Abstract
Antagonists selectively inhibiting activation of the nociceptin/orphanin FQ (N/OFQ) receptor reduce motor symptoms in experimental models of Parkinson’s disease, and genetic deletion of the ppN/OFQ gene offers partial protection of mid-brain dopamine neurons against the neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP increased ppN/OFQ mRNA expression in the substantia nigra. We have evaluated the temporal relationship of dopamine cell loss to increased ppN/OFQ mRNA expression in the substantia nigra after MPTP treatment, and characterized the cellular locations in which increased ppN/OFQ mRNA expression was observed after MPTP treatment. MPTP increased by about 5-fold the number of neurons expressing ppN/OFQ mRNA in the pars reticulata of SN (SNr) by 24 hours after treatment and the elevation remained significant for at least 7 days. This period coincided with the timing of the loss of dopamine neurons from the pars compacta of substantia nigra (SNc) after MPTP. The increased expression of ppN/OFQ mRNA co-localized with a neuronal marker in the SNr. MPTP treatment resulted in a small increase in the numbers of neurons expressing ppN/OFQ in the SNc in mice from one mouse colony but the increase did not reach statistical significance in mice from another colony. No changes in ppN/OFQ-mRNA expression were observed in the VTA, the caudate-putamen, the subthalamic nucleus, or in two other brains areas. These results demonstrate that increased N/OFQ expression in the SNr is closely associated with the MPTP-induced loss of dopamine neurons in the SNc in a widely used animal model of Parkinson’s disease.
Keywords: dopamine, GABA, nociceptin/orphanin FQ, Parkinson’s disease
Nociceptin/orphanin FQ (N/OFQ) is an endogenous seventeen amino acid peptide that activates the NOP receptor (NOPr, also termed the opioid-like receptor -1, ORL1, a member of the opioid peptide receptor family of G-protein coupled receptors; Meunier, 1997, Cox et al., 2009; New & Wong, 2002). The N/OFQ-NOPr system is a potential pharmacological target for the treatment of neuropsychiatric disorders, including anxiety, depression and drug addiction (Lambert, 2008). The N/OFQ precursor peptide, ppNOFQ, and NOPr are both expressed in the substantia nigra (SN) and the ventral tegmental area (VTA) (Norton et al., 2002; Maidment et al., 2002). N/OFQ modulates motor activity and reduces dopamine release in the striatum of rodents and non-human primates (Marti et al., 2004a; Viaro et al., 2008). Blockade of the endogenous N/OFQ-NOPr pathway has been proposed as a potential therapeutic strategy for the symptomatic relief of motor deficits associated with Parkinson’s disease (PD; Marti et al., 2005; 2007). Antagonists acting at the NOPr reduce the motor deficits induced by impaired dopamine (DA) release in the caudate-putamen (CPu) (Marti et al., 2005; Viaro et al., 2008). Haloperidol induces hypokinesia and muscle rigidity resembling the symptoms of PD, and elevates the release of Glu in the SNr; N/OFQ antagonists normalize this elevated release and alleviates these motor symptoms (Marti et al., 2004b). Deletion of the ppN/OFQ gene attenuates MPTP-induced dopaminergic deficits in both the SN and CPu (Marti et al., 2005; Brown et al., 2006). This protective effect was not due to impaired conversion of MPTP to MPP+ (1-methyl-4-phenyl-2,3-dihydropyridinium, the neurotoxic metabolite of MPTP) or reduced uptake of MPP+ into DA neurons (Marti et al., 2005). Moreover, it has also been reported that dopaminergic deficits induced by the DA neurotoxins 6-hydroxydopamine (6-OHDA), MPTP, and MPP+ all cause persistent increases in ppN/OFQ expression within the SN (Norton et al., 2002; Marti et al., 2005; di Benedetto et al., 2009). ppN/OFQ mRNA can be expressed in both neurons (Buzas et al., 1998, Norton et al., 2002) and glial cells (Buzas et al., 2002). Activation of primary astrocytes in culture with hydrogen peroxide or inflammatory mediators stimulates ppN/OFQ mRNA expression in the astrocytes (Buzas et al., 2002). Within the brain, an acute stab-wound injury induces expression of ppN/OFQ in neurons adjacent to the site of injury (Witta et al., 2003). Collectively these data suggest that N/OFQ expression is increased in response to some neuronal insults and may participate in neurotoxic or neurodegenerative processes.
In the present study, we have evaluated the temporal relationship between DA cell loss after MPTP administration and the increase in the numbers of neurons expressing ppN/OFQ mRNA in the SN. We have also characterized the cellular location in which increased ppN/OFQ mRNA expression is observed after MPTP treatment. The lack of availability of antisera against N/OFQ with adequate sensitivity and specificity for immunohistochemical analysis made it impossible to evaluate the effects of MPTP on the expression of the peptide in this study. Our results are consistent with the concept that increased N/OFQ expression in SNr is closely associated with MPTP-induced loss of DA neurons in a widely used animal model of PD, and support earlier studies pointing to the NOP receptor as a potential target for therapeutic intervention in PD.
METHODS
Subjects
Male C57BL/6 mice (Taconic Farms, Germantown, NY) aged 2 – 3 months (20-25 g) were housed in shoebox plastic cages and kept on a 12 hour light-dark cycle. Mice were housed individually after MPTP or vehicle treatment since it was noted in preliminary experiments that there was a higher drug-induced mortality in group-housed MPTP-treated animals. Food and water were provided ad libitum. One study was conducted using wild-type (ppN/OFQ+/+) male mice, approx 20 - 25g, from a colony of the ppN/OFQ knock-out mice described by Koster et al. (1999). Two N/OFQ−/− mice were used as controls for the in situ hybridization studies. All experiments were approved by the USU Animal Care and Use Committee and conducted according to the principles set forth in the “Guide for Care and Use of Laboratory Animals”, Institute of Animal resources, National Research Council, National Academy Press, Washington DC (1996).
Materials
An N/OFQ probe corresponding to nucleotides 225-671 of mouse N/OFQ cDNA (accession number NM_010932) was used for all in situ hybridization procedures; this corresponds to approximately 63% of the open reading frame for ppN/OFQ mRNA (Nothacker et al., 1996). The following antisera were used for immunohistochemical studies: rat anti-tyrosine hydroxylase (TH) antiserum, catalog number 65702, from Calbiochem (San Diego, CA) and mouse anti-NeuN (neuronal nuclear marker; catalog number MAB377) from Chemicon (Temecula, CA). NeuN was detected using the ABC immunohistochemical method with biotinylated horse anti-mouse IgG secondary antibody and ABC complex (Vector Laboratories, Burlingame, CA). The chromagen 3,3′-diaminobenzidine (DAB, Vector Laboratories) was used to detect labeled cells. Fluorescence (Alexa Flour®) conjugated antibodies were obtained from Molecular Probes (Eugene, OR). Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). Hybridized sections were cover-slipped using Cytoseal 60 (Kalamazoo, MI). Emulsion, developer, and fixer were purchased from Eastman Kodak. Unless otherwise noted, other chemicals were purchased from Sigma (St. Louis, MO).
Drug treatments
Mice were injected with either MPTP (20 mg/kg/90 min, i.p.) or saline vehicle (10 ml/kg/90 min, i.p.), for a total of four injections over a period of 270 mins. Groups of mice were euthanized by decapitation under anesthesia (ketamine 85 mg/kg, xylazine 12 mg/kg, i.p.) at 24 hours, 48 hrs, or 7 days after the last MPTP or saline injection. Following decapitation, the brains were removed from the skulls, frozen on solid CO2, and stored at −70°C until sectioned. All brains were sectioned using a cryostat as previously described (Marti et al., 2005). Control mice receiving vehicle instead of MPTP were also analyzed at each time point; since neither the total cell counts nor counts of ppN/OFQ mRNA-expressing cells did not differ across time points for these animals, data from all vehicle treated animals was averaged to determine the control values for each time point.
In situ hybridization and immunohistochemistry
The DNA template was prepared and stored in plasmid. Plasmid was restriction-digested (EcoR1, Invitrogen, Carslbad, Ca) and the DNA template was gel purified using MinElute® Gel Extraction Kit (Qiagen, Valencia, Ca.) then PCR amplified and purified a second time. 35S-UTP–labeled riboprobes were generated from the template using Maxiscript kit (Ambion, Austin, TX). Riboprobes were synthesized using T7 (antisense) RNA polymerase. After incubation at 37°C for 1 h, probes were treated with DNase I, precipitated and resuspended. Sections were fixed in 4 % formaldehyde followed by two 5 minute washes in PBS. They were then placed in 0.25 % acetic anhydride/triethanolamine (1.5%) for 10 min and rinsed in 2 × SSC twice for 5 minutes each. Sections were dehydrated in ascending ethanol concentrations (70, 80, 95, and 100 %) and then air-dried. Antisense-labeled probes (2.04 × 106 dpm / 100 μl) were hybridized to tissue sections overnight at 55°C in hybridization buffer (20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 300 mM NaCl, 50% formamide, 10% dextran sulfate, 1× Denhardt’s). Slide-mounted sections including SN (from −2.7 to −3.5 mm caudal to bregma), STN (from −1.34 to −2.30 mm caudal to bregma), or CPu (from 1.18 to −0.34 mm caudal to bregma) were rinsed in 4× SSC at room temperature to remove coverslips and then washed four times for 5 min each in 4× SSC containing 1 mM DTT. Free probe was removed using 20 mg/mL RNase (Sigma, St. Louis, MO) in buffer (0.5 M NaCl, 0.01 M Tris-HCl, 0.25 mM EDTA) at 37°C for 30 min. After rinsing twice in 2 × SSC/1mM DTT, then once in 1 × SSC/1mM DTT and once in 0.5 × SSC/1mM DTT (all rinses for 5 mins), sections were washed twice for 30 min in 0.1× SSC, 1 mM DTT at 65°C. The slides were then cooled to room temperature and placed in 0.02 M PBS.
Avidin–biotin (ABC) immunohistochemistry was used to visualize NeuN positive (NeuN+) cells. Sections were pre-incubated in carrier solution (3% normal goat serum (NGS) containing 0.3% Triton X-100) in 0.02 M PBS for 30 min, rinsed, and incubated overnight at room temperature with NeuN (1:100) primary antibody in carrier solution. Biotinylated horse anti-mouse IgG secondary antibody and ABC complex was prepared according to manufacturer’s instructions and applied to the slides for 1 hour each with a PBS wash in between. DAB was prepared according to manufacturer’s instructions and applied to sections to visualize the labeled neurons. Sections were then dehydrated in ethanol, and coated with Kodak NTB emulsion. After 18 days of exposure at 4°C, the slides were developed in Dektol and fixed. Slides were then cover-slipped using Cytoseal 60. Sections from control and MPTP-treated animals were always exposed and processed together.
Immunohistochemical staining for TH was used to visualize the dopaminergic neurons as described by Brown et al. (2006). To identify all neurons, the neuronal marker NeuN was used. Slide-mounted serial coronal sections (20 μm) of the midbrain containing the central SNr (from −2.7 to −3.5 mm caudal to bregma; Paxinos and Franklin, 2001) were collected and stored at −70°C. Slides containing sections were fixed in 4 % paraformaldehyde for 10 minutes and then blocked in 3.0 % NGS containing 0.3 % Triton X-100 in 0.02 M PBS (pH 7.4) for 30 minutes, and finally incubated overnight at room temperature with anti-TH (1:1000) and anti-NeuN (1:100) antisera. After rinsing (2 × 10 min) in PBS (0.02 M, pH 7.4), sections were incubated for 3 hours in Alexa Flour® 555 labeled goat anti-rabbit IgG (1:200, for TH) and/or Alexa Flour® 488 labeled goat anti-mouse IgG1 (1:200 for NeuN) in 3 % NGS. Sections were rinsed in PBS (2× 10 min.), dried, and cover slipped with Vectashield Hard Set mounting medium containing DAPI (1.5 μg/ml: Vector Labs, Servion, Switzerland) to permit visualization of nuclei. Sections from control and MPTP-treated animals were processed together.
Image capturing for combined immunohistochemistry/in situ hybridization
Sections were visualized and scanned using a Fluorescent Leica DM-RXA Microscope equipped with a motorized stage. Specific brain atlas areas (at AP levels −2.98, −3.08, −3.16), according to Paxinos and Franklin (2001), were identified, scanned and subsequently evaluated as described by Brown et al., (2006). Briefly, using bright field microscopy and under 2.5× objective, an area which included the SNc and SNr was outlined. This area was scanned at 40× magnification (requiring 90-100 frames). A montage was then created using these frames so the entire area could be viewed. Using these montages, the SNc and SNr were then outlined. Background density measurements (14 in the SNr and 10 in the SNc) were then taken by placing an 8 μm diameter circle over NeuN positive cells where the silver grain density was at background levels. The 8 μm circle was then placed sequentially over NeuN- labeled neurons within the outlined region of the SNr or SNc where the density of overlying silver grains was higher than the background level and a density measurement was then taken for each NeuN labeled neuron or neuron cluster. Neurons (or clusters) were counted as positive for N/OFQ expression if the silver grain density in the 8 μm circle centered on the neuron was 2.5 or more standard deviations above the mean background silver grain density for each tissue section. The location of each positive neuron cluster in each tissue section was recorded.
A similar approach was taken for analysis of ppN/OFQ mRNA expression in the ventral tegmental area (VTA). Analyses were conducted at the same AP levels as for the SN. Because of the lack of distinct boundaries for the VTA, a region of interest in the central VTA was defined by a rectangle placed over the central VTA, medial to the SNc, lateral to the fasciculus retroflexus and dorsal to the medial mammillary nucleus, at each AP level analyzed. For analyses of ppN/OFQ mRNA+/NeuN+ cells, the rectangles measured 680 × 484 μm (height × width) at AP level −2.98, 672 × 307 μm at AP −3.08, and 561 × 322 μm at AP −3.16. Fifteen background density measurements were taken in each region of interest of the VTA to estimate background densities. Neurons (or clusters) were counted as positive for N/OFQ expression if the silver grain density in the 8 μm circle centered on the neuron was 2.5 or more standard deviations above the mean background silver grain density for each tissue section. Coronal sections at the level of the caudate-putamen (CPu), globus pallidus, and the sub-thalamic nucleus were also examined for ppN/OFQ expression; no significant clusters of silver grains were observed in these structures. To evaluate possible effects of MPTP treatment on ppN/OFQ mRNA expression in brain regions not directly associated with the mid-brain dopaminergic nuclei, the numbers of neurons expressing ppN/OFQ mRNA in two other brain regions (based on Neal et al., 1999) were also quantified unilaterally at one AP level for each structure. The nucleus of Darkschewitz (DK) close to the periaqueductal gray region contains many neurons expressing ppN/OFQ mRNA (Neal et al., 1999). These were counted as described above at AP level −3.08 in a region of interest defined by the outline of the nucleus according to Paxinos & Franklin (2001) in sections of brains from mice treated with MPTP or vehicle 7 days prior to analysis. Similar measurements were made of ppN/OFQ mRNA expression in cells of the molecular, granular layer and polymorphic layers of the dentate gyrus (DG) of hippocampus at AP level −2.70, outlined as defined by Paxinos & Franklin (2001). Background measurements (7 in the DK and 15 in the DG) were taken across the regions of interest for the analyses. Neurons (or neuron clusters) were counted as positive for N/OFQ expression if the silver grain density in the 8 μm circle centered over the cells of interest was 2.5 or more standard deviations above the mean background silver grain density for each tissue section.
Image capturing for combined TH and NeuN immunohistochemistry
Images were captured and the number of cells showing both TH and NeuN immunoreactivity (TH+/NeuN+) in the SNc and the VTA were quantified as described previously (Marti et al., 2005; Brown et al., 2006). Briefly, using a Leica (Nussloch, Germany) DM-RXA fluorescence microscope, every fifth 20 μm section through SNc and VTA from −2.85 to −3.88 mm to bregma (Paxinos and Franklin, 2001) on one side of the brain was viewed with and scanned at 20× magnification using appropriate selective fluorescent filters to yield 36-46 frames. Montages were created for each scanned section using these frames permitting the entire cross section of the SNc to be viewed at 100 μm intervals from the anterior to posterior extent of the nucleus. The boundaries of the right SNc (as defined by Paxinos and Franklin, 2001) were outlined using surrounding anatomical structures. A similar outline was defined for the SNr in each section. There was no significant difference in the total areas analyzed between treatments. Counts of TH+/NeuN+ cells were made at 3 AP levels, −2.98, −3.08 and −3.16 within the defined outlines of the SNc. For the VTA, regions of interest were defined by placing a rectangle over the central VTA measuring 279 − 226 μm (height × width) at AP −2.98; 469 − 185 μm at AP −3.08; and 357 − 243 μm at AP −3.16. All TH+/NeuN+neurons were counted at 1520 × magnification. The diameters of SNc and VTA neurons are substantially smaller than the tissue section thickness (20 μm), ensuring that any counted cell (with DAPI-stained nucleus fully visible) was contained within the section under analysis, avoiding cell counting bias. In this study, TH+/NeuN+ cell counts in the SNc or VTA were averaged from three sections through the SNc at the same AP levels that were used in the in situ hybridization analyses of ppN/OFQ mRNA expression. The same sections were also analyzed to estimate the total number of NeuN+/DAPI+ cells within the outlines defined for the SNc.
Data analysis
Counts of “clusters” of silver grains reflecting the expression of ppN/OFQ mRNA within the defined regions of interest (SNc, SNr, VTA) were estimated on one side of the brain at three AP levels through the SN in saline and MPTP treated mice at each time point after MPTP of vehicle treatment. Cell counts for TH+ and NeuN+ cells (both TH+/NeuN+ or NeuN+/DAPI+) within the defined area of the SNc were also determined for three AP levels from saline and MPTP treated mice at each time point. Data were analyzed separately for each AP level; additionally, mean values averaged across the 3 AP levels for each mouse were calculated. Counts of cells expressing ppN/OFQ mRNA in the DK and the DG were made on one side of the brain at one AP level. Effects of treatments on the numbers of cells/clusters expressing ppN/OFQ mRNA were compared by a 2 (treatment groups) × 3 (time points) factorial ANOVA and Bonferroni post-hoc comparisons. Mean numbers of TH+/NeuN+or NeuN+/DAPI+ neurons in the SNc at 3 AP levels were compared by a one-way ANOVA with Newman-Keuls test for significances. All other comparisons were made by t-test. An alpha level of < 0.05 denoted a significant difference. Mean values ± standard errors of the means are reported in the text.
RESULTS
MPTP treatment reduces the number of neurons expressing TH in the SNc and VTA
Confirming previous results (Marti et al., 2005; Brown et al., 2006), the MPTP treatment used in this study resulted in a loss of TH-expressing neurons in the SNc. In C57BL/6 mice, the number of TH+/NeuN+ cells was significantly reduced (P<0.05) by about 40 to 50% at 24 and 48 hrs after MPTP treatment, and by almost 80% at 7 days (Table 1). MPTP treatment produced a similar reduction in TH+/NeuN+ cells in the SNc of wild-type mice (ppN/OFQ+/+) of the ppN/OFQ knock-out colony (Koster et al., 1999) used in our previous study of the effects of N/OFQ deletion on the neurotoxic actions of MPTP (Marti et al., 2005). To confirm that the reduction of TH+-cells at 7 days was due to a loss of neurons and not simply a temporary reduction in TH expression in surviving neurons, NeuN+ cells were also counted at three AP levels through the SNc of CV57BL/6 mice. The total number of NeuN+ cells declined significantly at 24 hr, 48 hr and 7 days after MPTP treatment (55 ± 11% of control at 24 hrs; 63 ± 14% at 48 hrs; 42 ± 3% at 7 days; n was between 5 and 8 for each time point; P< 0.05 for each time point by ANOVA). The effects of MPTP treatment on the numbers of TH+/NeuN+ cells in the VTA were also evaluated in the same mice. MPTP treatment resulted in a 51% reduction in the number of TH+/NeuN+ cells in the central region of the VTA at 7 days after MPTP treatment; (Table 1).
Table 1.
Effects of MPTP Treatment on Number of TH+-Neurons in SNc and VTA of C57BL/6 mice.
| Treatment | TH+/NeuN+ Cells | ||
|---|---|---|---|
| In SNc Number % of Control |
In VTA Number % of Control |
||
| Experiment 1: | |||
| Control | 89 ± 12 (8) | - | not determined |
| MPTP, 24 hrs | 46 ± 2 (6) 52% | not determined | |
| MPTP, 48 hr | 52 ± 11 (5) 58% | not determined | |
| Experiment 2: | |||
| Control | 114 ± 3 (6) | - | 27 ± 3 (6) |
| MPTP, 7 days | 25 ± 1 (8) 22% | 13 ± 2 (8) 49% | |
This table reports the number of TH+ and NeuN+ cells within the defined boundaries of the SNc or VTA (based on Paxinos & Franklin, 2001) of C57BL/6 mice, in 20 μm sections cut through the SNc at AP levels −2.98, −3.08, −3.16 (levels corresponding to the levels at which ppN/OFQ mRNA expression was evaluated). The data from two experiments with different MPTP treatment times are reported separately. Data are presented as the mean number of TH+ cells in the SNc or VTA averaged across the three AP levels (mean ± s.e.m), with the number of animals indicated in parentheses. The reduction in TH+/NeuN+ cell number in the SNc after MPTP treatment at 24 and 48 hrs was significant by analysis of variance [F(2,18) = 4.59, p< 0.0265]; the reductions in TH+/NeuN+ cell number after MPTP treatment at 7 days were also significant (t-test; p<0.0001 for SNc and VTA).
MPTP treatment increases the numbers of neurons expressing ppN/OFQ mRNA in the SNr but is less effective in increasing the number of neurons expressing ppN/OFQ mRNA in the SNc
In situ hybridization for ppN/OFQ mRNA was used to identify cells in the SN expressing N/OFQ. Neurons were identified by immunohistochemistry for the selective neuronal marker NeuN and in some studies TH was visualized by immunohistochemistry. In both MPTP and vehicle treated mice, cells expressing ppN/OFQ mRNA were identified by dense aggregates or “clusters” of silver grains that lay over cells expressing NeuN (Fig 1A-H). The density of the ppN/OFQ mRNA silver grain clusters in the C57BL/6 mice, and the close packing of some NeuN-labeled neurons in both the SNr and SNc made it impossible to tell in all cases if each ppN/OFQ silver grain cluster reflected the presence of a single cell or groups of two or even three ppN/OFQ-expressing cells. For this reason, we refer in this manuscript to “clusters” of ppN/OFQ mRNA expressing neurons, although in most cases a “cluster” probably represents a single N/OFQ+ neuron surrounded by non-N/OFQ expressing cells. No clusters of silver grains were observed in brain sections from N/OFQ−/− mice processed in parallel with the C57BL/6 mice and wild-type N/OFQ+/+ mice used in this study. All ppN/OFQ mRNA clusters co-localized with NeuN, but the clusters did not co-localize with TH-expressing DA neurons in the SNc (Fig 1G,H).
Fig. 1.
In situ hybridization autoradiography for ppN/OFQ mRNA in selected brain regions in representative control and MPTP-treated C57BL/6 mice.
Panels A, C, E, and G show results from vehicle (saline) treated control animals; panels B, D, F and H show results 7 days after MPTP or vehicle treatments (except for panels E and F which show results at 48 hrs after treatment) for the following brain regions: panels A and B, SNr; panels C and D, CPu and lateral septum (LS); panels E and F, STN and zona incerta ventral (ZIV) and panels G and H, SNc and SNr, showing TH immunofluorescence . In panels A through F, brown staining indicates NeuN+-neurons; the black dots show silver grains reflecting ppN/OFQ mRNA expression; clusters of silver grains lying over NeuN+ cells are indicated by black arrows. The dashed lines indicate the approximate boundaries between the CPu and LS in panel C and D, and between STN and ZIV in panels E and F. Panels G and H show silver grains reflecting ppN/OFQ mRNA as blue dots (white arrows); green fluorescence indicates the location of TH expression; the dashed lines in panels G and H indicate the approximate boundaries between the SNc and the SNr. Note the loss of TH+ cells after MPTP treatment, the absence of ppN/OFQ mRNA in the SNr of control mice and the increase in ppN/OFQ expression in SNr of an MPTP-treated mouse. Similar results were obtained in equivalent sections from at least 4 control or MPTP treated mice. Scale bar in panel A represents 50 μm; all images are at same magnification.
MPTP treatment did not significantly affect the numbers of ppN/OFQ mRNA cell clusters observed in SNc of C57BL/6 mice at three AP levels at any tested time point after MPTP treatment [24 hrs, 112 ± 17% of control (mean ± sem); 48 hrs, 88 ± 11%; 7 days, 92 ± 12%; the control mean was 40 ± 11 ppN/OFQ clusters/mm2 in SNc; values summed across the 3 tested AP levels within SNc, n = 4 to 6 per group; treatment effects not significant, p > 0.05]. The absence of effect of MPTP treatment on the number of ppN/OFQ mRNA-expressing cells in SNc was surprising in view of earlier studies reporting that 6-hydroxydopamine treatment increased ppN/OFQ mRNA levels in both the SNr and SNc of rats (Marti et al., 2005). We therefore also evaluated the effects of the MPTP treatment on SNc expression of N/OFQ in mice from another colony. In wild-type mice derived from the ppN/OFQ knock-out mouse line (Koster et al., 1999) used in our previous studies of MPTP effects on nigral function (Marti et al., 2005), MPTP treatment increased the number of ppN/OFQ clusters in the SNc by about 2.5-fold at 48 hrs after treatment (control 26 ± 2.6 clusters/mm2; MPTP, 64 ± 4.9 clusters/mm2; p < 0.01, n = 5). In both mouse colonies, ppN/OFQ mRNA-expressing clusters were found throughout the SNc at all three AP levels examined.
In vehicle-treated mice the numbers of ppN/OFQ clusters in the SNr was markedly lower than in the SNc. However, in contrast to the results obtained in the SNc, the number of neurons expressing ppN/OFQ mRNA in neurons of the SNr was consistently elevated following MPTP treatment in both mouse colonies. MPTP treatment caused an approximately 5 to 7-fold increase in the number of ppN/OFQ-expressing clusters in SNr in C57BL/6 mice (Fig 1A,B,G,H, & Fig 2). An increase in ppN/OFQ clusters was already apparent at 24 hrs after MPTP treatment; a further increase in ppN/OFQ mRNA clusters was observed at 48 hrs and 7 days after MPTP treatment (Fig 2). A 4-fold increase in the number of ppN/OFQ mRNA clusters at three AP levels in the SNr was also observed in wild-type mice (N/OFQ+/+) of the ppN/OFQ knock-out colony (Koster et al., 1999) at 48 hr after MPTP treatment (control 2.6 ± 0.4 clusters/mm2; MPTP, 48 hrs, 10.4 ± 1.2; p < 0.01; n = 5). No ppN/OFQ expression was observed in structures lateral and ventral to the SNr.
Fig. 2.
Effects of MPTP treatment on the number of neurons expressing ppN/OFQ mRNA at three time points after MPTP or vehicle treatments. The numbers (means ± sem) of ppN/OFQ clusters in the SNr region (one side) summed across sections at 3 AP levels through the rostral to caudal axis of the SN in each mouse are plotted against the time after MPTP treatment (n = 4 to 8 mice per treatment and time point). Treatment effects were significant [F(1,27) = 29.94, p < 0.0001]; MPTP effects differed significantly from vehicle at 48 hrs (p<0.05, Bonferoni post-test) and 7 days (p<0.001).
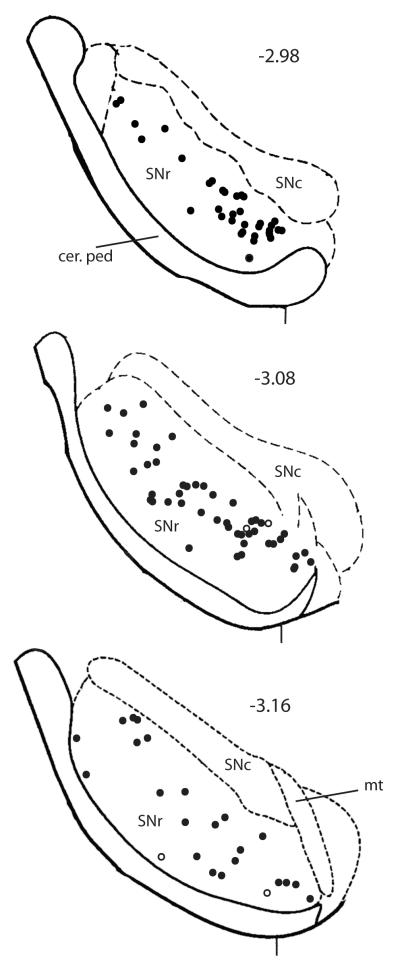
Fig. 3 shows a montage of the locations within the SNr of the ppN/OFQ clusters in C57BL/6 mice that had received MPTP treatment 7 days earlier. The ppN/OFQ clusters in SNr were distributed relatively evenly throughout the SNr after MPTP treatment. TH+ neuronal processes were observed coursing laterally from the SNc into the SNr when sections were stained for TH, but there was no indication that the numbers of ppN/OFQ expressing neurons were any greater in these regions of SNr than more distally (Fig 1G, H). The ppN/OFQ mRNA clusters did not co-localize with TH in the very few TH+-expressing cells in the SNr.
Fig. 3.
Location of ppN/OFQ mRNA-expressing NeuN+-neurons at three AP levels of the SNr at 7 days after MPTP treatment.
The approximate locations of ppN/OFQ expressing neurons at 7 days after MPTP treatment at AP levels −2.98 (upper panel), −3.08 (middle panel) and −3.16 (lower panel) summed for 6 mice/treatment; black circles, MPTP treatment; open circles, vehicle treated animals. SNc, SN pars compacta; SNr, SN pars reticulata; cer ped, cerebral peduncle; mt, medial terminal nucleus of the accessory optic tract. The vertical bar at the bottom of each section indicates a position 1 mm lateral to the mid-line for each section. The regional outlines at each level are based on the drawing of Paxinos and Franklin, 2001. The ppN/OFQ mRNA-expressing neurons are distributed relatively evenly across the entire SNr after MPTP treatment; few SNr neurons express ppN/OFQ mRNA in vehicle treated mice.
ppN/OFQ mRNA expression in the VTA was not affected by MPTP treatment
As reported by Norton et al. (2002), N/OFQ mRNA expression is also observed in the VTA. MPTP treatment resulted in a 51% reduction of TH expressing cells in the VTA (Table 1). This depletion of TH+ cells was less marked than the MPTP-induced reduction in the numbers of TH+ neurons in the SNc of the same mice (78%). In contrast to the effects of MPTP on ppN/OFQ expression in the SNc, there was no change in the numbers of clusters of ppN/OFQ-mRNA expressing neurons in the VTA of MPTP-treated mice (Table 2).
Table 2.
Lack of effect of MPTP treatment on ppN/OFQ mRNA-expressing neurons in the VTA, nucleus of Darkschewitsch (DK) and in dentate gyrus (DG) of hippocampus.
| ppN/OFQ+ cells/mm2 | |||
|---|---|---|---|
| Treatment | VTA | DK | DG |
| Controls (6) | 7.0 ± 1.7 | 220 ± 50 | 34.8 ± 3.1 |
| MPTP, 7 days (8) | 6.9 ± 2.6 | 150 ± 34 | 28.9 ± 4.00 |
| P value | P = 0.949 | P = 0.253 | P = 0.294 |
The table reports the numbers of ppN/OFQ mRNA-expressing neuronal cells or cell clusters/mm2 in three brain regions, the VTA, the DK, and the DG of hippocampus (see Methods for details). MPTP treatment 7 days previously did not significantly alter the numbers of cells expressing ppN/OFQ mRNA in any of these brain regions (analysis by t-test). The numbers in parentheses after each treatment indicate the numbers of animals receiving this treatment.
Neurons projecting to the SNr express little ppN/OFQ mRNA in both control and MPTP-treated mice
The SNr is heavily innervated by axons of the striato-nigral pathway originating from a major subset of GABA neurons in the CPu and from the globus pallidus (Alexander & Crutcher, 1990; Bolam et al., 2000). There is also a major glutamatergic pathway from the subthalamic nucleus (STN) to SNr (Bolam et al., 2000; Blandini et al., 2000). Sections through the CPu (at 3 AP levels), or through the STN, of control and MPTP-treated mice were subjected to in situ hybridization autoradiography for ppN/OFQ mRNA and immunohistochemistry for NeuN to identify neuronal cell bodies at 48 hrs after MPTP treatment. Our results confirmed previous studies in rats indicating that there is very little ppN/OFQ mRNA expression in the CPu (Neal et al., 1999); we also observed very few cells expressing ppN/OFQ mRNA silver grain clusters in the globus pallidus, Additionally, we now demonstrate that neurons in the STN do not express ppN/OFQ mRNA. Furthermore, there was no induction of ppN/OFQ mRNA expression in either CPu or STN after MPTP treatment. Representative autoradiograph images of sections through the CPu (Fig 1C,D) and STN (Fig 1E,F), demonstrate the absence of any ppN/OFQ mRNA clusters in either CPu or STN in either control or MPTP-treated animals. Silver grain clusters indicating cells expressing ppN/OFQ mRNA were clearly apparent overlying NeuN positive neurons in several structures adjacent to the CPu (e.g., lateral septum, Fig 1C,D) or the STN (e.g., zona incerta ventral, Fig 1E,F), in both control and MPTP-treated mice.
MPTP treatment does not affect ppN/OFQ mRNA expression in neurons in the nucleus of Darkschewitsch or in the dentate gyrus of hippocampus
To determine if MPTP treatment affected ppN/OFQ mRNA expression in brain regions that do not have significant dopaminergic innervation, counts of ppN/OFQ mRNA expressing neurons were also made in two structures contained within the sections used for analyses of expression in SN and CPu, the nucleus of Darkschewitsch (DK), and the dentate gyrus (DG) of hippocampus (see Methods). Many neurons in the DK express ppN/OFQ mRNA in untreated mice, while the ppN/OFQ mRNA-expressing neurons in the DG, although readily detectable particularly in the granule cell layer, are less densely expressed than in DK. In neither region did MPTP treatment 7 days earlier significantly affect the number of ppN/OFQ mRNA-expressing neurons (P > 0.05; Table 2).
DISCUSSION
The major finding of this study is that MPTP treatment of mice causes a significant increase in the number of neurons in the SNr expressing ppN/OFQ mRNA. The time course of increase in ppN/OFQ mRNA expressing neurons in SNr coincided with the time course for loss of DA neurons in the SNc after MPTP treatment. A significant reduction in DA neurons is apparent by 24 hours after MPTP treatment, and the reduction continues to be observed at 48 hours and at 7 days. In untreated mice, few SNr neurons express ppN/OFQ mRNA at a detectable level; the number of ppN/OFQ mRNA-expressing cells measurably increased at 24 hrs after MPTP treatment, increased further at 48 hrs and remained elevated approximately 5-fold at 7 days after treatment.
The absence of effect of MPTP on N/OFQ neurons in the SNc of C57BL/6 mice was unexpected. Although there are no previous studies reporting the numbers of neurons expressing ppN/OFQ in discrete regions of the SN of any species after drug treatments that damage DA neurons of the SNc, it has previously been reported that an increase in ppN/OFQ mRNA levels in both the SNc and SNr of rats was observed after 6-hydroxydopamine-induced loss of SNc DA neurons (Norton et al., 2002; Marti et al., 2005). However, in contrast to our results with C57BL/6 mice, we found that MPTP treatment induced a smaller approximately 2.5-fold increase in the number of neurons expressing ppN/OFQ mRNA in the SNc of wild-type mice (ppN/OFQ+/+) of a ppN/OFQ knock-out mouse colony developed on a 129/Ola X C57BL/6 background (Koster et al., 1999). The difference between mouse colonies is not obviously related to differences in the loss of SNc TH+-neurons caused by the MPTP treatment because both groups of mice showed equivalent sensitivity to this action of MPTP, and the two colonies have a similar C57BL/6 genetic background. However, the ppN/OFQ knock-out mouse colony of Koster et al. (1999) has been selectively inbred over a period of more than 10 years; some genetic variation from the out-bred C57BL/6 mice must be expected. There are also differences between colonies in diet, housing, handling, and transportation history.
Di Benedetto et al. (2009) reported an approximately 50% increase in overall ppN/OFQ mRNA expression in the SN of Sprague-Dawley rats, measured by RT-PCR analysis of extracts of the micro-dissected SN region as a whole after intra-cerebroventricular administration of the MPTP toxic metabolite, MPP+. This result is quantitatively consistent with the data reported in the current study in mice when considering the combined number of ppN/OFQ expressing neurons in the SNc and SNr of either C57BL/6 or ppN/OFQ mice after MPTP treatment, assuming that the number of N/OFQ-expressing neurons is approximately proportional to ppN/OFQ mRNA content.
Our results confirm previous studies showing that MPTP-treatment in mice reduces the number of DA neurons in the VTA, although the MPTP-induced reduction is less marked that in the SNc (Jackson-Lewis et al., 1995; German et al., 1996, Ahmad et al., 2009). Similar relative reductions in the number of TH+-neurons of the VTA and SNc were observed after MPTP-treatment in the present study. However, despite the significant loss of DA neurons in the VTA, we found that MPTP treatment did not affect the number of neurons expressing ppN/OFQ mRNA in the VTA. If the elevation in N/OFQ expression in the SNr neurons is caused by MPTP-induced injury to closely adjacent DA neurons, the absence of an MPTP-induced increase in ppN/OFQ mRNA expression in the VTA might be related to the lower density of DA neurons in the VTA than in the SNc; there are simply fewer damaged DA neurons in the vicinity of the VTA ppN/OFQ mRNA-expressing neurons. However, our results show that there is less elevation of ppN/OFQ expression in the SNc than in the SNr following MPTP treatment, yet MPTP induces DA neuron death in the SNc while the predominant elevation of ppN/OFQ mRNA expression occurred in the SNr, with no differences in ppN/OFQ mRNA expression in neurons close to or further from the damaged DA neurons of the SNc. These observations point to a role for the unique circuitry involving the SNc and SNr that is not directly replicated in the VTA. Levels of ppN/OFQ mRNA expression in the caudate-putamen, globus pallidus, and sub-thalamic nucleus were low and not affected by MPTP treatment. Thus if endogenous ppN/OFQ gene products play a role in mediating insults to the nigral DA neurons, as suggested by the partial protective effects of deletion of the ppN/OFQ gene (Morari et al., 2005), this action is likely to be mediated through the SNr N/OFQ-expressing neurons, not from N/OFQ neurons in known projection pathways to the SN. MPTP treatment also did not alter ppN/OFQ mRNA expression in other brain regions where N/OFQ is expressed, including the nucleus of Darkschewitsch and the dentate gyrus of hippocampus. MPTP is not a universal stimulator of ppN/OFQ mRNA expression.
The neurochemical phenotype of the SNr cells in which increased ppN/OFQ mRNA expression is observed after MPTP administration is still not totally resolved. In neither region of SN was ppN/OFQ mRNA expression co-localized with TH-expression, confirming the observation by Norton et al. (2002) that ppN/OFQ mRNA is not expressed by the DA neurons of the SNc. However, the silver grain clusters were always observed over NeuN+ cells in both regions of the SN (and at other sites throughout the brain), indicating an exclusively neuronal location for ppN/OFQ mRNA, even after MPTP treatment. Previous studies have shown that inflammatory mediators and oxidative stress can both increase ppN/OFQ mRNA expression in glial cells in culture (Rosenberger et al., 2001; Buzas et al., 2002) but no evidence of non-neuronal N/OFQ expression was obtained in our studies of mid-brain N/OFQ expression after MPTP treatment. The reasons for the absence of observable expression of ppN/OFQ mRNA in non-neuronal cells of the SN in either MPTP-treated or control mice are not known, but may relate to differences in assay sensitivity in the in vivo and cell culture studies. The non-DA neurons of SNc are predominantly GABAergic; thus ppN/OFQ mRNA expression in SNc probably occurs in a subset of these GABA neurons.
The GABA neurons of the SNr are organized in a laminar onion-like structure in which neurons receive GABAergic inputs from topographically organized striatal sectors that in turn receive topographically organized cortical afferents (Mailly et al., 2001; 2003). However, neurons in the SNr expressing ppN/OFQ mRNA after MPTP treatment were uniformly distributed; they were not limited to discrete layers within the SNr. This suggests that whatever function elevated ppN/OFQ mRNA expression may have in the SNr, it is mediated generally across all SNr neurons. The complex axon-collateral network in the SNr offers an anatomical basis for a widespread influence of elevated N/OFQ release from a relatively small number of SNr neurons.
NOPr are expressed widely in the central nervous system where their activation leads to neuronal hyperpolarization (New & Wong, 2002). About 50% of SNc DA neurons express the mRNA for NOPr (Norton et al., 2002); application of N/OFQ to the SNr reduces local DA release and this effect can be reversed by NOPr antagonists (Marti et al., 2004). However, it is not obvious that loss of DA neuron hyperpolarization by locally released N/OFQ could account for the partial neuroprotection against MPTP toxicity provided by deletion of the ppN/OFQ gene.
Increased activation of the STN glutamatergic innervation of SNr (Alexander & Crutcher, 1990; Bolam et al., 2000) is a feature both of experimental Parkinsonism induced by 6-hyroxydopamine or MPTP, and of clinical Parkinson’s disease (Bergman et al., 1990; Blandini et al., 2000; Alvarez et al., 2005). The NOPr antagonists J113397 or Trap-101 increase GABA release in the SNr, and this action is associated with a reduction of GABA release in the ventromedial thalamus, thus decreasing the impairment of motor function (Marti et al., 2007; 2008). These reports lead us to postulate that elevated release of N/OFQ from SNr neurons after MPTP treatment inhibits local GABA release from SNr neurons via the dense SNr axon collateral network, thus disinhibiting the STN glutamatergic pathway to SNr and exacerbating the motor symptoms resulting from the depletion of DA (Marti et al., 2007). NOPr antagonists are effective in reducing motor impairments in MPTP-induced Parkinsonism even when given 7 days after the MPTP treatment (Viaro et al., 2008). Elevated release of endogenous N/OFQ after MPTP treatment must therefore be exacerbating the motor impairment induced by the loss of DA neurons, probably by modulating the balance of inhibitory and excitatory transmitters regulating the residual SNc DA neurons. Persistent local elevation of glutamate release in SNr from the STN pathway after MPTP treatment may also play a part in the loss of SNc DA neurons in view of the high sensitivity of these neurons to excitotoxicity (Nafia et al., 2008). Finally, it is also possible that elevated local release of N/OFQ after MPTP inhibits SN GABA neurons that directly inhibit the SNc DA neurons (Hajos & Greenfield, 1994), again leaving the DA neurons more sensitive to glutamate-induced excitotoxicity.
In conclusion, we suggest that the increase in expression of ppN/OFQ mRNA and a resulting increase in the release of N/OFQ from SNr GABA neurons after MPTP treatment facilitates the loss of SNc DA neurons that follows as a consequence of the initial DA depletion induced by MPTP. These results support earlier results suggesting that sustained antagonism of endogenous N/OFQ might provide symptomatic relief in PD and slow the loss of SN DA neurons.
ACKNOWLEDGMENTS
We thank Dr. Fereshteh Nugent and Dr. David Turk for their critical readings of earlier drafts of the manuscript, and Julia Silveira BS and Gregory Bull, BS, for their assistance with the microscopy analyses of the VTA, DK and DG. The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the Government of the United States. All animal studies were approved by the USUHS Institutional Animal Care and Use Committee, and were conducted in accordance with the NRC Guide to the Care and Use of Laboratory Animals (National Academy Press, Washington DC 1996). This work was supported by funding from the National Institute on Drug Abuse, National Institutes of Health, grant number DA 03102.
ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- ABC
avidin-biotin complex
- ANOVA
analysis of variance
- AP
anterior to posterior location
- CPu
caudate-putamen
- DA
dopamine
- DAPI
4′-6-diamidino-2-phenylindole (a nuclear stain)
- DG
dentate gyrus of hippocampus
- DK
nucleus of Darkschewitsch
- DTT
dithiothreitol
- MPP+
1-methyl-4-phenyl-2,3-dihydropyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mRNA
messenger RNA
- NeuN
a neuron nuclei-specific immunochemical marker (Neuronal Nuclei)
- N/OFQ
nociceptin/orphanin FQ
- NOPr
nociceptin/orphanin FQ receptors (also known as ORL-1), a member of the opioid family of G protein coupled receptors
- ORL-1
opioid-like receptor-1 (see NOPr)
- PBS
phosphate buffered saline
- PD
Parkinson’s disease
- ppN/OFQ
prepro-nociceptin/orphanin FQ (the precursor peptide)
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- SSC
standard sodium citrate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmad SO, Park J-H, Stenho-Bittel L, Lau Y-S. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-treated mice. Neurosci Let. 2009;450:102–105. doi: 10.1016/j.neulet.2008.11.065. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, Juncos JL, Maragoto C, Guridi J, Litvan I, Tolosa ES, Koller W, Vitek J, DeLong MR, Obeso JA. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog in Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PAJ, Bevan MD. Synaptic organization of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Gouty S, Iyer V, Rosenberger J, Cox BM. Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J Neurochem. 2006;98:495–505. doi: 10.1111/j.1471-4159.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- Buzas B, Rosenberger J, Cox BM. Activity and cAMP-dependent regulation of nociceptin/orphanin FQ gene expression in primary neuronal and astrocyte cultures. J Neurochem. 1998;71:556–563. doi: 10.1046/j.1471-4159.1998.71020556.x. [DOI] [PubMed] [Google Scholar]
- Buzas B, Rosenberger J, Kim K-W, Cox BM. Inflammatory mediators increase the expression of nociceptin/orphanin FQ in rat astrocytes in culture. Glia. 2002;39:237–246. doi: 10.1002/glia.10106. [DOI] [PubMed] [Google Scholar]
- Cox BM, Borsodi A, Caló G, et al. [Accessed on 2010-03-07];Opioid receptors. IUPHAR database (IUPHAR-DB) 2009 Last modified on 2009-10-29. http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=50.
- Di Benedetto M, Cavina C, D”Addario C, Leoni G, Candeletti S, Cox BM, Romualdi P. Alterations of N/OFQ and NOP receptor gene expression in the substantia nigra and caudate putamen of MPP+ and 6-OHDA lesioned rats. Neuropharmacology. 2009;56:761–767. doi: 10.1016/j.neuropharm.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK. The neurotoxoin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration. 1996;5:299–312. doi: 10.1006/neur.1996.0041. [DOI] [PubMed] [Google Scholar]
- Hajós M, Greenfield SA. Synaptic connections between pars compacta and pars reticulata neurons: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 1994;660:216–224. doi: 10.1016/0006-8993(94)91292-0. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nature Rev Drug Disc. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Chen Y, Tan AM, Murphy NP, Leslie FM. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport. 2002;13:1137–1140. doi: 10.1097/00001756-200207020-00013. [DOI] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau J-M. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci. 2003;23:5247–5257. doi: 10.1523/JNEUROSCI.23-12-05247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Thierry AM, Glowinski J, Deniau J-M. Dendritic arborizations of the rat substantia nigra pars reticulata neurons: spatial organization and relation to the lamella compartmentation of striato-nigral projections. J Neurosci. 2001;21:6874–6888. doi: 10.1523/JNEUROSCI.21-17-06874.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Guerrini R, Beani L, Bianchi C, Morari M. Nociceptin/orphanin FQ receptors modulate glutamate extracellular levels in the substantia nigra pars reticulata. A microdialysis study in the awake freely moving rat. Neurosci. 2002;112:153–160. doi: 10.1016/s0306-4522(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Marti M, Mela F, Veronesi C, Guerrini R, Salvadori S, Federici M, Mercuri NB, Rizzi A, Franchi G, Beani L, Bianchi C, Morari M. Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J Neurosci. 2004a;24:6659–6666. doi: 10.1523/JNEUROSCI.0987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Mela F, Guerrini R, Caló G, Bianchi C, Morari M. Blockade of nociceptin/orphanin FQ transmission in rat substantia nigra reverses haloperidol-induced akinesia and normalizes nigral glutamate release. J Neurochem. 2004b;91:1501–1504. doi: 10.1111/j.1471-4159.2004.02843.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S, Guerrini R, Romualdi P, Candeletti S, Simonato M, Cox BM, Morari M. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J Neurosci. 2005;25:9591–9601. doi: 10.1523/JNEUROSCI.2546-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Trapella C, Morari M. The novel nociceptin/orphanin FQ receptor antagonist Trap-101 alleviates experimental Parkinsonism through inhibition of the nigro-thalamic pathway: positive interaction with L-DOPA. J Neurochem. 2008;107:1683–1696. doi: 10.1111/j.1471-4159.2008.05735.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Trapella C, Viaro R, Morari M. The nociceptin/orphanin FQ receptor antagonist J-113397 and L-DOPA additively attenuate experimental parkinsonism through overinhibition of the nigrothalamic pathway. J Neurosci. 2007;27:1297–1307. doi: 10.1523/JNEUROSCI.4346-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Viaro R, Guerrini R, Franchi G, Morari M. Nociceptin/orphanin FQ modulates motor behavior and primary motor cortex output through receptors located in substantia nigra reticulata. Neuropharmacol. 2009;34:341–355. doi: 10.1038/npp.2008.56. [DOI] [PubMed] [Google Scholar]
- Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- Nafia I, Re DB, Masmejean F, Melon C, Kachidian P, Kerkerian-LeGoff L, Nieoullon A, Had-Aissouni H. Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J Neurochem. 2008;105:484–496. doi: 10.1111/j.1471-4159.2007.05146.x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker H-P, Civelli O, Watson SJ. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- New DC, Wong YH. The ORL1 receptor: molecular pharmacology and signaling mechanisms. Neurosignals. 2002;11:197–212. doi: 10.1159/000065432. [DOI] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- Nothacker H-P, Reinscheid RK, Mansour A, Henningsen RA, Ardati A, Monsma FJ, Jr, Watson SJ, Civelli O. Primary structure and tissue distribution of the orphanin FQ precursor. Proc Natl Acad Sci USA. 1996;93:8677–8682. doi: 10.1073/pnas.93.16.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed Academic Press; San Diego: 2001. [Google Scholar]
- Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NK-κB. J Neurochem. 2001;79:35–44. doi: 10.1046/j.1471-4159.2001.00520.x. [DOI] [PubMed] [Google Scholar]
- Viaro R, Sanchez-Pernaute R, Marti M, Trapella C, Isacson O, Morari M. Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonsism. Neurobiol of Dis. 2008;30:430–438. doi: 10.1016/j.nbd.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta J, Buzas B, Cox BM. Traumatic brain injury induces nociceptin/orphanin FQ expression in neurons of the rat cerebral cortex. J Neurotrauma. 2003;20:523–532. doi: 10.1089/089771503767168456. [DOI] [PubMed] [Google Scholar]