Abstract
Ocular exposure to ultraviolet irradiation (UVR) induces photokeratitis, a common environmental concern that inflames ocular tissues and causes pain. The central neural mechanisms that contribute to the sensory aspects of photokeratitis after UVR are not known. In awake male rats, ocular surface application of hypertonic saline evoked eye wipe behavior that was enhanced 2–3 days after UVR and returned to control levels by 7 days. Similarly, under isoflurane anesthesia, hypertonic saline-evoked activity of ocular neurons in superficial laminae at the trigeminal subnucleus caudalis/cervical (Vc/C1) region was enhanced 2 days, but not 7 days, after UVR. By contrast, the response of neurons at the interpolaris/caudalis (Vi/Vc) transition region to hypertonic saline was not affected by UVR. The background activity and convergent cutaneous receptive field areas of Vc/C1 or Vi/Vc neurons were not affected by UVR. Aqueous humor protein levels were elevated 2 and 7 days after UVR.
UVR enhanced nociceptive behavior, after a latent period, with a time course similar to that of ocular neurons in superficial laminae at the Vc/C1 region. The Vc/C1 region plays a key role in primary hyperalgesia induced by UVR, while the Vi/Vc region likely mediates other aspects of ocular function.
Keywords: eye wipe behavior, inflammation, ocular pain, trigeminal subnucleus caudalis, UV irradiation
The eye is vulnerable to most forms of radiant energy. Exposure to ultraviolet irradiation (UVR) is particularly harmful and nearly all short wavelength (UVA, below 280 nm) and middle wavelength (UVB, 280–320 nm) UVR is absorbed by the cornea and other compartments of the anterior eye segment (Sliney, 2002). Solar energy is the main environmental source of UVA and UVB irradiation for most individuals; however, artificial sources from arc welding and tanning salons also contribute (Rieke, 1943; Tenkate, 1999). Photokeratitis is the major clinical condition following acute exposure to UVR and is characterized by anterior eye segment inflammation, reduced visual acuity and burning-like pain of the ocular surface that develops slowly over several hours (Schein, 1992; Cullen, 2002). The neural basis for sensory changes in photokeratitis is not well defined. Most animal studies have focused on peripheral histological and biochemical aspects of UVR-induced damage to the anterior eye segment (Bergmanson, 1990; Doughton and Cullen, 1989; Zuclich, 1989), while less is known concerning the behavioral and central neural correlates of this injury.
The ocular surface is the most densely innervated tissue in the body (Rozsa and Beuerman, 1982) and trigeminal sensory neurons that supply this structure terminate mainly in two spatially discrete regions of trigeminal subnucleus caudalis (Vc), a rostral caudalis/interpolaris transition region (Vi/Vc) and more caudally at the Vc junction with the upper cervical spinal cord (Vc/C1) (Marfurt, 1981; Marfurt and del Toro, 1987; Panneton and Burton, 1981). Previous recording studies, based mainly on response properties in naïve rats (Hirata et al., 1999; Hirata et al., 2000; Hirata et al., 2004; Meng et al., 1997; Meng et al., 1998), have led to the proposal that neurons at the Vi/Vc transition and in the superficial laminae at the Vc/C1 region serve different aspects of trigeminal function in ocular pain (Bereiter et al., 2000). Although ocular inflammation is a common clinical problem that causes pain and can develop from a variety of conditions such as refractive surgery, infection or trauma (Dargin and Lowenstein, 2008; Leibowitz, 2000), most animal models of ocular pain have been relied on approaches that involve direct nerve damage (see Belmonte et al., 2004; Wenk and Honda, 2003). Recently we reported that endotoxin-induced uveitis (EIU), an animal model that mimics some of the early signs of anterior uveitis in humans, produced time-dependent changes in responsiveness of neurons in the caudal trigeminal sensory brainstem complex (Bereiter et al., 2005). However, EIU also produces fever with widespread effects on immune, endocrine and cardiovascular systems (Romanovsky, 2004) that confound the delineation of mechanisms underlying the observed neural plasticity. In addition to serving as a model for photokeratitis, UVR offers several advantages for studies of ocular pain in general. As described for studies of cutaneous pain, UVR is non-invasive, produces localized inflammation, uses stimulus variables that can be readily controlled, and since it can be applied to humans, is well suited for translational research (Davies et al., 2005; Bishop et al., 2007; Bishop et al., 2009).
The goal of this study was to develop a rodent model for photokeratitis and determine the time course and intensity-related effects of UVR on behavioral and neurophysiological correlates of ocular nociception. The ocular surface was exposed to narrowband UV-B irradiation and behavioral and electrophysiological responses were followed for one week. Hypertonic saline was used to activate ocular surface nociceptors, since it reliably evokes eye wipe behavior in animals and can be applied daily without causing persistent sensitization (Farazifard et al., 2005).
Experimental Procedures
The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and conformed to the established guidelines set by The National Institutes of Health guide for the care the use of laboratory animals (PHS Law 99–158, revised 2002).
Animals and ultraviolet irradiation (UVR)
Male Sprague-Dawley rats (250–390 g, Harlan, Indianapolis, IN) were briefly anesthetized with pentobarbital sodium (50 mg/kg, ip) and narrow band (302 nm) UV-B radiation was delivered from a light source (UVP Co., Upland, CA) at varying intensities (100, 200 or 300 mJ/cm2). The intensity of stimulation was calculated as: mJ/cm2 = mW/cm2 × duration in seconds. The actual mW/cm2 delivered by the light source was measured directly with a UV meter at the corneal surface (model UV-340, Mannix Co., New York, NY). The rat’s head, except for the eye, was covered with a UV opaque material. Each rat received a single exposure to UVR and survived for 2–7 days after irradiation. Rats that did not receive UVR were defined in the text as “controls”.
Eye wipe behavior
The eye wipe test was performed using hypertonic saline as the inducing stimulus. This is a sensitive test for acute ocular irritation-related behavior in awake rats that is non-invasive, non-inflammatory by itself, and requires little or no conditioning (Farazifard et al., 2005). Rats were placed in a Plexiglass box for 1 h to habituate to the testing chamber. Test stimuli consisted of a single drop of NaCl (0.15, 2.5 and 5 M, 20 µl) applied to the ocular surface from a micropipette. Test solutions of NaCl were applied in ascending order of concentration at 30 min intervals. The number of eye wipes, defined as purposeful wiping of the face by the forelimb, was counted over 5 min on days 1, 2, 3, 5 and 7 after UVR. In preliminary experiments we determined the threshold concentration of NaCl to evoke eye wiping was ~1 M and suprathreshold concentrations (5 M) could be applied daily for up to 2 weeks while evoking consistent responses with no visible signs of ocular inflammation. Controls were handled daily and tested at the same times as UVR-treated rats. Rats were tested without prior knowledge of UV treatment and 6–8 rats were included in each group. Eye wipe behavior was assessed by ANOVA corrected for repeated measures. Significant treatment effects were further assessed by Newman-Keuls after ANOVA. In several cases (n = 4–5 rats per treatment group: controls, 2 days and 7 days after UVR) an aqueous humor sample was collected and total protein was measured by the Bradford assay (Pierce BCA Kit, Rockford, IL) as an indicator of ocular inflammation.
Electrophysiology procedures
Rats were anesthetized initially with pentobarbital sodium (70 mg/kg, ip) and catheters were positioned in the right femoral artery and jugular vein for monitoring blood pressure and drug infusion, respectively. After tracheotomy animals were respired artificially with oxygen-enriched room air and anesthesia was maintained with isoflurane (1.2–2.0 %). Rats received an infusion of the short-acting paralytic agent, gallamine triethiodide (25 mg/kg/h), at the time of neural recording. Expiratory end-tidal CO2 (3.5–4.5%), mean arterial pressure (MAP, 100–120 mmHg) and body temperature (38°C) were monitored continuously and kept within normal range. Animals were placed in a stereotaxic frame and portions of the C1 vertebra were removed to expose the lower brainstem and upper cervical dorsal horn. The exposed brainstem surface was bathed in warm mineral oil. Single neurons were recorded at the ventrolateral trigeminal subnucleus interpolaris/caudalis (Vi/Vc) transition and superficial laminae (laminae I–II) at the subnucleus caudalis/cervical (Vc/C1) junction regions (see Fig 2). Neurons recorded at the Vi/Vc transition were approached at an angle of 28° off vertical and 45° off midline, and 1.5–2.5 mm below the brainstem surface. For neurons in superficial laminae at the caudal Vc/C1 junction region, the electrode was directed at an angle of 43° off vertical, 60° off midline and the depth of recording was within 250 µm of the dorsal brainstem surface. Unit activity was recorded extracellularly using tungsten microelectrodes (9 Mohm, Frederick Haer Inc., Bowdoinham, ME) and amplified, discriminated (model DIS-1, BAK Electronics, Mount Airy, MD), stored and analyzed offline on a Apple (G4) computer using a DAQ interface board and LabVIEW software (National Instruments, Austin, TX).
Figure 2.
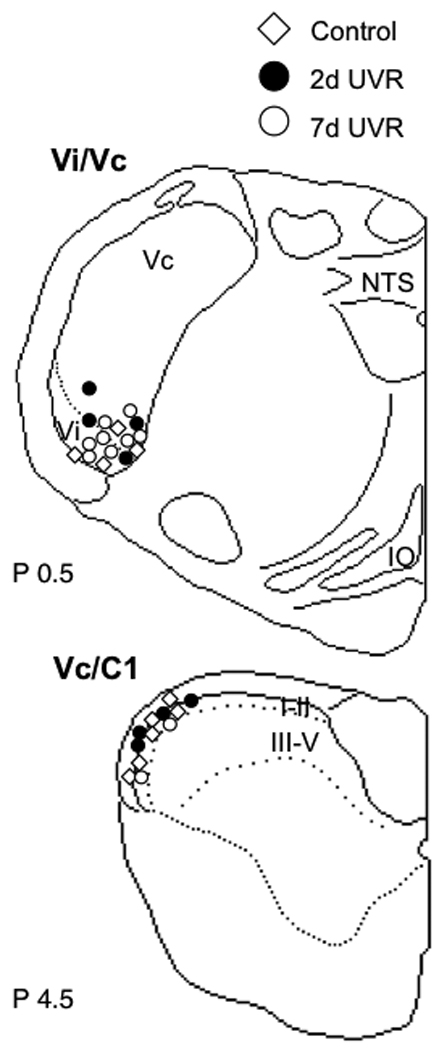
Recovered lesion sites for ocular units recorded at the Vi/Vc transition and Vc/C1 junction regions in controls, and 2 day and 7 days after UVR. Abbreviations: IO, inferior olive; NTS, nucleus tractus solitarius; Vc, trigeminal subnucleus caudalis; Vi, trigeminal subnucleus interpolaris. I–II and III–V indicate laminar boundaries in caudal Vc. Numbers to lower left of each outline refer to the distance in mm caudal to the obex.
Characterization of ocular-responsive units and experimental design
The encoding properties of units at the Vi/Vc transition and Vc/C1 junction regions were compared across three treatment groups: controls, 2 days after UVR and 7 days after UVR. UVR was delivered at 300 mJ/cm2 since this intensity reliably enhanced hypertonic saline-evoked eye wipe behavior. A single neuron was recorded from each rat. The search stimulus consisted of gently swiping a fine camelhair brush across the ocular surface (e.g., cornea surface and conjunctiva). Since all units included in this study had a receptive field (RF) on the cornea and/or the conjunctiva, for convenience, they are referred to in the text as “ocular units”. Most ocular units also received convergent cutaneous input from periorbital skin and could be further classified as nociceptive specific (NS, pinch only) or wide dynamic range (WDR, pinch plus touch) based on the responses to a low force von Frey filament (1.2 g) and pinch with blunt forceps. A similar number of ocular neurons were classified as NS and WDR, based on cutaneous RF properties, in each treatment group and were not analyzed separately. Several ocular units at Vi/Vc transition region were classified as “complex” and displayed an inhibitory cutaneous RF that was contiguous with the ocular surface or had no apparent cutaneous RF and were classified as cornea only. All units were activated by mechanical stimulation of the conjunctiva and the threshold for this input was determined with a calibrated series of von Frey filaments applied to the palpebral conjunctiva below the lower eyelid. After establishing the ocular surface and periorbital skin RF characteristics to mechanical stimulation, NaCl was applied to ocular surface in a cumulative concentration regimen (0.15, 2.5 and 5 M, 20 µl) with an interstimulus interval of 30 min. Saline solutions were applied at 22–25 °C.
Neural recording data analyses
Neural recording data were acquired and displayed as peristimulus time histograms (PSTH) of spikes per 1-s bins, exported to a spreadsheet and analyzed off-line. Neural responses were analyzed as a total response magnitude (Rmag) for each stimulus period defined as the cumulative sum of spikes for contiguous bins in which the spike count exceeded the mean+2SD of the background activity (Hirata et al., 1999). The total Rmag was calculated for each stimulus period and can be thought of as equivalent to the “area under the curve”. Units were defined as NaCl-responsive if the total Rmag exceeded a value of 10 after 2.5 or 5 M NaCl. The high-threshold convergent cutaneous RF area of each unit was determined with a small blunt forceps mapped onto standardized drawings of the rat face, digitized and quantified by a planimetric method using NIH software (ImageJ). Treatment effects on cutaneous RF areas and unit activity were assessed by ANOVA corrected for repeated measures. Treatment effects on aqueous humor total protein concentrations were assessed by ANOVA. Individual comparisons were assessed by Newman-Keuls after ANOVA. The data were presented as mean ± SEM and significance set at P = 0.05.
Histology
At the end of the recording session the rat was given a bolus of pentobarbital sodium (60 mg/kg, i.v.) and perfused through the heart with saline followed by 10% formalin. Brainstem sections were cut at 40 µm on a freezing microtome and stained with cresyl violet. Recording sites at the Vi/Vc transition and Vc/C1 junction regions were marked electrolytically (5 µA, 10 s).
Results
General effects of UVR
There were no visible signs of ocular inflammation immediately and for several hours after UVR regardless of intensity. By 1 day after moderate or high intensity UVR (200 or 300 mJ/cm2) the irradiated eye displayed a dilated pupil and signs of inflammation such as hyperemia of the sclera and corneal opacities that gradually diminished over 7 days. After low intensity UVR (100 mJ/cm2) the pupil was dilated slightly for 1–2 days, with some hyperemia of the sclera and corneal opacity that resolved by 5 days. Total protein content in aqueous humor samples was measured in controls and after 300 mJ/cm2 UVR at the end of several experiments. Total protein concentration in the irradiated eye was significantly (F2,11 = 8.11, P < 0.01) elevated 2 days (14.56 ± 4.48 mg/ml, n = 5) and 7 days after UVR (36.28 ± 7.97 mg/ml, n = 5) compared to controls (3.75 ± 0.04 mg/ml, n = 4). Body weight gain was not affected by UVR and similar across all groups during the week after irradiation (3 ± 1 g per day, n = 80).
Effects of UVR on eye wipe behavior
There was no spontaneous eye wipe behavior during the 1 h habituation period and physiological saline (0.15 M) also did not cause eye wiping in any group suggesting that UVR alone was not sufficient to induce this behavior. However, both 2.5 M (Fig 1A) and 5 M NaCl (Fig 1B) evoked consistent increases in eye wiping after moderate (200 mJ/cm2) and high intensity (300 mJ/cm2) UVR exposure (F7,50 = 18.7, P < 0.001). Low intensity UVR (100 mJ/cm2) did not alter the eye wipe counts to 2.5 or 5 M NaCl above that seen prior to inflammation. Moderate intensity UVR (200 mJ/cm2) also did not affect eye wipe behavior evoked by 2.5 M NaCl (P > 0.05), whereas the response to 5 M NaCl was significantly increased 2 days after UVR (F5,221 = 3.40, P < 0.005). High intensity UVR (300 mJ/cm2) enhanced eye wiping to 2.5 M NaCl 2 days after UVR (F5,221 = 3.35, P < 0.005) and to 5 M NaCl both 2 and 3 days after UVR (F5,221 = 8.66, P < 0.001). By 5 days after 200 or 300 mJ/cm2 hypertonic saline-induced eye wipe behavior was similar that seen prior to UVR. These results indicated that UVR caused a transient increase, after a latent period of 1 day, in ocular surface-evoked eye wipe behavior that depended on UV intensity and the concentration of the saline stimulus.
Figure 1.
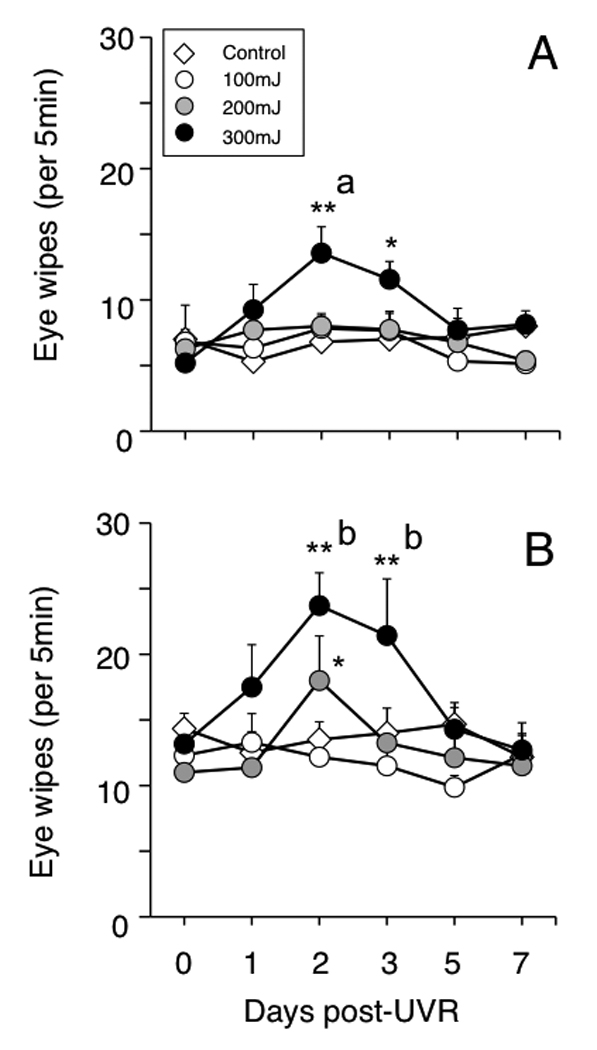
UVR induces an intensity-dependent increase in eye wipe behavior evoked by hypertonic saline that peaks at 2–3 days and returns to control values by 7 days. A). Response to 2.5 M NaCl. B). Response to 5 M NaCl. A single drop (~20 µl) of NaCl was applied to the ocular surface and eye wipes were counted for 5 min. Sample size: n = 6–8 rats in each group; *P < 0.05, **P< 0.01 versus prior to UVR; a = P < 0.05, b = P < 0.01 versus control group. Test periods separated by 30 min.
Effects of UVR on ocular neurons in superficial laminae at Vc/C1 region
A total of 29 ocular neurons were recorded in superficial laminae at the Vc/C1 region and tested with hypertonic saline. All recovered Vc/C1 recording sites were within the 250 µm of the dorsal brainstem surface and located similarly for each treatment group (Fig 2, lower panel). All Vc/C1 neurons were classified as either NS (control, n = 10; 2 day UVR, n = 4; 7 day UVR, n = 4) or WDR (control, n = 6; 2 day UVR, n = 3; 7 day UVR, n = 2) based on cutaneous RF properties. Hypertonic saline evoked a concentration-dependent increase in Vc/C1 unit activity in all units as seen in the examples in Fig 3. The total Rmag to hypertonic saline was enhanced 2 days after UVR compared to units from controls or 7 days after UVR (Fig 4A, F2,26 = 10.39, P < 0.001). Similarly, response duration to hypertonic saline was significantly prolonged 2 days after UVR compared to controls and 7 days after UVR (Fig 4B, F2,26 = 6.08, P < 0.01). The background activity of Vc/C1 units from controls, 2 day UVR and 7 day UVR groups averaged 2.5 ± 0.5, 1.7 ± 0.8 and 0.5 ± 0.2 spikes/s, respectively and were not significantly different (F2,52 = 1.07, P > 0.05). The threshold for mechanical stimulation of the lower conjunctiva was not affected by UVR (0.24 ± 0.04, 0.25 ± 0.1 and 0.33 ± 0.11 g, for controls, 2 day UVR and 7 day UVR rats, respectively). There was a trend towards enlarged convergent cutaneous RF areas for units from 2 day UVR (1.43 ± 0.23 cm2, n = 7) and 7 day UVR groups (1.17 ± 0.26 cm2, n = 6) compared to controls (0.79 ± 0.14 cm2, n = 16) that was not significant (F2,26 = 2.92, P > 0.05) when assessed across all cell classes. However, analysis by cell class revealed a significant increase in cutaneous RF area 2 days after UVR for WDR units (F3,19 = 5.01, P < 0.01), while NS units were not affected compared to controls. The VF mechanical threshold at the conjunctiva was similar between cells classes (NS versus WDR) in controls and not changed 2 days after UVR regardless of cell class (F 3,22 = 0.76, P > 0.05).
Figure 3.
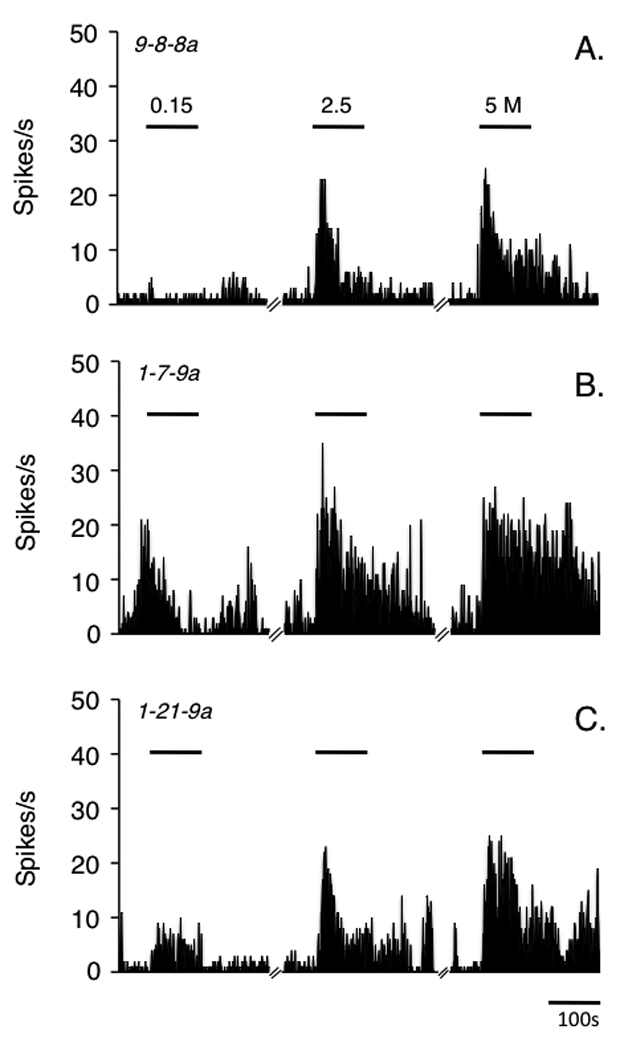
Peristimulus time histograms of the response of ocular units recorded in superficial laminae at the caudal Vc/C1 junction region to hypertonic saline. A) controls, B) 2 days after UVR and C) 7 days after UVR. UVR intensity was 300 mJ/cm2. Horizontal bars indicate stimulus periods (100 s) and each stimulus is separated by 30 min.
Figure 4.
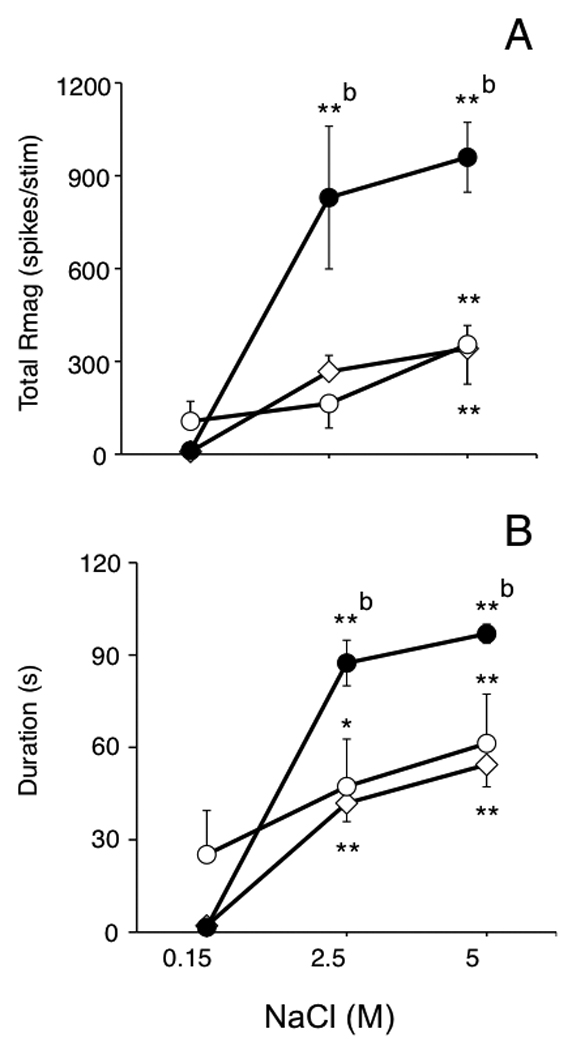
UVR enhances the total response magnitude (A) and response duration (B) to hypertonic saline applied to the ocular surface for units in superficial laminae at the Vc/C1 junction region. Symbols: controls = open diamonds, 2 day UVR = solid circles, 7 day UVR = open circles. Sample sizes: control (n = 16), 2 day UVR (n = 7) and 7 day UVR rats (n = 6). *P < 0.05, **P< 0.01 versus response to 0.15 M saline; b = P < 0.01 versus control group. Test periods separated by 30 min.
Effects of UVR on ocular neurons at the Vi/Vc transition region
A total of 22 ocular surface-responsive neurons were recorded at the Vi/Vc transition region and tested with hypertonic saline. All recovered recording sites were located in the ventrolateral portion of the Vi/Vc transition region (Fig 2, upper panel). Most Vi/Vc neurons were classified as either NS (controls, n = 7; 2 day UVR, n = 4; 7 day UVR, n = 4) or WDR (controls, n = 1; 2 day UVR, n = 1; 7 day UVR, n = 1) based on cutaneous RF properties. In addition, several Vi/Vc units were classified as complex units with a large convergent inhibitory RF (controls, n = 1; 2 day UVR, n = 1; 7 day UVR, n = 1), while one unit in a 7 day UVR rat lacked a convergent cutaneous RF and was classified cornea only. Complex and cornea only units were found exclusively at the Vi/Vc transition region. Hypertonic saline (2.5 or 5 M) evoked a significant increase in Vi/Vc unit activity independent of NaCl concentration in all groups (Fig 5A; F2,38 = 38.5, P < 0.001). Similarly, hypertonic saline evoked significant, and similar, increases in response duration in all three treatment groups (Fig 5B, F2,38 = 91.2, P < 0.001). However, comparisons across treatment groups revealed no significant differences in hypertonic saline-evoked total Rmag or response duration. The mean background activity for ocular units at the Vi/Vc transition was increased slightly after UVR (2 day UVR, 12 ± 6 spikes/s; 7 day UVR, 8.8 ± 2.2 spikes/s) compared to controls (4 ± 1.6 spikes/s); however, these differences were not significant (F2,38 = 2.77, P > 0.05). The threshold for mechanical stimulation of the lower conjunctiva was not significantly affected by UVR (0.39 ± 0.09, 0.26 ± 0.1 and 0.35 ± 0.09 g, for controls, 2 day UVR and 7 day UVR groups, respectively). The convergent cutaneous RF area for NS and WDR units combined from 2 day UVR (1.43 ± 0.6 cm2, n = 6) and 7 day UVR groups (1.19 ± 0.3 cm2, n = 7) were not different from controls (0.74 ± 0.31 cm2, n = 9, F2,17 = 0.99, P > 0.05).
Figure 5.
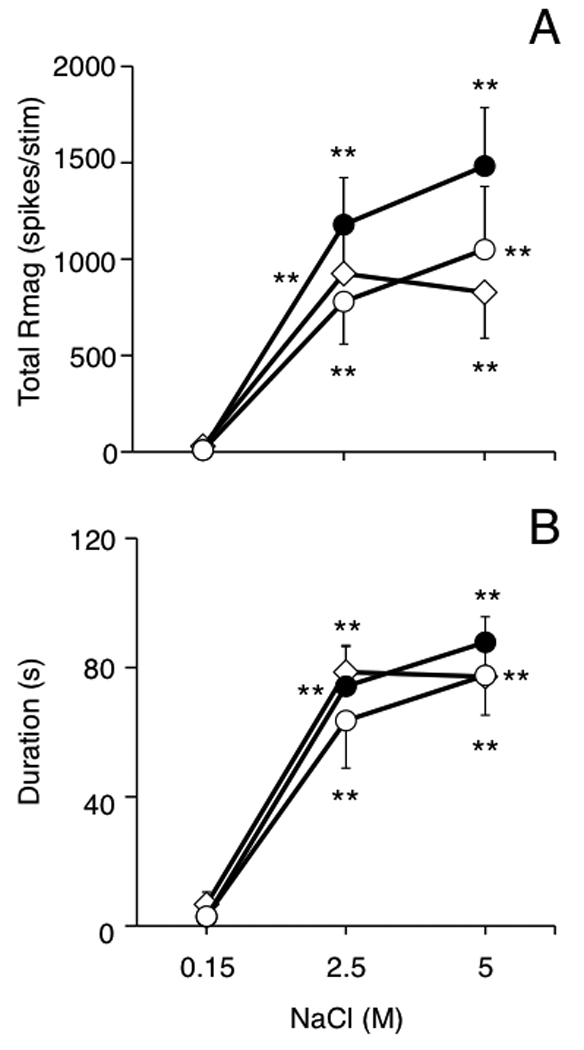
UVR has no significant effect on the total response magnitude (A) or response duration (B) to hypertonic saline applied to the ocular surface for units at the Vi/Vc transition region. Symbols: controls = open diamonds, 2 day UVR = solid circles, 7 day UVR = open circles. Sample sizes: control (n = 9), 2 day UVR (n = 6) and 7 day UVR rats (n = 7). **P< 0.01 versus response to 0.15 M saline. Test periods separated by 30 min.
Discussion
These data indicated that acute exposure of the eye to UVR produced, after a 1 day latent period, a transient increase in ocular-specific nociceptive behavior evoked by hypertonic saline applied to the ocular surface. The time course for UVR-induced changes in behavior matched well the increase in responsiveness of neurons in superficial laminae at the Vc/C1 region to hypertonic saline. By contrast, although ocular neurons at the Vi/Vc transition region, located 4–5 mm rostral to the Vc/C1 recording region, also displayed vigorous responses to hypertonic saline, this activity was not influenced by UVR. The total protein content in the aqueous humor was elevated 2 days and 7 days after UVR. This suggested that inflammation per se contributed to the induction of nociceptive responses after UVR but was not sufficient to maintain enhanced ocular-induced eye wipe behavior or Vc/C1 neural activity.
The pattern and time course of these responses agreed generally with the clinical presentation of photokeratitis (also termed actinic keratitis) in which overexposure to UVR produced few signs of ocular inflammation or sensory disturbance until several hours later (Rieke, 1943; Schein, 1992). Similarly, in a detailed study in rabbits UVR-induced ocular inflammation and tissue damage after exposure to 125 mJ/cm2 caused corneal edema, pupillary dilatation and photophobia-like behavior that peaked at 2 days and resolved by 6 days (Doughty and Cullen, 1989). The lowest intensity of UVR used in the present study (100 mJ/cm2) exceeded the reported threshold for corneal inflammation in the rabbit (55 mJ/cm2, Gallar et al 1995) and monkey (80 mJ/cm2, Bergmanson, 1990). Since we noted clear signs of ocular surface and intraocular inflammation such as hyperemia of the sclera, increased protein in aqueous humor, pupillary dilatation and corneal opacity, it was somewhat unexpected to find that the 100 mJ/cm2 UVR dose was not sufficient to enhance eye wipe behavior. The reason for this was not certain. A mismatch in the time course for erythema and cutaneous hyperalgesia after UVR has been noted in human studies (Hoffman and Schmelz, 1999; Benrath et al., 2001). We cannot exclude that differences in methodology (e.g., UV wavelength, use of hypertonic saline as a test stimulus) or animals (e.g., species or rodent strain) may have influenced our results. However, the dose of UVR required to observe enhanced eye wipe behavior (200 mJ/cm2) was similar to that required to cause cutaneous hyperalgesia in the rat of the hindlimb (~250 mJ/cm2, Bishop et al., 2007). This suggested that, at least in the rat, the minimal UVR dose needed to produce ocular surface-related nociceptive behavior was similar to that for cutaneous hyperalgesia.
Some features of UVR differed from other more common models of ocular inflammation and hyperalgesia. Often described as a sterile injury model, UVR activates the immune system in a manner consistent with tissue repair (Davies et al. 2005) and the resulting inflammation derives mainly from apoptosis of epithelial cells after DNA damage (Matsumura and Ananthaswamy, 2004) or the formation of reactive biochemical products (Cejkova et al. 2004). By contrast, ocular inflammation caused by endotoxin in the EIU model (Smith et al., 1998; Bereiter et al., 2005) or by direct physical damage of the ocular surface (Wenk and Honda, 2003) recruits immune cells to the anterior eye segment and activates the immune system in a manner more consistent with the destruction of pathogens. Thus, the hyperalgesia induced by each model may involve different underlying neural mechanisms. This notion was supported by the differences in time course for changes in ocular-specific behavior with each method. UVR did not affect hypertonic saline-induced eye wipe behavior until 2 days later, whereas eye blink behavior evoked by CO2 gas was slightly depressed at 2 days and not enhanced until 7 days after endotoxin in the EIU model (Bereiter et al. 2005). Cauterization of the ocular surface resulted in a maximum increase in eye blinks evoked by dilute capsaicin after 6–12 h and returned to control levels by 24 h (Wenk and Honda, 2003). Thus, although signs of ocular inflammation were elevated in all 3 models (e.g., UVR, EIU and eye damage) at or before 2 days post-treatment, the time courses for induced ocular hyperalgesia were markedly different. Interestingly, in this and our previous endotoxin study (Bereiter et al., 2005), the change in responsiveness of ocular units in superficial laminae at the Vc/C1 region matched well the time course for the change in behavior. This provided further support for the hypothesis that the Vc/C1 region was critical for nociceptive processing relevant for ocular pain regardless of the experimental model.
In cutaneous pain studies it has been reported that UVR differs from other models since only primary, but not secondary, hyperalgesia is induced (Koppert et al., 2004; Bishop et al., 2009). This conclusion was based on psychophysical results in humans (Bishop et al. 2009) or indirect estimates of nociceptor sensitization in animals (Rukwied et al., 2008). Several findings of the present study supported the notion of primary, but not secondary, hyperalgesia after eye exposure to UVR. Consistent with primary hyperalgesia, increasing UVR intensity reduced the concentration of hypertonic saline necessary to enhance eye wipe behavior and ocular-evoked Vc/C1 unit activity. Consistent with a failure to induce secondary hyperalgesia, Vc/C1 unit responses to stimuli applied to periocular tissue outside the field of irradiation (e.g., conjunctiva mechanical thresholds, convergent cutaneous RF areas) displayed small or no enhancement after UVR. It is generally accepted that an increase in cutaneous RF area of dorsal horn units (Cook et al., 1987; Jinks and Carstens, 1998) reflects secondary hyperalgesia and requires central neural plasticity (Klede et al. 2003). By contrast, some aspects of the present data did not agree with traditional concepts of primary hyperalgesia (see Meyer et al., 2006). For example, background activity of ocular units was not increased and spontaneous eye wipe behavior was not seen after UVR alone. Others have reported increased background activity in spinal dorsal horn neurons after UVR of the hindlimb; however, units were recorded only from deep laminae (Urban et al., 1993; Chapman and Dickenson, 1994), while we recorded only in superficial laminae. Recent evidence suggests that, when compared directly, units in superficial and deep laminae have different properties and may serve different aspects of nociception (Craig, 2004; Eckert et al., 2006; Tashiro et al., 2007). Also, our study relied mainly on hypertonic saline as the provocative stimulus after UVR, while other studies used mechanical and/or thermal stimuli to test for primary or secondary hyperalgesia. Nearly all corneal nociceptors activated by thermal stimuli also responded to hypertonic saline in the cat (Belmonte et al., 1991; Gallar et al., 1993). The exact mechanism for hypertonic saline-evoked nociceptor activity is not known; however, it may involve neuropeptide-containing afferents (Baraniuk et al., 1999) and has been linked to both TRPV1 (Rinder et al., 1994) and TRPV4 channels (Alessandri-Haber et al., 2005).
A unique feature of the trigeminal sensory innervation of the eye is the dual representation of ocular tissues at rostral (Vi/Vc transition) and caudal (Vc/C1 junction) regions of Vc (see Bereiter et al., 2000; Bereiter et al., 2009). The basis for multiple representation is not certain; however, these and previous data strongly suggest that ocular neurons in each region serve different aspects of ocular function. The properties of ocular neurons at the Vc/C1 region were most consistent with a role in sensory aspects of nociception. Vc/C1 units displayed a marked enhancement to hypertonic saline after UVR that matched the time course for increases in eye wipe behavior. Similarly, in the EIU model modulation of CO2-evoked responses of Vc/C1 units and eye blink behavior occurred at the same times (Bereiter et al. 2005). It is well established that the caudal portions of Vc are critical for long term changes in behavior and sensitization of central trigeminal neurons after craniofacial tissue injury (Chiang et al., 2002; Sessle, 2000). By contrast, the function of ocular neurons at the Vi/Vc transition is less certain. One possible interpretation is that the Vi/Vc transition region serves mainly ocular specific functions and is supported by several lines of evidence. The responsiveness of Vi/Vc neurons was not significantly affected by UVR (present study) or endotoxin (Bereiter et al., 2005). Repetitive noxious thermal stimulation of the ocular surface sensitized Vc/C1 units, whereas Vi/Vc units were inhibited (Meng et al., 1997). The Vi/Vc transition is necessary for reflex lacrimation and ocular units in this region encode the moisture status of the ocular surface (Hirata et al., 2004). Alternatively, the Vi/Vc transition may play a significant role in recruiting descending controls during pain conditions, including ocular pain as supported by the high percentage of ocular units at the Vi/Vc transition that were excited by opioid analgesics, while nearly all units at the Vc/C1 region were inhibited (Hirata et al., 2000; Meng et al., 1998). The eye is arguably our most complex sense organ. The available evidence suggests that a specialized central organization of the trigeminal system has developed to support this complexity.
Acknowledgements
The authors thank Randall Thompson for excellent technical assistance. This work was supported in part by a grant from the NIH (NS26137).
Abbreviations
- UVR
ultraviolet irradiation
- Vc
trigeminal subnucleus caudalis
- Vi
trigeminal subnucleus interpolaris
- Vc/C1
trigeminal subnucleus caudalis/upper cervical spinal cord transition
- Vi/Vc
trigeminal subnucleus interpolaris/ subnucleus transition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, Ali M, Yuta A, Fang SY, Naranch K. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med. 1999;160:655–662. doi: 10.1164/ajrccm.160.2.9805081. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benrath J, Gillardon F, Zimmermann M. Differential time courses of skin blood flow and hyperalgesia in the human sunburn reaction following ultraviolet irradiation of the skin. Eur J Pain. 2001;5:155–167. doi: 10.1053/eujp.2001.0229. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Basbaum A, Bushnell MC, editors. Science of Pain. New York: Elsevier; 2009. pp. 435–460. [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005;94:3815–3825. doi: 10.1152/jn.00616.2005. [DOI] [PubMed] [Google Scholar]
- Bergmanson JP. Corneal damage in photokeratitis--why is it so painful? Optom Vis Sci. 1990;67:407–413. doi: 10.1097/00006324-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Bishop T, Ballard A, Holmes H, Young AR, McMahon SB. Ultraviolet-B induced inflammation of human skin: characterisation and comparison with traditional models of hyperalgesia. Eur J Pain. 2009;13:524–532. doi: 10.1016/j.ejpain.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Bishop T, Hewson DW, Yip PK, Fahey MS, Dawbarn D, Young AR, McMahon SB. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Cejkova J, Stipek S, Crkovska J, Ardan T, Platenik J, Cejka C, Midelfart A. UV Rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 2004;53:1–10. [PubMed] [Google Scholar]
- Chapman V, Dickenson AH. Enhanced responses of rat dorsal horn neurons after UV irradiation of the hindpaw; roles of the NMDA receptor. Neurosci Lett. 1994;176:41–44. doi: 10.1016/0304-3940(94)90866-4. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Hu B, Hu JW, Dostrovsky JO, Sessle BJ. Central sensitization of nociceptive neurons in trigeminal subnucleus orlais depends on the integrity of subnucleus caudalis. J Neurophysiol. 2002;88:256–264. doi: 10.1152/jn.00944.2001. [DOI] [PubMed] [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- Craig AD. Lamina I, but not lamina V, spinothalamic neurons exhibit responses that correspond with burning pain. J Neurophysiol. 2004;92:2604–2609. doi: 10.1152/jn.00385.2004. [DOI] [PubMed] [Google Scholar]
- Cullen AP. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int J Toxicol. 2002;21:455–464. doi: 10.1080/10915810290169882. [DOI] [PubMed] [Google Scholar]
- Dargin JM, Lowenstein RA. The painful eye. Emerg Med Clin North Am. 2008;26:199–216. doi: 10.1016/j.emc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Davies SL, Siau C, Bennett GJ. Characterization of a model of cutaneous inflammatory pain produced by an ultraviolet irradiation-evoked sterile injury in the rat. J Neurosci Methods. 2005;148:161–166. doi: 10.1016/j.jneumeth.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, Cullen AP. Long-term effects of a single dose of ultraviolet-B on albino rabbit cornea--I. in vivo analyses. Photochem Photobiol. 1989;49:185–196. doi: 10.1111/j.1751-1097.1989.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Eckert WA, 3rd, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Farazifard R, Safarpour F, Sheibani V, Javan M. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res Brain Res Protoc. 2005;16:44–49. doi: 10.1016/j.brainresprot.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gallar J, Garcia de la Rubia P, Gonzalez GG, Belmonte C. Irritation of the anterior segment of the eye by ultraviolet radiation: influence of nerve blockade and calcium antagonists. Curr Eye Res. 1995;14:827–835. doi: 10.3109/02713689508995805. [DOI] [PubMed] [Google Scholar]
- Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24:4224–4232. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Takeshita S, Hu JW, Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioid agonists and projections to thalamus and brainstem. J Neurophysiol. 2000;84:1050–1061. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- Hoffmann RT, Schmelz M. Time course of UVA- and UVB-induced inflammation and hyperalgesia in human skin. Eur J Pain. 1999;3:131–139. doi: 10.1053/eujp.1998.0106. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Spinal NMDA receptor involvement in expansion of dorsal horn neuronal receptive field area produced by intracutaneous histamine. J Neurophysiol. 1998;79:1613–1618. doi: 10.1152/jn.1998.79.4.1613. [DOI] [PubMed] [Google Scholar]
- Klede M, Handwerker HO, Schmelz M. Central origin of secondary mechanical hyperalgesia. J Neurophysiol. 2003;90:353–359. doi: 10.1152/jn.01136.2002. [DOI] [PubMed] [Google Scholar]
- Koppert W, Brueckl V, Weidner C, Schmelz M. Mechanically induced axon reflex and hyperalgesia in human UV-B burn are reduced by systemic lidocaine. Eur J Pain. 2004;8:237–244. doi: 10.1016/j.ejpain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Leibowitz HM. The red eye. New Engl J Med. 2000;343:345–351. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203:785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J Neurophysiol. 1998;79:2593–2602. doi: 10.1152/jn.1998.79.5.2593. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th Edition. Philadelphia, PA: Elsevier; 2006. pp. 3–34. [Google Scholar]
- Panneton WM, Burton H. Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;199:327–344. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- Rieke F. Arc flash conjunctivitis. J Am Med Assoc. 1943;122:734–736. [Google Scholar]
- Rinder J, Stjarne P, Lundberg JM. Capsaicin de-sensitization of the human nasal mucosa reduces pain and vascular effects of lactic acid and hypertonic saline. Rhinology. 1994;32:173–178. [PubMed] [Google Scholar]
- Romanovsky AA. Signaling the brain in the early sickness syndrome: are sensory nerves involved? Front Biosci. 2004;9:494–504. doi: 10.2741/1247. [DOI] [PubMed] [Google Scholar]
- Rozsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Dusch M, Schley M, Forsch E, Schmelz M. Nociceptor sensitization to mechanical and thermal stimuli in pig skin in vivo. Eur J Pain. 2008;12:242–250. doi: 10.1016/j.ejpain.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Schein OD. Phototoxicity and the cornea. J Natl Med Assoc. 1992;84:579–583. [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21:501–509. doi: 10.1080/10915810290169927. [DOI] [PubMed] [Google Scholar]
- Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76:497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Tenkate TD. Occupational exposure to ultraviolet radiation: a health risk assessment. Rev Environ Health. 1999;14:187–209. doi: 10.1515/reveh.1999.14.4.187. [DOI] [PubMed] [Google Scholar]
- Urban L, Perkins MN, Campbell E, Dray A. Activity of deep dorsal horn neurons in the anaesthetized rat during hyperalgesia of the hindpaw induced by ultraviolet irradiation. Neuroscience. 1993;57:167–172. doi: 10.1016/0306-4522(93)90118-y. [DOI] [PubMed] [Google Scholar]
- Wenk H, Honda CN. Silver nitrate cauterization: characterization of a new model of corneal inflammation and hyperalgesia in the rat. Pain. 2003;105:393–401. doi: 10.1016/S0304-3959(03)00295-1. [DOI] [PubMed] [Google Scholar]
- Zuclich JA. Ultraviolet-induced photochemical damage in ocular tissues. Health Phys. 1989;56:671–682. doi: 10.1097/00004032-198905000-00012. [DOI] [PubMed] [Google Scholar]


