Abstract
The mammalian Copper Transporter 1 (CTR1) is responsible for the uptake of copper (Cu) from the extracellular space, and has been shown to play a major role in the initial accumulation of platinum-based drugs. In this study we re-expressed wild type and structural variants of hCTR1 in mouse embryo fibroblasts in which both alleles of mCTR1 had been knocked out (CTR1−/−) to examine the role of the N-terminal extracellular domain of hCTR1 in the accumulation of cisplatin (cDDP). Deletion of either the first 45 amino acids or just the 40MXXM45 motif in the N-terminal domain did not alter subcellular distribution or the amount of protein in the plasma membrane but it eliminated the ability of hCTR1 to mediate the uptake of Cu. In contrast it only partially reduced cDDP transport capacity. Neither of these structural changes prevented cDDP from triggering the rapid degradation of hCTR1. However, they did alter the potency of the cDDP that achieved cell entry, possibly reflecting the fact that hCTR1 may mediate the transport of cDDP both through the pore it forms in the plasma membrane and via endocytosis. We conclude that cDDP interacts with hCTR1 both at 40MXXM45 and at sites outside the N-terminal domain that produce the conformational changes that trigger degradation.
Keywords: Cisplatin, Copper transporter 1, Cytotoxicity, Structure, Transport
1. Introduction
Copper (Cu) is a critical element in the normal function of all cells. Cu plays a key role in controlling not only metabolism but is also instrumental in redox regulation and p53 activity, and may even be involved in cellular trafficking (reviewed in [1]). Cu homeostasis involves multiple transporters and chaperones all working to maintain adequate levels of intracellular Cu but at the same time to protect the cell from the toxicity of this metal (reviewed in [2–4]). The importance of Cu homeostasis is evidenced by the fact that mutations that disturb the distribution of Cu cause serious disorders such as Menkes and Wilson’s diseases [5, 6].
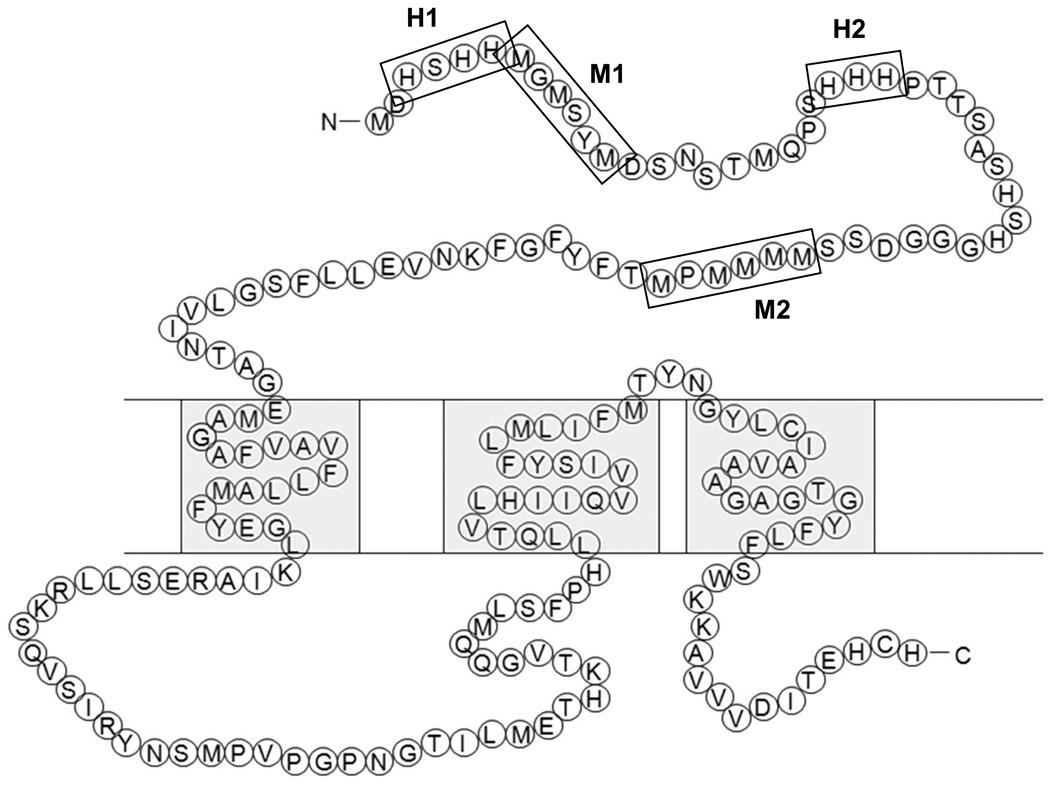
Copper transporter 1 (CTR1) is the major high affinity Cu influx transporter [7] and is essential for embryonic development [8]. Human and mouse CTR1 exhibit 92% sequence homology [9]. In both species CTR1 monomers assemble to form a homotrimeric structure that contains a small flexible pore that allows Cu+1 to pass into the cell down a concentration gradient [10–14]. Ionic interactions between Cu+1 and methionines, histidines and cysteines in the pore appear to determine both the selectivity of the pore for Cu+1 and the rate of transport [15]. As shown in Figure 1, additional clusters of methionines and histidines capable of interacting with Cu are found in the 67 amino acid extracellular hydrophobic N-terminal domain of CTR1. This domain contains 4 such clusters the first of which is the H1 region encompassing residues 3–6 and containing 3 histidines. The second is the M1 region that encompasses amino acids 7–12 and includes 3 methionines. The H2 cluster encompasses 3 histidines at positions 22–24, and the M2 region contains 5 methionines clustered together at positions 40 – 45. Prior studies have shown that, both in yeast and mammalian cells, the M2 region is required for Cu transport when environmental levels of Cu are low [16–19], but when normal levels of Cu are available even truncation of the entire N-terminus of yCTR1 does not disable its ability to transport Cu [19].
Figure 1.
Schematic diagram of the amino acid sequence of hCTR1. Boxes highlight the H1, M1, H2 and M2 motifs.
CTR1 is of interest with respect to cancer chemotherapy because it mediates the cellular accumulation and efficacy of cDDP and the other platinum-containing drugs carboplatin and oxaliplatin [20–22]. Knockout of both alleles of CTR1 markedly reduces the uptake of cDDP in both yeast and mammalian cells and renders them resistant to the cytotoxic effects of this drug [21, 23, 24]. Re-expression of wild type CTR1 in cells in which both alleles of CTR1 have been knocked out (CTR1−/− cells) restores cDDP accumulation and cytotoxicity both in vitro and in vivo [20]. In many types of cells high levels of Cu trigger the degradation of CTR1, an effect capable of limiting further Cu accumulation. cDDP likewise causes the down-regulation of CTR1 but does so at much lower concentrations and substantially more rapidly than Cu [25, 26].
In the current study we examined the role of the N-terminus of hCTR1, including the M2 region, with respect to the ability of CTR1 to control the cellular accumulation and cytotoxicity of cDDP. We used the approach of re-expressing wild type and variant forms of hCTR1 in mouse embryonic fibroblasts in which both alleles of endogenous CTR1 had been deleted. We report here that loss of the M2 cluster, either as a result of deletion of just this region or through deletion of the entire first 45 amino acids, reduced the ability of hCTR1 to transport cDDP into the cell but paradoxically increased its cytotoxicity.
2. Materials and methods
2.1 Drugs and reagents
The commercial formulation of cDDP was purchased from the Moores Cancer Center pharmacy; it contains cDDP at a concentration of 3.33 mM in 0.9% NaCl. The cDDP was diluted into DMEM-RS Reduced Serum Media (HyClone, Logan, UT) to produce a final concentration of 30 µM. Bradford reagent was purchased from BioRad Laboratories, Inc. (Hercules, CA) and sulforhodamine B was obtained from Sigma-Aldrich (St. Louis, MO) and 0.4% SRB (w/v) was solubilized in 1% (v/v) acetic acid solution. Anti-myc primary antibody 9B11 was obtained from Cell Signaling Technology, Inc. (Danvers, MA). Secondary anti-mouse, HRP-conjugated antibody was obtained from GE Healthcare (Piscataway, NJ). Hoechst 33342 nuclear stain and secondary AlexaFluor 488-conjugated anti-mouse antibody were obtained from Invitrogen (Carlsbad, CA).
2.2 Cell types, culture and engineering
Mouse embryonic fibroblast cell line in which both copies of CTR1 had been somatically knocked out (CTR1−/−) was kindly provided by Dr. Dennis Thiele [8]. The myc-CTR1−/−/wt subline was constructed by infecting the CTR1−/− cells with a lentivirus expressing a wild type human CTR1 cDNA, tagged with the myc-epitope on the N-terminus of the protein, using the ViraPower Lentiviral Induction kit (Invitrogen, Carlsbad, CA). Mutations to the hCTR1 molecule were created using the GeneTailor Site-Directed Mutagenesis Kit (Invitrogen, Carlsbad, CA) using the following primers: for the myc-CTR1−/−/M2 deletion (residues 40–45) (cccatggtggaggagacagcagcaccttctactttggctttaagaa, ttcttaaagccaaagtagaaggtgctgctgtctcctccaccatggg), for the myc-CTR1−/−/Truncated (deletion of residues 1–45) mutation (caccatgggcggcagggaacaaaaacttatttctgaagaagatctgg, cttatttctgaagaagatctgggcggcaccttctactttgg, tcaatggcaatgctctgtg).
2.3 Cell Survival Assay
Cell survival following exposure to increasing concentrations of drugs was assayed using the sulforhodamine B assay system [27]. The optimal number of cells seeded per well was determined to be 5,000 cells in preliminary experiments. Five thousand cells were seeded into each well of a 96-well tissue culture plate. Cells were incubated overnight at 37°C, 5% CO2 and then exposed for 5 min by the addition of 200 µl Pt drug-containing DMEM-RS medium. After 5 min the drug-containing media was aspirated off, cells were washed once with 37°C PBS, PBS was aspirated off and cells were covered in 200 µl complete medium. Cells were allowed to grow for 5 d after which the media was removed, the protein was precipitated with 50% trichloroacetic acid and stained using 100 µl of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 minutes. Following washing the absorbance of each well at 515 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA). All experiments were repeated at least 3 times using 3 cultures for each drug concentration.
2.4 Immunocyto Chemistry
All images were analyzed using a Delta Vision Deconvolution Microscope System utilizing a Nikon TE-200 Microscope (Applied Precision, Inc., Issaquah, WA). Deconvolution and analysis was done using the softWoRx software suite (Applied Precision, Inc., Issaquah, WA).
2.5 Measurement of cellular drug accumulation
Drug accumulation was measured by ICP-MS as previously described [28] with the only modification being the use of 1 ml of medium and the use of DMEM-RS instead of OptiMEM.
2.6 qRT-PCR
CTR1 mRNA levels were measured by qRT-PCR. cDNA was generated from mRNA isolated using Trizol (Invitrogen, Carlsbad, CA). Purified mRNA was converted to cDNA using Oligo(dT)20 priming and the SuperScript III First-Strand Kit (Invitrogen, Carlsbad, CA). qRT-PCR was performed on a Bio-Rad MyIQ qPCR machine (Hercules, CA). The forward and reverse primers for hCTR1, mCTR1 and GAPDH were, respectively: gatgatgatgcctatgacct, tcttgagtccttcatagaac, actgttgggcaacagatgct, ctgctgctactgcaatgcag, tcaccaccatggagaaggc, gctaagcagttggtggtgca. Reactions were prepared using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), according to manufacturer’s recommendations. Samples were prepared in quadruplicate and at least 3 independent RNA isolates were used in independent experiments. Analysis was done using the Bio-Rad iQ5 system software (Hercules, CA).
2.7 Statistical Analysis
All multi-group analyses were done utilizing one way analysis of variance testing with Tukey’s test post hoc analysis, where p ≤ 0.05 was determined to be statistically significant. All data is expressed as mean ± SEM values.
3. Results
3.1 Expression of hCTR1 in CTR1−/− mouse embryo fibroblasts
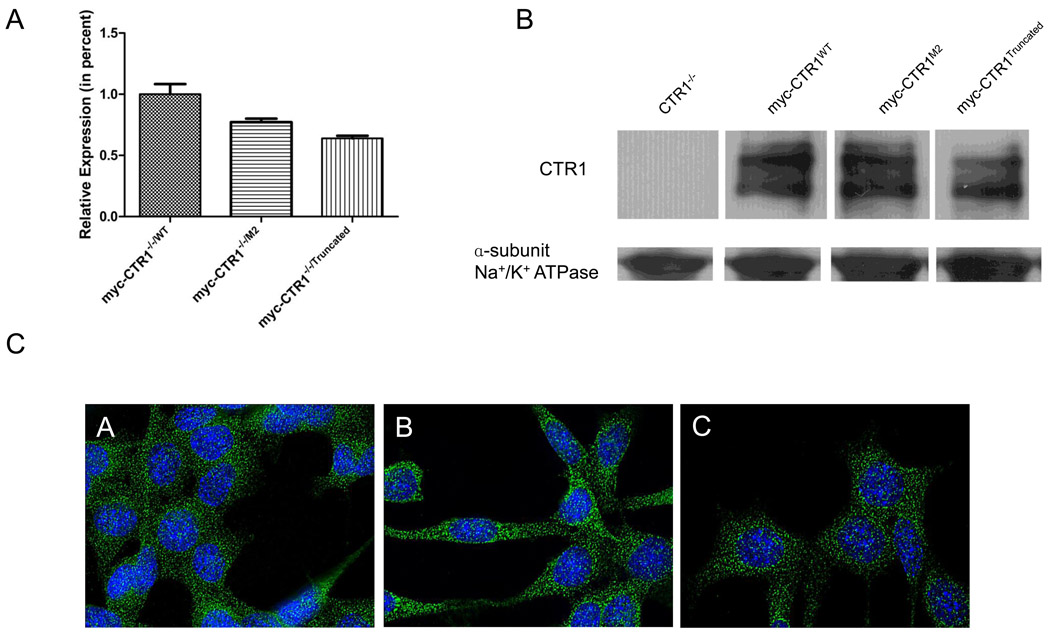
Prior studies of the importance of the histidine and methionine motifs in the N-terminal end of hCTR1for the transport of Cu and cDDP have been confounded by the presence of endogenous CTR1. To avoid this problem, wild type and variant forms of hCTR1 were re-expressed in mouse embryo fibroblasts in which both alleles of mCTR1 had been knocked out (CTR1−/− cells). qRT-PCR verified complete absence of the expression of mCTR1 in the CTR1−/− cell line. Lentiviral vectors containing a blastocidin resistance marker were constructed to express either wild type hCTR1 (CTR1−/−/WT), a variant in which the 40MXXXM45 motif was deleted (CTR1−/−/M2), or a variant in which the first 45 amino acids were deleted (CTR1−/−/Truncated). Deletion of the first 45 amino acids removes all of the histidine and methionine clusters in the N-terminal domain. All 3 forms of hCTR1 contained a myc tag at the N-terminal end. Cells were infected, selected with blastocidin and then characterized with respect to the expression of each form of exogenous CTR1. Figure 2A shows that the wild type hCTR1 was re-expressed at a 30% higher level than either of the two variants at the mRNA level as determined by qRT-PCR. To assess the level of CTR1 protein expression at the plasma membrane, the cell surface proteins were biotinylated by exposure to sulfo-NHS-SS-biotin before lysis, then recovered on streptavadin-coated beads, and subjected to western blot analysis using an antibody to the myc tag. Figure 2B shows that the wild type and both variant forms of hCTR1 were correctly localized to the plasma membrane. The CTR1−/−/M2 cells expressed plasma membrane CTR1 at a mean of 94 ± 3% of than in the CTR1−/−/WT cells and the CTR1−/−/Truncated cells expressed it at mean of 92 ± 5% of than in the CTR1−/−/WT cells. Wild type CTR1 was detected as a band migrating at 37 kDa corresponding to the glycosylated form of the myc-tagged monomer that has been observed in prior studies [16, 18]. The CTR1 in which the 40MXXXM45 was deleted, referred to as the M2 variant, also migrated at ~37 kDa and the truncated protein migrated somewhat more rapidly consistent with its smaller size.
Figure 2.
Re-expression of wild type and variants forms of myc-hCTR1 in CTR1−/− cells. A. Relative mRNA levels measured by qRT-PCR. B. Representative western blot showing the expression of myc-tagged hCTR1. C. Micrograph demonstrating the uniform expression of the myc-tagged hCTR1 protein (60× magnification). Panel A, CTR1−/−/wt cells; panel B, CTR1−/−/M2 cells; panel C, CTR1−/−/Truncated cells. Vertical bars, ± SEM.
To further assess the subcellular distribution of the CTR1 variants, the CTR1−/−/WT, CTR1−/−/M2 and CTR1−/−/Truncated cells were examined by deconvoluting microscopy using an antibody to the myc tag and a secondary fluorochrome-labeled anti-mouse antibody. Figure 2C shows that the distribution of CTR1 was similar for all 3 forms with only a minority of the protein found at the plasma membrane. Thus, the N-terminal domain of CTR1 does not contain key signals required for trafficking from the ER to the Golgi and then to the plasma membrane and internal membranous compartments.
3.2 CTR1 regulation of Cu uptake and cytotoxicity
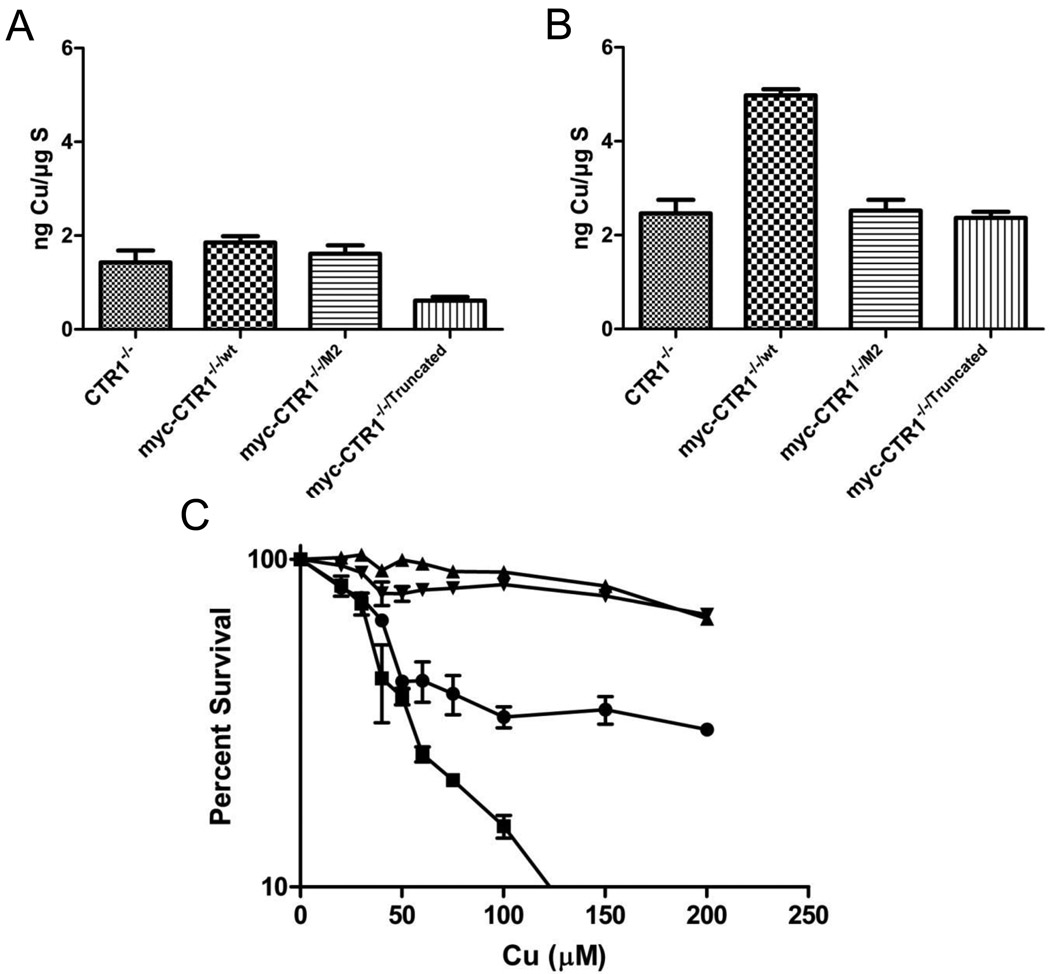
The basal Cu content of the CTR−/−, CTR1−/−/WT, CTR1−/−/M2 and CTR1−/−/Truncated cells was measured by ICP-MS while the cells were growing in complete DMEM medium that contains ~0.3 µM Cu. Figure 2A shows that re-expression of the wild type hCTR1 increased basal Cu content by 1.3-fold, whereas expression of the M2 variant increased it by 1.1-fold. Of interest was the fact that expression of the truncated variant actually decreased the basal copper level to 43% of that in the CTR1−/− cells. This suggests that the truncated form of CTR1 interferes with the uptake or enhances the efflux of Cu mediated by other transporters. The rate of Cu uptake was determined by measuring the Cu content following exposure of the cells to 100 µM Cu for 1 hour. As shown in Figure 2B, re-expression of wild type hCTR1 increased Cu uptake by 2-fold (p < 0.001) while neither the M2 deletion variant nor the N-terminal truncation variant enhanced Cu accumulation at all. These results indicate that, even under Cu replete conditions, the M2 component of the N-terminal domain is required for optimal Cu accumulation. The fact that deletion just the M2 motif disabled Cu transport to the same extent as deletion of the whole N-terminal domain identifies this motif as essential to optimal function of the N-terminal domain but does not exclude the possibility that individual deletion of the M1, H1 or H2 motifs might not have some effect.
To determine whether the differences in Cu accumulation translated into different tolerances for the cytotoxic effect of Cu, the growth rate of the CTR−/−, CTR1−/−/WT, CTR1−/−/M2 and CTR1−/−/Truncated cells was measured during a 96 h exposure to increasing concentrations of Cu. As shown in Figure 2C, the CTR1−/−/WT cells were 1.2-fold more sensitive to Cu than the CTR1−/− cells (IC50 37 ± 1 versus 46 ± 2 µM; p < 0.05). In contrast, the M2 and N-terminal truncation variants were less sensitive. The IC50 for the CTR1−/−/M2 cells was 5.2-fold higher (238 ± 9 µM; p < 0.01), and that for the CTR1−/−/Truncated cells was 6.5-fold higher (301 ± 14 µM; p<0.001), than that of the CTR1−/−/WT cells. Thus, consistent with the failure of the two variants to mediate as much Cu uptake as the wild type form, the cells expressing these variants were quite resistant to the cytotoxic effects of Cu. However, the finding that both variants rendered cells even more resistant than the CTR1−/− cells suggests that the variants are either disabling other Cu influx transporters or otherwise limiting the access of Cu to key targets capable of triggering impaired proliferation.
3.3 CTR1 regulation of cDDP uptake and cytotoxicity
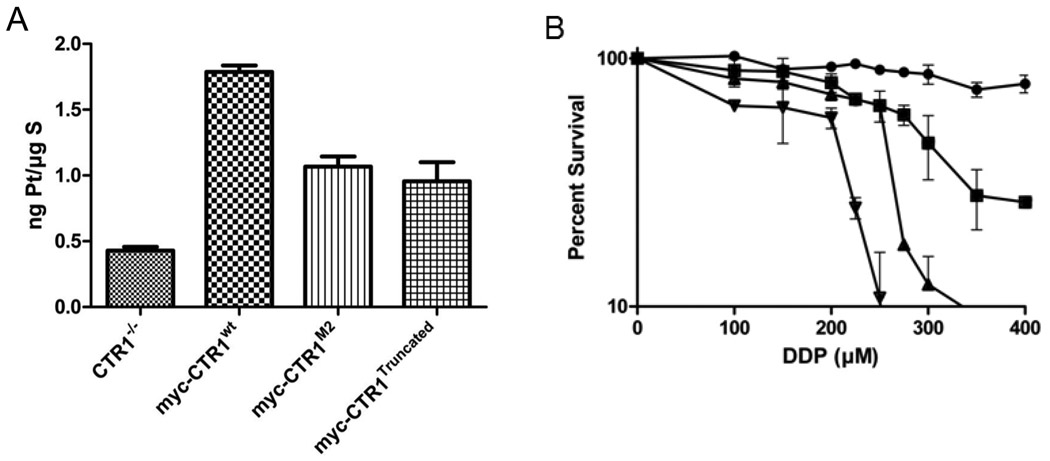
To assess the effect of structurally modifying the N-terminal end of hCTR1on the initial transport of cDDP, the 4 cell lines were exposed to 30 µM cDDP for 5 min, washed thoroughly and the Pt content measured by ICP-MS. Drug accumulation was measured at 5 min because the greatest effect of hCTR1 appears to be on the initial phase of uptake; the concentration of 30 uM was selected as being clinically relevant to intraperitoneal therapy. As shown in Figure 4A, expression of wild type hCTR1 in the CTR1−/− cells increased the Pt content by 4.2-fold (p < 0.001). Expression of the M2 variant increased the Pt content by 2.5-fold or 60% of the incremental increase in Pt content produced by the wild type hCTR1 (p < 0.001). Expression of the truncated variant produced a very similar enhancement. The Pt content was increased 2.3-fold or 55% of the incremental increase produced by wild type CTR1 (p < 0.001). Thus, both variant forms of hCTR1 retained substantial although muted ability to enhance cDDP uptake despite their inability to enhance the uptake of Cu.
Figure 4.
cDDP accumulation and cytotoxicity. A, Net accumulation of Pt in MEF cells following 5 min exposure to 30 µM cDDP. B, Inhibition of growth of MEF cells following 5 minute exposure to varying concentrations of cDDP. (●), CTR1−/−; (□), myc-CTR1−/−/wt; (▲), myc-CTR1−/−/M2; (▼), myc-CTR1−/−/Truncated. All values represent means of 6 independent experiments each containing triplicate cultures. Vertical lines, ± SEM.
Figure 3B shows the effect of a 5 minute exposure to increasing concentrations of cDDP on the growth rate of the CTR−/−, CTR1−/−/WT, CTR1−/−/M2 and CTR1−/−/Truncated cells during a subsequent period of 96 h as measured by sulforhodamine B staining. As anticipated, expression of wild type hCTR1 increased the sensitivity of the CTR1−/− cells by a factor of 2.2-fold, reducing the IC50 from 650 ± 40 to 291 ± 7 µM (p < 0.001). Interestingly, expression of either variant form of hCTR1 increased sensitivity by an even larger degree. Expression of the M2 variant increased sensitivity by 2.5-fold (IC50 255 ± 10 µM; p < 0.001), and expression of the truncated variant by 3.2-fold (IC50 204 ± 8 µM; p < 0.001). Because of the discordance between the effect of re-expressing the variant forms of CTR1 on the cellular accumulation versus the cytotoxicity of cDDP, these experiments were repeated a total of 6 times and were found to be consistent across the entire set of experiments. Thus, despite the fact that neither variant mediated cDDP accumulation as well as the wild type CTR1, the CTR1−/−/M2 and CTR1−/−/Truncated cells were less able to withstand attack by cDDP.
Figure 3.
Cu accumulation and cytotoxicity. A, total basal Cu; B, total Cu following 1 h exposure to 100 µM Cu; C, inhibition of growth of MEF cells during 96 h continuous exposure to varying concentrations of Cu. (●), CTR1−/−; (□), myc-CTR1−/−/wt; (▲), myc-CTR1−/−/M2; (▼), myc-CTR1−/−/Truncated. Each value represents the mean of no less than 3 independent experiments each performed with 3 separate cultures. Vertical bars, ± SEM.
3.4 Effect on cDDP-induced down-regulation of CTR1
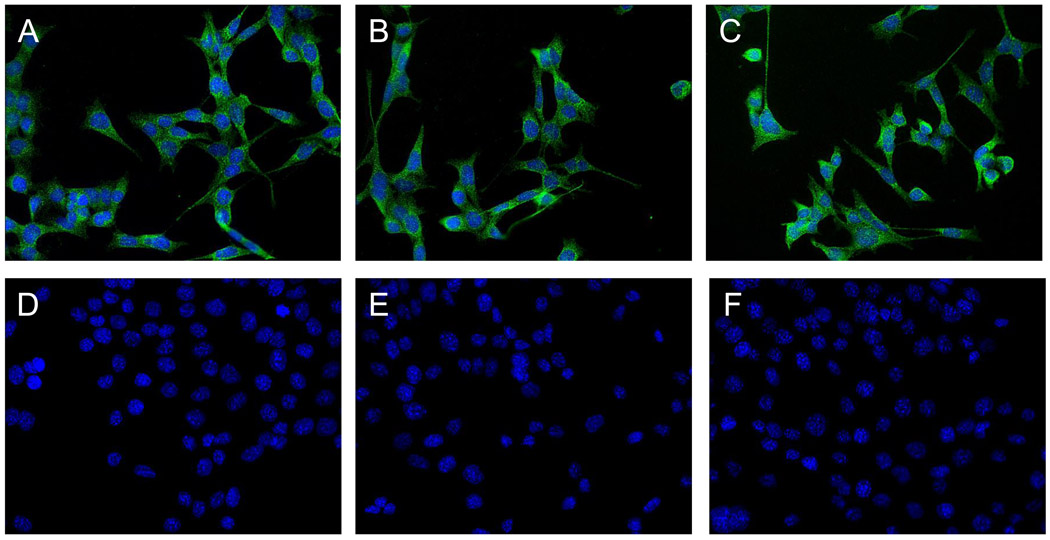
One of the most striking features of the interaction between cDDP and CTR1 in many cell types is the ability of cDDP to trigger rapid and extensive degradation of this transporter. This has been documented by western blotting and flow cytometric analysis, but the most dramatic effects are observed by immunohistochemical staining [26, 29, 30]. To determine whether this reaction was mediated by interaction of cDDP with either the M2 domain or some other component of the first 45 amino acids of the N-terminal end of hCTR1, the CTR−/−, CTR1−/−/WT, CTR1−/−/M2 and CTR1−/−/Truncated cells were exposed to cDDP for 15 min, stained with an antibody to the myc tag and examined by quantitative deconvoluting microscopy. Although degradation can be detected after 5 min of exposure, maximum differences were observed at 15 min. As shown in panels A and D of Figure 5, cDDP produced a marked decrease in immunocytochemically detectable hCTR1 in the CTR1−/−/WT cells. As shown in panels B and E the M2 variant of hCTR1 also showed a near complete disappearance of detectable hCTR1. Similar results were shown in panels C and F with regard to the truncation variant of hCTR1. These results indicate that the methionine and histidine motifs located in the first 45 amino acids of hCTR1 are not required for the ability of cDDP to down-regulate hCTR1. Thus, although cDDP may interact with motifs in the N-terminal domain, it must also bind elsewhere in the protein at a site that triggers the degradative process.
Figure 5.
Micrographs of MEF cells expressing hCTR1. All panels are 20×. Panels A–C, no treatment; panels D – F, cells exposed to 30 µM CDDP treatment for 15 minutes prior to fixing. Panels A and D, CTR1−/−/wt cells; panels B and E, CTR1−/−/M2 cells; panels C and F, CTR1−/−/Truncated cells.
4. Discussion
Crystallographic analysis of hCTR1 did not identify structure for the N-terminal domain of hCTR1, but this region contains multiple methionine and histidine motifs similar to those found in the interior of the pore formed by trimeric hCTR1 that have been proposed to mediate and regulate Cu influx by virtue of their ability to chelate Cu and cDDP [13–15]. The results of the current study provide new details regarding the role of the N-terminal domain in both Cu and cDDP accumulation. Neither the M2 motif nor the entire first 45 amino acids appear to be important to the intracellular distribution of hCTR1. Consistent with most prior studies [16, 19, 31], when expressed at equivalent levels in the CTR1−/− cells, there were no discernable differences in the subcellular distribution as detected by confocal microscopy. In addition, nearly equal amounts of the wild type and variant proteins were found on the cell surface. Thus, differences in the ability of the alternate forms of the protein to mediate Cu and cDDP uptake and cytotoxicity can be confidently attributed to the difference structure of these proteins rather than to differential levels of expression or subcellular distribution. Despite a prior report to the contrary [17], the results of the current study do not provide support for the presence of a signal essential to plasma membrane localization in the first 45 amino acids of hCTR1.
The first finding of interest is that re-expression of the N-terminal truncated variant further reduced the steady-state cellular Cu level below that found in the CTR1−/− cells when they were grown in standard tissue culture medium containing ~0.3 µM Cu, an effect not observed with the variant missing just the M2 motif. Cells that lack CTR1 clearly acquire Cu from their environment via other transporters, likely including the second mammalian Cu transporter CTR2 [32]. One possibility is that the function of these other transporters is impaired by interaction with truncated CTR1. Alternatively, the N-terminal domain of CTR1 may be required to prevent rapid efflux of Cu, a possibility given support by our previously reported finding that knockout of mCTR1 results in a large increase in the initial efflux of cDDP [33].
Prior studies performed with mutant forms of CTR1 expressed in yeast and insect cells suggested that the M2 motif in the N-terminal domain was required for uptake of Cu when environmental Cu was low, but that the M2 motif, and indeed the entire N-terminal domain, was dispensable when Cu was abundant [16, 19, 31]. However, we found that, whereas re-expression of wild type hCTR1 in the CTR1−/− cells enhanced Cu uptake, hCTR1 containing a deletion of either the M2 motif or the first 45 amino acids N-terminal was ineffective. In addition, whereas re-expression of wild type hCTR1 enhanced the cytotoxicity of Cu, the M2 and N-terminal deletion variants actually rendered the CTR1−/− cells significantly more resistant to Cu. Liang et al. [17] also found that deletion of the first 45 amino acids obviated the ability of hCTR1 to enhance Cu uptake and rendered the cells resistant the cytotoxic effect of Cu and ascribed this to a transdominant effect of the exogenously expressed mutant hCTR1 on endogenous hCTR1. However, since the CTR1−/− cells contain no endogenous CTR1 another explanation must be sought. Although CTR1 has been characterized as a Cu influx transporter, these data are actually more consistent with the concept that hCTR1 functions to retain Cu in the cell, and that the M2 motif and N-terminal domain is important to this function. Both the inability of the M2 and N-terminal truncation variants to enhance Cu accumulation, and the enhanced resistance to Cu, may be a result of enhanced efflux. The finding that deletion of just the M2 motif produced the same impairment of Cu uptake as deletion of the entire N-terminal domain is consistent with prior studies indicating the much greater importance of the M2 motif than the other histidine and methionine-rich motifs in the N-terminal domain with respect to Cu uptake [16, 19, 31].
The results of the current study establish that the N-terminal domain is important but not essential to the ability of hCTR1 to mediate cDDP uptake. A recent study by Liang et al. [17] also showed that the M2 motif is important for cDDP uptake in small cell lung cancer cells. Truncation of entire N-terminal domain removes N15 glycosylation site whereas deletion of just the M2 motif does not; glycosylation at this site is not a determinant of Cu uptake [17, 18] and the similar effect of these changes on cDDP uptake indicates that it is also not a key determinant of cDDP uptake by hCTR1.
While there are some parallels between the effect of deleting the M2 motif and the entire N-terminal domain on Cu and cDDP uptake, there are also some clear differences. As for Cu, re-expression of wild type hCTR1 enhanced the initial uptake of cDDP; in fact the magnitude of the effect was substantially larger for cDDP than for Cu. This interesting in light of prior studies showing that small changes in the sensitivity of cells to Cu are associated with substantially larger changes in sensitivity to cDDP [34]. However, whereas the variant forms of hCTR1 failed to enhance the accumulation of Cu and actually rendered the CTR1−/− cells resistant to Cu, both variants were still partially effective in enhancing cDDP uptake and rendered the cells more rather than less sensitive to the cytotoxic effect of cDDP. The former observation is consistent with the concept that, as for Cu, the M2 motif is involved in chelating cDDP during the transport process. Conversion in yCTR1 of the methionine corresponding to M45 in hCTR1 to alanine has previously been reported to block cDDP uptake in yeast [35], and conversion of either M43 or M45 to glutamine in hCTR1 has the same effect [17]. A recently proposed model suggests that, when assembled as a trimer, these motifs form a set of stacked rings of methionines and histidines that loosely chelate cDDP and pass it sequentially through the pore [13–15]. Such a set of transchelation reactions has the potential to provide a path through the plasma membrane that is highly selective for metals that form weak bonds with methionine. There is now evidence supporting the concept that cDDP does in fact bind to hCTR1. Incubation of cDDP with cells increases the fraction of hCTR1 that is found in the trimeric rather than monomeric state [16, 17]. In addition, cDDP binds to a peptide containing the first 20 amino acids of hCTR1 that includes the H1 and M1 motifs [36], and to an peptides with similar methionine motifs [37, 38] as well as to the CXXC motif in ATOX1 [39], although it remains unclear whether the kinetics of binding are consistent with a role for the interaction in the relatively rapid process of cDDP uptake. Both the binding of DDP to the N-terminal domain and the multimerization of CTR1 occur only after prolonged periods of incubation at concentrations of DDP that are far higher than those found in patient plasma and the relevance of these reactions to the regulation of DDP transport is not currently apparent.
The results of the current study provide clear evidence that none of the motifs in the first 45 amino acids of hCTR1 are required for cDDP-induced degradation of hCTR1. Thus, in addition to interacting with the N-terminal domain, cDDP must be binding at another site in hCTR1. Chelation by the rings of methionines putatively contributed by M40 and M45 is a strong candidate for the mechanism by which conformational changes that trigger endocytosis and degradation are initiated [13–15].
The M2 and N-terminal deletion variants were less effective than the wild type hCTR1 at enhancing the initial influx of cDDP, but curiously produced a greater enhancement of the cytotoxic effect of cDDP. Thus, per unit of cDDP take up by the CTR1−/−/M2 and CTR1−/−/Truncated cells there was a greater degree of cell kill. While we have argued the case for movement of cDDP through the plasma membrane pore created by trimeric hCTR1 [15], there remains the possibility that hCTR1 also delivers cDDP bound to the N-terminal domain into cells by endocytosis and that deletion of M2 motif or the N-terminal domain alters the ratio of drug entering by the two routes. Loss of the histidine and methionine-rich N-terminal domain might be expected to favor entry of cDDP via the pore. Since such entry would deliver cDDP directly into the cytoplasm, whereas entry by endocytosis leaves cDDP still inside a vesicular structure, we speculate that drug entering via the pore might be more potent. It is of interest that it has been noted previously that there are quite large differences among cell lines in the fraction of the amount of cellular oxaplatin that actually reaches critical targets such as DNA [40], a feature that may reflect differences in the route of entry.
Acknowledgements
Grant support: This work was supported by grant CA095298 from the National Institutes of Health and grant W81XWH-08-1-0135 from the Department of Defense. Additional support for core laboratories was from grants P30 NS047101 for the UCSD Neuroscience Microscopy Shared Facility and the UCSD Cancer Center Specialized Support Grant P30 CA23100.
The authors wish to thank Dr. Dennis Thiele for kindly providing the CTR1−/− cells used in this study. The authors also wish to thank Gerald Manorek, Dr. Paolo Abada, Dr. Xinjian Lin, and Dr. Xiaoqin Yuan for assistance, technical expertise and valuable discussion.
Non-standard abbreviations
- cDDP
cisplatin
- CTR1
copper transporter 1
- ICP-MS
inductively coupled plasma mass spectrometry
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- PBS
phosphate buffered saline
- TBS
tris buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madsen E, Gitlin JD. Copper deficiency. Curr Opin Gastroenterol. 2007;23:187–192. doi: 10.1097/MOG.0b013e32801421bb. [DOI] [PubMed] [Google Scholar]
- 2.Balamurugan K, Schaffner W. Copper homeostasis in eukaryotes: teetering on a tightrope. Biochim Biophys Acta. 2006;1763:737–746. doi: 10.1016/j.bbamcr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Bertinato J, L'Abbe MR. Maintaining copper homeostasis: regulation of copper-trafficking proteins in response to copper deficiency or overload. J Nutr Biochem. 2004;15:316–322. doi: 10.1016/j.jnutbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 5.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the Ctr1 high affinity copper transporter. J Biol Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aller SG, Eng ET, De Feo CJ, Unger VM. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem. 2004;279:53435–53441. doi: 10.1074/jbc.M409421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A. 2006;103:3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Feo CJ, Aller SG, Unger VM. A structural perspective on copper uptake in eukaryotes. Biometals. 2007;20:705–716. doi: 10.1007/s10534-006-9054-7. [DOI] [PubMed] [Google Scholar]
- 14.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci U S A. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharm. 2010 doi: 10.1124/mol.109.063172. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279:17428–17433. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 17.Liang ZD, Stockton D, Savaraj N, Kuo MT. Mechanistic Comparison of Human Copper Transporter hCtr1-Mediated Transports between Copper Ion and Cisplatin. Mol Pharmacol. 2009 doi: 10.1124/mol.109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277:29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 19.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277:26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 20.Larson CA, Blair BG, Safaei R, Howell SB. The Role of the Mammalian Copper Transporter 1 in the Cellular Accumulation of Platinum-based Drugs. Mol Pharmacol. 2008;75:324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 22.Song I, Savaraj N, Siddik Z, Liu P, Wei Y, Wu C, et al. Roles of copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and resistant cells. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- 23.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson C, Chung N, Howell S. Role of mammalian copper transporter (CTR1) in the cellular accumulation of cisplatin, carboplatin and oxaliplatin [abstract]. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 2008 Apr 12–16; San Diego, CA. Philadelphia (PA). 2008. [Google Scholar]
- 25.Holzer AK, Varki NM, Le QT, Gibson MA, Naredi P, Howell SB. Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem. 2006;54:1041–1049. doi: 10.1369/jhc.6A6970.2006. [DOI] [PubMed] [Google Scholar]
- 26.Jandial DD, Farshchi-Heydari S, Larson CA, Elliot GI, Wrasidlo WJ, Howell SB. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clinical Cancer Research. 2009;15:553–560. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–765. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 28.Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75:324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzer AK, Katano K, Klomp LW, Howell SB. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res. 2004;10:6744–6749. doi: 10.1158/1078-0432.CCR-04-0748. [DOI] [PubMed] [Google Scholar]
- 30.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–10952. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]
- 31.Eisses JF, Kaplan JH. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J Biol Chem. 2005;280:37159–37168. doi: 10.1074/jbc.M508822200. [DOI] [PubMed] [Google Scholar]
- 32.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2007;409:731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 33.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res. 2009;15:4312–4321. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safaei R, Holzer AK, Katano K, Samimi G, Howell SB. The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem. 2004;98:1607–1613. doi: 10.1016/j.jinorgbio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for CTR1-mediated copper and cisplatin transport. J Biol Chem. 2007;282:26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Liu Q, Liang X, Yang X, Wang N, Wang X, et al. Reactivity of platinum-based antitumor drugs towards a Met- and His-rich 20mer peptide corresponding to the N-terminal domain of human copper transporter 1. J Biol Inorg Chem. 2009;14:1313–1323. doi: 10.1007/s00775-009-0576-7. [DOI] [PubMed] [Google Scholar]
- 37.Arnesano F, Scintilla S, Natile G. Interaction between Platinum Complexes and a Methionine Motif Found in Copper Transport Proteins. Angew Chem Int Ed Engl. 2007 doi: 10.1002/anie.200703271. [DOI] [PubMed] [Google Scholar]
- 38.Sze CM, Khairallah GN, Xiao Z, Donnelly PS, O'Hair RA, Wedd AG. Interaction of cisplatin and analogues with a Met-rich protein site. J Biol Inorg Chem. 2009;14:163–165. doi: 10.1007/s00775-008-0452-x. [DOI] [PubMed] [Google Scholar]
- 39.Boal AK, Rosenzweig AC. Crystal structures of cisplatin bound to a human copper chaperone. J Am Chem Soc. 2009;131:14196–14197. doi: 10.1021/ja906363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishima M, Samimi G, Kondo A, Lin X, Howell SB. The cellular pharmacology of oxaliplatin resistance. Eur J Cancer. 2002;38:1405–1412. doi: 10.1016/s0959-8049(02)00096-5. [DOI] [PubMed] [Google Scholar]