice cold radio-immuno-precipitation assay (RIPA) buffer. The lysates were centrifuged at 14,000g for 10 minutes at 4°C and the supernatant protein lysates were stored at −70°C for long term storage. The lysates were assayed for protein concentration by the Bradford method (Bio-Rad Laboratories, CA). Proteins were separated by gel electrophoresis and the membranes were probed using the following antibodies: PI3K, Akt ½ (Santa Cruz Biotech), pPI3k (Upstate), pPKB (ser473) (Cell Signaling) and IGF-1R (Abcam). Band intensity was measured using UVP Bioimaging system (UVP, Minneapolis, MN) and the data was analyzed in five separate blotting experiments by Labworks image acquisition and analysis software.
Cellular Activation of Signaling ELISA (CASE)
CASE (SuperArray Bioscience Corporation, MD) is an assay method to detect protein kinase cascade activated by an extracellular stimulus in whole cells by monitoring the phosphorylated status of the protein. The method was followed according to the manufacturer’s protocol. Human SV and IMA SMCs were cultured in 96 well plates. For each treatment point two wells of cells at the same density and treated in identical fashion were used. Each assay included a blank control (with no cells), null controls (cells incubated with the secondary antibody but no primary antibody) and experimental controls (cells not subjected to the experimental conditions). Prior to the assay, the cells were synchronized by serum withdrawal for 24 hr and treated with IGF-1 (100 ng/ml) over a time course and then fixed in the wells using a formaldehyde- based fixing reagent. The wells were washed three times with washing buffer followed by the addition of quenching buffer for 20 min at room temperature. The wells were further washed and incubated overnight at 4°C with phosphor-protein or pan-protein specific antibody (1:100). The wells were washed three times and incubated with secondary antibody (1:500) for 1 hr at room temp., further washed and treated with developing solution for the formation of end-point blue coloration, which was terminated using the stop solution provided. The absorbance was read on a multi-well plate reader at 450 nm.
Cell Proliferation Assay
SMCs were plated at a density of 50,000 cells/well in 96-well culture plates with complete media. The BrdU incorporation assay was performed using a cell proliferation ELISA BrdU kit (Roche Applied Science, Germany). The cells were labeled with 10 µM of BrdU solution and incubated for 8 hr at 37°C. The cells were dried and fixed, and the cellular DNA was denatured with FixDenat solution for 30 min at room temperature. A mouse anti-BrdU monoclonal antibody conjugated with peroxidase was added to each well and the plates were incubated again at room temperature for 2 hr. Tetramethylbenzidine was added and the cells were incubated for 30 min at room temperature. Finally, the absorbance of the samples was measured by a microplate reader at 450 nm.
Statistical analysis
All data are represented as mean ± SEM. Data was analyzed using one-way ANOVA with Tukey’s post test for analysis of intra- and inter-sample groups. The p value of <0.05 was considered significant.
Results
IGF-1 receptor expression
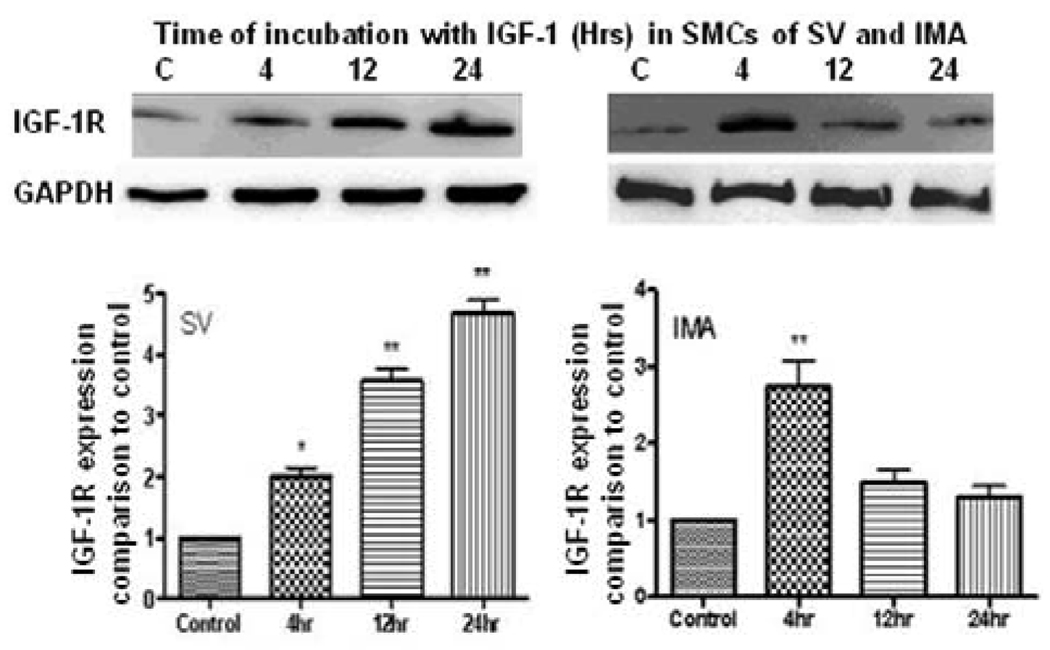
We previously reported that IGF-1 with an empty plasmid vector could significantly increase IGF-1R expression at 4 and 12 hrs, but not at 2 hrs in SV SMCs (Mitra et al., 2009). So we chose 4, 12, and 24 hrs as the incubation times in this experiment of IGF-1-induced IGF-1R in SV and IMA SMCs. Therefore, the expression of IGF-1R was determined by immunoblotting in IGF-1-stimulated SMCs of SV and IMA. In the SV SMCs there was a significant increase in the density of IGF-1R, which was almost five-fold and lasted up to 24 hrs. While in the IMA SMCs, quantitatively the increase was just under three-fold at 4 hrs but was not sustained beyond 4 hrs (Fig.1). Thus, the pattern of IGF-1R expression suggests a greater and sustained sensitivity of SV SMCs to IGF-1 activation than that of IMA.
FIGURE 1. Effect of IGF-1 on the expression of IGF-1R in SV and IMA SMCs.
Cultured SMCs were incubated with IGF-1 (100 ng/ml) for 4, 12 and 24 hrs. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. Each bar represents the ratio of IGF-1R/GAPDH (mean ±SEM) from five independent experiments. **p <0.001 vs control; * p <0.05 vs control.
Expression of phosphorylated PI3K, Akt and IGF-1R in SV and IMA SMCs from vascular explants
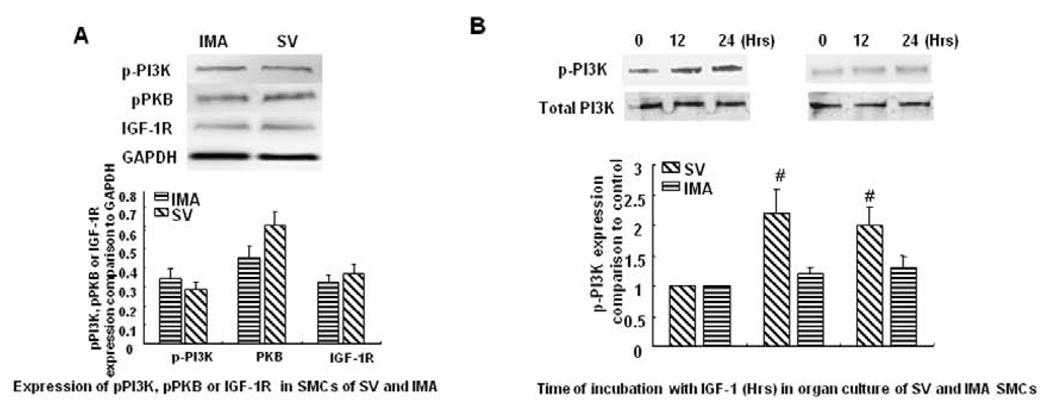
We examined the basal expression of phosphorylated PI3K, Akt and IGF-1R in SV and IMA SMCs from human bypass conduits. There were no significant difference in the basal expression of phosphorylated PI3K, Akt and IGF-1R in SV and IMA SMCs from human bypass conduits (Fig. 2A). However, IGF-1 induced phosphorylation of PI3K in SMCs of SV, but the SMCs of IMA failed to elicit a similar response (Fig. 2B).
FIGURE 2. Expression of phosphorylated PI3K, Akt and IGF-1R in SMCs of SV and IMA.
A: Expression of phosphorylated PI3K, Akt and IGF-1R in SV and IMA SMCs from human bypass conduits. B: Effect of IGF-1 on the expression of phosphorylated PI3K in organ culture SMCs of SV and IMA. The vascular explants were incubated with IGF-1 (100 ng/ml) for 12 and 24 hrs. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. Each bar represents the ratio of phosphorylated PI3K, Akt and IGF-1R to control (mean ±SEM) from five independent experiments. # p <0.01 vs control.
Expression of PI3K, pPI3K, PKB and pPKB in human SV and IMA smooth muscle cells following IGF-1 treatment
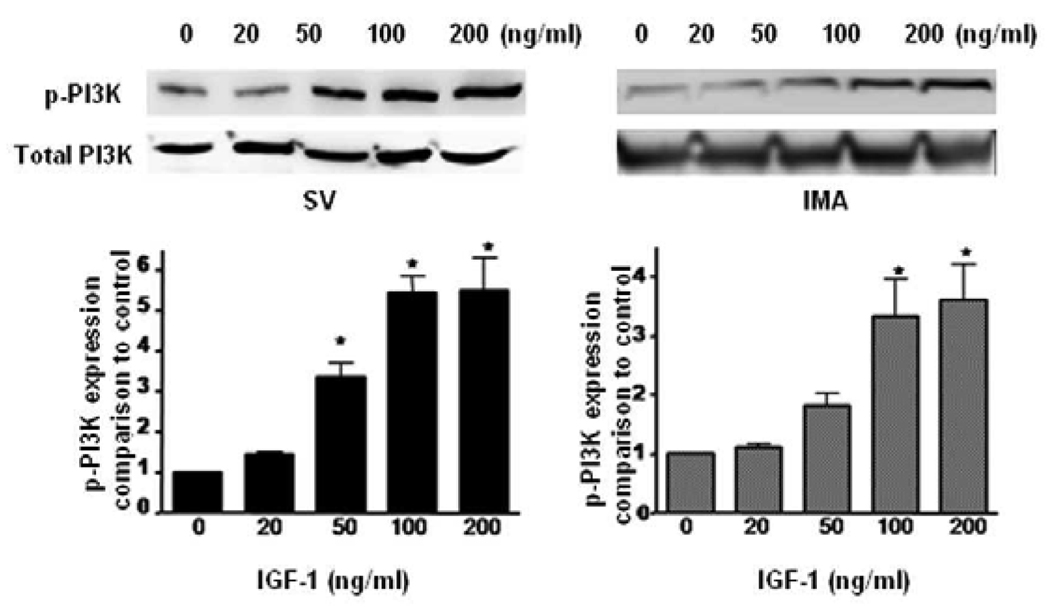
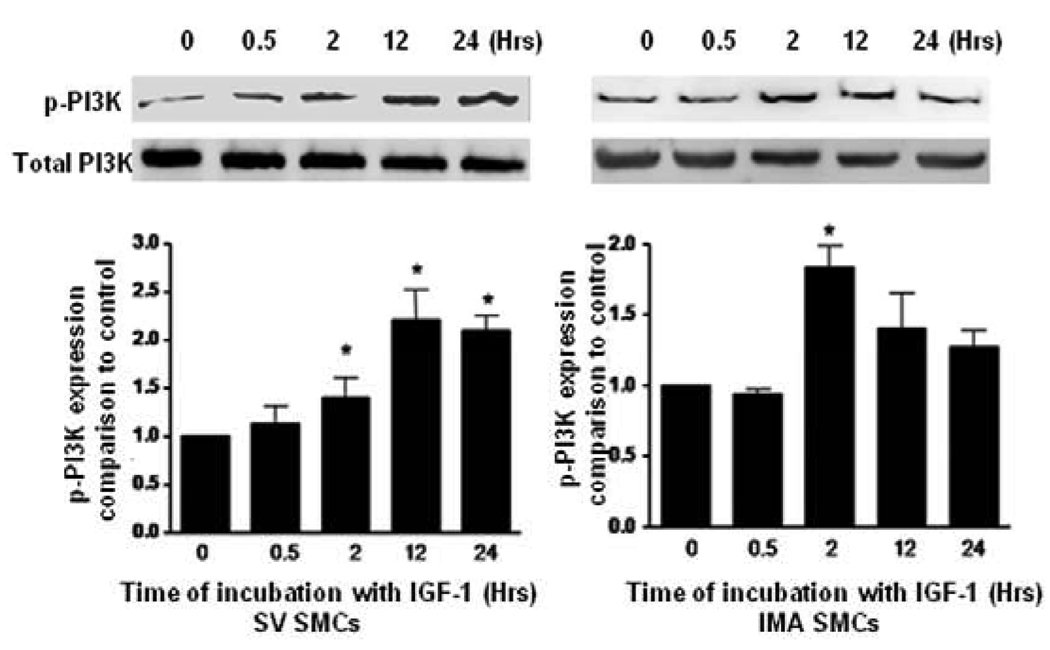
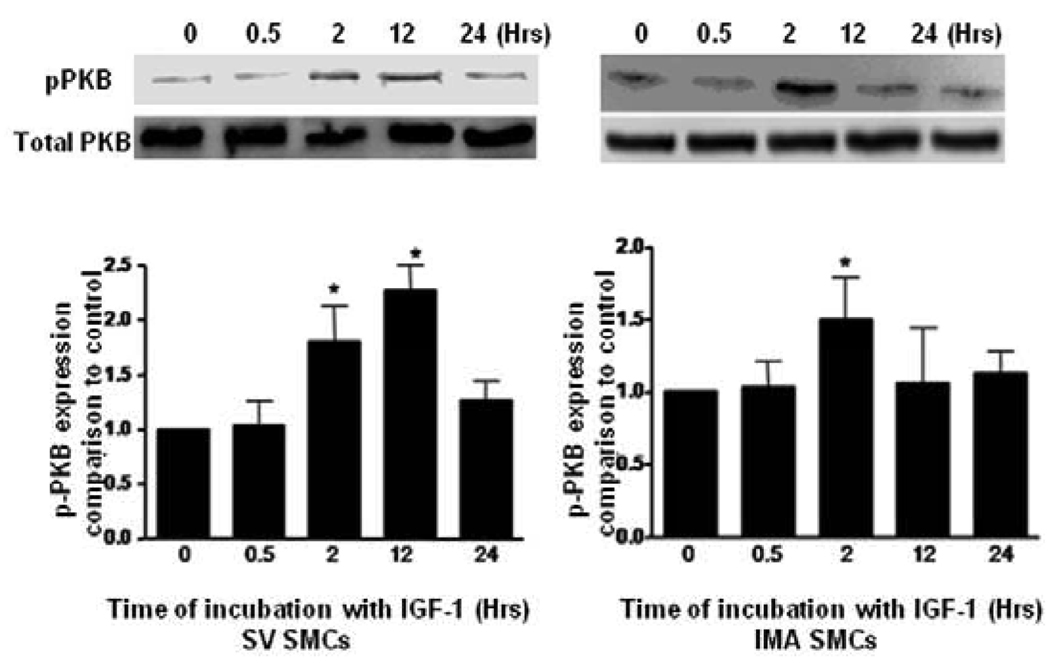
We examined the expression of PI3K, PKB and their phosphorylated forms after time course treatment with IGF-1 by Western blotting. As shown in (Fig. 3, 4), IGF-1-stimulated phosphorylation of PI3K in SV SMCs reaching to the maximum response at 12 hrs and is sustained even at 24 hrs. However, in IMA SMCs, although it reaches a significant level at 2 hrs, the phosphorylation of PI3K and Akt was not sustained and returns to near normal at 12 hrs and beyond. Similarly, exposure of the cells to IGF-1 promotes phosphorylation of Akt lasting up to 24 hrs in the SV SMCs (Fig. 4). IGF-I also stimulated phosphorylation of PI3K in a dose-dependent manner after 2 hrs incubation (Fig.5). Both SV and IMA SMCs present similar kinetics at various doses. However, SV SMCs are more sensitive since the significant response starts at lower doses. Taken together, these results indicate that IGF-1 induces phosphorylation of both SV and IMA SMCs. However, in the SV SMCs, the action is sustained over a longer period than in the IMA SMCs.
FIGURE 3. Effect of IGF-1 on the expression of phosphorylated PI3K in SV and IMA SMCs.
Cultured SMCs were incubated with IGF-1 (100 ng/ml) for 0.5, 2, 4, 12 and 24 hrs. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. Each bar represents the ratio of p-PI3K/PI3K (mean ±SEM) from five independent experiments. p <0.05 vs control.
FIGURE 4. Effect of IGF-1 on the expression of phosphorylated Akt in SV and IMA SMCs.
Cultured SMCs were incubated with IGF-1 (100 ng/ml) for 0.5, 2, 4, 12 and 24 hrs. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. Each bar represents the ratio of pPKB/PKB (mean ±SEM) from five independent experiments. p <0.05 vs control.
FIGURE 5. Dose-dependent response of IGF-1 on the expression of phosphorylated PI3K in SV and IMA SMCs.
IGF-1 induced phosphorylation of PI3K in SV and IMA SMCs at 2 hrs with a dose-dependent manner. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. Each bar represents the ratio of pPKB/PKB (mean ±SEM) from five independent experiments. p <0.05 vs control.
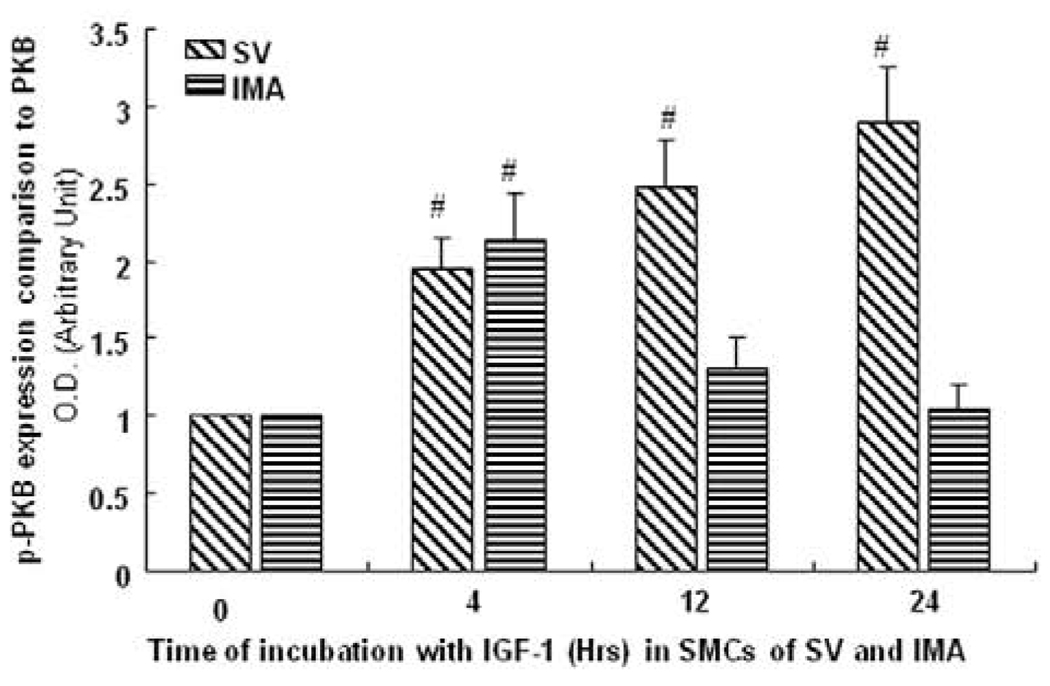
Akt/PKB activity by CASE assay
Akt plays a key role in proliferation. Therefore, levels of total and pPKB were determined by CASE assay. SV and IMA SMCs were treated with IGF-1 over 0–24 hrs time course and the phosphorylation of Akt was determined in the whole cell. We observed that pPKB was increased almost 3-fold in a sustained manner lasting up to 24 hrs in the SV SMCs (Fig. 6). However in the IMA SMCs, the increase was less that 1.5 times the control value and did not last beyond 12 hrs (Fig. 6). These results support the immunoblotting data. These findings suggest that different Akt levels and their activity determine the different proliferative rates in SV and IMA SMCs.
FIGURE 6. CASE assay to measure total and phosphorylated Akt activity in SV and IMA SMCs.
The cells were stimulated with IGF-1 (100ng/ml) over a time course. Akt activity was measured in both SV and IMA VSMCs. Values are mean + SEM (n = 5). # p <0.01 vs control.
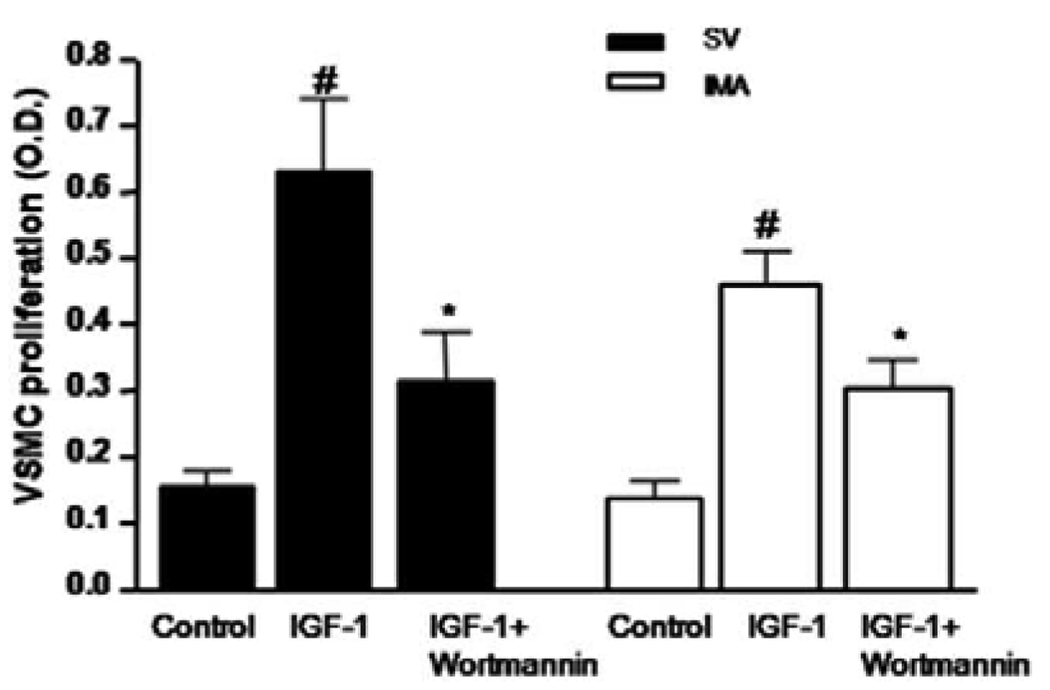
Wortmannin inhibit IGF-I-induced proliferation in VSMCs
To determine whether the IGF-I-stimulated VSMCs growth is mediated by the PI3-kinase signaling pathway, a selective PI3-kinase inhibitor, wortmannin, was used to inhibit PI3-kinase activity. As shown in Fig. 7, pretreatment of both SV and IMA SMCs with wortmannin (20 µM) attenuated IGF-I-induced proliferation.
FIGURE 7. Effect of PI3K blocker on IGF-1-induced proliferation of SV and IMA SMCs.
Serum-starved cells were pretreated with wortmannin (20 µM) for 2 hrs prior to 24 hrs stimulation with IGF-I (100 ng/ml) and BrdU incorporation was analyzed by photometric immunoassay. Data represent mean ± SEM (n = 4). #p <0.05 vs control, *p< 0.05 vs IGF-I stimulation in the absence of wortmannin.
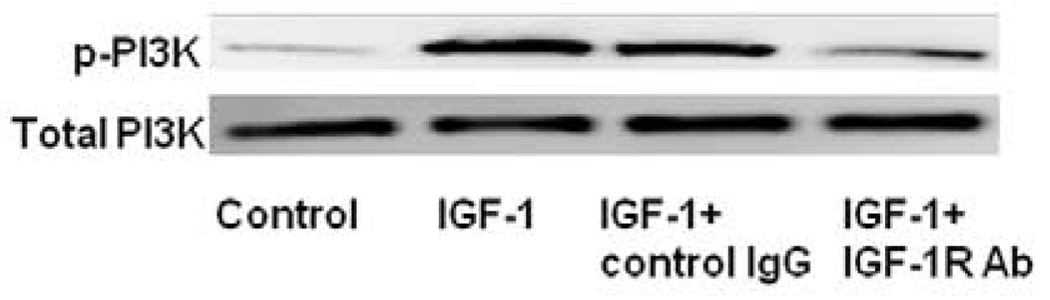
Effect of IGF-1R antibody on IGF-1-induced PI3-kinase phosphorylation in SV SMCs
To study whether the IGF-I-stimulated phosphorylation of PI-3-kinase is mediated by IGF-1R, IGF-1R antibody was used to block binding of IGF-1 to its receptor. The expression of IGF-1R in SV SMCs was about five-fold and lasted up to 24 hrs and was not sustained beyond 4 hrs in IMA SMCs (Fig.1). So, we only investigated the effect of IGF-1R antibody on IGF-1-induced PI3-kinase phosphorylation in SV SMCs. As shown in Fig. 8, pretreatment of SV SMCs with IGF-1R (20 µg/ml) inhibited IGF-I-induced phosphorylation of PI3-kinase.
FIGURE 8. Effect of IGF-1R antibody on the up-regulation of phosphorylated PI3K by IGF-1 in SV SMCs.
Cultured SV SMCs were exposed to a blocking IGF-1R antibody (20 µg/ml) for 30 minutes before the addition of IGF-1 (100 ng/ml) for 24 hrs. Protein concentration was determined in the cell lysate and each well was loaded with 20 µg of protein. This is a representative Western blot of total PI3K and p-PI3K expression in SV SMCs stimulated with IGF-1 (100ng/ml) pretreated with IGF-1R Ab.
Discussion
IGF-1 is involved in protein synthesis, cell migration and mitogenesis (Bayes-Genis, et al., 2000) and is a survival factor for VSMCs, fibroblasts, and macrophages. Activation of IGF-1 receptor triggers several signaling pathways which induce cell growth, differentiation and migration of SMCs. In this study, we observed significantly greater upregulation of IGF-1-induced IGF-1R in SV SMCs than IMA SMCs. To our knowledge, this is the first report demonstrating a direct comparison of IGF-1R expression in human SV and IMA SMCs. Neo-intimal cells are more sensitive to growth factor-induced activation as a result of higher expression of IGF-1R (Myit et al., 2003) and that decreased expression of IGF-1R in atherosclerotic plaque SMCs predispose to potential plaque rupture (Mitra et al., 2009).
There is increasing evidence that the PI3K-Akt pathway is involved in the downstream effector signaling of pro-hyperplasia effect of growth factors and mitogens on the medial SMCs. IGF-1 induced the DNA synthesis and SMC proliferation not only via PI3K-Akt/PKB pathway (Mitra et al., 2009), but also via ERK1/2 and p38 pathways (Jia et al., 2008). Thus, it is apparent that the IGF-1-PI3K-Akt/PKB axis is one of paramount importance in the proliferation of SMCs and subsequent formation of intimal hyperplasia and graft failure. In our previous study, we demonstrated a progressive increase in the phosphorylated form of PI3K which was increased at 4 h and persisted at 24 h in SV SMCs, but not in IMA SMCs (Mitra et al., 2009). These results indicate that PI3K, which is an early mediator in the IGF-PI3K-Akt axis, is quantitatively increased and exhibits greater temporal activation in SMCs of SV. In this study, we also observed quantitatively and temporally greater phosphorylation of Akt in response to IGF-1 activation in SV SMCs as compared to IMA SMCs. Thus, greater number of mitogen-induced IGF-1R together with sustained activation of Akt in SV SMCs than in the IMA could be the underlying mechanisms for the development of intimal hyperplasia in SV bypass conduits following CABG.
There have been concerns in the literature on the change in phenotype of the cells with increasing passage number. Indeed, the gene expression patterns in basal state between venous and arterial SMC subtypes were markedly different in passages 6 (Deng et al., 2006). In that study, molecular signatures that define the venous and arterial SMC subtypes including IGFBPs, β-chemokines, TIMPs, and some SMC marker genes, which were notably expressed higher in SV, and α-chemokines and endothelial marker genes, which were higher in human coronary artery. The relative expression level of these genes between venous and arterial SMCs was closely related to their responses to Ox-LDL and PDGF (Deng et al., 2006). Also, Turner and colleagues (Turner et al., 2007) found SV-SMCs in passages 2–4 to be inherently more proliferative and invasive than IMA-SMCs, most likely due to a relative increase in p44/42-MAPK activation and matrix metalloproteinase secretion. We recently reported that Cx43 expression, which could be a SMC differentiation marker (Chadjichristos et al., 2008), was significantly different in SV and IMA (Jia et al., 2007). In that study, although different cell events between passages 3 to 6 were not examined, we found stronger expression of Cx43 in SV than IMA after stimulation with Ang II and IGF-1 under both in vivo using organ culture and in vitro cultured cells in passages 3–6 (Jia et al., 2007). These inherent functional differences between SMCs of different origin may contribute to increased prevalence of intimal hyperplasia in SV grafts compared to IMA grafts.
There are many factors that could affect PI3K-Akt pathway. In CABG, shear stress is the initial event that can activate Akt phosphorylation in VSMCs (Turner et al., 2007; Chadjichristosl et al., 2008). Mechano-transduction represents an integral part of vascular homeostasis and is important in sensing shear stress and initiating an outside-to-inside signaling, which triggers the pro-hyperplasia pathways (Sedding et al., 2005). In this study, we found that IGF-1 significantly induced the proliferation of SMCs in SV and in IMA. However, the effect was much greater in SV than in IMA. But, wortmannin, a PI3K inhibitor, inhibited the effect of IGF-1-induced SMC proliferation in SV and IMA. This is consistent with our hypothesis that IGF-1 induces the proliferation and causes restenosis via IGF-1-PI3K signaling pathway in bypass conduits. Although the effect of proliferation of SMCs in SV and IMA was similar after stimulation with wortmannin, these data are not necessarily contradictory to our findings. Cytokines and other biomolecules have been implicated in the development of intimal hyperplasia, and their relationship to the PI3K-Akt pathway has been clearly elucidated (Jia et al., 2008). Cytokines are important mediators of inflammatory response. IL-18 has been shown to increase MMP9 transcription and could be a possible facilitator of SMC migration leading to intimal hyperplasia (Chandrasekar et al., 2006). Metformin, an established anti-diabetic drug, has recently been found to exhibit a novel anti-inflammatory action on VSMCs, by inhibiting NF-κB via blockade of the PI3K-Akt pathway (Isoda et al., 2006). Therefore, the inflammatory mediators and mitogens may elicit synergistic response in the activation of PI3K-Akt, and anti-inflammatory drugs could be useful in the treatment of intimal hyperplasia and restenosis following interventional procedures. Further studies are warranted to examine the effect of inflammatory cytokines.
In summary, vein graft failure is a common clinical problem, because it has greater incidence of closure than their arterial counterparts due to intimal hyperplasia. Numerous growth factors and cytokines cause the propagation of pro hyperplasia signaling pathways, culminating in cell cycle progression leading to SMC proliferation. It is, therefore, important to have a better understanding of the process of intimal hyperplasia and the key molecules involved. In this study, we present novel data showing a greater and longer-lasting activation of PI3K-Akt together with upregulation of IGF-1R in human SV SMCs than IMA SMCs. This could be one of the mechanisms for the development of intimal hyperplasia in SV bypass grafts, and for the resistance of IMA grafts to intimal hyperplasia. Thus, the PI3K-Akt pathway could be a potential therapeutic target for better management of vein-graft disease. Further understanding of the role of the expression of the PI3K-Akt signaling in human arterial and venous bypass vessels has great clinical significance and could provide the basis for new approaches to effective treatment to contain the morbidity and mortality associated with vein-graft disease.
Acknowledgment
This study was supported by National Institutes of Health grant R01HL090580 (to D.K.A.), and a LB692 Nebraska Tobacco Settlement Grant, Creighton University (to D.K.A.). We gratefully acknowledge the contributions of the Nebraska Heart Hospital, Lincoln, NE for providing the human tissue samples for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- Campbell M, Trimble ER. Modification of PI3K- and MAPK-dependent chemotaxis in aortic vascular smooth muscle cells by protein kinase CbetaII. Circ Res. 2005;96:197–206. doi: 10.1161/01.RES.0000152966.88353.9d. [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Morel S, Derouette JP, Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML, Kwak BR. Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ Res. 2008;102:653–660. doi: 10.1161/CIRCRESAHA.107.170472. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, Greene WC, Valente AJ. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281:15099–15109. doi: 10.1074/jbc.M600200200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- Chisalita SI, Johansson GS, Liefvendahl E, Bäck K, Arnqvist HJ. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF1 and IGF2. J Mol Endocrinol. 2009;43:231–239. doi: 10.1677/JME-09-0021. [DOI] [PubMed] [Google Scholar]
- Deng DX, Spin JM, Tsalenko A, Vailaya A, Ben-Dor A, Yakhini Z, Tsao P, Bruhn L, Quertermous T. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: distinct responses to stimuli. Arterioscler Thromb Vasc Biol. 2006;26:1058–1065. doi: 10.1161/01.ATV.0000208185.16371.97. [DOI] [PubMed] [Google Scholar]
- Furundzija V, Fritzsche J, Kaufmann J, Meyborg H, Fleck E, Kappert K, Stawowy P. IGF-1 increases macrophage motility via PKC/p38-dependent alphavbeta3-integrin inside-out signaling. Biochem Biophys Res Commun. 2010;394:786–791. doi: 10.1016/j.bbrc.2010.03.072. [DOI] [PubMed] [Google Scholar]
- Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Agrawal DK. Autophagy of vascular smooth muscle cells in atherosclerotic lesions. Autophagy. 2007;3:63–64. doi: 10.4161/auto.3427. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Involvement of connexin 43 in angiotensin II-induced migration and proliferation of saphenous vein smooth muscle cells via the MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 2008;44:882–890. doi: 10.1016/j.yjmcc.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Cheng G, Soundararajan K, Agrawal DK. Insulin-like growth factor-I receptors in atherosclerotic plaques of symptomatic and asymptomatic patients with carotid stenosis: effect of IL-12 and IFN-gamma. Am J Physiol Heart Circ Physiol. 2007;292:H1051–H1057. doi: 10.1152/ajpheart.00801.2006. [DOI] [PubMed] [Google Scholar]
- Jia G, Mitra AK, Cheng G, Gangahar DM, Agrawal DK. Angiotensin II and IGF-1 regulate connexin43 expression via ERK and p38 signaling pathways in vascular smooth muscle cells of coronary artery bypass conduits. J Surg Res. 2007;142:137–142. doi: 10.1016/j.jss.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis. 2003;17:41–47. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]
- Li Q, Li G, Lan X, Zheng M, Chen KH, Cao CM, Xiao RP. Receptor interacting protein 3 suppresses vascular smooth muscle cell growth by inhibition of the phosphoinositide 3-kinase-Akt axis. J Biol Chem. 2010;285:9535–9544. doi: 10.1074/jbc.M109.071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Clemmons DR. Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res. 2003;93:925–931. doi: 10.1161/01.RES.0000101754.33652.B7. [DOI] [PubMed] [Google Scholar]
- Meng F, D'Mello SR. NF-kappaB stimulates Akt phosphorylation and gene expression by distinct signaling mechanisms. Biochim Biophys Acta. 2003;1630:35–40. doi: 10.1016/j.bbaexp.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Jia G, Gangahar DM, Agrawal DK. Temporal PTEN inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits. J Cell Mol Med. 2009;13:177–187. doi: 10.1111/j.1582-4934.2008.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D, Joseph M, Libermann TA, Garcia-Echeverria C, Pearson MA, Hofmann F, Anderson KC, Kung AL. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- Myit S, Delafontaine P, Bochaton-Piallat ML, Giraud S, Gabbiani G, Brink M. Different growth properties of neointimal and medial smooth muscle cells in response to growth factors. J Vasc Res. 2003;40:97–104. doi: 10.1159/000070706. [DOI] [PubMed] [Google Scholar]
- Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol. 2001;33:1777–1789. doi: 10.1006/jmcc.2001.1441. [DOI] [PubMed] [Google Scholar]
- Radcliff K, Tang TB, Lim J, Zhang Z, Abedin M, Demer LL, Tintut Y. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res. 2005;96:398–400. doi: 10.1161/01.RES.0000157671.47477.71. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- Torella D, Gasparri C, Ellison GM, Curcio A, Leone A, Vicinanza C, Galuppo V, Mendicino I, Sacco W, Aquila I, Surace FC, Luposella M, Stillo G, Agosti V, Cosentino C, Avvedimento EV, Indolfi C. Differential regulation of vascular smooth muscle and endothelial cell proliferation in vitro and in vivo by cAMP/PKA-activated p85alphaPI3K. Am J Physiol Heart Circ Physiol. 2009;297:H2015–H2025. doi: 10.1152/ajpheart.00738.2009. [DOI] [PubMed] [Google Scholar]
- Turner NA, Ho S, Warburton P, O'Regan DJ, Porter KE. Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells. J Vasc Surg. 2007;45:1022–1028. doi: 10.1016/j.jvs.2007.01.061. [DOI] [PubMed] [Google Scholar]
- Wu S, Walenkamp MJ, Lankester A, Bidlingmaier M, Wit JM, De, Luca F. Growth hormone and insulin-like growth factor I insensitivity of fibroblasts isolated from a patient with an I{kappa}B{alpha} mutation. J Clin Endocrinol Metab. 2010;95:1220–1228. doi: 10.1210/jc.2009-1662. [DOI] [PubMed] [Google Scholar]
- Wu X, Cheng J, Li P, Yang M, Qiu S, Liu P, Du J. Mechano-sensitive transcriptional factor Egr-1 regulates insulin-like growth factor-1 receptor expression and contributes to neointima formation in vein grafts. Arterioscler Thromb Vasc Biol. 2010;30:471–476. doi: 10.1161/ATVBAHA.109.184259. [DOI] [PubMed] [Google Scholar]