Abstract
Objectives
The purposes of this work were to quantitate the affinity and binding capacity of chlorhexidine (CHX) digluconate to mineralized vs. demineralized dentin powder, and to determine how much debinding would result from rinsing with water, ethanol, hydroxyethylmethacrylate (HEMA) or 0.5 M NaCl in water.
Methods
Dentin powder was made from coronal dentin of extracted human third molars. Standard amounts of dentin powder were tumbled with increasing concentrations of CHX (0–30 mM) for 30 min at 37 C. After centrifuging the tubes, the supernatant was removed and the decrease in CHX concentration quantitated by UV-spectroscopy. CHX-treated dentin powder was resuspended in one of the four debinding solutions for 3 min. The amount of debound CHX in the solvents was also quantitated by UV-spectroscopy.
Results
As the CHX concentration in the medium increased, the CHX binding to mineralized dentin powder also increased up to 6.8 μmoles/g of dry dentin powder. Demineralized dentin powder took up significantly (p<0.01) more CHX, reaching 30.1 μmoles CHX/g of dry dentin powder. Debinding of CHX was in the order: HEMA < ethanol < 0.05 M NaCl < water. The highest CHX binding to demineralized dentin occurred at 30 mM (1.5 wt%).
Significance
As CHX is not debound by HEMA, it may remain bound to demineralized dentin during resin-dentin bonding. This may be responsible for the long-term efficacy of CHX as an MMP inhibitor in resin-dentin bonds.
Keywords: chlorhexidine, binding, debinding, dentin bonding
1. Introduction
Chlorhexidine (CHX) is an excellent antimicrobial agent that has been used as a cavity disinfectant [1] and root canal irrigant [2,3]. However, Gendron et al. [4] reported that CHX was also a potent inhibitor of matrix metalloproteinases (MMPs). Chlorhexidine inhibits MMP-2, -8 and -9 at very low concentrations (i.e. 0.01–0.02%). As dentin is known to contain these MMPs [5–8], Pashley et al. [9] treated acid-etched dentin with 0.2% CHX digluconate to determine if it could inhibit the endogenous MMPs of the dentin matrix. Untreated control mineralized dentin powder was able to hydrolyze fluorescein-labeled soluble type I collagen, while powder incubated with 0.2% CHX inhibited that collagenolytic activity by 99%. This led to several in vitro and in vivo resin-dentin bonding studies that confirmed the ability of 2% CHX to protect dentin collagen degradation in vivo using transmission electron microscopy [10–13] and measurements of in vivo bond strengths over 14 months [13].
Such bonding studies involved topically treating acid-etched dentin with either 0.2 or 2% CHX as a therapeutic primer just prior to resin-dentin bonding. The MMPs in dentin are bound to the collagen matrix but can slowly degrade that matrix over time. Questions remain on how well CHX binds to dentin and how long it may remain in place as an MMP inhibitor. The exact mechanism(s) responsible for CHX inhibiting dentin MMPs are not clear. It is not known whether CHX binds to demineralized dentin matrix or to the mineralized matrix or to both. Likewise, the optimal concentration of CHX necessary to saturate binding sites on mineralized versus demineralized dentin has not been fully elucidated. More importantly, it is not known how tightly CHX binds to demineralized dentin. That is, can hydroxyethyl methacrylate (HEMA) and ethanol, which are common constituents of dental adhesives, displace or extract CHX that has just bound to the matrix during bonding procedures?
The purpose of this work was to test the null hypotheses that CHX binding to demineralized vs. mineralized dentin is not different and that once bound, CHX is not removed by HEMA, ethanol, or 0.5 M NaCl.
2. Materials and Methods
2.1 Preparation of dentin powder
One hundred non-carious freshly unerupted human third molars were collected after obtaining informed consent under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. The teeth were ground with coarse diamond burs in a high-speed handpiece with air-water spray to remove the enamel. The roots were removed at the cementoenamel junction using an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA). The pulpal soft tissues were removed with a spoon excavator and the predentin was removed with a diamond bur. The resulting crown segments were cut into small fragments (4 × 4 × 3 mm) that were placed in 25-mL stainless steel screw-top jars, submerged in liquid nitrogen for 10 min and triturated at 30 Hz for 9 min in a ball-mill (MM301, Retsch, Newtown, PA, USA). This treatment reduced the dentin fragments to a fine powder (mean particle size < 50 μm). The powder was stored at −70°C until required.
2.2 Preparation of demineralized dentin powder
Half of the dentin powder was kept mineralized (MD), while the other half was divided in 10-g batches that were transferred to centrifuge tubes containing 30 mL of 0.1 M formic acid/sodium formate buffer, pH 2.5. The centrifuge tubes were tumbled at room temperature for 5 days, replacing the demineralizing solution with fresh solution every day. Complete demineralization was confirmed radiographically and by calculating the density of the powder that fell from 2.1 to 1.05 g/mL at the end of demineralization [14]. After centrifuging the demineralized dentin (DD) powder at 3000 rpm for 30 min, the supernatant demineralizing solution was removed and the powder was resuspended with 30 mL of phosphate buffered saline (pH 7.4) three times to rinse away all traces of formic acid/formate.
2.3 Rinsing of dentin powder to remove UV absorbing material
Both mineralized and especially demineralized dentin powder releases products that may give UV absorption at 225 nm, the wavelength that was used to quantitate CHX uptake. This “background” absorbance was removed to < 0.05 absorbance at 225 nm by multiple rinses with water. That is, 3 g of powder was suspended in 30 mL of water and tumbled for 1 hr, centrifuged and the absorbance of the supernatant measured against water in UV transparent 96-well plate reader (Costar 3635, UV Plate, Corning, NY, USA) in a Synergy HC plate reader (Biotek, Winooski, VT, USA). This was repeated 5–6 times/day for up to 10 days, until the absorbance of the supernatant was less than 0.05 A at 225 nm. Then the powder was immediately used for the CHX binding experiments.
2.4 Chlorhexidine binding experiments
Following the method of Blackburn et al. [15], 0.05 g of dentin powder were transferred to microcentrifuge tubes and mixed with 1 mL of standard solutions containing 0, 0.04, 0.10, 0.2, 0.39, 3.9, 19.7 or 29.6 mM of chlorhexidine digluconate (CHX) in 0.05 M phosphate buffer, pH 7.4. Preliminary work demonstrated that CHX binding was similar irrespective of whether the dentin powder was treated for 1 min or 30 min; so it was decided to use 30 min for convenience. The microcentrifuge tubes were then capped and tumbled for 30 min at 37°C. Then, they were centrifuged at 3000 rpm and the supernatant was removed. Three hundred μL of supernatant were placed in a UV transparent 96-well plate for measurement of the absorbance at 225 nm against water, in duplicate in the Synergy HC plate reader. From a standard CHX curve, the absorbance of the supernatant was converted to CHX concentration. If there was no dentin powder in the tube, there was no change in the CHX concentration of the standard. In the presence of dentin powder, the CHX concentration of the supernatant was always less than that of the standard solution, indicating that the dentin powder bound CHX. This was expressed in μmoles CHX/g dry powder. This value was designated as the bound CHX at equilibrium. No further binding occurred at longer incubation times. The binding of CHX by dentin powder was calculated as:
| (equation 1) |
where: CHXBound = μmoles CHX/g dry weight of powder; CHXSTD = CHX concentration in standard solutions before exposure to powder (μmoles/mL) and CHXequil = equilibrium CHX concentration in solution (μmoles/mL) after exposure to powder, divided by the weight of the dry dentin powder.
2.5 Chlorhexidine debinding experiments
The excess remaining CHX solution was removed from the microcentrifuge tubes with dry paper points and the powder pellet was then resuspended in 1 mL of water, 100% hydroxyethyl methacrylate (HEMA), 100% ethanol, or 0.5 M NaCl. The use of HEMA was meant to serve as a representative hydrophilic monomer that is commonly used in many adhesive blends. High sodium chloride concentrations are generally used to displace electrostatistically-bound materials from their substrates [16]. The microcentrifuge tubes were hand shaken at 3 Hz for 3 min and then centrifuged to repellet the powder. Two 300 μL aliquots of the supernatant were transferred to 96-well plates for quantitation of the amount of CHX that could be displaced or extracted from the dentin powder by these solvents.
2.6 Hydroxyapatite (HA) binding and debinding experiments
Pure hydroxyapatite powder was used as a model bindnig substrate to simulate the expected binding characteristics of mineralized dentin powder. If binding of CHX to HA was similar to that of mineralized dentin powder than that binding would be predominately due to the mineral phase of dentin instead of the organic phase. Pure hydroxyapatite (reagent grade, Sigma-Aldrich, St. Louis, MO, USA, Catalog No. 289396) was used as a reference binding material. Fifty mg of HA was placed in separate microcentrifuge tubes and suspended in 1 mL of CHX standards for 30 min to obtain maximum CHX binding. As pilot studies showed that similar CHX binding occurred at 1 vs. 30 min, we used 30 min for these studies. The decrease in the CHX concentration of the standards (measured by decreases in absorbance at 225 nm) was used to calculate the degree of CHX binding to HA. Debinding experiments using water, HEMA, 100% ethanol, or 0.5 M NaCl were done as described above in chlorhexidine debinding experiments.
2.7 Hoy’s solubility parameters for HEMA, ethanol, water and demineralized dentin
We have used Hoy’s solubility parameters in our previous work to rank the ability of solvents to hydrogen bond (H-bond) by their Hoy’s solubility parameters for hydrogen bonding (δh) [17–21]. For the substrates (DD and MD), the Hoy’s solubility parameters for hydrogen bonding (δh) for HEMA, ethanol and water were plotted against the CHX binding concentration yielded at different CHX-applied concentrations.
2.8 FTIR absorption spectra of dentin powder
To determine whether there was any interaction between CHX and dentin substrates, the FTIR absorption spectra of these moist substrates were obtained before and after exposure to CHX. This was accomplished using a Nicolet 6700 FT-IR spectrophometer (Thermo Scientific Inc., Waltham, MA, USA) with a single-reflection diamond attenuated total reflection (ATR) setup (Smart OMNI-Sampler). The anvil of the back pressure tower of the Smart OMNI-Sampler was pressed against the dentin powder and the slip clutch of the back pressure tower was turned clockwise until an audible click was heard. This ensured that correct pressure was applied to each powder aliquot, independent of the size, shape or mineralization status of the powder particles. Infrared spectra were obtained over the range of 4000–700 cm−1 at 4 cm−1 resolution using 32 scans.
2.9 Statistical analyses
Polynomial regression was used to model the relationship between medium CHX concentration and CHX uptake separately for the three substrates: demineralized dentin (DD), mineralized dentin (MD) and hydroxyapatite (HA) powder. The maximum CHX uptake (Bmax) was estimated based on the fitted curve (or line) and the interpolation method was used to estimate the concentration K½ that would yield an uptake of Bmax/2. K½ is the concentration of CHX required to half-saturate each binding substrate. Since Bmax is the maximum binding capacity on the y axis, Bmax/2 represents half-saturation. Extrapolation of Bmax/2 to the binding curve permits determination of the CHX medium concentration by drawing a vertical line to intersect the x axis (CHX medium concentration). This permits comparisons of the concentration of CHX required to half-saturate the three different binding substrates. Approximate standard errors were then calculated and used to determine an approximate 95% confidence interval for K½ The Wald test was used to compare K½ values across the three media. Two-tailed tests with a significance level of 0.05 were used for all statistical comparisons.
3. Results
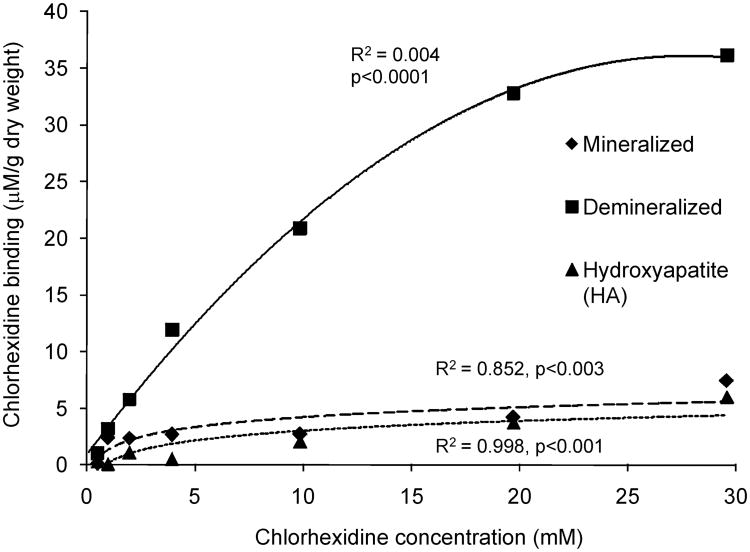
For the substrate DD, a quadratic model provided an excellent fit for the relationship between CHX concentration and CHX uptake, with highly significant omnibus F-tests (p<0.001) and large adjusted R2 values (0.994). For the substrates MD and HA, the quadratic terms were not statistically significant (p=0.476 and 0.561, respectively), so simple linear regression models were used instead. These provided excellent fits to the observed data, with highly significant omnibus F-tests (p=0.003 for MD and p<0.001 for HA) and large R2 values (0.852 for MD, 0.978 for HA). The fitted curves are plotted in Figure 1. Simultaneous tests of linear contrasts comparing the coefficients of the quadratic model across DD yielded significant differences in the quadratic [F(2, 14) = 102.25, p<0.001] and linear [F(2, 14) = 41.92, p<0.001] terms in the model. For the two substrates (MD, HA) for which a linear relationship was more appropriate, there was no significant difference between the slopes of the fitted lines [F(1, 10) = 0.03, p=0.857].
Figure 1.
Chlorhexidine (CHX) binding curve to hydroxyapatite (HA), mineralized dentin (MD) and demineralized dentin (DD) after incubation in CHX solutions.
Table I contains the details of the estimation of K½ for all three substrates. The K½ and maximum binding capacity (Bmax) for HA differed significantly from the K½ and Bmax for DD (p<0.05), but not from MD (p>0.05). DD reached a plateau in CHX binding of 30.1 μmoles/g (Fig. 1, Table I). MD exhibited a Bmax of 6.8 μmoles/g and the K½ for this substrate was 11.2 mmoles CHX/L (Fig.1, Table 1). HA had the lowest Bmax of all of the substrates (5.8 μmoles/g) and a K½ of 14.5 mmoles/L (Fig. 1, Table 1).
Table I.
Estimate of the amount of binding CHX to testing substrates.
| Substrate | Bmax μmoles/g | Bmax/2 μmoles/g | Estimate of K1/2 mmoles/L | Approx. S.E. | 95% CI(K½) |
|---|---|---|---|---|---|
| DD | 30.10b | 15.05 | 7.71a | 0.95 | 5.97 – 9.71 |
| MD | 6.84c | 3.42 | 11.15b | 8.70 | 0 – 28.20 |
| HA | 5.80c | 2.90 | 14.53b | 2.62 | 9.25 – 19.50 |
DD= demineralized dentin; MD= mineralized dentin; HA= hydroxyapatite; Bmax= maximum CHX uptake; Bmax/2= half of the maximum CHX uptake; K1/2=CHX concentration that yields estimated CHX binding of Bmax/2.
Surprisingly, the ability of debinding solvents to displace or extract CHX from the various substrates depended upon the CHX uptake. These results are summarized in Table 2 and show that water could not displace low concentrations of bound CHX as well as it could with higher CHX concentrations. Conversely, HEMA could displace low concentrations of bound (CHX) better than it could with higher CHX uptakes. However, relative to all of the other solvents, HEMA was very poor at displacing CHX. Ethanol was better than HEMA at displacing CHX from dentin powder but was not as good as water. Ethanol’s action was less influenced by CHX concentration compared to the other solvents. Half molar NaCl was used to attempt to displace bound CHX because Singh et al. [16] had found that solution effective to displacing electrostatically bound CHX to cellulose. In the present study, 0.5 M NaCl displaced matrix bound CHX from low CHX but not from high CHX concentrations (Table 2).
Table II.
Debinding of CHX (%) from various substrates by water, HEMA, ethanol and 0.5 M NaCl
| A. | % Debinding from Mineralized Dentin | |||
|---|---|---|---|---|
| CHX (mM) | Water | HEMA | Ethanol | NaCl |
| 0.1 | -- | -- | -- | -- |
| 0.5 | -- | 33.62% | -- | -- |
| 1.0 | 5.90% | 0.96% | 9.35% | 46.76% |
| 2.0 | 39.68% | 0.54% | 18.74% | 74.66% |
| 3.9 | 77.47% | 0.63% | 26.86% | -- |
| 9.9 | 82.00% | 0.42% | 19.38% | 73.46% |
| 19.7 | 89.00% | 0.39% | 47.08% | 28.38% |
| 29.6 | 93.00% | 0.18% | 24.19% | 24.70% |
| B | % Debinding from Demineralized Dentin | |||
| CHX (mM) | Water | HEMA | Ethanol | NaCl |
| 0.1 | -- | -- | -- | -- |
| 0.5 | 86.00% | 0.37% | -- | -- |
| 1.0 | 30.05% | 0.43% | 31.97% | 88.19% |
| 2.0 | 23.77% | 0.30% | 21.93% | 65.75% |
| 3.9 | 11.64% | 0.18% | 53.72% | 36.84% |
| 9.9 | 32.63% | 0.07% | 59.54% | 25.38% |
| 19.7 | 79.01% | 0.06% | 38.20% | 18.83% |
| 29.6 | 95.25% | 0.05% | 34.88% | 13.13% |
| C | % Debinding from Hydroxyapatite (HA) Powder | |||
| CHX (mM) | Water | HEMA | Ethanol | NaCl |
| 0.1 | -- | -- | -- | -- |
| 0.45 | 44% | 9.12% | −5% | 52% |
| 1.0 | -- | 7.20% | 1% | -- |
| 2.0 | 38% | 3.45% | −8% | 25% |
| 3.9 | -- | 7.32% | −9% | -- |
| 9.9 | 99% | 0.94% | −1% | 95% |
| 19.7 | -- | 0.49% | 88% | 79% |
| 29.6 | -- | 0.29% | 81% | 20% |
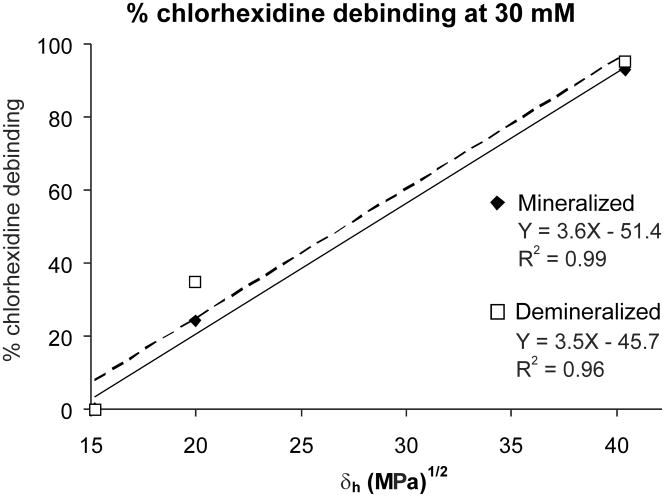
Figure 2 shows the relationship between the percent debinding of CHX from MD exposed to 30 mM CHX versus the Hoy’s δh of HEMA, ethanol and water. The R2 values were 0.99. When these relationships were examined using debinding results obtained at 1 mM CHX solutions, the R2 values were much lower. Note that the higher the δh values of the solvents, the greater the degree of debinding of CHX. Figure 2 shows the same relationship for CHX debinding from DD. The results showed that polar solvents (HEMA, ethanol and water) were not as successful in displacing CHX bound from low medium concentrations as they were displacing CHX bound from high medium concentrations.
Figure 2.
Percent debinding of CHX bound to mineralized human dentin (MD) and demineralized human dentin (DD) at high (29.6 mM) medium concentration, by HEMA, ethanol or water. δh = Hoy’s solubility parameter for hydrogen bonding cohesive forces. The δh values for HEMA, ethanol and water are 15.2, 20.0 and 40.4 (MPa½), respectively [18–20].
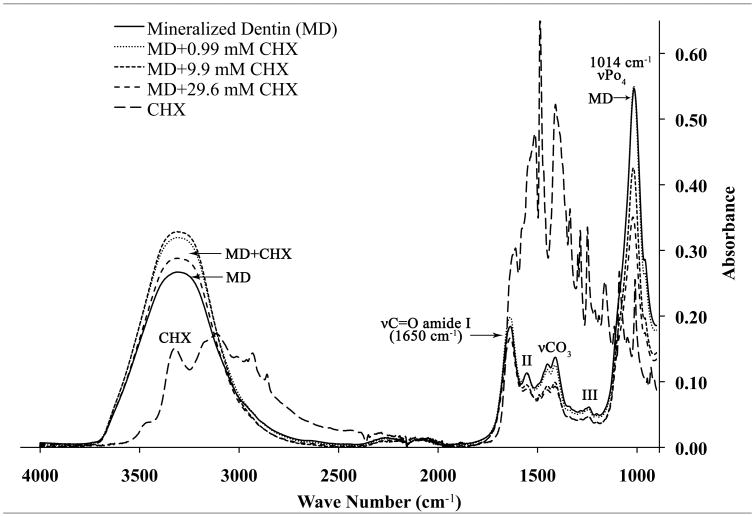
In Fig. 3A, the FTIR absorption spectra of MD from 4000 cm−1 to 900 cm−1 are shown. MD gave a broad symmetrical absorption peak between 3700–2700 cm−1 which represents the v1v3 OH stretching mode of absorbed water superimposed over CHX bands. Sharper amide bands were seen at 1650 cm−1 (amide I, C = O stretch), 1538 cm−1 (amide II, secondary N-H bend and C-N stretch) and a sharp, symmetrical PO4 absorption bands was seen at 1014 cm−1. Note that the highest absorbance at 1014 cm−1 was obtained for dentin alone, before being exposed to CHX. The absorbance peak height at 1014 cm−1 progressively fell when mineralized powder was incubated in 0.99, 9.9 and 29.6 mM CHX. When the mineralized dentin powder was incubated with increasing concentrations of CHX for 30 min, the phosphate peak progressively fell from 0.55 at zero CHX, to 0.50, 0.48 and 0.42 at 9.9 and 29.6 mM CHX. Smaller decreases in absorbance were seen in the amide I and II bands even though they were superimposed on CHX bonds at those same wave numbers. The opposite response (i.e. increased absorbance) was seen in the broad peaks between 3700–2700 cm−1, indicating that the superimposed CHX absorbance peaks produced increasing absorbance with increasing CHX concentration.
Figure 3.
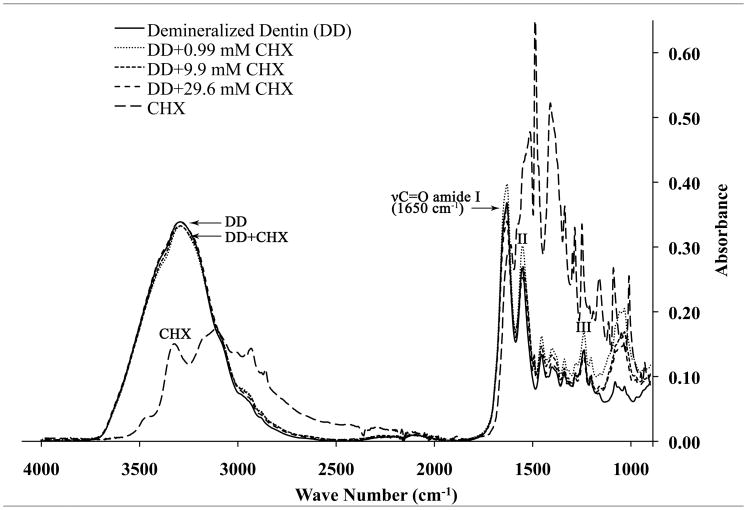
A: FTIR spectrum for mineralized dentin powder (MD) incubated with 0 or 29 mM CHX, and a reference spectrum of CHX by itself. B: FTIR spectrum for demineralized dentin powder alone, demineralized dentin powder incubated with 0 or 29 mM CHX and reference spectrum for CHX alone.
In Fig. 3B, the FTIR absorption spectra of DD from 4000 to 900 cm−1 are shown. Demineralized dentin alone gave a sharper, less symmetrical absorption peak between 3700-2700 cm−1 [21], suggesting that it was due to amide A and B peaks between 3400-2900 cm−1 overlapped with the broad water absorption band (3700–2500 cm−1) representing the v1v3 OH stretching mode of absorbed water [22]. The amide I, II and III bonds in DD gave higher absorbances (Fig. 3B) than were seen in MD (Fig. 3A). When DD powder was incubated with CHX, the absorbances of amide I–III peaks increased due to their superimposition with absorbance bands for CHX alone. Note in Fig. 3B that the phosphate band seen in Fig. 3A at 1014 cm−1 is missing from the FTIR spectrum as were the α and β carbonate peaks at 1453 and 1405 cm−1 [23], indicating that the collagen was completely demineralized. Neither frequency shifts nor the formation of new peaks were identified from both the mineralized and demineralized dentin specimens after treatment with different concentrations of CHX.
4. Discussion
Since the maximum CHX binding to demineralized human dentin was significantly greater than that of mineralized dentin powder, the first null hypothesis must be rejected. Because bound CHX could be displaced from both mineralized and demineralized dentin by water and ethanol and 0.5 M NaCl (but not HEMA), we must partially reject the second null hypothesis. This suggests that the mechanism(s) of CHX uptake in mineralized dentin is different from that of demineralized dentin.
At near neutral pH, CHX has two positive charges [15]. It is likely that these positive charges were electrostatically attracted to the negative charges in trivalent phosphate in the hydroxyapatite crystalline lattice [24] of mineralized dentin powder. Since CHX treatment of mineralized dentin powder attenuated the IR absorbance of the PO4 band at 1014 cm−1 more than it did the amide bands, we speculate that this provides support for binding of CHX to mineralized dentin as being primarily due to the inorganic phases of dentin. Hydroxyapatite (HA) powder binds CHX much about like mineralized human dentin (MD) powder (Fig. 2). Thus, it is likely that CHX binding by MD is due to HA binding. Presumably, CHX binding to the trivalent phosphate groups in HA crystallites only involves the surface mineral. The porosity of mineralized dentin is low because the spaces between collagen fibrils are occupied by mineral crystallites. The porosity of mineralized dentin is largely determined by the volume of water that fills the dentinal tubules and their lateral branches, occupying about 10 wt% of mineralized dentin [25].
In completely demineralized dentin, there are no apatite crystallites available for CHX binding. However, there are negative charges on pendent glutamic and aspartic acid amino acids in collagen and associated noncollagenous proteins. We speculate that such binding by CHX lowers the absorption band heights of amide II–III (Fig. 3B) even though the CHX bands at those wave numbers superimpose with the dentin spectrum and should increase absorbance rather than decrease it. Additionally, demineralized dentin has a much higher water content and porosity compared to mineralized dentin. As the amide A and B bands are masked by the broad water region (3700–2500 cm−1) in hydrated, demineralized dentin specimens, it is not surprising that this broad water peak remained unchanged after binding of CHX to hydrated demineralized dentin [26]. Demineralized dentin is 65% water, while mineralized dentin is only 10–15% water [13].
In demineralized dentin, the dentinal tubules become larger when peritubular dentin is lost, but the major increase in water content is due to the loss of mineral from around and within the collagen fibrils [14]. When CHX solution is applied to demineralized dentin, it can diffuse into the 65% water-filled spaces of that matrix [14]. In doing so, the CHX would bind to exposed collagen fibrils but would also tend to remain trapped within the interfibrillar spaces between the collagen fibrils. The net result is a more than 8-fold increase in CHX uptake by demineralized matrices relative to CHX binding to mineralized dentin matrices.
The issue of the substantivity of CHX bound to mineralized or demineralized matrices [2,3] as an MMP inhibitor is only important during resin-dentin bonding procedures because after bonding, the CHX is sealed in place and covered by adhesive that infiltrates the matrix. Substantivity implies that CHX is on a surface that is exposed to oral fluids. When CHX is used as a bonding primer [10–13], chlorhexidine-treated demineralized dentin becomes sealed off from the surface by subsequently applied adhesive resin. How much CHX remains bound to dentin depends upon how much CHX was originally taken up, how much CHX was extracted or rinsed from the matrix during bonding procedures, and how accessible it is to the solvents such as water, after resin-dentin bonding.
The assumption in this study is that CHX binding and debinding to the endogenous bound MMPs of dentin [6–9] is similar to that of CHX binding and debinding to dentin matrices. Several studies have shown that CHX inhibits MMP-2, 8 and 9 [4], the most abundant MMPs in dentin [6,7]. Even if CHX binding to matrix-bound MMPs differs from CHX binding to dentin matrices, the latter represent a large reservoir of bound CHX that may provide long-term saturation of adjacent dentin MMPs, thereby prolonging the durability of resin dentin bonds by inhibiting the MMPs in the hybrid layer.
The binding of cationic CHX to mineralized and demineralized dentin occurs via different mechanisms. In mineralized dentin, CHX seems to bind electrostatically to phosphate groups in HA hydroxyapatite crystallites. In demineralized dentin, although CHX could bind electrostatically to negative carboxyl groups on collagen, it could also hydrogen bond with carboxyl groups. In both mineralized and demineralized dentin, the affinity constants of CHX binding (K½) are millimolar (Table I) not micromolar, suggesting nonspecific binding.
The fact that neither HEMA nor ethanol extracted much bound CHX, while water did, indicates that adhesive monomers solvated in ethanol should not debind CHX from either mineralized or demineralized dentin. Clearly, after allowing CHX to bind to dentin, water rinses should be avoided. These differences in the debinding effectiveness of solvents correlated well with differences in solubility parameters for hydrogen bonding cohesive energy (δh). The evidence for this is shown in Figs. 2A (mineralized powder) and 2B (demineralized dentin powder), where the amount of CHX extracted in HEMA, ethanol and water are plotted against the δh values for those solvents.
It was expected that electrostatic forces would be the primary attractive force operating between CHX in solution and mineralized dentin, particularly at low CHX concentrations. If that assumption is correct then the sodium ions in 0.5 M NaCl solution should overwhelm the bound cationic CHX causing it to be displaced off anionic trivalent phosphate sites in mineralized dentin. The results of 0.5 M NaCl in debinding CHX from mineralized dentin (Table IIA) at CHX concentrations below 1 mM confirm that expectation. The fact that 0.5 M NaCl was less effective at higher CHX concentrations could mean that a second binding mechanism operates at higher CHX concentrations. That is, CHX binding to dentin may occur by a combination of both electrostatic and hydrogen-bonding forces.
One can rank the ability of solvents to hydrogen bond by their Hoy’s solubility parameters for hydrogen bonding (δh). Chlorhexidine contains strongly basic guanidine groups with a cationic charge distributed over five neighboring secondary amine nitrogen atoms [27] of each guanidine group. These groups can hydrogen bond (H-bond) to electronegative groups such as carboxyl groups in collagen peptides. After CHX H-bonds to collagen, the association remains relatively stable until a solvent with a higher Hoy’s solubility for H-bonding forces is applied. Hydrogen bonding solvents can be used as liquid energy probes [28]. If a solvent has a δh value that is below the strength of H-bonding between CHX and collagen, it will not be able to compete with CHX-collagen carboxyl H-bonding. This seems to be the case for HEMA which has a δh value of 15.2 (MPa)½. HEMA was unable to displace CHX from any dentin matrix or HA (Table IIC). Ethanol, with a δh value of 20 (MPa)½ displaces some CHX from demineralized dentin. This suggests that the strength of the CHX-collagen H-bonding is about 20 (MPa)½. When solvents have δh values close to that of a ligand, the probability of CHX H-bonding to collagen vs. ethanol is about the same. However, water, one of the strongest known H-bonding solvents, has a δh value of 40 (MPa)½ that is twice that of CHX-collagen H-bonding (estimated above to be about 20 MPa½). When water is used as the solvent, water H-bonds with collagen molecules rather than with CHX, thereby causing debinding. This is the reason why water should not be used to rinse CHX-treated dentin.
At higher CHX concentrations, the guanidine groups in CHX could hydrogen bond (H-bond) to carbonyl groups in the peptide bonds of collagen. Thus, it is likely that electrostatic attractions, as well as H-bonding are both contributing to CHX binding to dentin. These figures were obtained using the highest medium CHX (30 mM). When similar analyses were done at 1 mM CHX, the R2 values were much lower (not shown).
It is clear that if one applied 30 mM CHX to acid-etched dentin, that the matrix would become saturated by 7–30 mM CHX depending on the type of dentin powder. This CHX would remain bound to the mineralized matrix while the remainder (23 mM) of the CHX in mineralized dentin would remain within the open tubules and interfibrillar spaces. When dental adhesives are applied, it is logical to speculate that their constituents could displace, extract or solubilize that excess, unbound CHX. The debinding results indicate that HEMA, and ethanol were not very effective in this regard while water is very effective.
Much can be learned about the adsorption of many compounds of dental interest to dentin by measuring their uptake by dentin powder. This model demonstrated that mineralized dentin binds far less chlorhexidine (CHX) than does demineralized dentin. Once bound, CHX is relatively resistant to the displacing effects of HEMA or ethanol but is more easily removed by water. Thus, if CHX-treated acid-etched dentin is not rinsed with water, most of the CHX applied to the matrix will remain bound during the application of a solvated etch-and-rinse adhesive. However during bonding, the solvated comonomers may physically displace some of the debound CHX in the dentinal tubules and interfibrillar spaces. At the very least, they would dilute the CHX concentrations at these sites. That is, the CHX would mix with the solvated comonomers as they diffused into those regions thereby incorporating CHX into solvated comonomers in interfibrillar spaces. As these spaces are only 20 nm wide [29] and are initially filed with water in etch-and-rinse adhesive systems, the excess CHX may quickly saturate the water. As the solvated adhesives interact with the contents of the nanospaces, the mixture may undergo nanophase changes [30]. The rate of release of CHX from these extremely thin resins films may be quite different from that of bulk resin disks that have been studied previously [31–33]. We speculate that the CHX within the polymerized resin matrix may be able to slowly diffuse out [29–31] if the concentration of collagen-bound CHX falls below the CHX concentration in the resin. Thus, the incorporation of unbound CHX into surrounding adhesive resin may provide a reservoir of CHX that can contribute to MMP inhibition over time.
Acknowledgments
This work was supported, in part, by grants R01 DE015306-06 from the National Institute of Dental and Craniofacial Research (NIDCR) to DHP (PI); FAPESP (#07/54618-4), CNPq (#300615/2007-8) to Marcela Carrilho (PI) and Grant #8126472 from the Academy of Finland to Arzu Tevergil-Mutluay (PI). The authors are grateful to Michelle Barnes for secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohamadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal S, Spångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2004;98:488–492. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.deCastro FL, de Andrade MF, Duarte-Junior SL, Vaz LG, Ahid FJ. Effect of chlorhexidine on microtensile bond strength of composite to dentin. J Adhes Dent. 2003;5:129–138. [PubMed] [Google Scholar]
- 4.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diag Lab Immun. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45:757–765. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res. 2007;86:436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 7.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase 8 (MMP-8) is the major collagenase in human dentine. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Santos J, Carrilho M, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, Pashley D, Tay F, Ferraz C, Tjäderhane L. Determination of matrix metalloproteinases in human radicular dentin. J Endod. 2009;35:686–689. doi: 10.1016/j.joen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 10.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 11.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32:107–111. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 12.Brackett MG, Tay FR, Brackett WW, Dib FA, Mai S, Pashley DH. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent. 2009;34:381–385. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 13.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho RM, Yoshiyama M, Brewer PD, Pashley DH. Dimensional changes of demineralized human dentine during preparation for scanning electron microscopy. Arch Oral Biol. 1996;41:379–386. doi: 10.1016/0003-9969(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn RS, Harvey A, Kettle L, Manian AP, Payne JD, Russell SJ. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B. 2007;111:8775–8784. doi: 10.1021/jp070856r. [DOI] [PubMed] [Google Scholar]
- 16.Singh MP, Lumpkin JA, Rosenblatt J. Effect of electrostatic interactions on polylysine release rates from collagen matrices and comparison with model predictions. J Controlled Release. 1995;35:165–179. [Google Scholar]
- 17.Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001;56:273–281. doi: 10.1002/1097-4636(200108)56:2<273::aid-jbm1095>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Eddleston CL, Hindle AR, Agee KA, Carvalho RM, Tay FR, Rueggeberg FA, Pashley DH. Dimensional changes in acid-demineralized dentin matrices following the use of HEMA-water vs. HEMA-alcohol primers. J Biomed Mater Res. 2003;67A:900–907. doi: 10.1002/jbm.a.10151. [DOI] [PubMed] [Google Scholar]
- 19.Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol infiltration and solvent evaporation. J Biomed Mater Res. 2006;79A:349–358. doi: 10.1002/jbm.a.30752. [DOI] [PubMed] [Google Scholar]
- 20.Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Infiltration/evaporation-induced shrinkage of demineralized dentin by solvated model adhesives. J Biomed Mater Res (Appl Biomater) 2006;80B:156–175. doi: 10.1002/jbm.b.30580. [DOI] [PubMed] [Google Scholar]
- 21.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, García-Godoy F. From dry bonding to wet bonding to ethanol-wet bonding: A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 1007;20:7–20. [PubMed] [Google Scholar]
- 22.Payne KJ, Veis A. Fourier transform IR spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers. 1988;27:1749–1760. doi: 10.1002/bip.360271105. [DOI] [PubMed] [Google Scholar]
- 23.Eliades G, Palaghias G, Vongiouklakis G. Effect of acidic conditioners on dentin morphology, molecular composition and collagen confirmation in situ. Dent Mater. 1997;13:24–33. doi: 10.1016/s0109-5641(97)80005-x. [DOI] [PubMed] [Google Scholar]
- 24.LeGeros RZ. Calcium Phosphates in Oral Biology and Medicine. In: Myers Howard M., editor. Monographs in Oral Science. Vol. 15. Karger; Basal: 1991. p. 144. [PubMed] [Google Scholar]
- 25.van der Graaf ER, ten Bosch JJ. The uptake of water by freeze-dried human dentine sections. Arch Oral Biol. 1990;35:731–739. doi: 10.1016/0003-9969(90)90096-s. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann L, Baffa O, Zazell DM. Thermal degradation of dentin collagen evaluated with ESR, infrared and optical spectroscopy. Philos Mag. 2007;87:1033–1042. [Google Scholar]
- 27.Luthra AK, Sandhu SS. Methods and clinical devices for the inhibition or prevention of mammalian cell growth. 6,929,818B2. US Patent. 2005 Aug 16;
- 28.Hansen CM. Hansen Solubility Parameters: A User’s Handbook. CRC Press; Boca Raton: 2000. p. 2. [Google Scholar]
- 29.Carvalho RM, Mendonca JS, Santiago SL, Siliveria RR, Garcia FCP, Tay FR, Pashley DH. Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003;82:597–601. doi: 10.1177/154405910308200805. [DOI] [PubMed] [Google Scholar]
- 30.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nanoheterogeneous dentin adhesive. J Dent Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Anusavice KJ, Zhang N-Z, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–954. doi: 10.1177/154405910608501016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraishi N, Yiu CKY, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine incorporated hydrophobic/hydrophilic resins. Dent Mater. 2008;24:1391–1399. doi: 10.1016/j.dental.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]