Abstract
Thioredoxin (Trx) is an anti-oxidant and anti-apoptotic molecule, and its activity is regulated by post-translational modifications. Trx-1 has recently been reported to exert potent protective action against endotoxic liver injury. However, whether Trx-1 activity is affected by endotoxin has never been previously investigated. The aim of the present study was to determine endotoxic regulation of Trx-1, and the potential mechanism involved. In vitro co-incubation of Trx-1 with lipopolysaccharide (LPS) inhibited Trx-1 activity in a dose- and time-dependent fashion. The core (polysaccharide containing) region of LPS had greater inhibitory effect on Trx-1 activity than its Lipid A fragment, suggesting the involvement of sugar groups. Periodic acid-schiff staining and fructosamine assay demonstrated that Trx-1 was rapidly glycated by LPS. Aminoguanidine, a competitive glycation-inhibitor, completely blocked the inhibitory effect of LPS on Trx-1. Moreover, Trx-1 activity was also significantly inhibited by in vitro ribose incubation. Finally, in vivo administration of Trx-1, but not glycated Trx-1, reduced LPS-induced hepatic injury. Taken together, these results demonstrated for the first time that Trx-1 is susceptible to glycative inactivation. This novel post-translational Trx-1 modification contributes to LPS cytotoxicity, suggesting that blockading protein glycation might be a new therapeutic strategy against endotoxic organ injury.
Keywords: Sepsis, Organ Injury, Post-Translational Protein Modification
Introduction
Thioredoxin (Trx, including both cytosolic Trx-1 and mitochondrial Trx-2) is a ubiquitous protein with a conserved redox-active disulfide/dithiol center (Cys-Gly-Pro-Cys)[1]. Trx is an essential cell survival molecule and protects cells through two distinctive mechanisms. Atop its strong anti-oxidant activity, Trx reduces cell death through its direct interactions with various proteins such as apoptosis signal-regulation kinase-1 (ASK-1)[2] and thioredoxin-interacting protein (TXNIP)[3]. Pathological reduction of Trx activity contributes to organ injury in many diseases, whereas exogenous supplementation or genetic overexpression of Trx protects multiple organs from various injurious stimulations[4–6].
Recent studies have demonstrated that beyond upregulation or downregulation of Trx expression at the gene level, Trx activity is regulated by post-translational modification. Four forms of Trx post-translational modifications have been previously identified, and each modification type affects Trx differently. Oxidation of the thiol groups of Cys-32 and -35 forms a disulfide bond, and inhibits Trx anti-oxidative activity in a reversible manner. Glutathionylation selectively occurs at Cys-73. This post-translational modification significantly inhibits Trx antioxidant activity[7], but its impact upon thioredoxin’s cellular protective ability remains unknown. S-nitrosylation occurs at Cys-69 or Cys-73. We and others have previously demonstrated that S-nitrosylation, in contrast to oxidation and glutathionylation, markedly enhances Trx’s anti-oxidant as well as anti-apoptotic/organ-protective activity[8–10]. Finally, we recently reported the novel discovery that, unlike the three other forms of post-translational Trx modification which all occur at the cysteine residue, Trx can undergo nitrative modification at the tyrosine residue (Tyr49)[11]. Dissimilar to oxidative modification, nitration inhibits Trx anti-oxidative and cellular protective activity in irreversible fashion. Therefore, directional regulation of post-translational Trx modification may represent a novel therapeutical strategy against oxidative stress and organ injury.
As the tenth most common cause of death in the United States, sepsis constitutes a serious clinical problem and therapeutic challenge[12]. Lipopolysaccharide (LPS) release from the degradation of gram-negative bacterium cell walls is responsible for many sepsis symptoms, ultimately causing multiple organ failure[13]. Several recent studies have demonstrated that Trx might be a novel therapeutic target against LPS-induced liver injury. Specifically, low-dose LPS pretreatment upregulates Trx protein expression, and consequently enhances endotoxin tolerance in mice[6]. Moreover, overexpressing Trx attenuates thioacetamide-induced hepatic fibrosis and acute hepatitis, and reduced LPS-induced liver injury[14,15]. However, whether and how LPS might alter Trx activity remains completely unknown.
Therefore, the aims of the present study were to determine whether LPS may interact with and alter Trx activity, and if so, to dissect the molecular mechanism by which Trx activity is altered by LPS.
Materials and Methods
In vitro incubation of Trx-1 with LPS or its components
Although both Trx-1 and Trx-2 possess similar anti-oxidant and cellular protective effects, Trx-1 is better characterized, and the effect of LPS on Trx-1 activity was thus investigated in the current study. Trx-1 (100 μg/ml) was incubated with different doses of LPS for various time periods at 37°C. After incubation, excess LPS was removed, and Trx-1 anti-oxidant activity and Trx-1 protein modification were determined.
Trx-1 activity assay
Trx-1 activity was determined by using the well established insulin disulfide reduction assay[16]. In brief, 75 μl of sample was pre-incubated with 4 μl activation buffer (Solution A: 50 mM HEPES pH7.6, 1 mM EDTA, 1 mg/ml BSA, and 2 mM DTT) at 37 °C for 20 min to convert oxidized thioredoxin to its reduced form. 35 μl reaction buffer (Solution B: 250 mM HEPES, 10 mM EDTA, 2.4 mM NADPH, and 6.4mg/ml insulin) was then added, and the reaction was initiated by addition of 0.05U mammalian Trx-1 reductase. After 20 min incubation at 37 °C, the reaction was terminated by addition of 125 μl stopping solution (0.2 M Tris–HCL, 6 M guanidine–HCl, and 1.7 mM DTNB). Absorption (412 nm) was measured with a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA), and Trx-1 activity was expressed as U/mg protein or mU/mg protein (for liver tissue Trx activity only).
Western blotting
To determine Trx-LPS direct interaction, Trx-1 (100 μg/ml) was incubated with vehicle or LPS (100 μg/ml) for 1 h at 37° C. After removing excess LPS, proteins were separated using native gels (Invitrogen, Carlsbad, CA) in native running buffer, transferred to nitrocellulose membranes, and incubated with primary antibodies (anti-LPS or anti-Trx) and HRP-conjugated secondary antibody. The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL), and bands were visualized with a Kodak Image Station 400 (Rochester, NY). Each experiment was repeated at least 5 times.
Determination of Trx glycation
Trx-1 was incubated with vehicle or LPS as described above. Trx-1 glycation was determined by two established methods (fructosamine and Periodic Acid-Schiff) for detection of early glycative reactions. Fructosamine assay was performed using the Glycated Serum Protein Enzymatic Test Kit (Diazyme, San Diego, CA) as described previously[17]. For Periodic Acid-Schiff (PAS) staining[18,19], vehicle or LPS-treated Trx was separated by 4–20% SDS-PAGE gel (Invitrogen). Gels were stained with either GelCode® Glycoprotein Staining Kit (Pierce) or GelCode® Blue Stain Reagent (Coomassie Blue staining), and visualized with a Kodak Image Station 400. Each experiment was repeated at least 5 times.
In vivo experiments
Adult male mice (20–25g) were treated with lipopolysaccharide (20 μg/g, IP injection). Tissue Trx-1 activity was determined as described in our previous study[20]. Plasma ALT/AST levels were determined using ALT/AST assay kit, and plasma glucose was determined by ACCU-CHEK® Blood glucose monitor kit (Roche, Indianapolis, IN). All experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals, and were approved by the Thomas Jefferson University Committee on Animal Care.
Statistical analysis
All values in the text and figures are presented as means ± SEM of n independent experiments. All data (except Western blot density) were subjected to one-way ANOVA followed by Bonferroni correction for post-hoc t test. Western blot densities were analyzed with the Kruskal-Wallis test followed by Dunn’s post-hoc test. Probabilities of 0.05 or less were considered to be statistically significant.
Results
In vitro LPS incubation significantly inhibited Trx-1 activity
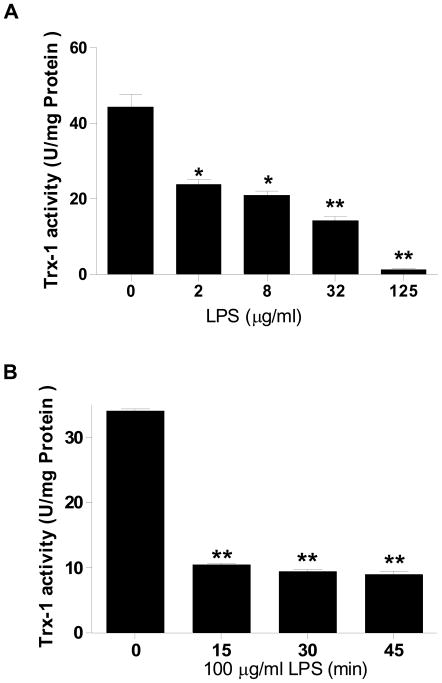
As summarized in Figure 1A, in vitro incubation of Trx-1 with LPS decreased Trx-1 activity in a dose-dependent fashion, with the minimal significant effect observed at LPS concentration as low as 2 μg/ml. When exposed to LPS concentration 125 μg/ml, Trx-1 activity was virtually abolished. Moreover, incubation of Trx-1 with LPS dose 100 μg/ml for merely 15 min markedly inhibited Trx-1 activity (10.4±0.28 vs. 34.1±0.25 U/mg protein, P<0.01). Extension of the incubation period up to 30 min further inhibited Trx-1 activity slightly (8.9±0.45 U/mg protein). To determine whether decreased Trx-1 activity was caused by incomplete removal of non-reacted LPS that may react with other component present in the assay system (such as Trx-1 reductase and insulin), Trx-1 reductase or insulin was pre-incubated with LPS (125 μg/ml) for 30 minutes before adding into the assay system. Our results showed that pre-incubation of Trx-1 reductase or insulin had no effect on Trx-1 activity (data not shown).
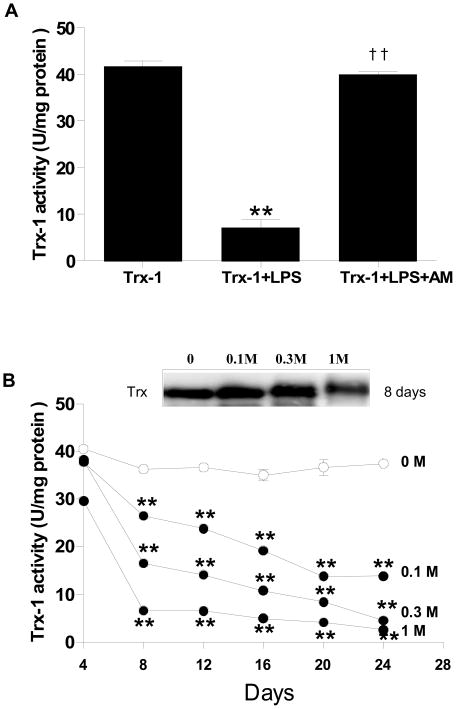
Figure 1.
Concentration- (A, N=4–6/group. Incubation time = 1 h) and time-dependent (B, n=4–6/group) effect of LPS on Trx activity. Human recombinant Trx-1 (100 μg/ml) was incubated with different concentrations of LPS (0 to 125 μg/ml) for 1 h at 37° C, or with LPS (100 μg/ml) for different time periods (0 to 45 min). Trx-1 activity was then determined. *P<0.05, **P<0.01 vs. control.
The polysaccharide component had more inhibitory effect on Trx-1 than the Lipid A component
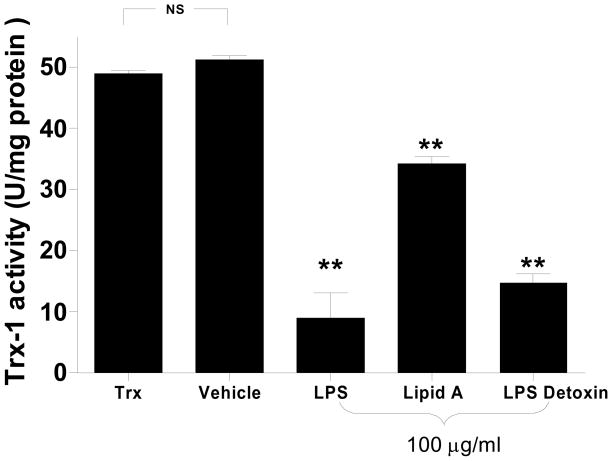
LPS is comprised of three parts: 1) Lipid A, 2) core polysaccharide, and 3) polysaccharide (O) side chains, with Lipid A being the primary toxic component. To determine whether Lipid A is also the most important LPS component responsible for Trx-1 inhibition, the lipid component (Lipid A) and saccharide component (Detoxin, containing LPS core region and polysaccharide side chains) were individually incubated with Trx-1 at 37°C for 1 h, and their effect on Trx-1 activity was determined. Surprisingly, the saccharide component had more inhibitory effect than the Lipid A component (69.9% versus 30.1% inhibition, P<0.01), and the saccharide component inhibited Trx-1 activity to comparable extent seen with LPS incubation of the same concentration (Figure 2). This result suggested that LPS may inhibit Trx-1 activity through sugar-protein interaction, a recently identified post-translational modification termed protein glycation.
Figure 2.
Comparison of the inhibitory effect of different LPS components on Trx-1 activity. Lipid A: hydrophobic lipid component of LPS; Detoxin: polysaccharide component of LPS; Vehicle: 0.05% Triethlamine. LPS or its components were all purchased from Sigma-Aldridge. N=4–6/group. **P<0.01 vs. vehicle.
LPS incubation caused Trx-1 modification and molecular size alteration
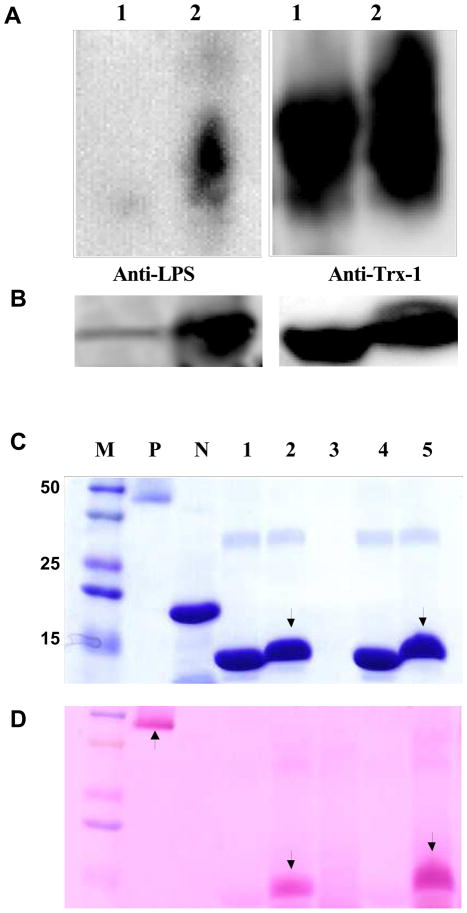
To explore the possibility that LPS may directly interact with Trx-1 via its reduced sugar component and cause Trx-1 glycation, the following experiments were performed. Vehicle or LPS incubated Trx-1 (non-reducing condition) was separated with native gel and Western blotted with antibody against LPS or Trx-1. As illustrated in Figure 3A, LPS was detected in LPS-treated Trx-1 (lane 2 of left panel), but not in vehicle-treated Trx-1 (lane 1 of left panel). Moreover, Western blot in native gel using anti-Trx-1 antibody demonstrated greater smear bands in the LPS incubated Trx-1 (lane 2 of right panel) than vehicle-incubated Trx-1 (lane 1 of right panel). To obtain more evidence supporting that LPS interacts with Trx-1, LPS-incubated Trx-1 was loaded to SDS-PAGE gel and stained with GelCode® Blue Stain Reagent (Pierce, IL). As expected, no protein band was detected when only LPS was loaded (Figure 3B, lane 3), and a clear single band with a molecular weight corresponding to recombinant human Trx-1 is visualized when Trx-1 was loaded (Figure 3B, lanes 1, 4). Importantly, incubation with LPS caused Trx-1 shifting to a higher location (Figure 3B, lanes 2, 5), indicating that LPS or its constituent component(s) bound with Trx-1, and reduced its mobility. Taken together, these results demonstrated that LPS indeed directly binds with Trx-1.
Figure 3.
Representative Western blots using native-gel (A) and SDS-PAGE gel (B). Experiment was repeated at least 5 times. Lane 1: Trx-1+vehicle; lane 2: Trx-1+LPS (100 μg/ml). (C) Representative SDS-PAGE gel stained with Coomassie blue (experiment was repeated at least 5 times). M: Marker; P: Positive control; N: negative control; lanes 1 and 4: vehicle incubated Trx-1; lanes 2 and 5: LPS (100 μg/ml) incubated Trx-1; lane 3: LPS only. Please note Trx-1 molecular weight change after LPS treatment (lanes 2 and 5). (D) Representative SDS-PAGE gel stained with GelCode® Glycoprotein Staining Kit (experiment was repeated at least 5 times). Periodic acid-schiff (PAS) positive staining (arrow) indicates the protein glycation. Labels are the same as B.
LPS caused significant Trx-1 glycation
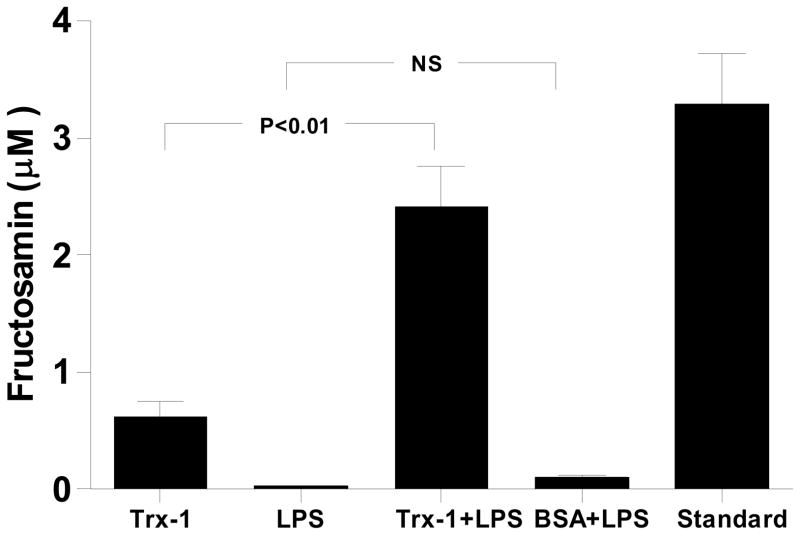
To determine whether the LPS-Trx-1 interaction resulted in Trx-1 glycation, two established methods that specifically detect early protein glycation were used. LPS-incubated Trx-1 was loaded to SDS-PAGE gel and stained with GelCode® Glycoprotein Staining Kit (Pierce, IL). As illustrated in Figure 3B, protein bands were detected when either positive (P) or negative (N) glycative protein markers (manufacturer-provided) were loaded (GelCode® Blue Stain Reagent). However, PAS staining was detected when positive (Figure 3C, P lane), but not negative (Figure 3C, N lane), glycative protein markers was loaded (GelCode® Glycoprotein Staining). Most importantly, a significant band of “glycated protein” (PAS positive staining) was detected when LPS-treated (Figure 3C, lanes 2, 5), but not vehicle-treated Trx-1 (Figure 3C, lanes 1, 4), was loaded. To further determine Trx-1 glycation in a quantitative manner, the content of fructosamine, the first step reaction product in protein glycation, was determined. As summarized in Figure 4, incubation of Trx-1 with LPS significantly increased fructosamine concentration. In contrast, incubation of bovine serum protein (BSA) with LPS failed to yield any fructosamine formation, indicating that LPS induced protein glycation is protein selective. Taken together, the observed patterns of PAS staining and fructosamine production support the conclusion that Trx-1 was glycated by LPS treatment.
Figure 4.
Fructosamin (early glycation product) assay indicating that LPS caused significant Trx-1 glycation. BSA: bovine serum albumin; Standard: Fructosamin standard provided by the company.
Glycative modification is responsible for Trx-1 inhibition
The data presented above clearly demonstrated that Trx-1 is glycated and its activity is inhibited after LPS treatment. To determine whether glycative modification of Trx-1 is responsible for Trx-1 inhibition, two experiments were performed. Firstly, the effect of aminoguanidine, a competitive inhibitor of protein glycation[21], on LPS-induced Trx-1 inhibition was determined. As summarized in Figure 5A, the addition of aminoguanidine (final concentration 100 mM) during LPS treatment completely reversed the inhibitory effect of LPS and restored Trx-1 activity to levels observed in control. Secondly, Trx-1 was incubated with ribose, the most commonly utilized monosaccharide in protein glycation research[22]. Trx-1 activity was determined after ribose incubation at different doses (0 to 1M) for different time periods (0 to 24 days). As summarized in Figure 5B, incubation with ribose decreased Trx-1 activity in a dose and time-dependent fashion. Specifically, incubation of Trx-1 with ribose at concentration as low as 0.1M for as short as 8 days markedly decreased Trx-1 activity, a time point during which no advanced glycation end products (AGE, determined by formation of N epsilon-[carboxymethyl)lysine) were detected (data not shown). Moreover, incubation of Trx-1 with a higher concentration of ribose (1 M) for a longer period of time (24 days) caused significant AGE formation, and completely inactivated Trx-1. Finally, incubation with ribose caused a Trx-1 molecular shift in a dose-dependent fashion (Figure 5B, insert). When Trx-1 was incubated with 1M ribose for 8 days, a Trx-1 molecular shift, comparable with that seen after LPS treatment, was observed (Figure 5B, insert).
Figure 5.
(A) Aminoguanidine, a competitive glycative-inhibitor (100 μM), completely blocked the inhibitory effect of LPS (100 μg/ml, 45 min) on Trx-1 (100 μg/ml) activity. **P<0.01 vs. vehicle incubated Trx-1; ††P<0.01 vs. LPS incubated Trx-1 without addition of aminoguanidine. N=5–7/group. (B) Concentration- and time-dependent inhibitory effect of ribose on Trx-1 activity. **P<0.01 vs. 4 days. Insert: representative Western blots showing that ribose caused concentration-dependent Trx-1 molecular weight augmentation.
Glycated-Trx-1 unable to prevent LPS liver toxicity
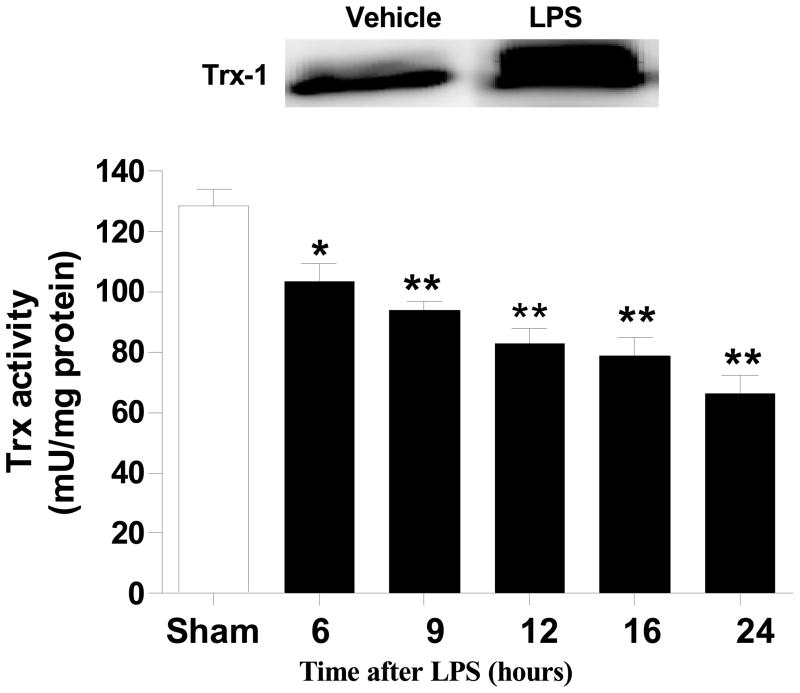
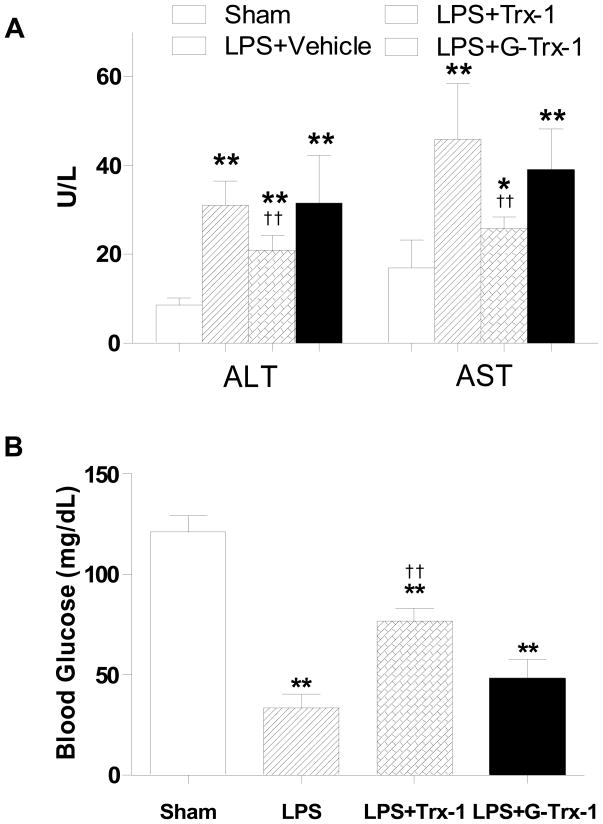
Our in vitro experiments (presented in Figures 1–5) demonstrated that LPS caused glycation and inactivation of Trx-1. To obtain more evidence supporting that LPS-induced Trx-1 glycative inactivation is pathologically important, two additional in vivo experiments were performed. In the first series of experiments, adult male mice treated with LPS were sacrificed 6, 9, 12, 16 and 24 h after LPS injection, and liver Trx-1 activity was determined. As summarized in Figure 6, liver Trx-1 activity was significantly decreased as early as 6 h after LPS injection, and continuously decreased thereafter. After 24 h LPS injection, liver Trx-1 activity decreased to approximately 50% of control value. Moreover, liver tissue Trx-1 expression was significantly increased 24 hours after LPS injection, and an upward shifting of Trx-1 molecule was observed (Figure 6, insert). In the second series of experiments, adult male mice were pre-treated with vehicle, vehicle-incubated Trx-1, or ribose-incubated Trx-1 (4 μg/g, i.p.,) 10 min prior to LPS injection, and LPS-induced liver injury was determined 12 h after LPS injection. In vehicle-treated animals, LPS caused significant liver injury characterized by 3.6 and 2.7 fold elevation in plasma ALT and AST levels respectively (Figure 7A) with dramatic reduction (70%) in plasma glucose, an early marker of hepatic dysfunction in sepsis[23](Figure 7B). Treatment with vehicle-incubated Trx-1 significantly attenuated LPS-induced liver injury as evidenced by 43 and 45 percent reduction in ALT and AST respectively (Figure 7A), and significantly increased blood glucose levels (Figure 7B, 76.8±6.7 mg/dL vs. 33.4±6.8 mg/dL, P<0.01). However, treatment with the same dose of ribose-incubated Trx-1 neither reduced ALT/AST activity (Figure 7A) nor increased blood glucose level (Figure 7B) significantly in LPS-treated mice. This in vivo experimental result provides additional evidence that the glycation of Trx-1 causes irreversible inactivation, rendering it unable to provide hepato-protection against LPS-induced injury.
Figure 6.
Time course of hepatic Trx-1 activity after LPS administration. N= 4–20/group, *P<0.05, **P<0.01 vs. sham control. Insert: A typical Western blot of 6 repeated experiments showing that liver Trx-1 expression is increased 24 h after LPS injection, and Trx-1 band is upward shifted.
Figure 7.
Effect of in vivo administration of native Trx-1 and ribose glycated Trx-1 on LPS-induced liver injury determined by serum ALT and AST (A, N=14–19/group) and blood glucose concentration (B, N=14–19/group). *P<0.05, **P<0.01 vs. sham control; †† P<0.01 vs. LPS alone without Trx-1 treatment.
Discussion
Distinct from glycosylation, which refers to enzymatically controlled addition of a glycosyl group to either asparagine, hydroxylysine, serine, or threonine (resulting in a glycoprotein), glycation (sometimes called non-enzymatic glycosylation) is the result of a reduced sugar molecule, such as fructose or glucose, bonding directly to a protein or lipid molecule without the controlling action of an enzyme[24]. Glycation occurs in several steps. In a brief initial step taking minutes to hours, the reducing sugar reacts with the protein and a Schiff base, which is a covalent double bond between the carbon atom of the glucose and the nitrogen atom of the lysine, is formed. After several days or weeks, Amadorial rearrangement commences, and advanced glycated end-products (AGEs) are formed[25]. In recent years, the pathogenic roles of AGE have been the source of extensive investigation. Increased AGE accumulation and subsequent tissue injury have been found in many human diseases, such as type 2 diabetes and the aging process itself [26–28]. However, whether initial protein modification by sugars prior to actual AGE formation may have protein function alteration ramifications remains largely unknown. McCarthy et al[22] reported that incubation of alkaline phosphatase (ALP) with reducing sugars reduced enzyme activity that was associated with an increase in fructosamine levels, indicating that early glycation may alter protein function. In two more recent studies, human Cu-Zn-superoxide dismutase[29] and esterase[30] have been found to be glycated by methylglyoxal, and their actions subsequently inhibited.
In the current study, we have demonstrated for the first time that glycative Trx-1 inhibition is a novel post-translational modification for this critical cell survival molecule. We have obtained several lines of evidence supporting that Trx-1 is susceptible to glycative inactivation by LPS. Firstly, we have demonstrated that LPS directly bonds to Trx-1, resulting in its molecular weight shift in SDS-PAGE gel (Figure 3B), consistent with reports by Sen et al (ALP)[30] and Kang (SOD)[29]. Secondly, we have utilized two well-accepted methods that specifically detect early stage protein glycation[17–19], and demonstrated that incubation of Trx-1 with LPS significantly increased fructosamine level (Figure 4), and caused Trx-1 to stain PAS-positive (Figure 3C). Thirdly, we have demonstrated that the competitive glycative-inhibitor aminoguanidine, a second generation glycation inhibitor that blocks protein glycation by trapping reactive carbonyl compounds[31,32], completely blocked the inhibitory effect of LPS on Trx-1 (Figure 5A). Fourthly, we have demonstrated that incubation of Trx-1 with ribose, a traditionally utilized molecule in protein glycation studies, caused Trx-1 molecular weight shift and activity inhibition similar to that caused by LPS, albeit after a longer incubation period (Figure 5B). Finally, we have demonstrated that the protective effects of Trx-1 against LPS hepatic injury were abolished after ribose pre-exposure (Figure 7).
Lipopolysaccharide release from the degradation of gram-negative bacterium cell walls is responsible for many sepsis symptoms[13], which remains a challenging disease process to treat. The release of inflammatory cytokines such as TNFα, IL-1, and IL-6 rovoked by LPS has important physiologic and pathologic implications. Several excellent studies by Yodoi and colleagues have demonstrated that overexpressing Trx attenuates thioacetamide-induced hepatic fibrosis, acute hepatitis, and reduced (LPS)-induced liver injury[14,15]. Moreover, LPS-induced neutrophil infiltration, cartilage destruction, and chondrocyte apoptosis within joints can be dramatically suppressed in Trx transgenic overexpression. The 8-hydoxy-2′-deoxyguanosine (8-OHdG) expression seen in wild-type mice after LPS injection was almost completely inhibited in Trx transgenic mice. The administration of recombinant Trx suppressed LPS-induced joint swelling[33]. Taken together, these results suggest that Trx protects against LPS-induced unfavorable health response. We currently investigated the effects of LPS upon thioredoxin, a cytoprotective molecule whose inactivation plays a critical role in tissue injury, in an attempt to define a novel mechanism responsible for LPS cytotoxicity. We have demonstrated that administration of LPS markedly reduced hepatic Trx-1 activity (Figure 6) and caused significant liver injury. Since the liver is an organ of front line defense against LPS toxicity, it is conceivable that hepatic Trx-1 is inhibited, and significant hepatic cellular damage occurs before other organs are affected. Moreover, we have demonstrated that, unlike Trx-1 oxidation, which is fully reversible in vivo (by Trx-1 reductase), Trx-1 glycation is irreversible, resulting in permanent Trx-1 inactivation (Figure 7). Therefore, glycative modification of Trx-1 may play a critical role in LPS related cell death, and overexpression or exogenous supplementation of Trx may overwhelm LPS-induced Trx-1 glycation, thus protecting organs from LPS injury.
The physiologic and pathologic implications of LPS glycative inactivation of Trx-1 are not restricted to sepsis only. Trx-1 is a critical cell survival molecule whose inactivation plays a causative role in many human diseases, such as myocardial ischemia/reperfusion[11] and degenerative neurological disorders[34]. Emerging evidence indicates that proteins can be glycatively modified by many molecules. Of particular interest is methylglyoxal, a highly reactive compound derived from glycolysis[35]. Methylglyoxal has been shown to cause rapid (within 3 to 6 h) and significant protein glycation with inhibitory effect[29]. Its concentration is significantly increased under many pathologic conditions, including diabetes, inflammation, and the aging process[35]. As such, Trx-1 is likely to be glycatively modified, and its cytoprotective ability inhibited under these pathologic conditions. The glycative inactivation of Trx-1 may thus have broad pathologic significance. Moreover, although we only observed LPS causing rapid Trx-1 glycation and inactivation in the current study, the potency with which LPS modified Trx-1 through glycation suggests the likelihood that LPS may glycate and modify the functions of other important signaling proteins due to its rich saccharide composition. This might represent a novel signaling mechanism through which LPS induces tissue injury. Therefore, glycation inhibitors, such as aminoguanidine and newly developed compounds (e.g., ALT-711)[36], may have great therapeutic potential in the treatment of endotoxic organ injury.
Although it is well-recognized that Trx-1 is secreted by various cell types and presents in plasma, it is generally accepted that Trx-1 exerts its biological functions as an intracellular protein. However, emerging evidence indicates that extracellular Trx-1 as well as membrane-bound Trx-1 is biologically active and cytoprotective[37,38]. As such, LPS in circulation may cause extracellular Trx-1 and membrane-bound Trx-1 glycation and subsequent loss of its cytoprotective function. On the other hand, it has been known for many years that LPS can enter living cells through endocytosis and activates intracellular signaling[39]. Therefore, LPS may also cause intracellular protein glycation and functional modification.
Limitations
Firstly, the specific amino acid residuals of Trx-1 that are responsible for glycative modification remains unknown and is currently under investigation. However, our preliminary data indicated that cysteine residues are not involved in glycative modification because mutations of any or all of the 5 cysteine residues failed to block Trx-1 glycation. Secondly, we were unable to directly measure hepatic Trx-1 glycation after in vivo LPS administration because a method sensitive enough to detect in vivo early protein glycation is currently unavailable. Nonetheless, our in vitro results demonstrating that Trx-1 function is glycatively inhibited, together with our in vivo results showing that hepatic Trx-1 activity is reduced in LPS-treated animals, and administration of native but not glycated Trx-1 reduced LPS liver toxicity, strongly summarily suggest that glycative Trx-1 inactivation may contribute to LPS liver toxicity.
Acknowledgments
Grant Support: This research was supported by the following grants: NIH 2R01HL-63828, American Diabetes Association Research Award 7-08-RA-98, and American Heart Association Grant-in-Aid 0855554D (to X.L.M.).
List of Abbreviations
- ALT
alanine Aminotransferase
- ANOVA
analysis of variance
- ASK
apoptosis signal-regulation kinase-1
- AST
aspartate aminotransferase
- BSA
bovine serum protein
- DTNB
5,5′-dithiobis (2-nitrobenzoic acid)
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LPS
lipopolysaccharide
- NADPH
nicotinamide adenine dinucleotide phosphate
- Trx
thioredoxin
- TXNIP
thioredoxin-interacting protein
Footnotes
Author Disclosure Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakamura H, Nakamura K, Yodoi J. REDOX REGULATION OF CELLULAR ACTIVATION. Annual Review of Immunology. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 4.Kondo N, Ishii Y, Kwon YW, Tanito M, Horita H, Nishinaka Y, Nakamura H, Yodoi J. Redox-Sensing Release of Human Thioredoxin from T Lymphocytes with Negative Feedback Loops. J Immunol. 2004;172:442–448. doi: 10.4049/jimmunol.172.1.442. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui T, Koide H, Fukahori H, Isoda K, Higashiyama S, Maeda I, Tashiro F, Yamato E, Miyazaki JI, Yodoi J, Kawase M, Yagi K. Adenoviral transfection of hepatocytes with the thioredoxin gene confers protection against apoptosis and necrosis. Biochemical and Biophysical Research Communications. 2003;307:765–770. doi: 10.1016/s0006-291x(03)01253-1. [DOI] [PubMed] [Google Scholar]
- 6.Sano H, Sata T, Nanri H, Ikeda M, Shigematsu A. Thioredoxin is associated with endotoxin tolerance in mice. Crit Care Med. 2002;30:190–194. doi: 10.1097/00003246-200201000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci U S A. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 9.Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of Statins via S-nitrosylation and activation of thioredoxin in endothelial cells. A novel vasculoprotective function of Statins. Circulation. 2004;110:856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- 10.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of S-nitrosation. PNAS. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao L, Jiao X, Gao E, Lau WB, Yuan Y, Lopez B, Christopher T, Ramachandrarao SP, Williams W, Southan G, Sharma K, Koch W, Ma XL. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006;114:1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- 12.Martin GS, Mannino DM, Eaton S, Moss M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 13.Opal SM. The clinical relevance of endotoxin in human sepsis: a critical analysis. J Endotoxin Res. 2002;8:473–476. doi: 10.1179/096805102125001109. [DOI] [PubMed] [Google Scholar]
- 14.Okuyama H, Nakamura H, Shimahara Y, Uyama N, Kwon YW, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol. 2005;42:117–123. doi: 10.1016/j.jhep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Okuyama H, Nakamura H, Shimahara Y, Araya S, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology. 2003;37:1015–1025. doi: 10.1053/jhep.2003.50203. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. Journal of Clinical Investigation. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 18.Misenheimer TM, Hahr AJ, Harms AC, Annis DS, Mosher DF. Disulfide connectivity of recombinant C-terminal region of human thrombospondin 2. J Biol Chem. 2001;276:45882–45887. doi: 10.1074/jbc.M104218200. [DOI] [PubMed] [Google Scholar]
- 19.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 20.Tao L, Gao E, Hu A, Coletti C, Wang Y, Christopher TA, Lopez BL, Koch W, Ma XL. Thioredoxin reduces post-ischemic myocardial apoptosis by reducing oxidative/nitrative stress. Br J Pharmacol. 2006;149:311–318. doi: 10.1038/sj.bjp.0706853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ota K, Nakamura J, Li W, Kozakae M, Watarai A, Nakamura N, Yasuda Y, Nakashima E, Naruse K, Watabe K, Kato K, Oiso Y, Hamada Y. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem Biophys Res Commun. 2007;357:270–275. doi: 10.1016/j.bbrc.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy AD, Cortizo AM, Gimenez SG, Bruzzone L, Etcheverry SB. Non-enzymatic glycosylation of alkaline phosphatase alters its biological properties. Mol Cell Biochem. 1998;181:63–69. doi: 10.1023/a:1006857309142. [DOI] [PubMed] [Google Scholar]
- 23.Deutschman CS, Andrejko KM, Haber BA, Bellin L, Elenko E, Harrison R, Taub R. Sepsis-induced depression of rat glucose-6-phosphatase gene expression and activity. Am J Physiol. 1997;273:R1709–R1718. doi: 10.1152/ajpregu.1997.273.5.R1709. [DOI] [PubMed] [Google Scholar]
- 24.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 25.Lapolla A, Traldi P, Fedele D. Importance of measuring products of non-enzymatic glycation of proteins. Clinical Biochemistry. 2005;38:103–115. doi: 10.1016/j.clinbiochem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 27.Furber JD. Extracellular Glycation Crosslinks: Prospects for Removal. Rejuvenation Research. 2006;9:274–278. doi: 10.1089/rej.2006.9.274. [DOI] [PubMed] [Google Scholar]
- 28.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JH. Modification and inactivation of human Cu,Zn-superoxide dismutase by methylglyoxal. Mol Cells. 2003;15:194–199. [PubMed] [Google Scholar]
- 30.Sen S, Bose T, Roy A, Chakraborti AS. Effect of non-enzymatic glycation on esterase activities of hemoglobin and myoglobin. Mol Cell Biochem. 2007;301:251–257. doi: 10.1007/s11010-007-9418-5. [DOI] [PubMed] [Google Scholar]
- 31.Monnier VM. Intervention against the Maillard reaction in vivo. Arch Biochem Biophys. 2003;419:1–15. doi: 10.1016/j.abb.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji G, Koshiba M, Nakamura H, Kosaka H, Hatachi S, Kurimoto C, Kurosaka M, Hayashi Y, Yodoi J, Kumagai S. Thioredoxin protects against joint destruction in a murine arthritis model. Free Radic Biol Med. 2006;40:1721–1731. doi: 10.1016/j.freeradbiomed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Oka Si, Masutani H, Nakamura H, Yodoi J. The Role of Thioredoxin in the Aging Process: Involvement of Oxidative Stress. Antioxidants & Redox Signaling. 2003;5:563–570. doi: 10.1089/152308603770310211. [DOI] [PubMed] [Google Scholar]
- 35.Ramasamy R, Yan SF, Schmidt AM. Methylglyoxal comes of AGE. Cell. 2006;124:258–260. doi: 10.1016/j.cell.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura H, Masutani H, Yodoi J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Seminars in Cancer Biology. 2006;16:444–451. doi: 10.1016/j.semcancer.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Cowan DB, Noria S, Stamm C, Garcia LM, Poutias DN, del Nido PJ, McGowan FX., Jr Lipopolysaccharide Internalization Activates Endotoxin-Dependent Signal Transduction in Cardiomyocytes. Circ Res. 2001;88:491–498. doi: 10.1161/01.res.88.5.491. [DOI] [PubMed] [Google Scholar]